Abstract
Background:
Short arm deletions of the X-chromosome are challenging issues for genetic counseling due to their low penetrance in population. Female carriers of these deletions have milder phenotype than male ones, considering the intellectual ability and social skills, probably because of the X-chromosome inactivation phenomenon.
Case report:
A female patient with a 10Mb distal Xp deletion and an Xq duplication, showing mild intellectual disability, is described in this report. While the deletion arose from a maternal pericentric inversion, the duplication was directly transmitted from the mother who is phenotypically normal.
Conclusion:
This report underlines the usefulness of molecular cytogenetic technics in postnatal diagnosis.
Keywords: Xp deletion, Pericentric inversion, Array CGH, Classical cytogenetics, Genetic counseling, Xq duplication
1. Introduction
Pericentric chromosome inversions have usually no or minor effects on their carriers, unless the breakpoint disrupts a critical gene; the latter can lead to pathological consequences or abnormal phenotypes [1]. In human, pericentric inversions are found in a range of 1-2% of the general population [2]. A single crossing over between the inverted chromosome and its normal homolog within an inversion loop would lead to the formation of recombinant chromosomes (rec) with reciprocal terminal deletions and duplications; thus, the carrier of an inversion may produce chromosomally unbalanced gametes. Carriers of autosomal pericentric inversions in families with no history of recombinants have an overall risk of around 1% to give birth to an abnormal liveborn child, whereas this risk increases to 5-15% for families who have already had an abnormal child due to the rearrangement [3]. Pericentric inversions of chromosome X are relatively rare rearrangements with about 30 published families as summarized by Madariaga and Rivera (1997) [4].
Regarding the rec(X) phenotype, female subjects with lost Xp or Xq segments a short stature is typically seen in del(Xp), and ovarian failure is observed in del(Xq) [3]. In male individuals, beside short stature, small deletions of distal Xp can be associated with several genetic diseases including X-linked recessive chondrodysplasia punctata (MIM: 302950), X-linked mental retardation, X-linked Ichthyosis (MIM:308100), and Kallmann syndrome (MIM:308700). The short stature observed in del(Xp) individuals is due to haploinsufficiency of the PAR1 (Pseudoautosomal Region 1) gene SHOX (Short Stature Homeobox), and is on the one hand responsible for idiopathic short stature (MIM:300582) and also for the growth failure observed in Turner syndrome patients [5]. Defects in SHOX gene are also seen in Leri-Weill dyschondrosteosis (LWD, MIM:127300), a skeletal dysplasia characterized by short stature, mesomelia, and Madelung wrist deformity. Prenatal diagnosis of subtle X-chromosome structural abnormalities after ultrasound assessment of Intrauterine Growth Retardation (IUGR) is uncommon, although this finding has been reported by Yaegashi et al. (2000) who reviewed the birth records of a cohort of Turner syndrome patients [6].
Here, we report the clinical and molecular studies in a child diagnosed with partial deletion of chromosome Xp resulting from a pericentric inversion of chromosome X present in the mother.
2. Clinical report
We report on a term female born to unrelated healthy parents after a 39 weeks pregnancy by normal delivery. Her body weight was 2.550 g. (10th - 25th centile), body length 47cm (10th centile) and head circumference 33 cm (10th centile). Apgar scores were 8/1 min and 9/5min and her perinatal period was normal. Her motor milestones are reported normal as she sat unsupported at the age of 6 months and walked unaided at the age of 15 months, but her language development was delayed as she uttered her first words after the age of 3 years.
At the age of 6 years she was referred for developmental assessment because of speech and language delay. She was a sociable child with developmental delay, severe behavioral “contact-type”-disorders and mild dysmorphic facial / body features such as: frontal bossing, moderate micrognathia, short thin nose, small mouth with long flat philtrum, low set ears with auricle abnormalities, widely spaced nipples, broad thumbs, and long tapering fingers. She showed good ability for symbolic play, but her comprehension was limited to simple commands, while her speech was limited to “three-word phrases”. Her Developmental Quotient (D.Q.) was 55, which corresponds to moderate mental retardation level. On neurological examination she showed global trunk and limps hypotonia without focal neurological signs. Her height was 110cm (10th centile), weight 25 kg (75th percentile) and H.C. 50cm (10th percentile).
Laboratory investigation including, audiological, visual, biochemical, metabolic, endocrine (thyroid, GH, LH, FSH, ACTH, Prolactin), bone age, kidney/liver and triplex ultrasound proved normal. Brain MRI and electroencephalogram proved also normal.
3. Results
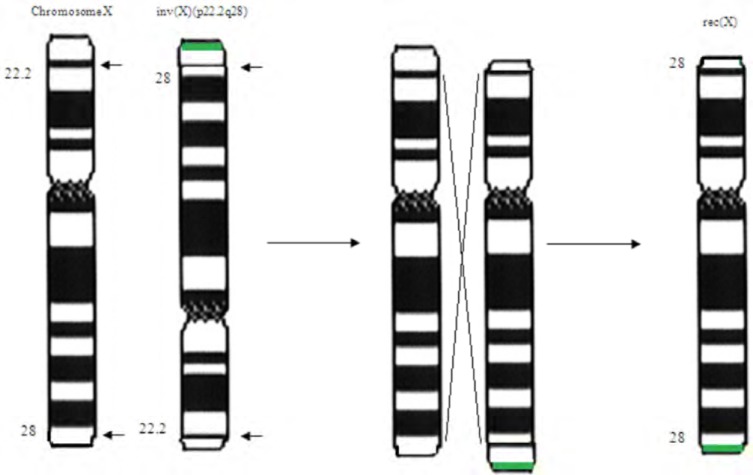
Cytogenetic analysis from lymphocytes according to standard procedures with GTG-banded chromosomes showed a 46,X,der(X) karyotype. Subsequent chromosome analysis of lymphocyte cultures of both parents was performed. Maternal karyotype showed an inversion in one X-chromosome with breakpoints at Xp22.1 and Xq28: 46,X,inv(X)(p22.1q28). The paternal karyotype was normal. Since the finding of an inverted X-chromosome in the maternal karyotype, it was suggested that the derivative chromosome in the child is a rec(X) chromosome with partial deletion of Xp and duplication of Xq (Fig. 1).
Fig. (1).
The possible mechanism of the rec(X) chromosome generation. The normal chromosome and the inverted one with breakpoints at p22.1q28 are shown at the left. A recombination between the normal and the invert chromosome had as a result the rec(X). The recombination should have happened in a region between the breakpoints of the inverted chromosome. The green line shows the maternal duplication which was inherited to the current patient.
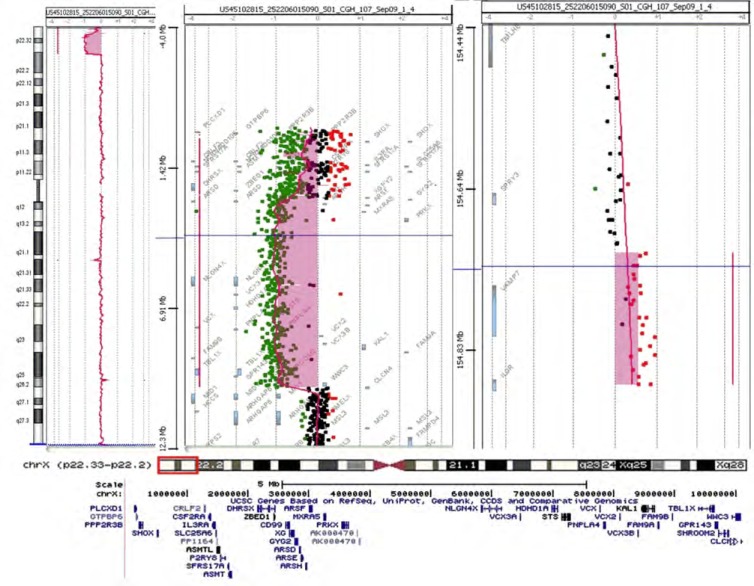
To better characterize the rearrangement and to evaluate the exact size of the Xp deletion, Array Comparative Genomic Hybridization (Array-CGH) analysis was performed on DNA extracted from peripheral blood of the patient and both her parents. Array-CGH was performed by using a 180K platform (Agilent Technologies, Santa Clara, CA) as previously reported [7]. Array-CGH analysis revealed a 10.1 Mb deletion of the Xp22.33-p22.2 region and a 161 kb duplication of Xq28 (Fig. 2): arr[hg19] Xp22.33-p22.2(61, 091-10,156,319)x1, Xq28(155,059,343-155,232,863)x3. The deleted Xp region included 42 transcribed genes (8 disease-associated genes). As to the Xq duplication, the same copy number gain was found in the mother and was considered not pathogenic on the basis of the gene content.
Fig. (2).
aCGH of the current patient (above). On the left side and in the middle is the deletion, while the duplication is on the right side. At the bottom are the genes deleted at the current patient.
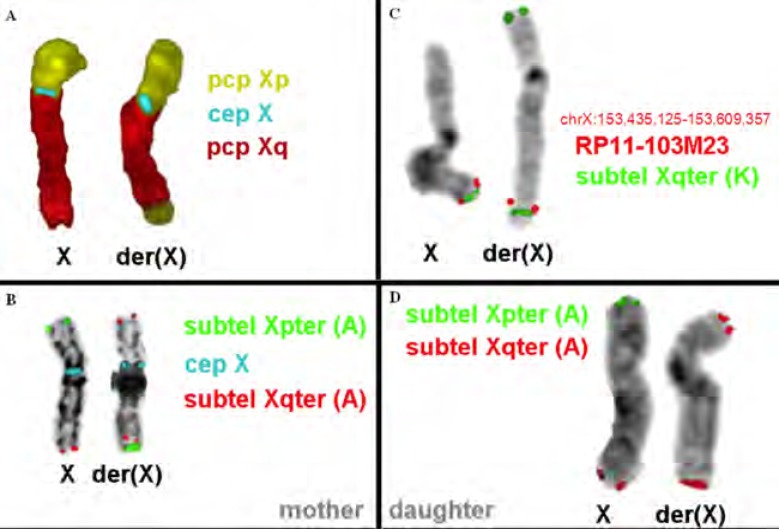
FISH experiments were performed on metaphase spreads for further confirmation of the diagnosis. Multicolor banding (MCB) for the X-chromosome was performed as previously described [8]. FISH analysis of metaphases with XYpter and XYqter subtelomeric probes (Kreatech) along with CEP X (Abbott Laboratories, IL) was performed according to the manufacturers’ instructions (Fig. 3). The experiments showed the absence of signal from the subtelomeric Xp probes from the patient’s rec(X), whereas it was present in the mother’s inv(X) chromosome (Fig. 3).
Fig. (3).
FISH analysis of the current patient’s and her mother’s X chromosome. (A) and (B) The normal and the derivative X chromosome of the mother are shown. The derivative chromosome is inverted and has a qter duplication. (C) and (D) The normal and the derivative X chromosome of the current patient are shown. The derivative chromosome has a qter duplication and a pter deletion.
4. Discussion
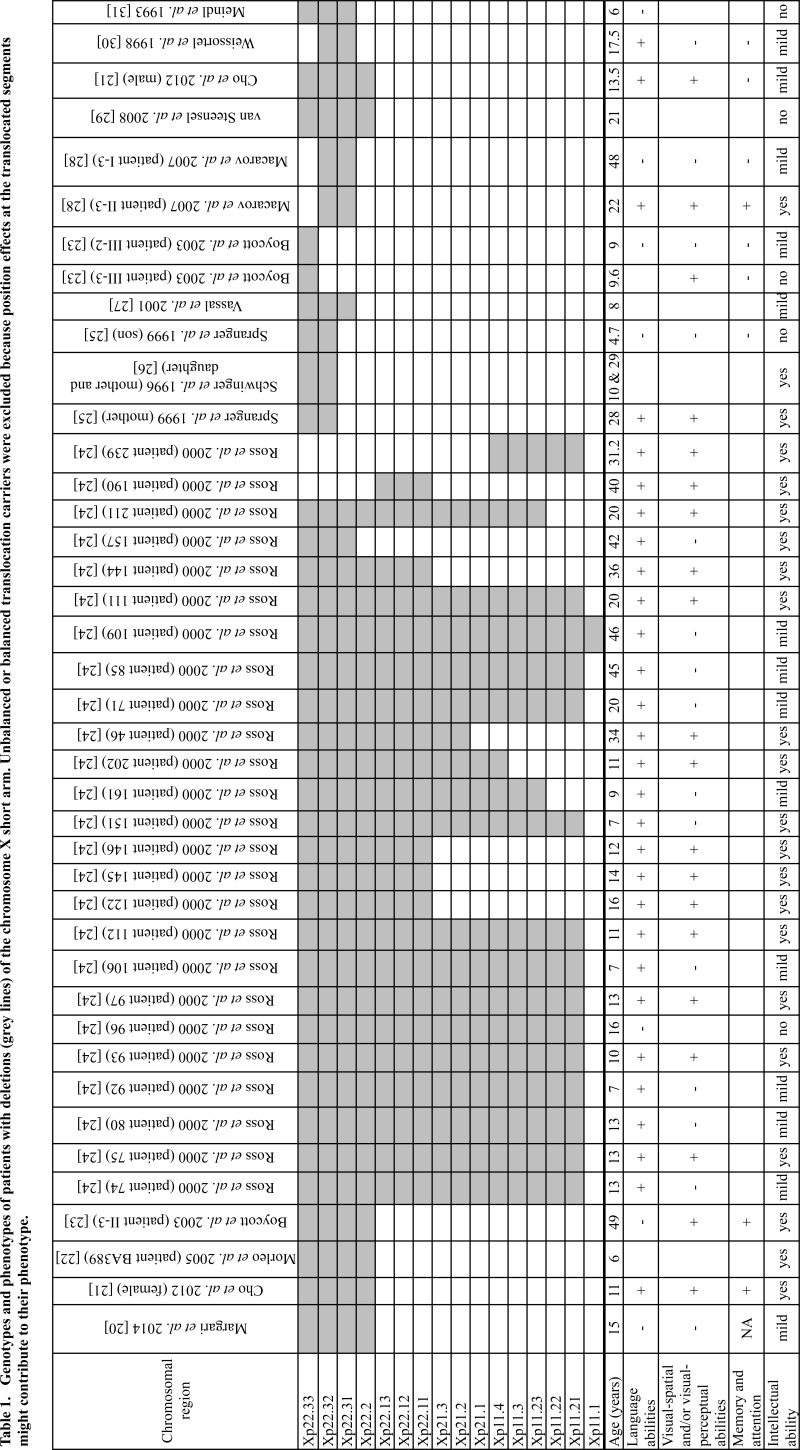
In this report, a female patient with a 10.1 Mb Xp22.3p22.2 deletion and a 161 kb inherited Xq28 duplication. The Xp22.3p22.2 deletion correspond to Xp22.3 microdeletion syndrome, which is a contiguous syndrome, encompassing Leri-Weill syndrome, X-linked recessive chondrodysplasia punctata, X-linked mental retardation with attention deficit hyperactivity disorder, X-linked ichthyosis, Kallmann syndrome, X-linked recessive ocular albinism and microthalmia with linear skin defects syndrome. Apart from her short stature and the mild intellectual disability, she does not present any characteristics of the previous syndromes. This is in agreement with other reports which present affected males and mildly affected females from this syndrome (Table 1 for X-linked mental retardation). Furthermore, more female than male patients were found in literature. Female patients have various deletions in extend, while none of the male patients have any deletion beyond Xp22.2. This suggests a lower penetrance of this syndrome to females than to males.
The low penetrance of Xp22.3 microdeletion syndrome in females is probably due to X-chromosome inactivation. Most X-linked maternally or paternally inherited genes are inactivated early in embryogenesis, and the maternal or the paternal X-chromosome is inactivated randomly in each cell. There is probably a strong selection of cells that inactivate the abnormal chromosome during embryonic development in tissues except from the brain; however the mechanism of this selection is still unknown.
Among the genes deleted in our patients, NLGN4 is related to X-linked intellectual disability and autism, [9, 10]. This gene encodes the synaptic cell adhesion protein Neuroligin-4, playing a role in synapse formation between neurons [11-13]. A loss-of-function mutation in the murine ortholog Nlgn4, causes highly selective deficits in reciprocal social interactions and communication, which are reminiscent of autism spectrum conditions in humans [14]. Our patient has mild mental retardation with autistic aspects probably due to the needs of both copies of NLGN4 for the correct formation of synapses in brain, as this gene is reported to escape X-inactivation [15, 16].
The current patient presented in utero growth deficiency at the third trimester of pregnancy. To date, only three prenatal cases have been documented in the literature. Chen et al. (2003) reported a 27-year-old woman who at routine antenatal care at 23 weeks’ gestation showed an in utero growth deficiency of 3 weeks [17]. Cytogenetic analysis of umbilical cord lymphocytes reveled a rearranged X chromosome: 46,X,der(X)(qter->q22.1::p22.3->qter) de novo. During referral at 34 weeks’ gestation, level II sonographic examinations revealed a single live female fetus with a biparietal diameter of 7.3 cm (equivalent to 28 weeks’ gestation). In this case several clinical sings were evident such as IUGR, short stature and developmental delay (at the age of 6 months). Orellana et al. (2006) described a 39-year-old patient referred for prenatal diagnosis due to advanced maternal age. Cytogenetic analysis from cultured amniocytes showed a recombinant chromosome X: 46,X,rec(X), dup(Xq)del(Xp)(p22.1->pter),inv(X)(p22q11) mat. The high-resolution sonography performed at 19 weeks of gestation did not show apparent malformations. A female baby was born at 35 weeks of gestation: birth weight 2,400 g, height 43.5 cm. Clinical examination at 3 months of age showed absence of dysmorphic features [18]. Horikoshi et al. 2010 reported a 34-year-old woman, referred to prenatal cares at 33 weeks of gestation because of polyhydramnios, shortening of the long bones and hypoplastic nasal bone. Chromosome analysis performed on amniotic fluid after amnioreduction showed a 46,Y,del(X)(p22.3) karyotype. SNP array analysis allowed the authors to define the extent of Xp deletion, that involved the first 8.33 Mb of chromosome X and included SHOX, CSF2RA, XG, ARSE, NLGN4 and STS genes. After cesarean section at 35 weeks’ gestation, a male infant was delivered. The baby presented with a complex phenotype characterized by shortening of the long bones, nasal hypoplasia and stippled calcification outside of the lumbar, proximal femur and ankle, likely to be related to deletion of the ARSE gene. The infant also manifested a mild respiratory problem and poor sucking and developed dry ichthyotic skin on the 7th day after birth, as a consequence of STS gene deletion [19].
CONCLUSION
In conclusion, this new case of pericentric inversion of chromosome X further supports the meiotic recombination risk for parental rearrangement. Moreover, it underlines the usefulness of molecular cytogenetics techniques in complements to high resolution banding in the study of chromosome X aberrations, and provides further elements for genotype phenotype correlation in chromosome X aneusomies.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of “P. & A. Kyriakou” Children’s Hospital, Athens, Greece.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
CONSENT FOR PUBLICATION
The family of the patient approved the study to be published, having provided a written consent form.
ACKNOWLEDGEMENTS
The authors would like to thank the patient and her family for their collaboration. All authors are funded by Access to Genome P.C. for this study.
LIST OF ABBREVIATION
- Array-CGH
Array Comparative Genomic Hybridization
- CNV
Copy Number Variations
- H.C
Head Circumference
- IUGR
Intrauterine Growth Retardation
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Shashi V., Golden W.L., Allinson P.S., Blanton S.H., von Kap-Herr C., Kelly T.E. Molecular analysis of recombination in a family with Duchenne muscular dystrophy and a large pericentric X chromosome inversion. Am. J. Hum. Genet. 1996;58(6):1231–1238. [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiser P. Pericentric inversions: Problems and significance for clinical genetics. Hum. Genet. 1984;68(1):1–47. doi: 10.1007/BF00293869. [DOI] [PubMed] [Google Scholar]
- 3.Gardner R.J.M., Sutherland G.R. Chromosome abnormalities and genetic counseling. 3rd ed. Oxford: 2004. [Google Scholar]
- 4.Madariaga M.L., Rivera H. Familial inv(X) (p22q22): Ovarian dysgenesis in two sisters with del Xq and fertility in one male carrier. Clin. Genet. 1997;52(3):180–183. doi: 10.1111/j.1399-0004.1997.tb02541.x. [DOI] [PubMed] [Google Scholar]
- 5.Rao E., Weiss B., Fukami M., Rump A., Niesler B., Mertz A., Muroya K., Binder G., Kirsch S., Winkelmann M., Nordsiek G., Heinrich U., Breuning M.H., Ranke M.B., Rosenthal A., Ogata T., Rappold G.A. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat. Genet. 1997;16(1):54–63. doi: 10.1038/ng0597-54. [DOI] [PubMed] [Google Scholar]
- 6.Yaegashi N., Uehara S., Ogawa H., Hanew K., Igarashi A., Okamura K., Yajima A. Association of intrauterine growth retardation with monosomy of the terminal segment of the short arm of the X chromosome in patients with Turner’s syndrome. Gynecol. Obstet. Invest. 2000;50(4):237–241. doi: 10.1159/000010323. [DOI] [PubMed] [Google Scholar]
- 7.Vetro A., Manolakos E., Petersen M.B., Thomaidis L., Liehr T., Croci G., Franchi F., Marinelli M., Meneghelli E., Dal Bello B., Cesari S., Iasci A., Arrigo G., Zuffardi O. Unexpected results in the constitution of small supernumerary marker chromosomes. Eur. J. Med. Genet. 2012;55(3):185–190. doi: 10.1016/j.ejmg.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Weise A., Mrasek K., Fickelscher I., Claussen U., Cheung S.W., Cai W.W., Liehr T., Kosyakova N. Molecular definition of high-resolution multicolor banding probes: First within the human DNA sequence anchored FISH banding probe set. J. Histochem. Cytochem. 2008;56(5):487–493. doi: 10.1369/jhc.2008.950550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamain S., Quach H., Betancur C., Rastam M., Colineaux C., Gillberg I.C., Soderstrom H., Giros B., Leboyer M., Gillberg C., Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003;34:27–29. doi: 10.1038/ng1136. https://www.nature.com/articles/ng1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laumonnier F., Bonnet-Brilhault F., Gomot M., Blanc R., David A., Moizard M.P., Raynaud M., Ronce N., Lemonnier E., Calvas P., Laudier B., Chelly J., Fryns J.P., Ropers H.H., Hamel B.C., Andres C., Barthelemy C., Moraine C., Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am. J. Hum. Genet. 2004;74(3):552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chih B., Engelman H., Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. 2005 doi: 10.1126/science.1107470. mag.org/content/307/5713/1324 [DOI] [PubMed]
- 12.Graf E.R., Zhang X., Jin S.X., Linhoff M.W., Craig A.M. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119(7):1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varoqueaux F., Aramuni G., Rawson R.L., Mohrmann R., Missler M., Gottmann K., Zhang W., Sudhof T.C., Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51(6):741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Jamain S., Radyushkin K., Hammerschmidt K., Granon S., Boretius S., Varoqueaux F., Ramanantsoa N., Gallego J., Ronnenberg A., Winter D., Frahm J., Fischer J., Bourgeron T., Ehrenreich H., Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA. 2008;105(5):1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrel L., Willard H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434(7301):400–404. doi: 10.1038/nature03479. https://www.nature.com/articles/nature03479 [DOI] [PubMed] [Google Scholar]
- 16.Zinn A.R., Roeltgen D., Stefanatos G., Ramos P., Elder F.F., Kushner H., Kowal K., Ross J.L. A Turner syndrome neurocognitive phenotype maps to Xp22.3. Behav. Brain Funct. 2007;3:24. doi: 10.1186/1744-9081-3-24. https://behavioralandbrainfunctions.biomedcentral.com/articles/ 10.1186/1744-9081-3-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C.P. Prenatal diagnosis of de novo partial trisomy Xq (Xq22.1-->qter) and terminal Xp deletion following sonographic detection of intrauterine growth restriction. Prenat. Diagn. 2003;23(6):518–519. doi: 10.1002/pd.621. [DOI] [PubMed] [Google Scholar]
- 18.Orellana C., Badía L., Martínez F., Oltra J.S., Monfort S., Roselló M., Cervera J.V., García Z., Prieto F. Recombinant X chromosome in a prenatal diagnosis. Cytogenet. Genome Res. 2006;112(3-4):337–340. doi: 10.1159/000089890. [DOI] [PubMed] [Google Scholar]
- 19.Horikoshi T., Kikuchi A., Tamaru S., Ono K., Kita M., Takagi K., Miyashita S., Kawame H., Shimokawa O., Harada N. Prenatal findings in a fetus with contiguous gene syndrome caused by deletion of Xp22.3 that includes locus for X-linked recessive type of chondrodysplasia punctata (CDPX1). J. Obstet. Gynaecol. Res. 2010;36(3):671–675. doi: 10.1111/j.1447-0756.2010.01193.x. [DOI] [PubMed] [Google Scholar]
- 20.Margari L., Colonna A., Craig F., Gentile M., Giannella G., Lamanna A.L., Legrottaglie A.R. Microphthalmia with linear skin defects (MLS) associated with Autism Spectrum Disorder (ASD) in a patient with familial 12.9Mb terminal Xp deletion. BMC Pediatr. 2014;14:220. doi: 10.1186/1471-2431-14-220. https://bmcpediatr.biomedcentral.com/articles/ 10.1186/1471-2431-14-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho E.H., Kim S.Y., Kim J.K. A case of 9.7 Mb terminal Xp deletion including OA1 locus associated with contiguous gene syndrome. J. Korean Med. Sci. 2012;27(10):1273–1277. doi: 10.3346/jkms.2012.27.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morleo M., Pramparo T., Perone L., Gregato G., Le Caignec C., Mueller R.F., Ogata T., Raas-Rothschild A., de Blois M.C., Wilson L.C., Zaidman G., Zuffardi O., Ballabio A., Franco B. Microphthalmia with linear skin defects (MLS) syndrome: Clinical, cytogenetic, and molecular characterization of 11 cases. Am. J. Med. Genet. A. 2005;137(2):190–198. doi: 10.1002/ajmg.a.30864. [DOI] [PubMed] [Google Scholar]
- 23.Boycott K.M., Parslow M.I., Ross J.L., Miller I.P., Bech-Hansen N.T., MacLeod P.M. A familial contiguous gene deletion syndrome at Xp22.3 characterized by severe learning disabilities and ADHD. Am. J. Med. Genet. A. 2003;122A(2):139–147. doi: 10.1002/ajmg.a.20231. [DOI] [PubMed] [Google Scholar]
- 24.Ross J.L., Roeltgen D., Kushner H., Wei F., Zinn A.R. The Turner syndrome-associated neurocognitive phenotype maps to distal Xp. Am. J. Hum. Genet. 2000;67(3):672–681. doi: 10.1086/303039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spranger S., Schiller S., Jauch A., Wolff K., Rauterberg-Ruland I., Hager D., Tariverdian G., Troger J., Rappold G. Leri-Weill syndrome as part of a contiguous gene syndrome at Xp22.3. Am. J. Med. Genet. 1999;83(5):367–371. doi: 10.1002/(sici)1096-8628(19990423)83:5<367::aid-ajmg5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Schwinger E., Kirschstein M., Greiwe M., Konermann T., Orth U., Gal A. Short stature in a mother and daughter with terminal deletion of Xp22.3. Am. J. Med. Genet. 1996;63(1):239–242. doi: 10.1002/(SICI)1096-8628(19960503)63:1<239::AID-AJMG41>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 27.Vassal H., Medeira A., Cordeiro I., Santos H.G., Castedo S., Saraiva C., da Silva P.M., Monteiro C. Terminal deletion of Xp22.3 associated with contiguous gene syndrome: Leri-Weill dyschondrosteosis, developmental delay, and ichthyosis. Am. J. Med. Genet. 2001;99(4):331–334. doi: 10.1002/1096-8628(20010401)99:4<331::aid-ajmg1175>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Macarov M., Zeigler M., Newman J.P., Strich D., Sury V., Tennenbaum A., Meiner V. Deletions of VCX-A and NLGN4: A variable phenotype including normal intellect. J. Intellect. Disabil. Res. 2007;51(Pt 5):329–333. doi: 10.1111/j.1365-2788.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- 29.van Steensel M.A., Vreeburg M., Engelen J., Ghesquiere S., Stegmann A.P., Herbergs J., van Lent J., Smeets B., Vles J.H. Contiguous gene syndrome due to a maternally inherited 8.41 Mb distal deletion of chromosome band Xp22.3 in a boy with short stature, ichthyosis, epilepsy, mental retardation, cerebral cortical heterotopias and Dandy-Walker malformation. Am. J. Med. Genet. A. 2008;146A(22):2944–2949. doi: 10.1002/ajmg.a.32473. [DOI] [PubMed] [Google Scholar]
- 30.Weissortel R., Strom T.M., Dorr H.G., Rauch A., Meitinger T. Analysis of an interstitial deletion in a patient with Kallmann syndrome, X-linked ichthyosis and mental retardation. Clin. Genet. 1998;54(1):45–51. doi: 10.1111/j.1399-0004.1998.tb03692.x. [DOI] [PubMed] [Google Scholar]
- 31.Meindl A., Hosenfeld D., Bruckl W., Schuffenhauer S., Jenderny J., Bacskulin A., Oppermann H.C., Swensson O., Bouloux P., Meitinger T. Analysis of a terminal Xp22.3 deletion in a patient with six monogenic disorders: Implications for the mapping of X linked ocular albinism. J. Med. Genet. 1993;30(10):838–842. doi: 10.1136/jmg.30.10.838. [DOI] [PMC free article] [PubMed] [Google Scholar]