Summary
MTN-017, a phase 2 expanded safety and acceptability study of the reduced-glycerin 1% tenofovir (TFV) gel, showed that rectal application was safe, with adherence and likelihood to use it at least twice weekly similar to daily oral emtricitabine/TFV disoproxil fumarate.
Keywords: rectal, microbicide, HIV, prevention, tenofovir.
Abstract
Background.
Human immunodeficiency virus (HIV) disproportionately affects men who have sex with men (MSM) and transgender women (TGW). Safe and acceptable topical HIV prevention methods that target the rectum are needed.
Methods.
MTN-017 was a phase 2, 3-period, randomized sequence, open-label, expanded safety and acceptability crossover study comparing rectally applied reduced-glycerin (RG) 1% tenofovir (TFV) and oral emtricitabine/TFV disoproxil fumarate (FTC/TDF). In each 8-week study period participants were randomized to RG-TFV rectal gel daily, or RG-TFV rectal gel before and after receptive anal intercourse (RAI; or at least twice weekly in the event of no RAI), or daily oral FTC/TDF.
Results.
MSM and TGW (n = 195) were enrolled from 8 sites in the United States, Thailand, Peru, and South Africa with mean age of 31.1 years (range 18-64). There were no differences in ≥grade 2 adverse event rates between daily gel (incidence rate ratio [IRR], 1.09; P = .59) or RAI gel (IRR, 0.90; P = .51) compared to FTC/TDF. High adherence (≥80% of prescribed doses assessed by unused product return and Short Message System reports) was less likely in the daily gel regimen (odds ratio [OR], 0.35; P < .001), and participants reported less likelihood of future daily gel use for HIV protection compared to FTC/TDF (OR, 0.38; P < .001).
Conclusions.
Rectal application of RG TFV gel was safe in MSM and TGW. Adherence and product use likelihood were similar for the intermittent gel and daily oral FTC/TDF regimens, but lower for the daily gel regimen.
Clinical Trials Registration:
Globally, men who have sex with men (MSM) and transgender women (TGW) are disproportionately affected by human immunodeficiency virus (HIV) infection [1–3] associated with receptive anal intercourse (RAI) without a condom. Randomized, placebo-controlled clinical studies of the oral antiretroviral combination emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) tablet taken daily or around the time of sexual intercourse have demonstrated high efficacy in reducing incident HIV infection in MSM [4–6]. Oral FTC/TDF is available by prescription for at-risk individuals in the United States, with licensure recently approved in France, Australia, Peru, South Africa, and Kenya.
Oral FTC/TDF preexposure prophylaxis (PrEP) requires established healthcare services to ensure safe prescribing and monitoring, and it remains to be seen if it will be acceptable and used in the longer term by at-risk MSM and TGW. While topical PrEP will also require monitoring, a potential advantage is its use in an event-driven manner. Lubricating gel is frequently used to facilitate anal intercourse [7], and topical HIV prevention candidates formulated as lubricants are likely to be acceptable and easily incorporated into the sexual practices of populations having RAI, with less behavior modification. Topical rectal microbicide (RM) PrEP has been in development for more than 15 years. Recently, this effort has focused on development of the 1% formulation of tenofovir (TFV) gel, initially using the vaginal formulation and later the reduced glycerin (RG) gel with lower osmolality [8]. The Microbicide Trials Network (MTN) 007 phase 1 study demonstrated that this formulation was safe and acceptable to men and women following daily rectal application for up to 7 consecutive days [9]. Two phase 1 studies of the RG formulation, CHARM 01 and CHARM 02, also confirmed safety as well as favorable colon pharmacokinetics (PK), including lower systemic exposure and reduced mucosal permeability associated with simulated RAI, compared to the vaginal formulation [9, 10]. This RG product was taken into the phase 2 MTN-017 study.
Our objective was to compare the safety profiles of daily oral FTC/TDF tablet, daily rectal TFV RG 1% gel, and RAI-associated rectal TFV RG 1% gel and to evaluate and compare their acceptability as potential HIV prevention methods.
METHODS
Study Design
MTN-017 was a phase 2, randomized sequence, open-label, expanded safety and acceptability crossover study of the oral FTC/TDF tablet and rectally applied TFV RG 1% gel (ClinicalTrials.gov number NCT01687218). Participants were randomized to 1 of 6 sequences to ensure equal likelihood of dosing sequence that consisted of three 8-week periods with different product use regimens: daily oral FTC/TDF (daily oral regimen), daily TFV RG 1% gel (daily rectal regimen), or TFV RG 1% gel used before and after RAI (RAI rectal regimen) and not exceeding 2 doses within 24 hours, consistent with the method used for vaginal application of 1% TFV gel in the CAPRISA 004 study in South African women (Table 1) [11]. If participants did not engage in RAI, they were instructed to use 2 doses of the TFV RG 1% gel at least once weekly. This ensured adequate product exposure to TFV RG 1% gel to allow progression to an effectiveness study. Participants were evaluated at weeks 0, 4 (mid-period), and 8 (end-period). There was a 1-week washout between periods, and a safety call was made 1 week after the last visit in period 3 to collect data on adverse events (AEs). A comprehensive package of HIV prevention counseling and sexually transmitted infection (STI) testing was administered throughout the study.
Table 1.
Study Regimens
| Sequence |
Period 1
(8 weeks)a |
Washout (~1 week) |
Period 2
(8 weeks)a |
Washout (~1 week) |
Period 3
(8 weeks)a |
|---|---|---|---|---|---|
| 1 | Daily oral | Daily rectal | RAI rectal | ||
| 2 | RAI rectal | Daily oral | Daily rectal | ||
| 3 | Daily rectal | RAI rectal | Daily oral | ||
| 4 | Daily rectal | Daily oral | RAI rectal | ||
| 5 | Daily oral | RAI rectal | Daily rectal | ||
| 6 | RAI rectal | Daily rectal | Daily oral |
Abbreviation: RAI: receptive anal intercourse.
aDaily rectal, daily tenofovir (TFV) reduced glycerin (RG) 1% gel. Daily oral, daily emtricitabine/TFV disoproxil fumarate. RAI rectal, RAI-associated TFV RG 1% gel.
The primary study objectives were to assess both safety and acceptability of daily oral FTC/TDF, daily rectal TFV RG 1% gel, and RAI-associated rectal TFV RG 1% gel. Secondary objectives were to compare systemic and local PK and to evaluate and compare adherence between the 3 product use regimens. The MTN-017 study protocol is available at http://www.mtnstopshiv.org/studies/4495.
Study Sites
There were 8 study sites: 4 in the United States (Boston, Pittsburgh, San Francisco, and San Juan), 2 in Thailand (Bangkok and Chiang Mai), and 1 each in Peru (Lima) and South Africa (Cape Town).
Ethical Considerations
Prior to implementation, the study protocol was reviewed and approved by the institutional review boards/ethics committees at each participating site. All participants provided written informed consent.
Participants
Healthy HIV-uninfected MSM and TGW aged ≥18 years with a history of RAI at least once in the previous 3 months were recruited via social and traditional media, online advertising, flyers, community engagement, and word of mouth. Individuals with abnormalities of the colorectal mucosa, significant gastrointestinal symptoms, rectal STI requiring treatment, chronic hepatitis B infection, hepatitis C exposure, a requirement to use drugs that were likely to increase the risk of bleeding following mucosal biopsy, or symptoms suggestive of HIV seroconversion were excluded from the study. Full inclusion and exclusion criteria are available at http://www.mtnstopshiv.org/studies/4495.
Study Products
CONRAD (Arlington, Virginia) supplied the TFV RG 1% gel, which was provided in prefilled applicators (HTI Plastics, Lincoln, Nebraska) containing 4 mL of gel. Oral FTC/TDF was supplied by Gilead Sciences (Foster City, California). Participants were asked to take either 1 oral FTC/TDF tablet with water daily or to deliver intrarectally the content of an applicator filled with TFV RG 1% gel using a sachet of lubricant to facilitate insertion (Good Clean Love Inc., Eugene, Oregon) either daily or before and after RAI.
Clinical Safety
AEs were graded using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0, December 2004, as well as Addendum 3 (Rectal Grading Table for Use in Microbicide Studies; http://rsc.tech-res.com/safetyandpharmacovigilance/). In cases where an AE was covered in both tables, the Rectal Grading Table for Use in Microbicide Studies was the grading scale used.
Laboratory Assessments
Routine safety laboratory evaluations included testing for renal and liver function, hematology including coagulation, and STIs including hepatitis B and HIV. HIV drug resistance was assessed using an in-house Sanger sequencing-based population genotyping assay. A “real-time” qualitative (detectable/undetectable) plasma TFV assessment using the lower limit of quantitation of the assay (0.31 ng/mL) was made at each mid- and end-period study visit. This allowed for on-study adherence monitoring and provided context for the behavioral assessments of adherence (product returns and Short Message System [SMS] text responses) described below. PK (plasma, rectal sponge, and tissue), Pharmacodynamic (PD; rectal sponge and tissue), mucosal T-cell phenotype, and microarray analysis are pending analyses [8, 12].
Product Acceptability
Product acceptability was assessed via a computer-assisted self-interview, administered at baseline and after each 8-week period, in the dominant languages of study participants (English, Spanish, Thai, Xhosa, and Afrikaans). After each period, participants were asked to indicate their overall liking of the product regimen they had just finished using (4-point scale from 1 = disliked very much to 4 = liked very much), overall ease of use (1 = very difficult to 4 = very easy), and likelihood of future use if the product regimen provided protection against HIV (1 = very unlikely to 4 = very likely).
Product Adherence
Adherence was assessed in 3 ways: daily SMS inquiring as to the number of doses taken since the last report, returned study product counts (unused pills and gel applicators) at mid- and end-period visits, and “real-time” qualitative drug detection using the lower limit of quantification of the assay (0.31 ng/mL) to indicate TFV detected or not detected in plasma taken at mid- and end-period visits when available (at the visit following the PK blood draw). A data convergence interview was conducted at mid- and end-period visits by trained adherence counselors in which any discrepancies between SMS reports of product use and returned study product counts were discussed. A client-centered approach was used to engage the participant’s collaboration with the counselor to estimate together the most accurate number of doses used. Once mid- and end-period visits for each regimen were complete, the final adherence rates for each 8-week period were summed to calculate the percentage of prescribed doses taken orally or administered rectally over the total number of days between initial and final period visits. High adherence was operationalized as ≥80% of prescribed doses as assessed by product return and SMS reports. A detailed description and analysis of the mixed-methods adherence measurement used in this study will be published separately.
Data Management and Analyses
The MTN-017 study replaced participants with no adherence data available in either the daily gel regimen or the RAI gel regimen until enrollment closed. Any participant with exposure to study product was included in safety analyses for the product regimens to which they were exposed.
Grade 2 or higher AEs were compared between study regimens using a generalized linear model with Poisson (log) link function; the model included product regimen as a covariate and adjusted for study period to account for the crossover design. A generalized estimating equations method was used with exchangeable correlation structure and robust standard error estimates to account for within-subject correlation due to repeated measures. The offset in the model was set to be the number of days a participant was intended to be exposed to the product.
For the 3 acceptability endpoints, the responses were dichotomized into positive (liked very much/a little, very easy/easy, very likely/likely) and negative (disliked very much/a little, very difficult/difficult, very unlikely/unlikely) responses. Because the distribution of the final adherence results for each regimen were skewed, they were dichotomized into <80% adherence vs ≥80% adherence. Mathematical modeling combining the results of a PK study following oral TDF and the Iniciativa Profilaxis Pre-Exposición study (iPrEx) data on HIV risk indicates that adherence to oral FTC/TDF for 4 days per week (ie, 4/7 = 57%) results in HIV risk reduction of 96% [13]. As rectal dosing with gel may result in more transient protection than oral dosing with FTC/TDF, a higher level of adherence (80%) was selected. To compare the study regimens for these behavioral endpoints, the same approach for analyzing AEs was used with the exception that a binomial (logit) link function was used. SAS software, version 9.4 (SAS Institute, Cary, North Carolina), was used for all analyses.
RESULTS
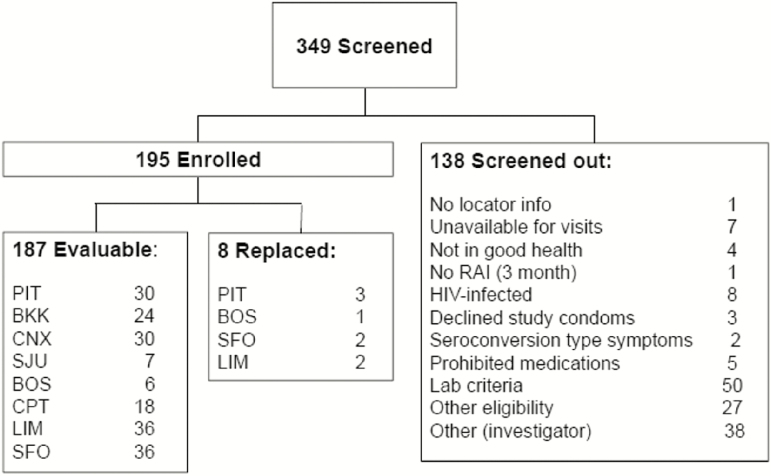
Between September 2013 and November 2014, 349 participants were screened and 195 enrolled (Figure 1). The mean participant age was 31.1 years (range 18–64; Table 2). Reasons for participants screening out were primarily for laboratory criteria (including STIs) and investigator discretion, with 8 individuals diagnosed as HIV infected. Eight study participants were replaced, up until full study accrual, due to declining further study participation or relocation. Of the 187 participants remaining, 185 (98.9%) were retained throughout the study, including their scheduled exit visit. More than 98% of all study visits and procedures were completed. Four participants became HIV infected while on study, 3 at the Cape Town site and 1 at a US site. Seroconversions occurred as described in the following text. Participant A (RAI, oral, daily rectal sequence) had symptoms consistent with and confirmed as seroconversion prior to starting oral product. He reported 100% gel use before and after RAI. Participant B (oral, daily rectal, RAI sequence) had a positive rapid HIV test at the end of the daily rectal regimen. Adherence on the oral regimen was 86%–100% by visit and on the daily rectal regimen was 79%–100% by visit. Participant C (RAI, oral, daily rectal sequence) had a positive HIV rapid test 110 days after his last HIV negative test at the mid-period visit in the daily rectal regime. Participant D (daily rectal, oral, RAI sequence) had a positive HIV rapid test at the oral end-period visit. Adherence on the oral regimen was 39%–93%, by visit.
Figure 1.
Consort diagram. Abbreviations: BKK, Bangkok; BOS, Boston; CNX, Chiang Mai; CPT, Cape Town; HIV, human immunodeficiency virus; LIM, Lima; PIT, Pittsburgh; RAI, receptive anal intercourse; SFO, San Francisco; SJU, San Juan.
Table 2.
Demographics
| Participant variables | Sites | All (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 a | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Participants enrolled | 38 | 7 | 18 | 24 | 30 | 7 | 33 | 38 | 195 |
| Age (mean/years) | 32.9 | 30.9 | 22.8 | 31.5 | 27.9 | 34.6 | 30.2 | 35.9 | 31.1 |
| College education (any) | 27 | 6 | 5 | 21 | 25 | 4 | 32 | 36 | 156 (80) |
| Gender | |||||||||
| Male | 24 | 7 | 13 | 15 | 7 | 7 | 32 | 36 | 141 (73) |
| Femaleb | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4 (2) |
| Transgender female | 3 | 0 | 4 | 2 | 9 | 0 | 0 | 1 | 19 (10) |
| Other | 6 | 0 | 1 | 6 | 13 | 0 | 0 | 1 | 27 (14) |
| Refused | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 (2) |
aGender data missing for 1 participant.
bAll participants were born male.
No participant had resistance to TFV or FTC, although 2 had a nonnucleoside reverse transcriptase resistance mutation indicating transmitted resistance.
Safety
Among the 195 enrolled participants, approximately one-third reported grade 2 or higher AEs for each study regimen (Table 3 and Supplemental Table 1), and there were no statistically significant differences between the regimens. Excluding rectal infection with Chlamydia trachomatis or Neisseria gonorrhoeae, the most common grade 2 AE when using the daily oral regimen was headache and when using the daily rectal regimen was diarrhea. There were 6 grade 3 and 4 grade 4 AEs reported, none of which were related to study product. No deaths were reported.
Table 3.
Comparison of Adverse Events by Study Regimen
| Study regimen | N Exposed | Total Number of All AEs Reported | N With at Least 1 ≥ Grade 2 AE | Estimated Incidence Rate Ratioa | 95% Confidence Interval | P Value | |
|---|---|---|---|---|---|---|---|
| Daily rectal | 192 | 139 | 64 | 1.09 | 0.79 | 1.53 | .59 |
| Receptive anal intercourse, rectal | 191 | 119 | 58 | 0.90 | 0.66 | 1.23 | .51 |
| Daily oral | 192 | 128 | 65 | Reference | |||
aThe analysis used a generalized linear model with Poisson (log) link function; the model included product regimen as a covariate and adjusted for study period to account for the crossover design. The generalized estimating equations method was used with exchangeable working correlation and robust standard error estimates to account for within-subject correlation due to repeated measures.
Acceptability
Participants reported “liking” product during the daily oral, RAI rectal, and daily rectal regimens with approximately 90%, 80%, and 70% frequencies, respectively, and liked oral product significantly more than daily gel (P < .001) or RAI gel (P = .002; Table 4). Regarding ease of use, participants reported that both the daily and RAI rectal gel regimens were “easy” less frequently compared to the daily oral regimen, though this was not statistically significant (P = .08 and .46, respectively). Participants less frequently reported that they were “likely” to use the daily rectal gel regimen compared to the daily oral regimen if it were found to provide protection against HIV (odds ratio [OR], 0.38; P < .001). There was no statistically significant difference when comparing likelihood to use daily oral regimen or RAI rectal regimen (Table 4). Participants rated liking the gel significantly higher than they rated liking the gel applicator in both the daily rectal and RAI rectal regimens (adjusted mean differences = 0.14, P = .008 and 0.16, P = .004, respectively).
Table 4.
Regression Analysis Comparing Acceptability of Study Regimen
| Study regimen | Unadjusted Proportion With Positive Rating |
Adjusted
Odds Ratio a |
95% Confidence Interval | P Value | |
|---|---|---|---|---|---|
| Outcome: Overall Liking [Liked (1) vs Disliked (0)] | |||||
| Oral | 0.91 | Reference | NA | NA | NA |
| Daily rectal | 0.74 | 0.28 | 0.15 | 0.50 | <.001 |
| RAI rectal | 0.79 | 0.37 | 0.20 | 0.70 | .002 |
| Outcome: Overall Ease of Use [Easy (1) vs Difficult (0)] | |||||
| Oral | 0.92 | Reference | NA | NA | NA |
| Daily Rectal | 0.87 | 0.56 | 0.29 | 1.08 | .08 |
| RAI Rectal | 0.90 | 0.76 | 0.37 | 1.56 | .46 |
| Outcome: Likelihood to Use [Likely (1) vs Unlikely (0)] | |||||
| Oral | 0.87 | Reference | NA | NA | NA |
| Daily Rectal | 0.72 | 0.38 | 0.22 | 0.65 | <.001 |
| RAI Rectal | 0.82 | 0.70 | 0.39 | 1.25 | .23 |
Abbreviation: RAI, receptive anal intercourse.
aThe analysis used a generalized linear model with binomial (logit) link function; the model included product regimen as a covariate and adjusted for study period to account for the crossover design. The generalized estimating equations method was used with exchangeable working correlation and robust standard error estimates to account for within-subject correlation due to repeated measures.
Adherence
Table 5 shows the proportion of participants with high adherence (≥80%) for each regimen. The daily oral and RAI rectal regimens had the highest percent of participants achieving ≥80% adherence (94% and 93%, respectively) based on the final adherence results, and the daily rectal regimen was lower (83%). Participants were significantly less likely to have been ≥80% adherent when they were on the daily rectal regimen compared to the daily oral regimen (OR, 0.35; P < .001). Participants’ odds of being adherent 80% or more were similar for the RAI rectal regimen and daily oral regimen. A separate publication will present a more detailed discussion of adherence measurement in this study.
Table 5.
Regression Analysis Comparing Adherence of Study Regimen
| Study regimen | Unadjusted Proportion With at Least 80% Adherence |
Adjusted
Odds Ratio a |
95% Confidence Interval | P Value | |
|---|---|---|---|---|---|
| Outcome: Product Adherence Based on Product Return and Short Message System Text [≥0.80 (1) vs <0.80 (0)] | |||||
| Oral | 0.94 | Reference | NA | NA | NA |
| Daily rectal | 0.83 | 0.35 | 0.19 | 0.63 | <.001 |
| Receptive anal intercourse, rectal | 0.93 | 0.89 | 0.43 | 1.81 | .74 |
aThe analysis used a generalized linear model with binomial (logit) link function; the model included product regimen as a covariate and adjusted for study period to account for the crossover design. The generalized estimating equations method was used with exchangeable working correlation and robust standard error estimates to account for within-subject correlation due to repeated measures. NA, not applicable.
DISCUSSION
In this study of MSM and TGW, rectal application of RG TFV gel was safe. The oral FTC/TDF and both rectal regimens were liked by more than 70% of participants with a significantly greater percent “liking” the daily oral compared to either rectal regimen. However, when acceptability was broken down into ease of use and likelihood to use if the rectal gel were shown to prevent HIV infection, there was no statistically significant difference between gel applied at least twice weekly compared to daily oral. This same comparison was demonstrated for adherence to gel product. The daily rectal gel was less frequently positively regarded in these categories compared to daily oral dosing and was associated with the least degree of product adherence compared to the RAI rectal and daily oral regimens.
Distinct from the important issues of acceptability and adherence, protection from HIV with TFV 1% gel may be achieved with less than single daily doses, but this remains to be tested. Active TFV diphosphate concentrations in colon tissue cells associated with protection in iPrEx are higher and achieved more rapidly after rectal dosing of TFV 1% gel (both vaginal formulation and RG formulations) compared to oral TDF dosing [9, 14, 15].
Participants found the daily rectal gel less acceptable and were less adherent during this study regimen. This is not surprising as there is very little context for this population to administer a rectal medication using any method, but particularly with an applicator, in the absence of a clear medical indication or need. It may therefore be more “usual” for this population to prefer an event-driven method of rectal product use such as applying anal lubricant prior to RAI. The investigational product was a rectal gel and, while most MSM and TGW are familiar with and frequently apply anal lubricant to facilitate RAI, an important distinction must be made. While the rectal product may look and feel like a lubricant, it has no known lubricating capacity when applied directly into the rectum via an applicator and clearly presented challenges to study participants that may relate to inconvenience or possible dose effect.
The context for oral dosing of prophylactic products such as vitamins or supplements may be more familiar to the study population, although less so with a product with the potential for significant adverse events that require medical monitoring. However, this may be balanced in the case of oral FTC/TDF that received a license for HIV prevention in the United States prior to MTN-017 starting and may have been perceived as being of proven benefit.
Similar to the topical and injectable female-controlled methods of contraception developed since the oral contraceptive pill became available, there is a need for alternatives to oral PrEP to satisfy user preferences [16–18]. One alternative may be an RM, ideally one that would lubricate the anal canal in order to facilitate RAI, protect the rectum from incident HIV infection, achieve satisfactory drug levels and mucosal coverage at the rectal site, and offer a delivery method that users would find acceptable. One small study demonstrated similar colon luminal distribution of gels when manual application as a lubricant was compared to intrarectal applicator dosing. However, only 3% of the gel volume was delivered manually compared to the applicator [19].
The safety, adherence, and acceptability profile of RG-TFV in this phase 2 study support further development of the product as an RM candidate; however, consideration needs to be given to dosing method and timing in relation to RAI. Specifically, future studies should explore the possibility of rectal dosing without an applicator by using the product as a “lubricant” prior to RAI with careful assessment of local tissue PK.
The limitations inherent to microbicide studies primarily concern issues surrounding adherence to product use. In MTN-017 this issue was comprehensively addressed by using multiple methods and providing feedback to participants on qualitative plasma PK results, and with similar SMS/product return and plasma TFV detection, may be a useful model for future studies.
In general, further study of any HIV prevention technology, whether it be a rectal gel, long-acting antiretroviral injection, or rectal enema, has obvious complexities and challenges in the context of efficacious oral PrEP. These challenges should be embraced and addressed by the scientific community in order to provide expanded options for those individuals who remain at risk of HIV regardless of oral PrEP availability.
Supplementary Material
Notes
Acknowledgments. We are grateful to the study participants for their participation and dedication. We thank the study team members at the research sites, the protocol management team, and the MTN leadership operations center for their contributions. We are grateful to Gilead Sciences who provided the FTC/TDF and CONRAD for providing RG-TFV.
Disclaimer. The authors designed and executed the study, had full access to the raw data, performed all analyses, wrote the manuscript, and had final responsibility for the decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the NIH.
Potential conflicts of interest. The authors declare the following: R. D. C., UpToDate medical encyclopedia royalties. B. A. R., Tobira Therapeutics, Inc., Theratechnologies, Inc., and Pepper Hamilton, LLC consultancy fees. C. W. H., University of California–Los Angeles consultancy fee. L. G. B., National Coordinator consultancy fee. A. L., International AIDS Society (IAS)-USA manuscript preparation and Medscape educational material preparation fees. I. M., Novicol Life Sciences board membership, ABIVAX and Aelix Therapeutics consultancy. J. L. S., CONRAD employment. J. R., Gilead Sciences employment and stock/options. J. P., AIDS Foundation of Chicago employment. C. Z., ViiV Healthcare consultancy, and Gilead Sciences and Pfizer grants pending. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS 2013; 27:2665–78. [DOI] [PubMed] [Google Scholar]
- 2. Baral S, Trapence G, Motimedi F, et al. HIV prevalence, risks for HIV infection, and human rights among men who have sex with men (MSM) in Malawi, Namibia, and Botswana. PLoS One 2009; 4:e4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13:214–22. [DOI] [PubMed] [Google Scholar]
- 4. Grant RM, Lama JR, Anderson PL, et al. ; iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Molina JM, Capitant C, Spire B, et al. ; ANRS IPERGAY Study Group On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 7. Carballo-Diéguez A, Stein Z, Sáez H, Dolezal C, Nieves-Rosa L, Díaz F. Frequent use of lubricants for anal sex among men who have sex with men: the HIV prevention potential of a microbicidal gel. Am J Public Health 2000; 90:1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGowan I, Hoesley C, Cranston RD, et al. A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007). PLoS One 2013; 8:e60147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mcgowan I, Cranston RD, Duffill K, et al. A phase 1 randomized, open label, rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of three formulations of tenofovir 1% gel (the CHARM-01 study). PLoS One 2015; 10:e0125363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiruy H, Fuchs EJ, Marzinke MA, et al. A phase 1 randomized, blinded comparison of the pharmacokinetics and colonic distribution of three candidate rectal microbicide formulations of tenofovir 1% gel with simulated unprotected sex (CHARM-02). AIDS Res Hum Retroviruses 2015; 31:1098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. ; CAPRISA 004 Trial Group Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hladik F, Burgener A, Ballweber L, et al. Mucosal effects of tenofovir 1% gel. Elife 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson PL, Glidden DV, Liu A, et al. ; iPrEx Study Team Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anton PA, Cranston RD, Kashuba A, et al. RMP-02/MTN-006: a phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 2012; 28:1412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang KH, Hendrix C, Bumpus N, et al. A multi-compartment single and multiple dose pharmacokinetic comparison of rectally applied tenofovir 1% gel and oral tenofovir disoproxil fumarate. PLoS One 2014; 9:e106196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newman PA, Cameron MP, Roungprakhon S, Tepjan S, Scarpa R. Acceptability and preferences for hypothetical rectal microbicides among a community sample of young men who have sex with men and transgender women in Thailand: a discrete choice experiment. AIDS Behav 2016; 20:2588–601. [DOI] [PubMed] [Google Scholar]
- 17. Marra E, Hankins CA. Perceptions among Dutch men who have sex with men and their willingness to use rectal microbicides and oral pre-exposure prophylaxis to reduce HIV risk—a preliminary study. AIDS Care 2015; 27:1493–500. [DOI] [PubMed] [Google Scholar]
- 18. Peinado J, Lama JR, Galea JT, et al. Acceptability of oral versus rectal HIV preexposure prophylaxis among men who have sex with men and transgender women in Peru. J Int Assoc Provid AIDS Care 2013; 12:278–83. [DOI] [PubMed] [Google Scholar]
- 19. Shieh E, Weld E, Fuchs EJ, et al. Gel applied as anal lube without applicator provides poor rectal mucosal HIV coverage. Conference on Retroviruses and Opportunistic Infections 2017, Boston. Abstract 169bLB2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.