Abstract
Pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α are central regulators of autoinflammatory diseases. While targeting these cytokines has proven to be a successful clinical strategy, the long-term challenges such as drug resistance, lack of efficacy and poor clinical outcomes in some patients are some of the limitations faced by these therapies. This has ignited strategies to reduce inflammation by potentially targeting a variety of molecules, including cell surface receptors, signalling proteins and/or transcription factors to minimize cytokine-induced inflammation and tissue injury. In this regard, transforming growth factor β activated kinase 1 (TAK1) is activated in the inflammatory signal transduction pathways in response to IL-1β, TNF-α or toll-like receptor stimulation. Because of its ideal position upstream of mitogen-activated protein kinases and the IκB kinase complex in signalling cascades, targeting TAK1 may be an attractive strategy for treating diseases characterized by chronic inflammation. Here, we discuss the emerging role of TAK1 in mediating the IL-1β, TNF-α and toll-like receptor mediated inflammatory responses in diseases such as RA, OA, gout and SS. We also review evidence suggesting that TAK1 inhibition may have potential therapeutic value. Finally, we focus on the current status of the development of TAK1 inhibitors and suggest further opportunities for testing TAK1 inhibitors in rheumatic diseases.
Keywords: inflammation, TAK1, inhibition, rheumatoid arthritis, osteoarthritis, gout, Sjögren’s syndrome
Rheumatology key messages
TAK1 has a central location in many inflammatory pathways suggesting that its inhibition may be therapeutic.
Despite there being many anti-inflammatory therapeutics, none of them target TAK1 directly.
More effort should be made to develop TAK1 inhibitors to be used in the clinic.
Introduction
Inflammation is the body’s first response to damage from physical injury, foreign bodies or xenobiotics. In a physiological situation, inflammation is a hallmark of the repair of damaged tissue and the process resolves once the repair is complete. However, chronic inflammation can eventually result in a degree of tissue damage that cannot be repaired. Chronic inflammation is characteristic of many diseases including autoimmune conditions and cancer. In the development of anti-inflammatory therapeutics, a reasonable goal is to develop agents that are effective for a range of inflammatory diseases.
Mitogen-activated protein kinases (MAPKs) are an evolutionarily conserved family of signalling kinases that mediate many cellular processes such as proliferation, differentiation and inflammation. They elicit their response through a series of phosphorylation steps that engage a cascade of kinases in cell signal transduction pathways [1]. One of these kinases within the MAPK family is transforming growth factor β-activated kinase-1 (TAK1). This MAPK family member was found to be activated by TGF-β and bone morphogenetic protein in yeast studies [2]. Deleting TAK1 results in embryonic lethality, indicating that TAK1 also plays a role in normal developmental processes [3–6]. Using conditional knockouts, TAK1 was shown to also have an important role in the immune responses, including the production of inflammatory cytokines such as IL-6 and IL-8 [7–11]. Almost a decade after its initial discovery, TAK1 is now known to be intimately involved in the IL-1β, TNF-α and toll-like receptor (TLR) signal transduction pathways, all of which are critical for a range of diseases associated with inflammation [12–14].
This review focuses on summarizing the emerging pathological role that TAK1 plays in numerous autoinflammatory and/or autoimmune diseases. We also highlight the potential of targeting TAK1 in the treatment of specific autoimmune diseases such as RA, OA, type I diabetes, SS and gout. We then highlight the current status and potential future applications of TAK1 inhibitors for use in the clinic.
Is TAK1 a key mediator in pro-inflammatory cytokine-driven signalling pathways?
TAK1 is a key cellular signalling molecule due to its position in the hierarchy of the IL-1β, TNF-α and TLR signalling cascades. Ligand-receptor binding introduces conformational changes in the transmembrane domains of cytokine receptors that alert signalling proteins proximal to the cellular membranes such as myeloid differentiation primary response gene 88 (MyD88) or TNF receptor-associated death domain (TRADD) to relay the signalling message. In a subsequent step, an E3 ubiquitin ligase family member, TNF receptor-associated factor (TRAF) 2 or 6, facilitates dissociation of the signalling complex from the receptor and its translocation to the cytoplasm to recruit and activate TAK1 [15, 16]. TRAF2 or TRAF6 undergoes K63 mono- or polyubiquitination in order to execute TAK1 activation at threonine 184/187 residues. This results in downstream phosphorylation of MAPK kinase (MKK) 3/6 and 4/7 to activate p38 and c-Jun N-terminal kinase (JNK) pathways, and inhibitor of nuclear factor-κB (IκB) kinase activation leading to IκBα degradation and nuclear factor-κB (NF-κB) activation [12, 17].
TAK1 activation requires association with TAK1-binding protein (TAB) 1 or 2. TAB1 homologues TAB2 and TAB3 bind in the same region of TAK1; however, TAB2 has been shown to mediate this activation process rather directly activating TAK1 [18, 19]. When TAB binds to TAK1, a conformational change occurs allowing an adenosine triphosphate (ATP) molecule to bind to TAK1. This conformation change also induces TAK1 autophosphorlyation at Thr184, Thr187 and Ser192 residues [20, 21]. However, phosphorylation of these residues is not required for TAB1 to bind to TAK1. On the other hand, a 30 amino acid sequence within TAB1/2 contains an α-helix that is centred on residue Phe484. Using a crystal structure analysis, Brown et al. showed that the α-helix region ensures correct alignment of aromatic residues of TAB1 with the hydrophobic binding pocket in TAK1. This study also showed that Phe484 is a critical amino acid for the TAK–TAB1 association due to its lipophilicity, thus stabilizing the TAK–TAB interaction [22]. TAB1 binding to TAK1 promotes the autophosphorylation of Ser192, which resides in the kinase activation loop. Upon removal of this residue, TAK1 is no longer able to autophosphorylate, thereby eliminating its kinase activity [23].
TAK1 activation also requires association with TNF receptor associated factors (TRAFs), a family of E3 ubiquitin ligases. There are seven members of the TRAF protein family all of which contain a RING finger domain. The RING domain is characterized by a series of cysteine and histidine residues capable of binding zinc and activating downstream signalling proteins [24, 25]. In TNF-α signalling, TNF receptor-associated death domain recruits TRAF2 or TRAF5. However, neither TRAF2 nor TRAF5 directly activates TAK1. Instead, TRAF2 and TRAF5 promote polyubiquitination of receptor–interacting protein 1 (RIP1), which initiates TAK1 activation [15].
In IL-1β and TLR signalling, TRAF6 is recruited and directly activates TAK1. Ubc13/Uev1A (also known as TRIKA1) is an E2 ubiquitin-conjugating enzyme that binds to TRAF6 and, in most cases, works together with E2 ligases to add ubiquitin to a substrate protein. However, in this case, Ubc13/Uev1A binding with TRAF6 results in its K63 autoubiquitination at Lys124 [16, 18]. Unlike K48 polyubiquitination that targets proteins for proteasome degradation, K63 polyubiquitination is involved in signalling functions including kinase activation [18].
Once the TAB–TAK–TRAF complex forms, TAK1 becomes mono- and polyubiquitinated at Lys34 [26]. There are other reports of TAK1 getting polyubiquitinated at Lys158 as well. The critical binding site remains unknown since mutating either lysine results in loss of TAK1 activation [26, 27]. The TAB–TAK–TRAF complex goes on to mediate NF-κB signalling by the degradation of IκBα by IκB kinase activation, or by activator protein 1 (AP-1) activation through MAP kinases in the same manner in IL-1β, TNF-α and TLR signalling [28, 29].
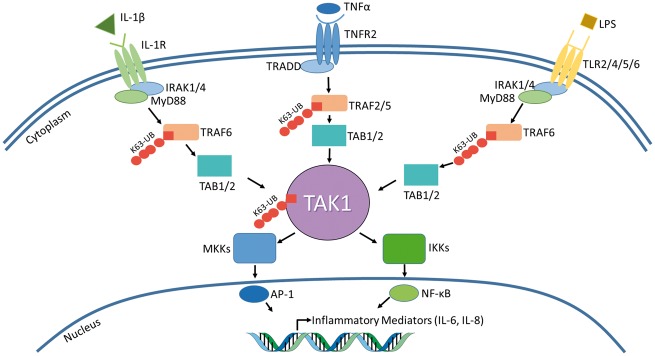
The IL-1β, TNF-α and TLR signalling pathways result in the activation of MAPK and NF-κB pathways, which in turn results in the production of inflammatory cytokines. Interestingly, a brief analysis of each pathway underlines the importance of TAK1 as a central signalling molecule in cytokine signalling networks (Fig. 1). Therefore, TAK1 dysregulation may have considerable impact on the pathogenesis of chronic inflammatory diseases, and thus may serve as a potential therapeutic target for such diseases.
Fig. 1.
IL-1β, TNF-α and TLR signalling pathways all converge at TAK1
TAK1 is a central signalling molecule. IL-1β, TNF-α and TLR signalling pathways all result in TAK1 activation and transcription of pro-inflammatory mediators. A brief summary of each pathway is illustrated above. Both IL-1 and TLR signalling use MyD88 in order to activate TRAF6, which undergoes K63 polyubiquitination. In TNF-α signalling, TRAF2 or TRAF5 is K63 polyubiquitinated. Despite different TRAFs being activated, they all result in TAB1/2 activation, which recruits TAK1. Upon ubiquitination, TAK1 phosphorylates its downstream targets, the MKKs and IKKs, resulting in IL-6, IL-8 and TNFα production. TAK1’s central role in these three pathways makes it a prime candidate as a druggable target to reduce the overall inflammatory response in order to treat diseases characterized by chronic inflammation. AP-1: activator protein 1; IKK: IκB kinase; IRAK: interleukin-1 receptor-associated kinase; K63-UB: ubiquitination at lysine residue at 63rd position; LPS: lipopolysaccharide; MKK: MAPK kinase; MyD88: myeloid differentiation primary response gene 88; TAB: TAK1-binding protein; TAK1: transforming growth factor β activated kinase 1; TLR: toll-like receptor; TNFR: tumour necrosis factor receptor; TRADD: TNF receptor-associated death domain; TRAF: TNF receptor-associated factor.
TAK1 in chronic inflammatory diseases
TAK1 in RA
RA is a chronic inflammatory disease characterized by hyperplasia of the synovial lining, which results in the progressive destruction of cartilage and underlying bone leading to joint dysfunction [1]. Recent findings have provided a better understanding of the pro-inflammatory cytokine-initiated molecular mechanisms that govern RA pathogenesis and a firmer rationale for developing novel targeted therapies [2]. The treatment regimen for RA has dramatically shifted from monotherapies involving DMARDs to combinations of DMARDs and highly targeted biologics such as anti-TNF, anti-IL-6R, anti-CD20 and CTLA4-Ig. While these pharmacological advances have advanced the treatment of RA, there continues to be partial or non-responders to some or even all of these therapies, and various adverse events associated with their long-term use [30]. While the rheumatology community currently relies on the success of these biologic therapies, the introduction of a pan-Janus kinase inhibitor, tofacitinib, has brought small-molecule therapeutics that inhibit signal transduction into prominent focus as a novel and distinct approach to the treatment of RA [31].
Recent studies suggest that TAK1 plays a critical role in inducing cartilage and bone destruction via its downstream signalling molecules such as JNK, p38 and NF-κB, which are involved in pro-inflammatory cytokine signalling [32]. A study by Hammaker et al. [33] showed TAK1 as one of the dominant MAP3K involved in JNK activation in RA fibroblast-like synoviocytes (FLSs). A knockdown of TAK1 in RA-FLSs was effective in inhibiting IL-1β-induced AP-1 activation, matrix metalloproteinase (MMP) 3 expression and IL-6 production. Another interesting study in RA-FLSs provides evidence that IL-1, TNF and TLR2, but not TLR4, mediated signalling is dependent on TAK1 [34]. An epistasis analysis in the study by Gottar-Guillier et al. [35] showed that small interfering RNA (siRNA) knockdown of the tyrosine kinase bone marrow kinase on chromosome X (BMX), which is downstream of TAK1 in the inflammatory signalling pathways, inhibited IL-8 production in primary human endothelial cells. In addition, BMX-deficient mice were protected from K/BxN serum-induced arthritis. The proposed possibility of BMX working with assistance from the TAK1–TAB1 complex points to a potential role of TAK1 in RA pathogenesis.
We recently provided a novel molecular insight regarding the post-translational mechanisms involved in IL-1 signalling in RA-FLSs [36]. We showed for the first time that inhibition of TAK1, but not IL-1 receptor-associated kinase (IRAK) 1 or TRAF6, completely abrogated IL-1β-induced IL-6 and IL-8 production in human RA-FLSs. Our findings also highlighted the role of ubiquitination in cytokine signalling networks in RA-affected cells. K63-linked autoubiquitination of TRAF6 was shown to be an essential step in inducing TAK1 kinase activity, and the inhibition of TAK1 phosphorylation at Thr184/187 was able to suppress downstream inflammatory mediators in RA. Using the rat adjuvant-induced arthritis model of inflammatory arthritis, we showed that K48-linked polyubiquitination and TAK1 phosphorylation were significantly upregulated with the severity of arthritis. The administration of epigallocatechin-3-gallate (EGCG), a potent anti-inflammatory molecule shown to selectively inhibit TAK1 activation in RA-FLSs, ameliorated arthritis in the treated rats. These findings provide evidence that TAK1 is a central regulator of cytokine signalling networks in RA and that therapeutic strategies that inhibit TAK1 involvement may therefore be beneficial in the treatment of RA.
TAK1 in OA
OA is the most common form of arthritis and is characterized by local inflammation coinciding with progressive cartilage destruction and bone remodelling. IL-1β has been shown to lead to the destruction of cartilage, resulting in the development of IL-1 blocking therapies for the treatment of OA [37]. This IL-1β effect is mediated by a class of matrix degrading collagenases (MMP-1, -3, -8 and -13). In particular, studies suggest MMP-1 and -13 have higher affinity to degrade the triple helical structure of collagen, thereby accelerating extracellular matrix degradation, proteoglycan release and induction of chondrocyte apoptosis [38]. In this regard, Klatt et al. showed that TAK1 is expressed in human knee cartilage and hypertrophic chondrocytes of the epiphyseal growth plate, suggesting a role for TAK1 in cartilage catabolism. Inhibition of TAK1 in SW1353 cells markedly reduced IL-1β-induced MMP-13, MMP-1 and TNF-α expression [39]. The results of these studies are further supported by another study where suppressor of cytokine signalling 1 (SOCS1) inhibited IL-1β-induced production of MMP-1, MMP-3 and MMP-13 in both SW1353 cells and primary human chondrocytes by downregulating TAK1 expression [32].
Oxidative stress in the OA joint has been shown to contribute to persistent pain, inflammation and progressive loss of cartilage [40]. Reactive oxygen species (ROS), mechanical overloading and a pro-inflammatory cytokine milieu in the joint leads to unwanted extracellular matrix remodelling, DNA damage and chondrocyte apoptosis [10]. While TAK1 has been shown to play an essential role in the morphogenesis, growth and maintenance of cartilage via bone morphogenetic protein signalling [41, 42], TAK1 also mediates ROS- and collagen type II-dependent induction of inflammatory gene cyclooxygenase-2 (Cox-2) expression and prostaglandin E2 production [43, 44]. Cox-2 overexpression is particularly evident in vascular endothelial cells, infiltrating mononuclear inflammatory cells and FLSs in patients with OA, and is further upregulated in the presence of IL-1β and TNF-α thereby further enhancing prostaglandin production [45]. A study done by Onodera et al. [44] connects TAK1 with ROS by showing that H2O2 was able to activate Cox-2 in bovine FLSs as evident by induced p38, extracellular signal-regulated kinase (ERK) and NF-κB expression, in a process that was directly mediated through TAK1 activation via Thr184/187 phosphorylation. These studies provide evidence of the importance of TAK1 in OA and suggest that TAK1 inhibition may be beneficial in OA by limiting the role of multiple inflammatory mediators in disease progression.
TAK1 in gout
Gout is one of the most severe and common arthritides, and is characterized by the deposition of monosodium urate crystals in musculoskeletal tissues, most commonly in joints of the foot. As a result of crystal deposition, the recruitment of neutrophils and monocytes to phagocytose the insoluble crystals triggers robust activation of the inflammasome, an intracellular protein complex that drives pain, swelling and inflammation of the affected joint [46]. Activation of the inflammasome further initiates the maturation and release of IL-1β through activation of caspase-1, which further amplifies vasodilation, recruitment of additional leucocytes and the production of pro-inflammatory cytokines and chemokines leading to the acute gout attacks. The management of gout starts with anti-inflammatory treatment such as NSAIDs or colchicine [47]. In addition, urate-lowering therapies such as allopurinol or febuxostat are recommended. However, with the increasing pathological role of IL-1 in gout, treating gout patients with anakinra (IL-1 receptor antagonist), rilonacept (IL-1 decoy receptor) or canakinumab (IL-1β antibody) has also proven effective at reducing inflammation and pain [48].
Recent studies suggest that IRAK-4, which is an indispensable protein in IL-1 and TLR signalling pathways, could be targeted to manage gout [49]. While this observation has sparked some interest in testing IL-1 signalling inhibitors for the treatment of gout, the lack of molecular mechanisms and the roles played by diverse signalling proteins such as MyD88, IRAK-1/4, TAK1 and TRAF2/6 in the IL-1 signalling pathway in response to monosodium urate crystals has restricted our therapeutic approaches and rationale for targeting IL-1 signalling protein(s) for the treatment of gout. However, a recent study suggests the TAK1–JNK pathway plays an important role in regulating NLRP3 inflammasome activation, an observation that may have pathological implication in acute gouty attacks [50]. Furthermore, this observation is experimentally supported by another study that showed that 5Z-7-oxozeanol, an inhibitor of TAK1, was effective in inhibiting caspase-1 activation induced by ATP plus other TLR ligands in THP-1 cells, suggesting that TAK1 is a critical mediator for TLR-sensitized inflammasome activation [51]. Considering that IL-1 inhibitors clinically tested for the management of gout are associated with an increased risk of adverse events [52], TAK1 may serve as an alternative, clinically valuable therapeutic target in the treatment of gout.
TAK1 in SS
SS is an autoimmune disease characterized by the infiltration of lymphocytes in the salivary and lacrimal glands, resulting in a decline of saliva and tears production. Because of this, many patients develop xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eyes). As in RA, there is no known cure for SS. Therefore, current treatment approaches focus on alleviating symptoms, such as moisture replacement therapies to deal with dryness, and NSAIDs and DMARDs to reduce the inflammatory response. There is also some recent interest in using biologics to treat SS [53].
Recent studies have elucidated the involvement of IL-1 signalling in SS [54]. Indeed Vijmasi et al. showed that topical administration of anakinra three times a day for 14 days improves tear secretion in aire-deficient mice. Lissamine green staining also indicated that the viability of corneal epithelial cells improved with anakinra treatment [55]. At the epigenetic level, microRNA (miR) 146a and 146b were found to be upregulated in peripheral mononuclear cells from patients with SS compared with healthy controls. As a result of miR-146a upregulation, TRAF6 was also overexpressed, as miR-146a regulates TRAF6 expression [56]. Since TRAF6 directly activates TAK1, perhaps TAK1 inhibition would also aid in treating SS. In a study by Zoukhri et al. the inhibition of JNK, one of the immediate downstream targets of TAK1 in the IL-1 signalling cascade, inhibited lacrimal gland secretion and tear production [57]. Recent approaches in identifying therapeutic targets for SS also point to the emerging role of IL-1, TLRs and the inflammasome, all of which rely on TAK1 in their signalling networks, to mediate catabolic events in disease pathogenesis. However, further studies are required to validate the role of TAK1 in this disease.
Inhibition of TAK1
Given the role of TAK1 in morphogenesis and developmental processes, TAK1 knockout using genetic models was found to be embryonically lethal [3, 58]. However, the overexpression of TAK1 in several autoimmune and autoinflammatory diseases has ignited research using conditional knockdown of TAK1 in understanding its role in the inflammatory response in the diseased states [7, 14, 33, 59]. In addition, the most common way of TAK1 inhibition so far studied is through the use of a small-molecule TAK1 inhibitor, 5Z-7-oxozeaenol. First discovered in fungi, this resorcyclic acid lactone-related compound has been shown to be an irreversible inhibitor of TAK1 [60, 61]. It inhibits inflammation when applied topically and in vitro in various cell lines. However, the studies using this molecule have been limited to primarily in vitro testing and there are currently no clinical trials in progress with this molecule [61]. In addition to this molecule, LYTAK1, developed by Eli Lilly, is the only known orally bioavailable TAK1 inhibitor. LYTAK1 has been shown to inhibit TAK1 by inhibiting Thr-187 phosphorylation in colorectal cancer cells. Interestingly, Zhou et al. [62] showed that the inhibition was effective only in KRAS-dependent colorectal cancer cell lines and not in KRAS-independent cell lines. Melisi et al. [63] also showed LYTAK1 reduces NF-κB activity in pancreatic cancer cell lines. The selectivity of LYTAK1 offers a therapeutic advantage in targeting KRAS-dependent cell lines. Despite in vitro success, LYTAK1 has not been tested for its efficacy in clinical trials.
Although there are no readily available TAK1 inhibitors, there are many in the making. For a more in-depth analysis regarding structure and development, we recommend a recent review by Kilty and Jones [64]. To summarize, TAK1’s role in TNF-α signalling has resulted in a push by pharmaceutical companies to develop small molecule inhibitors of TAK1 for the treatment of cancer. Companies such as OSI Pharmaceuticals, Eli Lilly and Company and AstraZeneca have recently developed TAK1 inhibitors (Table 1). Currently, none of these compounds have progressed to clinical trials for the testing of their efficacy in treating either cancer or chronic inflammatory diseases.
Table 1.
Current status of known TAK1 inhibitors
| TAK1 inhibitor | Current status | Reference |
|---|---|---|
| siRNA | Commercially available from Dharmacon, Santa Cruz Biotechology and CST for research purposes | [7, 14, 33, 59] |
| 5Z-7- oxozeaenol | Commercially available for research purposes | [60, 61] |
| LYTAK1 | Not in clinical trials or in oncology and autoimmune studies. Patent information unknown | [62, 63] (see Acknowledgements) |
| ABC-FP | Patented by OSI Pharmaceuticals. Patent US8378104 | [65] |
ABC-FP: trans-4-{4-[7-amino-2-(1,2,3-benzothiadiazol-7-yl)-3-chlorofuro[2,3-c]pyridin-4-yl]-1H-pyrazol-1-yl}cyclohexanol; LYTAK: TAK1 inhibitor from Eli Lilly Pharmaceuticals; siRNA: small interfering RNA; TAK1: transforming growth factor β activated kinase 1.
Future directions
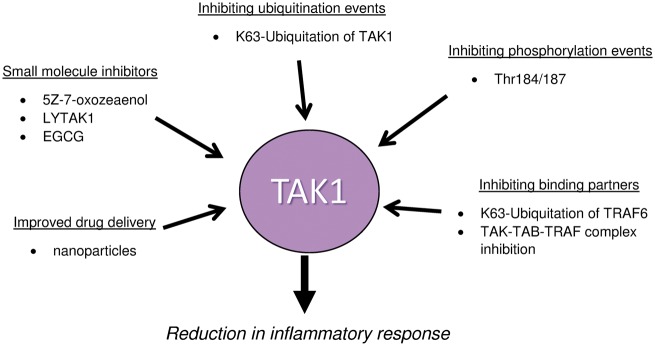
Targeting TAK1 has revealed a molecular specificity issue. TAK1 is an ATP-kinase, which shares many structural properties with several other ATP-kinases. The challenge lies in designing inhibitors that are specific to TAK1. In addition to specificity, TAK1’s central role in the inflammatory cascade poses an additional problem in clinical practice. Knocking out TAK1 is lethal, but inhibiting its overexpression may be beneficial in humans. Due to both the inherent toxicity of kinase inhibitors and TAK1’s central role in inflammation, determining the most effective dose of TAK1 inhibitor may be difficult in practice. One possible solution to avoid such toxicity is to design reversible TAK1 inhibitors with better efficacy-to-toxicity ratio to elicit desired pharmacological effects while allowing immune defence mechanisms to utilize TAK1. In addition to the reversible inhibitors, compounds that target the required mechanisms for TAK1 activation should also be a focus for drug discovery and development. Finally, toxicity challenges may be overcame by improving delivery mechanisms. These points are also summarized in Fig. 2.
Fig. 2.
Possible mechanisms for TAK1 inhibition
Several studies suggest that the inhibition of TAK1 may be beneficial in the treatment of diseases characterized by chronic inflammation. The schematic diagram shows multiple mechanisms, besides inhibiting TAK1 itself, that can potentially be exploited to inhibit TAK1 activation to reduce inflammatory response. EGCG: epigallocatechin-3-gallate; LYTAK: TAK1 inhibitor from Eli Lilly Pharmaceuticals; TAB: TAK1-binding protein; TAK1: transforming growth factor β activated kinase 1; TRAF: TNF receptor-associated factor.
One way of inhibiting TAK1 would be to inhibit the mechanisms that are required for activation. For instance, TNF-α and lipopolysaccharide (TLR4 agonist) stimulated cells require TAK1 ubiquitination at position lysine 34 (lysine linked) by K63 polyubiquitinated TRAF6. A study by Hamidi et al. [26] has shown that mutating the K34 site on TAK1 reduces polyubiquitination of TAK1, resulting in reduced NF-κB and IL-6 promoter activity. Rigel Pharmaceutics Inc. has identified the ubiquitin-conjugated enzymes that aid the K63 polyubiquitination of TRAF6 and has patented a ubiquitin ligase inhibitor that claims to inhibit TRAF6 polyubiquitination (patent WO2008115259) [66].
TAK1 activation also relies on a series of phosphorylation events. When stimulated by IL-1β, TAK1 is autophosphorylated at the Thr-184/187 sites that are located in TAK1’s kinase domain [67]. Stimulation with TNF-α results in Thr-187 and Ser-192 phosphorylation [68]. Blocking phosphorylation of TAK1 at one or more of these sites may inhibit or reduce the cellular functions of TAK1 and potentially its downstream signalling events. Kajino et al. [69] have already shown that protein phosphatase 6 is capable of dephosphorylating Thr-187 of TAK1. Therefore, the possibility of generating additional phosphorylation inhibitors remains feasible.
Another potential way to inhibit TAK1 is by regulating its interaction with its binding partners. TAK1 activation requires the formation of a complex with TAB1/2, which is activated by TRAF6 [15]. Therefore, inhibiting the effective formation of the TAK1–TAB1/2–TRAF6 complex would markedly inhibit TAK1’s activity and function. Some protein–protein inhibitors have been generated to inhibit mouse double minute 2 homologue (MDM2)–p53, hypoxia-inducible factor 1α and β-catenin–Tcf protein interactions [70]. On a similar note, a Bak–Bcl-X protein–protein interaction inhibitor has been shown to be effective in HEK293 cells [71]. In this regard, perhaps a small molecule could be designed to compete with TAB1/2 or TRAF6 in associating with TAK1 to limit TAK1’s downstream responses.
In addition to designing competitive inhibitors to compete with TAK1 binding partners, small molecule TAK1 inhibitors should continue to be developed with the overall goal of increased specificity. One strategy of doing so is the development of type II kinase inhibitors, which bind the ATP-binding pocket and an adjacent hydrophobic binding pocket of ATP kinases. Unlike type I inhibitors that only bind the ATP binding pocket, type II inhibitors offer more specificity to target a specific kinase with the additional binding site. Tan et al. [72] has recently reported a type II TAK1 inhibitor. The hydrophobic pocket is characterized by the DFG motif, which is flipped in when bound to ATP. These TAK1 inhibitors, reported as NG25 and GCK, bind to both TAK1 and MAP4K2 with high affinity and specificity. Likewise, Singh et al. [36] have recently shown that epigallocatechin-3-gallate, a potent anti-inflammatory molecule found in green tea, binds to TAK1 at the same position as 5Z-7-oxozeaenol. However, EGCG forms hydrogen bonds with TAK1 instead of covalent bonds suggesting the reversible nature of binding. Additional small molecules like NG25, GCK and EGCG should be developed and tried against TAK1 to promote the development of a small molecule inhibitor to be used in the clinic for treatment of chronic inflammatory diseases.
Finally, kinase inhibitors pose a toxicity challenge because of their lack of specificity. One major reason for this is the structural similarity of kinases, making it difficult to target one kinase specifically. In order to address this, research should also investigate more specificity in delivery methods. Such a method could utilize nanotechnology in order to deliver inhibitors directly to the site where TAK1 inhibition would be therapeutically beneficial. For instance, TAK1 inhibition using siRNA has already been shown to be effective in several models. However, one of the main issues with siRNA is its poor uptake into targeted cells. In this regard, Schiffelers et al. [73] have demonstrated the use of nanoparticles to deliver siRNA to human umbilical vein endothelial cells and inhibit VEGF receptor 2. Perhaps a similar delivery platform may be utilized to deliver TAK1 siRNA to the affected area such as the joint for the treatment of RA or gout.
Conclusion
Given the growing evidence for involvement of TAK1 in pro-inflammatory cytokine signalling networks, TAK1 inhibition may have a potential therapeutic benefit in the treatment of chronic inflammatory diseases. Future research in TAK1 inhibition should not only include the development of reversible small molecule inhibitors specific for TAK1, but also investigate the mechanisms of inhibition specific to TAK1 such as preventing it from forming activated complex with TRAF6 and TAB1. Finally, some novel ways of delivering TAK1 inhibitors should also be investigated to increase the bioavailability of the inhibitor and reduce toxicity.
Acknowledgements
We would like to thank Christina K. and Kathy Mybeck (Eli Lily, Indiana) for the information on LYTAK1 and Keith R. Hornberger (OSI Pharmaceuticals, Farmingdale, NY, USA) for providing information on ABC-FP. S.A. was supported by NIH grant AR063104 and an Arthritis Foundation Innovative Research Grant. S.F. was supported by the James & Diann Robbers Student Research Fund.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Plotnikov A, Zehorai E, Procaccia S, Seger R.. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta 2011;1813:1619–33. [DOI] [PubMed] [Google Scholar]
- 2. Yamaguchi K, Shirakabe K, Shibuya H. et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 1995;270:2008–11. [DOI] [PubMed] [Google Scholar]
- 3. Sato S, Sanjo H, Takeda K. et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 2005;6:1087–95. [DOI] [PubMed] [Google Scholar]
- 4. Shim JH, Xiao C, Paschal AE. et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 2005;19:2668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Komatsu Y, Shibuya H, Takeda N. et al. Targeted disruption of the Tab1 gene causes embryonic lethality and defects in cardiovascular and lung morphogenesis. Mech Dev 2002;119:239–49. [DOI] [PubMed] [Google Scholar]
- 6. Sanjo H, Takeda K, Tsujimura T. et al. TAB2 is essential for prevention of apoptosis in fetal liver but not for interleukin-1 signaling. Mol Cell Biol 2003;23:1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Courties G, Seiffart V, Presumey J. et al. In vivo RNAi-mediated silencing of TAK1 decreases inflammatory Th1 and Th17 cells through targeting of myeloid cells. Blood 2010;116:3505–16. [DOI] [PubMed] [Google Scholar]
- 8. Schuman J, Chen Y, Podd A. et al. A critical role of TAK1 in B-cell receptor-mediated nuclear factor kappaB activation. Blood 2009;113:4566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wan YY, Chi H, Xie M, Schneider MD, Flavell RA.. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol 2006;7:851–8. [DOI] [PubMed] [Google Scholar]
- 10. Sato S, Sanjo H, Tsujimura T. et al. TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int Immunol 2006;18:1405–11. [DOI] [PubMed] [Google Scholar]
- 11. Rajasekaran K, Chu H, Kumar P. et al. Transforming growth factor-beta-activated kinase 1 regulates natural killer cell-mediated cytotoxicity and cytokine production. J Biol Chem 2011;286:31213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narayanan KB, Park HH.. Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis 2015;20:196–209. [DOI] [PubMed] [Google Scholar]
- 13. Ninomiya-Tsuji J, Kishimoto K, Hiyama A. et al. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 1999;398:252–6. [DOI] [PubMed] [Google Scholar]
- 14. Blonska M, Shambharkar PB, Kobayashi M. et al. TAK1 is recruited to the tumor necrosis factor-alpha (TNF-alpha) receptor 1 complex in a receptor-interacting protein (RIP)-dependent manner and cooperates with MEKK3 leading to NF-kappaB activation. J Biol Chem 2005;280:43056–63. [DOI] [PubMed] [Google Scholar]
- 15. Adhikari A, Xu M, Chen ZJ.. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 2007;26:3214–26. [DOI] [PubMed] [Google Scholar]
- 16. Wang C, Deng L, Hong M. et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001;412:346–51. [DOI] [PubMed] [Google Scholar]
- 17. Ogura N, Kondoh T.. Molecular aspects in inflammatory events of temporomandibular joint: Microarray-based identification of mediators. Japanese Dental Sci Rev 2015;51:10–24. [Google Scholar]
- 18. Besse A, Lamothe B, Campos AD. et al. TAK1-dependent signaling requires functional interaction with TAB2/TAB3. J Biol Chem 2007;282:3918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takaesu G, Kishida S, Hiyama A. et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell 2000;5:649–58. [DOI] [PubMed] [Google Scholar]
- 20. Kishimoto K, Matsumoto K, Ninomiya-Tsuji J.. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem 2000;275:7359–64. [DOI] [PubMed] [Google Scholar]
- 21. Sakurai H, Miyoshi H, Mizukami J, Sugita T.. Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett 2000;474:141–5. [DOI] [PubMed] [Google Scholar]
- 22. Brown K, Vial SC, Dedi N. et al. Structural basis for the interaction of TAK1 kinase with its activating protein TAB1. J Mol Biol 2005;354:1013–20. [DOI] [PubMed] [Google Scholar]
- 23. Ono K, Ohtomo T, Sato S. et al. An evolutionarily conserved motif in the TAB1 C-terminal region is necessary for interaction with and activation of TAK1 MAPKKK. J Biol Chem 2001;276:24396–400. [DOI] [PubMed] [Google Scholar]
- 24. Zotti T, Vito P, Stilo R.. The seventh ring: Exploring TRAF7 functions. J Cell Physiol 2012;227:1280–4. [DOI] [PubMed] [Google Scholar]
- 25. Petroski MD, Deshaies RJ.. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 2005;6:9–20. [DOI] [PubMed] [Google Scholar]
- 26. Hamidi A, von Bulow V, Hamidi R. et al. Polyubiquitination of transforming growth factor beta (TGFbeta)-associated kinase 1 mediates nuclear factor-kappaB activation in response to different inflammatory stimuli. J Biol Chem 2012;287:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mao R, Fan Y, Mou Y. et al. TAK1 lysine 158 is required for TGF-β-induced TRAF6-mediated Smad-independent IKK/NF-κB and JNK/AP-1 activation. Cell Signal 2011;23:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hildebrand JM, Yi Z, Buchta CM. et al. Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions. Immunol Rev 2011;244:55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakurai H, Suzuki S, Kawasaki N. et al. Tumor necrosis factor-α-induced IKK phosphorylation of NF-κB p65 on Serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem 2003;278:36916–23. [DOI] [PubMed] [Google Scholar]
- 30. Lahiri M, Dixon WG.. Risk of infection with biologic antirheumatic therapies in patients with rheumatoid arthritis. Best Pract Res Clin Rheumatol 2015;29:290–305. [DOI] [PubMed] [Google Scholar]
- 31. Meier FM, McInnes IB.. Small-molecule therapeutics in rheumatoid arthritis: scientific rationale, efficacy and safety. Best Pract Res Clin Rheumatol 2014;28:605–24. [DOI] [PubMed] [Google Scholar]
- 32. Choi YS, Park JK, Kang EH. et al. Cytokine signaling-1 suppressor is inducible by IL-1beta and inhibits the catabolic effects of IL-1beta in chondrocytes: its implication in the paradoxical joint-protective role of IL-1beta. Arthritis Res Ther 2013;15:R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hammaker DR, Boyle DL, Inoue T, Firestein GS.. Regulation of the JNK pathway by TGF-beta activated kinase 1 in rheumatoid arthritis synoviocytes. Arthritis Res Ther 2007;9:R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geurts J, van den Brand BT, Wolf A. et al. Toll-like receptor 4 signalling is specifically TGF-beta-activated kinase 1 independent in synovial fibroblasts. Rheumatology 2011;50:1216–25. [DOI] [PubMed] [Google Scholar]
- 35. Gottar-Guillier M, Dodeller F, Huesken D. et al. The tyrosine kinase BMX is an essential mediator of inflammatory arthritis in a kinase-independent manner. J Immunol 2011;186:6014–23. [DOI] [PubMed] [Google Scholar]
- 36. Singh AK, Umar S, Riegsecker S, Chourasia M, Ahmed S.. Regulation of TAK1 activation by epigallocatechin-3-gallate in RA synovial fibroblasts: Suppression of K63-linked autoubiquitination of TRAF6. Arthritis Rheumatol 2016;68:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jotanovic Z, Mihelic R, Sestan B, Dembic Z.. Role of interleukin-1 inhibitors in osteoarthritis: an evidence-based review. Drugs Aging 2012;29:343–58. [DOI] [PubMed] [Google Scholar]
- 38. Burrage PS, Mix KS, Brinckerhoff CE.. Matrix metalloproteinases: role in arthritis. Front Biosci 2006;11:529–43. [DOI] [PubMed] [Google Scholar]
- 39. Klatt AR, Klinger G, Neumüller O. et al. TAK1 downregulation reduces IL-1β induced expression of MMP13, MMP1 and TNF-alpha. Biomed Pharmacother 2006;60:55–61. [DOI] [PubMed] [Google Scholar]
- 40. Ahmed S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: progress and promise. Arthritis Res Ther 2010;12:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shim JH, Greenblatt MB, Xie M. et al. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J 2009;28:2028–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gunnell LM, Jonason JH, Loiselle AE. et al. TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J Bone Miner Res 2010;25:1784–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klatt AR, Klinger G, Paul-Klausch B. et al. TAK1 mediates the collagen-II-dependent induction of the COX-2 gene and PGE2 release in primary human chondrocytes. Connect Tissue Res 2010;51:452–8. [DOI] [PubMed] [Google Scholar]
- 44. Onodera Y, Teramura T, Takehara T, Shigi K, Fukuda K.. Reactive oxygen species induce Cox-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio 2015;5:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crofford LJ. COX-2 in synovial tissues. Osteoarthritis Cartilage 1999;7:406–8. [DOI] [PubMed] [Google Scholar]
- 46. Cronstein BN, Sunkureddi P.. Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. Clin Rheumatol 2013;19:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khanna PP, FitzGerald J.. Evolution of management of gout: a comparison of recent guidelines. Curr Opin Rheumatol 2015;27:139–46. [DOI] [PubMed] [Google Scholar]
- 48. Dinarello CA, Simon A, van der Meer JWM.. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012;11:633–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bahia MS, Kaur M, Silakari P, Silakari O.. Interleukin-1 receptor associated kinase inhibitors: potential therapeutic agents for inflammatory- and immune-related disorders. Cell Signal 2015;27:1039–55. [DOI] [PubMed] [Google Scholar]
- 50. Okada M, Matsuzawa A, Yoshimura A, Ichijo H.. The lysosome rupture-activated TAK1-JNK pathway regulates NLRP3 inflammasome activation. J Biol Chem 2014;289:32926–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gong YN, Wang X, Wang J. et al. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell Res 2010;20:1289–305. [DOI] [PubMed] [Google Scholar]
- 52. Sivera F, Wechalekar MD, Andres M, Buchbinder R, Carmona L.. Interleukin-1 inhibitors for acute gout. Cochrane Database Syst Rev 2014;9:Cd009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sada PR, Isenberg D, Ciurtin C.. Biologic treatment in Sjogren's syndrome. Rheumatology 2015;54:219–30. [DOI] [PubMed] [Google Scholar]
- 54. Yamada A, Arakaki R, Kudo Y, Ishimaru N.. Targeting IL-1 in Sjögren's syndrome. Expert Opin Ther Targets 2013;17:393–401. [DOI] [PubMed] [Google Scholar]
- 55. Vijmasi T, Chen FY, Chen YT, Gallup M, McNamara N.. Topical administration of interleukin-1 receptor antagonist as a therapy for aqueous-deficient dry eye in autoimmune disease. Mol Vis 2013;19:1957–65. [PMC free article] [PubMed] [Google Scholar]
- 56. Zilahi E, Tarr T, Papp G. et al. Increased microRNA-146a/b, TRAF6 gene and decreased IRAK1 gene expressions in the peripheral mononuclear cells of patients with Sjögren's syndrome. Immunol Lett 2012;141:165–8. [DOI] [PubMed] [Google Scholar]
- 57. Zoukhri D, Macari E, Choi SH, Kublin CL.. c-Jun NH2-terminal kinase mediates interleukin-1beta-induced inhibition of lacrimal gland secretion. J Neurochem 2006;96:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci 2012;33:522–30. [DOI] [PubMed] [Google Scholar]
- 59. Zippel N, Malik RA, Fromel T. et al. Transforming growth factor-beta-activated kinase 1 regulates angiogenesis via AMP-activated protein kinase-alpha1 and redox balance in endothelial cells. Arterioscler Thromb Vasc Biol 2013;33:2792–9. [DOI] [PubMed] [Google Scholar]
- 60. Wu J, Powell F, Larsen NA. et al. Mechanism and in vitro pharmacology of TAK1 inhibition by (5Z)-7-oxozeaenol. ACS Chem Biol 2012;8:643–50. [DOI] [PubMed] [Google Scholar]
- 61. Ninomiya-Tsuji J, Kajino T, Ono K. et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 2003;278:18485–90. [DOI] [PubMed] [Google Scholar]
- 62. Zhou J, Zheng B, Ji J. et al. LYTAK1, a novel TAK1 inhibitor, suppresses KRAS mutant colorectal cancer cell growth in vitro and in vivo. Tumour Biol 2015;36:3301–8. [DOI] [PubMed] [Google Scholar]
- 63. Melisi D, Xia Q, Paradiso G. et al. Modulation of pancreatic cancer chemoresistance by inhibition of TAK1. J Natl Cancer Inst 2011;103:1190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kilty I, Jones LH.. TAK1 selective inhibition: state of the art and future opportunities. Future Med Chem 2015;7:23–33. [DOI] [PubMed] [Google Scholar]
- 65. Hornberger KR, Chen X, Crew AP. et al. Discovery of 7-aminofuro[2,3-c]pyridine inhibitors of TAK1: Optimization of kinase selectivity and pharmacokinetics. Bioorg Med Chem Lett 2013;23:4511–6. [DOI] [PubMed] [Google Scholar]
- 66. Petroski MD, Zhou X, Dong G. et al. Substrate modification with lysine 63-linked ubiquitin chains through the UBC13-UEV1A ubiquitin-conjugating enzyme. J Biol Chem 2007;282:29936–45. [DOI] [PubMed] [Google Scholar]
- 67. Yu Y, Ge N, Xie M. et al. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NFκB and AP-1 activation as well as IL-6 gene expression. J Biol Chem 2008;283:24497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shin M-S, Shinghirunnusorn P, Sugishima Y. et al. Cross interference with TNF-α-induced TAK1 activation via EGFR-mediated p38 phosphorylation of TAK1-binding protein 1. Biochim Biophys Acta 2009;1793:1156–64. [DOI] [PubMed] [Google Scholar]
- 69. Kajino T, Ren H, Iemura S. et al. Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J Biol Chem 2006;281:39891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guo W, Wisniewski JA, Ji H.. Hot spot-based design of small-molecule inhibitors for protein–protein interactions. Bioorg Med Chem Lett 2014;24:2546–54. [DOI] [PubMed] [Google Scholar]
- 71. Yin H, Lee G-i, Sedey KA. et al. Terephthalamide derivatives as mimetics of helical peptides: disruption of the Bcl-xL/Bak interaction. J Am Chem Soc 2005;127:5463–8. [DOI] [PubMed] [Google Scholar]
- 72. Tan L, Nomanbhoy T, Gurbani D. et al. Discovery of type II inhibitors of TGF beta-activated kinase 1 (TAK1) and mitogen-activated protein kinase kinase kinase kinase 2 (MAP4K2). J Medicinal Chem 2015;58:183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schiffelers RM, Ansari A, Xu J. et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res 2004;32:e149. [DOI] [PMC free article] [PubMed] [Google Scholar]