Abstract
STUDY QUESTION
Is adult adiposity associated with early menopause?
SUMMARY ANSWER
Overall and abdominal adiposity were non-linearly associated with odds for early natural menopause with elevated odds observed among women who were underweight in early or mid-adulthood compared to lean-normal weight women.
WHAT IS KNOWN ALREADY
High and low adiposity have been associated with reproductive function and may potentially impact timing of menopause. It is unclear whether various aspects of adiposity are associated with risk of early menopause.
STUDY DESIGN, SIZE, DURATION
Prospective cohort study that examined data from 78 759 premenopausal women from the Nurses’ Health Study II who were followed from 1989 to 2011 for incidence of early natural menopause.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Participants were aged 25–42 years and premenopausal at baseline in 1989, when information on menopausal status, height and weight was reported via questionnaire. Information on menopausal status, type of menopause (natural, surgical, radiation/chemotherapy), hormone therapy use and weight was updated every two years along with information on smoking, physical activity and other behavioral and health-related factors. Multivariable logistic regression was used to estimate odds ratios for early menopause, defined as natural menopause before age 45 years, by aspects of adiposity.
MAIN RESULTS AND THE ROLE OF CHANCE
Early natural menopause was reported by 2804 participants. Body mass index (BMI) was non-linearly associated with risk for early menopause. Compared to women with BMI = 18.5–22.4 kg/m2, those with BMI < 18.5 kg/m2 had a significant 30% higher odds of early menopause (95% confidence interval (CI) = 1.08, 1.57), while women with BMIs between 25.0–29.9 kg/m2 had significant 21–30% lower odds. Odds were not higher in women with BMI ≥ 35.0 kg/m2 in fully adjusted analysis. Non-linear associations with higher odds in underweight women were also observed for age 18 and age 35 BMI, though lower odds for overweight women was only observed for age 35 BMI. Odds were highest among women with age 18 BMI < 18.5 kg/m2 reporting severe weight cycling.
LIMITATIONS, REASONS FOR CAUTION
Though weight and early menopause status were self-reported, validation studies conducted among Nurses’ Health Study participants suggest that self-reported weight is highly correlated with directly measured weight, and prospective self-reported menopausal status is highly reproducible. It is possible that underweight women may have been misclassified with an earlier age at menopause if being underweight led to amenorrhea.
WIDER IMPLICATIONS OF THE FINDINGS
In one of the few studies to prospectively examine a variety of adiposity measures and risk for early menopause, our findings that women who were underweight in early or mid-adulthood had elevated risk for early menopause can assist in efforts to better understand the etiology of early menopause. Additional prospective research is needed to understand how low adiposity may physiologically impact timing of menopause.
STUDY FUNDING/COMPETING INTEREST(S)
This study was conducted with funding from NIH UM1CA176726 and R01HD078517. The authors declare no conflicts of interest.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: early menopause, adiposity, body mass index, weight, weight change
Introduction
Up to 10% of women experience early menopause, defined as the cessation of ovarian function before age 45 (Pelosi et al., 2015). Early menopause is associated with higher risk of cardiovascular disease, cognitive decline, osteoporosis and premature mortality (van Der Voort et al., 2003; Shuster et al., 2010; Gold, 2011; Wellons et al., 2012). In addition, early fertility decline has family planning consequences as women increasingly delay childbearing (Burger et al., 2007; Broekmans et al., 2009). Though genetic factors partially account for increased risk of early menopause, modifiable lifestyle, reproductive and environmental risk factors may also play a role (Gold, 2011; Pelosi et al., 2015).
Adiposity is associated with reproductive function and potentially also timing of menopause, yet findings of studies examining overall adiposity (e.g. body mass index [BMI], relative weight) and age at menopause have been inconsistent. Some have found earlier age at menopause among underweight women (Hardy et al., 2008). Others have found higher BMI associated with earlier menopause (Dratva et al., 2009) or ovarian insufficiency (Luborsky et al., 2003), while some report no association (Bromberger et al., 1997; Gold et al., 2001; Aydin, 2010). A recent meta-analysis found modest associations between being underweight and earlier age at menopause, and being overweight or obese and later age at menopause (Tao et al., 2015). However, the majority of studies included were limited by their cross-sectional design, with the authors noting the need for more studies better controlled for smoking (Tao et al., 2015).
Adiposity at different ages (e.g. early versus mid-adulthood) may have different effects on early menopause risk, because of changes in reproductive function over time, yet, few studies have directly examined this (Hardy et al., 2008). In addition, weight distribution is important to consider as it is more closely related to metabolic and cardiovascular conditions than overall adiposity (Cornier et al., 2011). Furthermore, weight change and weight cycling may be associated with menopause timing, though few studies have considered these (Bromberger et al., 1997; Dorjgochoo et al., 2008; Aydin, 2010), especially in the context of overall adiposity.
To better understand these relations, we prospectively examined associations of overall adiposity, weight distribution and weight change and early natural menopause among women participating in the Nurses’ Health Study 2 (NHS2). In addition, we compared associations between overall adiposity in early and mid-adulthood and early menopause.
Materials and Methods
Study design and population
The NHS2 is a prospective epidemiological study of 116 430 registered nurses aged 25–42 from 11 US states who responded to a mailed questionnaire in 1989. Participants provided information on their medical history and health-related behaviors, such as smoking and physical activity. Participants have completed questionnaires every two years to update information on health factors and to provide information on new disease diagnoses with a response rate for each questionnaire cycle ≥89%. The study protocol was approved by the Institutional Review Board at the Harvard Chan School of Public Health and Brigham and Women's Hospital.
Assessment of early menopause
The current analysis was limited to women who indicated that they were premenopausal at baseline in 1989 (n = 108 542; Fig. 1). On the initial questionnaire and each subsequent biennial questionnaire, participants were asked to report their menopausal status by indicating if their periods had ceased permanently, and if so, the age they ceased and the cause (natural, surgery, radiation, chemotherapy). Age at menopause was defined as age at which the menstrual cycle ceased, as confirmed by 12 subsequent consecutive months of amenorrhea. For the few women who inconsistently reported postmenopausal status (i.e. reported being premenopausal after reporting postmenopausal status), we defined age at menopause as age at which periods ceased followed by consistent reporting of this cessation on at least three consecutive questionnaires. Women were also asked to report on their hormone replacement therapy use on each questionnaire, including duration and type of use, which was used to categorize hormone therapy as prior to menopause, following menopause, or both.
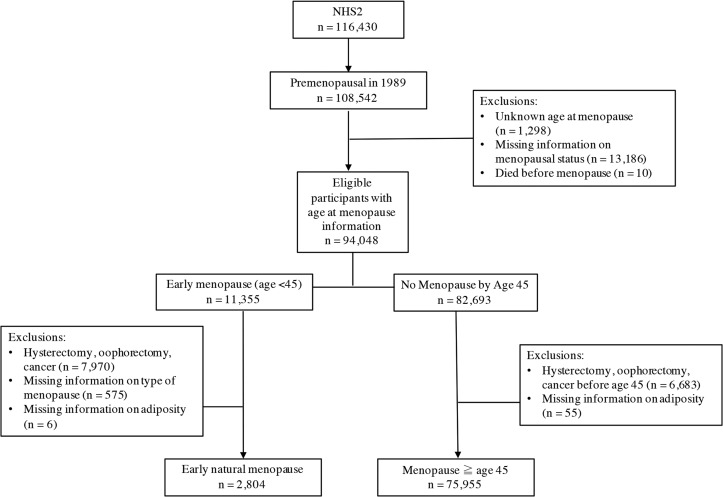
Figure 1.
Participant flow chart > NHS = Nurses Health Study.
Early natural menopause was defined as naturally occurring menopause before age 45, between return of the baseline questionnaire and June 2011 (i.e. the year in which the youngest NHS2 participants reached age 45). Women reporting menopause before age 45 and who had hysterectomy, bilateral oophorectomy, radiation or chemotherapy prior to menopause were excluded (n = 7970; Fig. 1). In addition, women who did not report menopause before age 45, but who had bilateral oophorectomy, hysterectomy or cancer (not including non-melanoma skin cancer) prior to age 45 were excluded (n = 6683) to maximize the comparability of comparison groups.
Assessment of adiposity
Weight at age 18 (lbs.), current weight, and height were reported at baseline. Current weight was then reported on each biennial questionnaire. BMI was calculated as weight (kg)/height (m)2. We then divided women into seven categories: <18.5 kg/m2, 18.5–22.49, 22.5–24.99, 25.0–27.49, 27.5–29.99, 30.0–34.99, ≥35.0. Underweight at age 18 was further subcategorized to <17.5 kg/m2 and 17.5–<18.5 kg/m2 to reflect the lower underweight cut-point of 17.5 kg/m2 for age 18 females recommended by the U.S. Centers for Disease Control and Prevention (Anonymous, 2014). Weight change from age 18 to 35 was calculated by subtracting age 18 weight from age 35 weight. In 1993, women were asked to measure and report their waist circumference at their navel and their hip circumference at the widest part (including buttocks) to the nearest 0.25 inch. Waist-to-hip ratio was calculated by dividing waist circumference by hip circumference. In 1993, women were also asked to report how many times they had intentionally lost 5–9, 10–19, 20–49 and ≥50 pounds between ages 18–30 excluding illness and pregnancy-related changes. We defined severe weight cyclers as women who lost ≥20 pounds three or more times between ages 18 and 30, and mild weight cyclers as women who did not meet this criteria, but lost ≥10 pounds three or more times during this timeframe (Field et al. 2009).
Covariates
Factors potentially associated with early menopause and adiposity were assessed as covariates. Information on age, pack-years of smoking, parity, and oral contraceptive (OC) use was collected and updated biennially. Age at menarche (1989), race/ethnicity (2005) and income (2001) were measured at a single time point over the course of the study. Dietary micro and macronutrient intake was assessed with a food frequency questionnaire (FFQ) in 1991, 1995, 1999, 2003, 2007 and 2011. Alcohol consumption was assessed on the initial questionnaire and on each FFQ. Breastfeeding history was assessed from all cohort members in 1997, and again in 2003 from the subgroup of women reporting pregnancies after 1997. Physical activity was assessed in 1989, 1991, 1997, 2005 and 2009, when participants were asked to indicate how much time they spent each week participating in a variety of recreational physical activities. This information was used to calculate metabolic equivalent task (MET)-hours per week (Ainsworth et al. 1993).
Statistical analysis
All statistical analyses were conducted with SAS version 9.4 (SAS Institute, Inc., Cary, NC). We compared age-standardized baseline characteristics between BMI categories. Logistic regression was used to estimate the odds ratio of early menopause for each BMI category, using lean-normal weight women (i.e. BMI 18.5–22.5 kg/m2) as the referent. Odds ratios (OR) and 95% confidence intervals (CIs) were calculated for BMI at baseline (1989) to assess the overall association between BMI and early menopause. For illustrative purposes, restricted cubic spline models were used to visualize the adjusted associations between early menopause and baseline BMI. Associations between waist circumference (n = 35 477) and waist-to-hip ratio (n = 35 522) and early menopause were also assessed among women who were premenopausal when this information was gathered to determine if central adiposity was associated with risk.
In sensitivity analyses, we used Cox proportional hazard regression to assess hazard ratios for early menopause by baseline BMI and by BMI updated throughout follow-up. Accrual of participant follow-up time (in months) began on the date of return of the 1989 questionnaire and continued until menopause or first report of hysterectomy, bilateral oophorectomy, cancer (not including non-melanoma skin cancer), loss to follow-up, death or 45th birthday. Analyses were stratified on age in months and questionnaire cycle.
Multivariable logistic regression included covariates selected a priori as factors associated with early menopause and/or BMI. Because the inclusion of other covariates in regression models did not substantively change the OR, we included only these a priori factors: age, pack-years cigarette smoking, physical activity, alcohol consumption, age at menarche, OC use, parity and breastfeeding duration. In addition, as reproductive variables such as parity may lie on the causal pathway between BMI and early menopause, we ran separate multivariable models adding these variables. To examine whether central adiposity was independently associated with early menopause risk, we additionally adjusted for BMI.
We then compared associations of BMI at ages 18 and 35 with early menopause. To maximize comparability of results across time periods, we limited this analysis to women premenopausal at age 35 who had BMI measures for both ages (n = 35 359).
We also examined whether risk of early menopause was associated with weight change from age 18 to age 35, and with weight cycling between ages 18 and 30; the weight cycling analysis was also limited to women who were premenopausal in 1993. In post hoc analysis, we stratified the analysis of weight cycling and early menopause by age 18 BMI to assess whether associations varied by early adulthood adiposity.
Because start of hormone therapy use could potentially precede the final menstrual period and lead to misclassification of early menopause, we conducted a sensitivity analysis excluding all women who reported hormone therapy use before age 45 if use was also prior to menopause (n = 12 216 excluded). We also conducted a sensitivity analysis excluding women who reported menopause between the ages of 45–47 (n = 10 298 excluded) to assess whether findings were impacted by potential misclassification of age at menopause. Finally, we conducted a sensitivity analysis excluding women who had gastrointestinal and connective tissue conditions that may be associated with both early menopause and adiposity (ulcerative colitis, Crohn's disease, rheumatoid arthritis and systemic lupus; n = 2655) to assess confounding.
Because some studies suggest that smoking may modify associations between adiposity and age at menopause (Willett et al. 1983) we assessed interaction between smoking status (ever smoker, non-smoker) and baseline BMI using the likelihood ratio test, and stratified associations between baseline BMI and early menopause by smoking status. We also assessed interaction between physical activity (<3 MET*h/week, ≥3–<49 MET*h/week, ≥49 MET*h/week) and baseline BMI.
Results
After exclusions, 78,759 participants had early natural menopause or had menopause at age ≥ 45 and had information on adiposity. Of these, 2804 had early natural menopause (Fig. 1). Baseline characteristics are presented by BMI in Table I. The mean age at baseline was 34.8 years (SD 4.6). Age was positively associated with BMI, while age at menarche and physical activity were inversely associated with adiposity. Alcohol consumption, duration of OC use, and duration of breastfeeding were lowest among obese women. Underweight women and obese women with BMI ≥ 35 kg/m2 had the lowest parity and were more likely to have been non-smokers.
Table I.
Age-standardized characteristics of the study population of premenopausal nurses in 1989 by BMI status, Nurses’ Health Study II, 1989–2011a. Data are mean (SD) or percent.
| Characteristic | BMI | ||||||
|---|---|---|---|---|---|---|---|
| <18.5 (n = 2650) | 18.5–22.4 (n = 35 539) | 22.5–25.0 (n = 17 581) | 25.0–27.4 (n = 9745) | 27.5–29.9 (n = 4413) | 30.0–34.9 (n = 5145) | ≥35.0 (n = 3237) | |
| Age | 33.3 (4.6) | 34.3 (4.6) | 35.0 (4.5) | 35.3 (4.5) | 35.6 (4.4) | 35.8 (4.4) | 36.0 (4.2) |
| Physical activity, 1989 (MET*h/week) | 29.3 (45.2) | 27.2 (38.5) | 24.0 (34.1) | 21.6 (31.8) | 19.7 (28.2) | 19.3 (30.4) | 15.0 (25.1) |
| Cigarette smoking (total pack-years)b | 11.6 (8.8) | 10.7 (7.9) | 11.5 (8.0) | 11.9 (8.2) | 12.2 (8.4) | 12.3 (8.4) | 12.8 (8.7) |
| Alcohol consumption (gm/day) | 3.0 (6.1) | 3.4 (5.8) | 3.2 (5.9) | 2.8 (5.8) | 2.4 (5.2) | 2.1 (5.5) | 1.7 (4.5) |
| Oral contraceptive use (total duration, months) | 42.2 (46.0) | 44.8 (45.4) | 45.5 (45.8) | 43.8 (45.1) | 42.5 (45.0) | 41.2 (43.2) | 37.8 (43.3) |
| Parityc | 2.0 (0.9) | 2.1 (0.9) | 2.1 (0.9) | 2.1 (0.9) | 2.1 (1.0) | 2.1 (0.9) | 2.0 (0.9) |
| Age at menarche | 13.1 (1.5) | 12.6 (1.4) | 12.4 (1.4) | 12.2 (1.4) | 12.1 (1.4) | 11.9 (1.4) | 11.8 (1.4) |
| Breastfeeding (total duration, months)c | 13.4 (14.0) | 14.0 (13.6) | 13.2 (13.5) | 12.7 (13.2) | 11.8 (13.1) | 11.2 (12.6) | 9.6 (11.8) |
| % | % | % | % | % | % | % | |
| Race/ethnicity | |||||||
| White, non-Hispanic | 92.8 | 94.7 | 93.9 | 93.6 | 93.5 | 94.0 | 94.0 |
| Cigarette smoking status | |||||||
| Never | 68.7 | 66.2 | 64.3 | 64.6 | 65.8 | 65.5 | 67.2 |
| Past | 16.2 | 21.9 | 23.0 | 22.3 | 21.6 | 21.5 | 21.0 |
| Current | 15.2 | 11.9 | 12.8 | 13.1 | 12.6 | 12.9 | 11.7 |
| Oral contraceptive use status | |||||||
| Never | 19.4 | 16.4 | 16.4 | 17.8 | 18.7 | 19.8 | 24.9 |
| Past | 68.5 | 70.3 | 71.1 | 71.1 | 70.3 | 70.6 | 67.4 |
| Current | 12.1 | 13.2 | 12.5 | 11.1 | 11.1 | 9.7 | 7.7 |
aAll characteristics except age are standardized to the age distribution of study population in 1989. Values are means (standard deviation [SD]) or percentages. MET, metabolic equivalent tasks; BMI, body mass index.
bAmong smokers.
cAmong parous women.
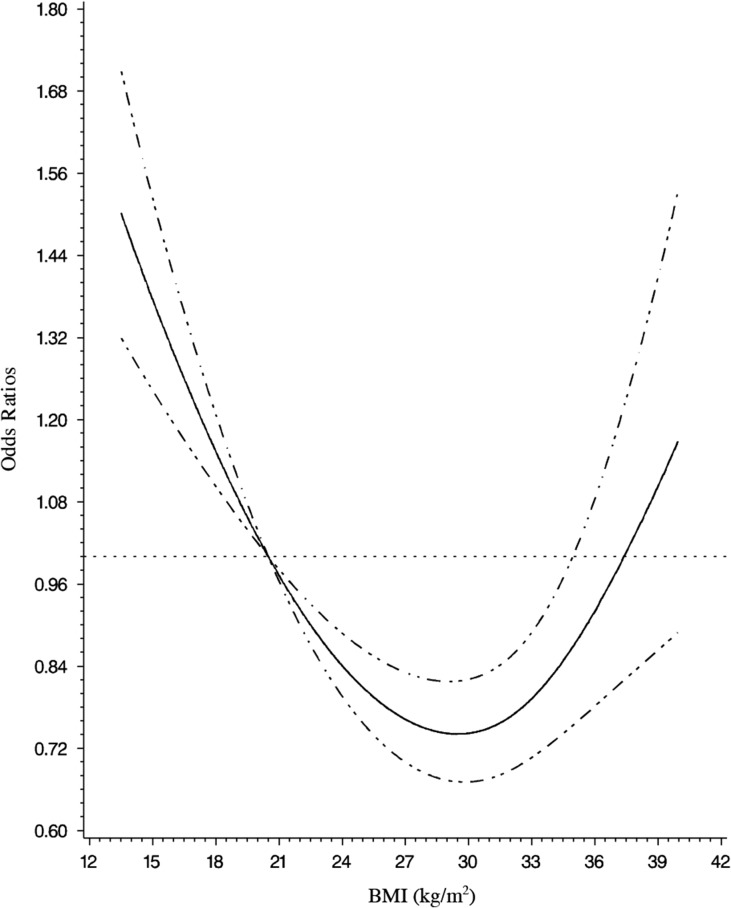
We observed a non-linear J-shaped association between BMI at baseline and risk of early menopause (Table II; Fig. 2). In age-adjusted and multivariable analyses, risk was significantly higher among underweight women (BMI < 18.5 kg/m2) compared with lean-normal weight women (BMI 18.5–22.4 kg/m2). Results were similar with and without adjustment for reproductive factors. In our fully adjusted model (Model 2), underweight women had 30% higher odds (95% CI = 1.08, 1.57), whereas, the odds were significantly lower among overweight women with BMI 25.0–27.4 kg/m2 (OR = 0.79, 95% CI = 0.69, 0.89) and BMI 27.5–29.9 kg/m2 (OR = 0.70, 95% CI = 0.58, 0.84) than lean-normal weight women. Similarly, obese women with a BMI of 30.0–34.9 kg/m2 had lower risk of early menopause compared to lean-normal weight women in fully adjusted analyses (OR = 0.83, 95% CI = 0.71, 0.98). Though BMI ≥ 35.0 kg/m2 had non-significantly elevated odds for early menopause in multivariable analysis that adjusted for nonproductive factors, the association was no longer elevated after adjustment for reproductive factors. Results from Cox proportional hazard regression models evaluating baseline BMI and BMI updated throughout follow-up were highly similar in shape and magnitude to our main analysis (Supplementary Table SI).
Table II.
Age-adjusted and multivariable odds ratios of early menopause by measures of adiposity, Nurses’ Health Study II, 1989–2011a.
| Adiposity measure | Total | Cases | Age-adjusted model | Multivariable-adjusted Model 1b | Multivariable-adjusted Model 2c | |||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| BMI, 1989 (kg/m2) | ||||||||
| <18.5 | 2650 | 125 | 1.30 | 1.07, 1.56 | 1.29 | 1.07, 1.56 | 1.30 | 1.08, 1.57 |
| 18.5–22.4 | 35 539 | 1299 | ref | ref | ref | |||
| 22.5–24.9 | 17 581 | 631 | 0.99 | 0.90, 1.09 | 0.96 | 0.87, 1.06 | 0.95 | 0.86, 1.04 |
| 25.0–27.4 | 9745 | 298 | 0.84 | 0.74, 0.95 | 0.81 | 0.71, 0.92 | 0.79 | 0.69, 0.89 |
| 27.5–29.9 | 4413 | 122 | 0.76 | 0.63, 0.92 | 0.73 | 0.60, 0.88 | 0.70 | 0.58, 0.84 |
| 30.0–34.9 | 5145 | 172 | 0.92 | 0.79, 1.09 | 0.89 | 0.76, 1.05 | 0.83 | 0.71, 0.98 |
| ≥35.0 | 3237 | 137 | 1.18 | 0.98, 1.42 | 1.13 | 0.94, 1.35 | 1.02 | 0.85, 1.23 |
| Waist circumference, 1993 (inches) | ||||||||
| <28.0 | 10139 | 325 | ref | ref | ref | |||
| 28.0–<30.0 | 7757 | 220 | 0.90 | 0.76, 1.08 | 0.89 | 0.75, 1.06 | 0.90 | 0.75, 1.07 |
| 30.0–<32.0 | 5774 | 154 | 0.87 | 0.71, 1.05 | 0.85 | 0.70, 1.03 | 0.84 | 0.69, 1.03 |
| 32.0–<36.0 | 6628 | 155 | 0.77 | 0.64, 0.94 | 0.74 | 0.61, 0.91 | 0.73 | 0.60, 0.89 |
| ≥36.0 | 5337 | 159 | 1.02 | 0.84, 1.23 | 0.97 | 0.80, 1.18 | 0.92 | 0.76, 1.12 |
| Waist-hip ratio, 1993 | ||||||||
| <0.75 | 12210 | 380 | ref | ref | ref | |||
| 0.75–<0.80 | 10669 | 300 | 0.90 | 0.77, 1.05 | 0.88 | 0.75, 1.02 | 0.89 | 0.76, 1.03 |
| 0.80–<0.85 | 6752 | 179 | 0.85 | 0.71, 1.02 | 0.81 | 0.68, 0.97 | 0.81 | 0.67, 0.97 |
| 0.85–<0.90 | 3474 | 79 | 0.74 | 0.58, 0.95 | 0.71 | 0.55, 0.91 | 0.70 | 0.55, 0.90 |
| ≥0.90 | 2372 | 74 | 1.03 | 0.80, 1.33 | 0.98 | 0.76, 1.26 | 0.96 | 0.74, 1.23 |
aBMI information in 1989; n = 78 310; waist circumference n = 35 635; waist-to-hip ratio n = 35 477.
bAdjusted for age at baseline (continuous), race/ethnicity (non-Hispanic White, Asian, Other), physical activity (<3, 3–49, >49 MET*h/week), pack-years cigarette smoking (0, 1–10, 10–15, >15 pack-years), alcohol consumption (0, 0–10, 10–30, ≥30 g of alcohol).
cModel 1 additionally adjusted for reproductive factors: oral contraceptive use and duration (nonuser, 1–71, ≥72 months), parity (0,1–2, ≥3 births), age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, ≥17 years of age), breastfeeding status and duration (0 −1, 1–3, 3–6, ≥6 months).
Figure 2.
Adjusted odds ratios of early menopause by BMI in 1989, multivariable odds ratios and 95% confidence intervals (CIs) of early natural menopause were derived from restricted cubic spline models. Data from Nurses Heatlth Study II 1989–2011, adjusted for age at baseline (continuous), race/ethnicity (non-Hispanic White, Asian, Other), physical activity (<3, 3–49, >49 metabolic equivalent task (MET)*h/week), pack-years cigarette smoking (0, 1–10, 10–15, >15 pack-years), alcohol consumption (0, 0–10, 10–30, ≥30 g of alcohol). The reference BMI is 20.5; a total of 21 knots were specified ranging from BMI of 17.7 to 37.5 with likelihood ratio tests comparing models with and without cubic spline terms to assess non-linearity yielding P < 0.0001.
When examining measures of central adiposity, relationships were also non-linear (Table II). Women with intermediate waist-to-hip ratios of 0.80–<0.85 and 0.85–<0.90 had a significantly lower risk of early menopause compared to women with the lowest waist-to-hip ratio (waist-to-hip ratio < 0.75). Risk was not higher among women with higher waist-hip ratio. Further adjustment for BMI only modestly attenuated results (Supplementary Table SII).
Table III presents results of analyses examining BMI at ages 18 and 35 as predictors of early menopause. Results differed somewhat by age. Women who were underweight at age 18 (<17.5 kg/m2) had a 50% higher odds compared to lean-normal weight women (OR = 1.54, 95% CI = 1.24, 1.90); however, odds were not elevated for BMI 17.5–18.5 kg/m2. When evaluating the effect of BMI at age 35 in fully adjusted models, compared to lean-normal weight women, odds were significantly higher among those who were underweight (<18.5 kg/m2) (OR = 1.59, 95% CI = 1.21, 2.09) and significantly lower among overweight women with BMI 27.5–29.9 (OR = 0.71, 95% CI = 0.54 0.92). Upon mutually adjusting for BMI at ages 18 and 35, the increased risk observed among underweight women was modestly attenuated but persisted at both ages (Supplemental Table SIII).
Table III.
Age-adjusted and multivariable odds ratios of early menopause by BMI at ages 18 and 35 and weight change between ages 18 and 35, Nurses’ Health Study II, 1989–2011a.
| Total | Cases | Baseline age-adjusted model | Multivariable-adjusted Model 1b | Multivariable Model 2c | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| BMI at age 18 (kg/m2) | ||||||||
| <17.5 | 1992 | 103 | 1.51 | 1.22, 1.87 | 1.50 | 1.22, 1.86 | 1.54 | 1.24, 1.90 |
| 17.5–18.4 | 3198 | 102 | 0.91 | 0.74, 1.13 | 0.91 | 0.74, 1.13 | 0.93 | 0.75, 1.15 |
| 18.5–22.4 | 21812 | 758 | ref | ref | ref | |||
| 22.5–24.9 | 4938 | 156 | 0.91 | 0.76, 1.08 | 0.89 | 0.75, 1.06 | 0.86 | 0.72, 1.02 |
| 25.0–27.4 | 1947 | 80 | 1.19 | 0.94, 1.51 | 1.16 | 0.92, 1.47 | 1.08 | 0.86, 1.38 |
| 27.5–29.9 | 641 | 27 | 1.22 | 0.82, 1.81 | 1.18 | 0.80, 1.75 | 1.07 | 0.72, 1.58 |
| 30.0–34.9 | 594 | 26 | 1.28 | 0.86, 1.90 | 1.25 | 0.84, 1.87 | 1.11 | 0.74, 1.66 |
| ≥35.0 | 237 | 13 | 1.60 | 0.91, 2.82 | 1.53 | 0.87, 2.69 | 1.34 | 0.76, 2.36 |
| BMI at age 35 (kg/m2) | ||||||||
| <18.5 | 1111 | 61 | 1.56 | 1.19, 2.05 | 1.56 | 1.19, 2.05 | 1.59 | 1.21, 2.09 |
| 18.5–22.4 | 15111 | 541 | ref | ref | ref | |||
| 22.5–24.9 | 7926 | 262 | 0.93 | 0.80, 1.08 | 0.91 | 0.79, 1.06 | 0.90 | 0.77, 1.04 |
| 25.0–27.4 | 4702 | 165 | 0.99 | 0.83, 1.18 | 0.97 | 0.81, 1.16 | 0.94 | 0.79, 1.13 |
| 27.5–29.9 | 2249 | 61 | 0.76 | 0.58, 1.00 | 0.74 | 0.57, 0.97 | 0.71 | 0.54, 0.92 |
| 30.0–34.9 | 2541 | 98 | 1.10 | 0.88, 1.37 | 1.07 | 0.86, 1.34 | 1.00 | 0.80, 1.25 |
| ≥35.0 | 1719 | 77 | 1.28 | 1.01, 1.64 | 1.26 | 0.99, 1.62 | 1.12 | 0.87, 1.43 |
| Weight change 18–35 (lbs.) | ||||||||
| lost ≥ 20.0 lbs. | 688 | 37 | 1.56 | 1.10, 2.21 | 1.48 | 1.04, 2.10 | 1.38 | 0.97, 1.96 |
| lost 5.0–19.9 lbs. | 3071 | 116 | 1.08 | 0.87, 1.34 | 1.06 | 0.86, 1.32 | 1.04 | 0.84, 1.30 |
| lost < 5 or gained up to 5.0 lbs. | 6468 | 234 | 1.04 | 0.87, 1.23 | 1.05 | 0.88, 1.24 | 1.05 | 0.88, 1.24 |
| gained 5.1–15.0 lbs. | 9172 | 320 | ref | ref | ref | |||
| gained 15.1–25 lbs. | 6112 | 206 | 0.97 | 0.81, 1.16 | 0.97 | 0.81, 1.16 | 0.96 | 0.81, 1.15 |
| gained 25.1–50 lbs. | 6833 | 232 | 0.99 | 0.83, 1.17 | 0.97 | 0.81, 1.15 | 0.96 | 0.80, 1.14 |
| gained > 50 lbs. | 3015 | 120 | 1.17 | 0.94, 1.45 | 1.15 | 0.93, 1.43 | 1.09 | 0.87, 1.35 |
aTotal n = 35 359; among women who had menopause ≥ age 35, and available data on BMI at age 18 and 35.
bAdjusted for age at baseline, race/ethnicity, physical activity in 1989, pack-years cigarette smoking in 1989 (pack-years at age 35 for age 35 BMI and weight change from 18 to 35), alcohol consumption 1989. See Table II footnote for categorization of covariates.
cAdditionally adjusted for reproductive factors:
BMI at age 18 additionally adjusted for age at menarche, total duration of OC use in 1989, parity in 1989 and breastfeeding in 1989.
BMI at age 35 and weight change additionally adjusted for age at menarche, duration of oc use at age 35, parity at age 35 and breastfeeding in 1989.
Women who lost 20 or more pounds from age 18 to 35 had marginally but not significantly higher risk for early menopause in fully adjusted models compared to women who gained 5.1–15.0 pounds (OR = 1.38, 95% CI = 0.97, 1.96) (Table III). Additionally, adjustment for age 18 BMI had minimal impact on results (Supplementary Table SIII).
We did not find weight cycling between ages 18 and 30 to be associated with early menopause in analyses including all women (Table IV). However, in post hoc analysis stratified by age 18 BMI, women with a BMI < 18.5 kg/m2 who were severe weight cyclers had more than double the odds of early menopause compared to noncyclers in adjusted analysis (OR = 2.40, 95% CI = 1.08, 5.33); of note is the small number of participants included in this analysis (n with early menopause = 7). No associations were observed between weight cycling and early menopause among normal weight or overweight/obese women at age 18.
Table IV.
Multivariable odds ratios of early menopause by weight cycling between ages 18–30, among all women and stratified by BMI at age 18, Nurses’ Health Study II, 1989–2011a.
| Total | Cases | Age-adjusted model | Multivariable-adjusted modelb | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Weight cycling between ages 18 and 30 | ||||||
| Noncycler | 53665 | 1901 | ref | ref | ||
| Mild cycler | 8232 | 281 | 0.96 | 0.85, 1.09 | 0.91 | 0.80, 1.03 |
| Severe cycler | 3412 | 141 | 1.18 | 0.99, 1.40 | 1.06 | 0.89, 1.27 |
| Weight cycling between ages 18–30 | ||||||
| BMI < 18.5 kg/m2 | ||||||
| Noncycler | 9006 | 354 | ref | ref | ||
| Mild cycler | 330 | 18 | 1.41 | 0.87, 2.29 | 1.31 | 0.80, 2.14 |
| Severe cycler | 76 | 7 | 2.47 | 1.13, 5.41 | 2.40 | 1.08, 5.33 |
| BMI 18.5–24.9 kg/m2 | ||||||
| Noncycler | 40827 | 1386 | ref | ref | ||
| Mild cycler | 6125 | 205 | 0.99 | 0.85, 1.15 | 0.94 | 0.81, 1.10 |
| Severe cycler | 2014 | 71 | 1.04 | 0.82, 1.33 | 0.96 | 0.75, 1.23 |
| BMI ≥ 25.0 kg/m2 | ||||||
| Noncycler | 3374 | 144 | ref | ref | ||
| Mild cycler | 1702 | 55 | 0.75 | 0.55, 1.03 | 0.75 | 0.55, 1.04 |
| Severe cycler | 1295 | 60 | 1.10 | 0.81, 1.50 | 1.09 | 0.80, 1.48 |
an = 65 309 with weight cycling information.
bMultivariable relative risks adjusted for age at baseline, race/ethnicity, physical activity, pack-years cigarette smoking, alcohol consumption, age at menarche, oral contraceptive use duration, parity and breastfeeding duration. See Table II footnote for categorization of covariates.
We observed similar J-shaped associations of BMI and early menopause in both smokers and non-smokers and interactions between BMI and smoking status were not significant (P = 0.34) (Table V). Among smokers, underweight women had significantly higher odds for early menopause (OR = 1.40, 95% CI = 1.03, 1.90), while odds were significantly lower among both categories of overweight women (ORBMI 25.0–27.4 = 0.70, 95% CI = 0.57, 0.86; ORBMI 27.5–30.0 = 0.58, 95% CI = 0.43, 0.80) when compared to lean-normal weight women. Risks were somewhat attenuated among non-smokers and not significant; underweight women had an OR of 1.22 (95% CI = 0.95, 1.55), while women with BMI of 27.5–29.9 had an OR of 0.80 (95%CI = 0.63, 1.02) when compared to lean-normal weight women. We also did not find effect modification of the association between BMI and early menopause by physical activity (P = 0.53).
Table V.
Age-adjusted and multivariable odds ratios of early menopause by BMI in 1989 stratified by smoking status, Nurses’ Health Study II, 1989–2011a.
| Adiposity measure | Total | Cases | Age-adjusted model | Multivariable-adjusted modelb | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Non-smokers | ||||||
| BMI, 1989 (kg/m2) | ||||||
| <18.5 | 1870 | 75 | 1.22 | 0.96, 1.55 | 1.22 | 0.95, 1.55 |
| 18.5–22.4 | 23677 | 772 | ref | ref | ||
| 22.5–24.9 | 11247 | 339 | 0.93 | 0.82, 1.06 | 0.92 | 0.80, 1.04 |
| 25.0–27.4 | 6232 | 179 | 0.89 | 0.76, 1.05 | 0.86 | 0.73, 1.02 |
| 27.5–29.9 | 2862 | 78 | 0.85 | 0.67, 1.08 | 0.80 | 0.63, 1.02 |
| 30.0–34.9 | 3323 | 100 | 0.95 | 0.76, 1.17 | 0.88 | 0.71, 1.09 |
| ≥35.0 | 2120 | 80 | 1.20 | 0.95, 1.51 | 1.06 | 0.83, 1.35 |
| Smokers | ||||||
| BMI, 1989 (kg/m2) | ||||||
| <18.5 | 777 | 50 | 1.48 | 1.09, 1.99 | 1.40 | 1.03, 1.90 |
| 18.5–22.4 | 11812 | 523 | ref | ref | ||
| 22.5–24.9 | 6310 | 291 | 1.05 | 0.90, 1.21 | 1.00 | 0.86, 1.15 |
| 25.0–27.4 | 3502 | 119 | 0.76 | 0.62, 0.94 | 0.70 | 0.57, 0.86 |
| 27.5–29.9 | 1543 | 44 | 0.64 | 0.47, 0.87 | 0.58 | 0.43, 0.80 |
| 30.0–34.9 | 1815 | 72 | 0.90 | 0.70, 1.16 | 0.78 | 0.61, 1.01 |
| ≥35.0 | 1116 | 57 | 1.18 | 0.89, 1.56 | 0.98 | 0.74, 1.31 |
aNon-smokers n = 51 331; smokers n = 26 875; interaction P = 0.34.
bAdjusted for age, race/ethnicity, physical activity, pack-years cigarette smoking until menopause, alcohol consumption, oral contraceptive use, parity, age at menarche and breastfeeding. See Table II footnote for categorization of covariates.
Findings were similar in sensitivity analyses excluding women who reported using hormone therapy prior to menopause and before age 45 (Supplementary Table SIV), excluding women who had menopause between the ages of 45–47 (Supplementary Table SV), and excluding women who had ulcerative colitis, Crohn's disease, rheumatoid arthritis and systemic lupus (Supplementary Table SVI).
Discussion
In our prospective study, we observed a non-linear, J-shaped association between BMI and risk of early natural menopause. Compared to lean-normal weight women with a BMI of 18.5–22.4 kg/m2, underweight women had significantly 30% higher odds of early menopause. In contrast, overweight women had a significant 21–30% lower odds. Intermediate abdominal adiposity was associated with lower risk when compared to the lowest level, which persisted after adjustment for BMI.
Ours is one of few prospective studies to assess a variety of adiposity measures and timing of menopause. Similar to our study, Hardy et al. found that being underweight at age 36 was associated with earlier age at menopause (HR = 1.30, 95% CI = 1.02, 1.65) in a prospective study of 1593 women (Hardy et al. 2008). Contrary to our findings, they did not observe increased risk among women who were underweight at younger ages, though this may be attributable to differences in the definition of underweight between studies. We also observed higher risk of early menopause among women with BMI ≥ 35 kg/m2 at age 18, though risk was attenuated and no longer significant after adjustment for reproductive factors. Hardy et al. examined risk among obese women as a whole (>30 kg/m2) and did not observe higher risk.
Our findings suggest that substantial weight loss may increase risk for early natural menopause. Women who lost ≥20 pounds from age 18 to 35 had a higher risk compared to women who gained 5–15 pounds, though findings were attenuated and marginally significant after adjusting for reproductive factors. In addition, weight cycling was also associated with higher risk. Our finding that weight loss may be associated with early menopause is consistent with the findings from several studies of age at menopause (Leidy, 1996; Kok et al., 2006).
Mechanisms underlying the association of underweight and early menopause are unclear. Low body weight increases risk for functional hypothalamic amenorrhea (FHA), which results from dysregulation of the hypothalamic-pituitary-gonadotropin (HPG) axis leading to anovulation, hypoestrogenism, and increased infertility risk (Meczekalski et al., 2014). While reproductive factors associated with fewer ovulatory cycles (e.g. greater parity, oral contraceptive use) have generally been associated with later menopausal age (Pelosi et al., 2015), it is possible that anovulation caused by HPG axis disruption is differentially associated with rate of reproductive aging. FHA also commonly leads to increased activation of the hypothalamic-pituitary-adrenal axis, leading to elevated levels of corticotrophin releasing hormone, aderenocorticotrophin, and cortisol (Meczekalski et al., 2014). It is possible that chronic elevation of these stress related hormones may increase early menopause risk, as some studies have found earlier age of menopause among women experiencing elevated stress levels (Bromberger et al., 1997).
Importantly, ‘underweight’ is a heterogeneous category that includes women with anorexia nervosa and women who are constitutionally thin, and some studies have found that amenorrhea and hormonal imbalances only occurred among women with anorexia nervosa (Estour et al., 2014). Unfortunately, we were unable to differentiate between these two groups in our study. Additionally, while irritable bowel syndrome and autoimmune disorders are potentially associated with BMI and with premature ovarian insufficiency (Pelosi et al., 2015), we did not observe evidence of confounding by these conditions.
Adult body size may be correlated with size at birth and consequently size of the ovarian pool, which is determined prenatally (Leidy, 1996). A smaller ovarian pool would potentially result in an earlier age of menopause. Additional studies should examine relationships between birth weight, early life adiposity and menopause timing.
We observed lower risk for early menopause among overweight women, which is consistent with findings from a meta-analysis by Tao et al. in which overweight women had a later menopausal age (Tao et al., 2015). Adipose tissue produces the endogenous estrogen estrone, which though produced at lower levels than estradiol in premenopausal years, at higher levels could potentially supplant declining estradiol levels in later reproductive years (Siiteri, 1975; Forney et al., 1981; Kershaw and Flier, 2004; Tao et al., 2015).
Conversely, we did not observe lower risk among women with BMI ≥ 35.0 kg/m2, especially for BMI measured at age 35. Premenopausal obesity is associated with anovulatory infertility, increased androgen production and decreased serum sex hormone binding-globulin level (Pasquali and Gambineri, 2006). In addition, some studies have found lower levels of Anti-Müllerian Hormone (AMH) among obese women compared to normal weight women (Freeman et al., 2007; Steiner et al., 2010), though others report no association (Bertrand et al., 2016; Jung et al., 2017). AMH, produced by granulosa cells in the ovary, is a marker of ovarian reserve highly correlated with antral follicle count (AFC) in older premenopausal women (van Rooij, Ilse et al., 2005; La Marca and Volpe, 2006). It is not clear what leads to lower AMH levels observed among obese women as a study by Su et al., found that AFC did not differ between obese and normal weight women despite lower AMH levels among obese women (Su et al., 2008). In addition, some studies have reported the highest AMH levels among women with the lowest BMI (Freeman et al., 2007; Bernardi et al., 2017); it remains unclear how AMH and AFC contribute to the higher risk we observed among underweight women.
In general, we did not find that the addition of reproductive risk factors (age at menarche, duration of OC use, parity, duration of breastfeeding) to regression models substantially affected ORs for adiposity and early menopause. However, the addition of these factors did attenuate the higher risk observed among women with BMI ≥ 35 kg/m2, and among those with large weight loss, suggesting that differences in these factors may explain some of the observed associations. We also adjusted for physical activity, which at high exercise levels has been found to be associated with menstrual cycle dysfunction and functional hypothalamic amenorrhea (Warren and Perlroth, 2001; Meczekalski et al., 2014). Women in our study generally had low to moderate levels of physical activity with very few exercising at levels that would disrupt menstrual cycle function.
Some studies have also found that associations between measures of adiposity and menopause timing are only found among smokers (Willett et al., 1983; Hardy et al., 2000). Compounds found in cigarette smoke may be toxic to the ovary and have been associated with both follicular loss and impaired follicular growth (Dechanet et al., 2011). Hardy et al. found that underweight smokers had the earliest age of perimenopause compared to other smokers, whereas no associations with BMI were observed among non-smokers (Hardy et al., 2000). We did not find that smoking status significantly modified the associations (P = 0.38), though the non-linear relation was more pronounced among smokers.
Our study has several potential limitations. First, BMI was self-reported, which could result in misclassification. However, as a validation study among Nurses’ Health Study participants found that prospectively gathered self-reported and measured weights were highly correlated (r = 0.97), we anticipate minimal misclassification of BMI (Rimm et al., 1990). Nonetheless, it is possible that a greater degree of misclassification occurred for age 18 BMI, because it was retrospectively reported at baseline, potentially biasing our findings to the null. Age at menopause was also self-reported. However, we do not anticipate that this resulted in misclassification that substantially impacted our findings because prospective reporting of menopause has been found to be highly reproducible (Colditz et al., 1987) and our findings were unchanged after excluding women who had menopause between the ages of 45 and 47.
Another potential source of misclassification of menopausal status is premenopausal hormone therapy use, which often leads to menstrual bleeding making it difficult to ascertain age at menopause. However, findings were unchanged after excluding women who reported using hormone therapy prior to menopause and before the age of 45. Yet, it is possible that underweight women with early menopause were more likely to be excluded in this analysis, as on average underweight women initiate hormone therapy at earlier ages (Hardy et al., 2008). It is also possible that underweight women may have been misclassified with an earlier age at menopause if their underweight status led to amenorrhea. Because our findings were similar in models that included and excluded hormone use, this is unlikely to have impacted our findings. However, it should be noted that our findings are specific to early natural menopause, and that associations and mechanisms may be different for early menopause occurring as a result of cancer, oophorectomy and/or underlying causes leading to surgical menopause.
We had limited power to assess whether associations varied by race/ethnicity because our study population was predominantly White. Studies have found that associations between adiposity and risk for health outcomes (WHO Expert Consultation, 2004; World Health Organization, 2008) and adiposity and age at menopause may vary by race/ethnicity (Gold, 2011). Additional studies are needed in diverse populations to assess whether associations vary by race/ethnicity.
Strengths of our study include the prospective design, large sample size, longitudinal assessment of BMI, assessment of both overall and abdominal measures of adiposity, and our adjustment for multiple confounders. In addition, this is among the first prospective studies to focus specifically on adiposity and early menopause, which has been associated with increased risk for cardiovascular disease and other health conditions.
Conclusion
In summary, our findings suggest that both overall and abdominal adiposity are non-linearly associated with risk of early natural menopause. Women who were underweight in early or mid-adulthood, especially those who reported severe weight cycling, had higher risk for early menopause compared to lean-normal weight women. Additional prospective research is needed to understand how low adiposity may physiologically impact the timing of menopause.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Authors’ roles
Conception and design of study: K.L.S., E.R.B.-J., B.W.W. J.E.M., S.E.H., B.A.R.; analysis and interpretation of data: K.L.S., E.R.B.-J., B.W.W., J.E.M., S.E.H., A.C.P.-S., M.E.B., B.A.R.; drafting of manuscript or revising critically for important intellectual content: K.L.S., E.R.B.-J., B.W.W., J.E.M., S.E.H., A.C.P.-S., M.E.B., B.A.R.; and final approval of version to be published: K.L.S., E.R.B.-J., B.W.W., J.E.M., S.E.H., A.C.P.-S., M.E.B., B.A.R.
Funding
This study was conducted with funding from NIH UM1CA176726 and R01HD078517.
Conflict of interest
None declared.
References
- Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, Paffenbarger RS. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- Aydin Z. Determinants of age at natural menopause in the Isparta Menopause and Health Study: premenopausal body mass index gain rate and episodic weight loss. Menopause 2010;17:494–505. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Carnethon M, de Chavez P, Ikhena D, Neff L, Baird D, Marsh E. Relationship between obesity and anti-Müllerian hormone in reproductive-aged African American women. Obesity (Silver Spring) 2017;25:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K, Baer H, Orav EJ, Klifa C, Kumar A, Hylton N, LeBlanc E, Snetselaar L, Van Horn L, Dorgan J. Early life body fatness, serum anti-müllerian hormone, and breast density in young adult women. Cancer Epidemiol Biomarkers Prev 2016;25:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev 2009;30:465–493. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol 1997;145:124–133. [DOI] [PubMed] [Google Scholar]
- Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women's Midlife Health Project. Hum Reprod Update 2007;13:559–565. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol 1987;126:319–325. [DOI] [PubMed] [Google Scholar]
- Cornier M, Després J, Davis N, Grossniklaus D, Klein S, Lamarche B, Lopez Jimenez F, Rao G St, Onge M, Towfighi A et al. . Assessing adiposity: a scientific statement from the American Heart Association. Circulation 2011;124:1996–2019. [DOI] [PubMed] [Google Scholar]
- Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, Hedon B, Dechaud H. Effects of cigarette smoking on reproduction. Hum Reprod Update 2011;17:76–95. [DOI] [PubMed] [Google Scholar]
- Dorjgochoo T, Kallianpur A, Gao Y, Cai H, Yang G, Li H, Zheng W, Shu X. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause 2008;15:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratva J, Gómez Real F, Schindler C, Ackermann Liebrich U, Gerbase M, Probst Hensch N, Svanes C, Omenaas E, Neukirch F, Wjst M et al. . Is age at menopause increasing across Europe? Results on age at menopause and determinants from two population-based studies. Menopause 2009;16:385–394. [DOI] [PubMed] [Google Scholar]
- Estour B, Galusca B, Germain N. Constitutional thinness and anorexia nervosa: a possible misdiagnosis? Front Endocrinol (Lausanne) 2014;5:175–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A, Malspeis S, Willett W. Weight cycling and mortality among middle-aged or older women. Arch Intern Med 2009;169:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney JP, Milewich L, Chen GT, Garlock JL, Schwarz BE, Edman CD, MacDonald PC. Aromatization of androstenedione to estrone by human adipose tissue in vitro. Correlation with adipose tissue mass, age, and endometrial neoplasia. J Clin Endocrinol Metab 1981;53:192–199. [DOI] [PubMed] [Google Scholar]
- Freeman E, Gracia C, Sammel M, Lin H, Lim L, Strauss J. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril 2007;87:101–106. [DOI] [PubMed] [Google Scholar]
- Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–874. [DOI] [PubMed] [Google Scholar]
- Gold E. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011;38:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R, Kuh D, Wadsworth M. Smoking, body mass index, socioeconomic status and the menopausal transition in a British national cohort. Int J Epidemiol 2000;29:845–851. [DOI] [PubMed] [Google Scholar]
- Hardy R, Mishra G, Kuh D. Body mass index trajectories and age at menopause in a British birth cohort. Maturitas 2008;59:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Allen N, Arslan A, Baglietto L, Brinton L, Egleston B, Falk R, Fortner R, Helzlsouer K, Idahl A et al. . Demographic, lifestyle, and other factors in relation to antimüllerian hormone levels in mostly late premenopausal women. Fertil Steril 2017;107:1012–1022.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw E, Flier J. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548–2556. [DOI] [PubMed] [Google Scholar]
- Kok H, van Asselt K, van der Schouw YT, van der Tweel I, PHM Peeters, PWF Wilson, Pearson P, Grobbee D. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol 2006;47:1976–1983. [DOI] [PubMed] [Google Scholar]
- La Marca A, Volpe A. Anti-Müllerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 2006;64:603–610. [DOI] [PubMed] [Google Scholar]
- Leidy LE. Timing of menopause in relation to body size and weight change. Hum Biol 1996;68:967–982. [PubMed] [Google Scholar]
- Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod 2003;18:199–206. [DOI] [PubMed] [Google Scholar]
- Meczekalski B, Katulski K, Czyzyk A, Podfigurna-Stopa A, Maciejewska-Jeske M. Functional hypothalamic amenorrhea and its influence on women's health. J Endocrinol Invest 2014;37:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Gambineri A. Metabolic effects of obesity on reproduction. Reprod Biomed Online 2006;12:542–551. [DOI] [PubMed] [Google Scholar]
- Pelosi E, Simonsick E, Forabosco A, Garcia Ortiz J, Schlessinger D. Dynamics of the ovarian reserve and impact of genetic and epidemiological factors on age of menopause. Biol Reprod 2015;92:130–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–473. [DOI] [PubMed] [Google Scholar]
- Shuster L, Rhodes D, Gostout B, Grossardt B, Rocca W. Premature menopause or early menopause: long-term health consequences. Maturitas 2010;65:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siiteri PK. Post-menopausal estrogen production. Front Horm Res 1975;3:40–44. [DOI] [PubMed] [Google Scholar]
- Steiner A, Stanczyk F, Patel S, Edelman A. Antimullerian hormone and obesity: insights in oral contraceptive users. Contraception 2010;81:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HI, Sammel M, Freeman E, Lin H, DeBlasis T, Gracia C. Body size affects measures of ovarian reserve in late reproductive age women. Menopause 2008;15:857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Jiang A, Yin L, Li Y, Tao F, Hu H. Body mass index and age at natural menopause: a meta-analysis. Menopause 2015;22:469–474. [DOI] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention Recommended BMI-for-Age Cut-Offs. 2014. Retrieved from https://www.cdc.gov/nccdphp/dnpao/growthcharts/training/bmiage/page4.html.
- van Der Voort DJM, van Der Weijer PHM, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int 2003;14:525–530. [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Broekmans FJ, Scheffer G, Looman CW, Habbema JDF, de Jong F, Fauser BJ, Themmen AP, te Velde E. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 2005;83:979–987. [DOI] [PubMed] [Google Scholar]
- Warren MP, Perlroth NE. The effects of intense exercise on the female reproductive system. J Endocrinol 2001;170:3–11. [DOI] [PubMed] [Google Scholar]
- Wellons M, Ouyang P, Schreiner P, Herrington D, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the multi-ethnic study of atherosclerosis. Menopause 2012;19:1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163. [DOI] [PubMed] [Google Scholar]
- Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH. Cigarette smoking, relative weight, and menopause. Am J Epidemiol 1983;117:651–658. [DOI] [PubMed] [Google Scholar]
- World Health Organization Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Geneva, SUI: World Health Organization, 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.