Summary
Reported are the results of the largest population pharmacokinetic study of intravenous colistin in various categories of critically ill patients, including those receiving different types of renal replacement therapy, and derived dosing guidance to achieve a desired plasma colistin concentration.
Keywords: Intravenous colistin, critically ill patients, population pharmacokinetics, influence of renal impairment and renal replacement modalities, dosing guidance.
Abstract
Background.
Intravenous colistin is difficult to use because plasma concentrations for antibacterial effect overlap those causing nephrotoxicity, and there is large interpatient variability in pharmacokinetics. The aim was to develop dosing algorithms for achievement of a clinically desirable average steady-state plasma colistin concentration (Css,avg) of 2 mg/L.
Methods.
Plasma concentration-time data from 214 adult critically ill patients (creatinine clearance, 0–236 mL/min; 29 receiving renal replacement therapy [RRT]) were subjected to population pharmacokinetic analysis. Development of an algorithm for patients not receiving RRT was based on the relationship between the dose of colistimethate that would be needed to achieve a desired Css,avg and creatinine clearance. The increase in colistin clearance when patients were receiving RRT was determined from the population analysis and guided the supplemental dosing needed. To balance potential antibacterial benefit against risk of nephrotoxicity the algorithms were designed to achieve target attainment rates of >80% for Css,avg ≥2 and <30% for Css,avg ≥4 mg/L.
Results.
When algorithm doses were applied back to individual patients not receiving RRT (including those prescribed intermittent dialysis on a nondialysis day), >80% of patients with creatinine clearance <80 mL/min achieved Css,avg ≥2 mg/L, but for patients with creatinine clearance ≥80 mL/min, the target attainment was <40%, even with the maximum allowed daily dose of 360 mg colistin base activity. For patients receiving RRT, target attainment rates were >80% with the proposed supplemental dosing. In all categories of patients, <30% of patients attained Css,avg ≥4 mg/L.
Conclusions.
The project has generated clinician-friendly dosing algorithms and pointed to circumstances in which intravenous monotherapy may be inadequate.
Colistin, a last-resort antibiotic against gram-negative infections, is administered intravenously as its inactive prodrug colistimethate [1–3]. Selection of a colistimethate dose for individual patients is extremely difficult because the apparent clearance of colistin depends on renal function [4] and is subject to extensive interpatient variability even at a given creatinine clearance [4, 5]. Furthermore, based on pharmacokinetic (PK)/pharmacodynamic data translated from infection models [6] and PK/toxicodynamic relationships for risk of nephrotoxicity in patients [7–9], there is substantial overlap in the plasma concentrations required for antibacterial effect and those that increase the likelihood of colistin-associated nephrotoxicity [10]. Based on these considerations, and keeping in mind that the actual minimum inhibitory concentration (MIC) may be unknown when therapy commences, an average plasma steady-state concentration of colistin (Css,avg) of 2 mg/L is a reasonable target when initiating therapy [10]. From the interim analysis of a population PK study in critically ill patients with a wide range of renal function including those receiving renal replacement therapy (RRT), Garonzik et al [4] have reported algorithms for individualized dosing to achieve a desired Css,avg. We report here an update to those dosing suggestions, based on the final analysis of data from more than double the number of patients in the interim analysis.
METHODS
Patients and Ethics
Patients enrolled into this multinational (United States, Thailand, and Greece), 4-center study on the population PK of colistimethate and formed colistin were adult (aged >18 years) critically ill patients (https://clinicaltrials.gov/ct2/show/NCT00235690). The ethics committee of each institution approved this open-label, nonrandomized study; informed consent was obtained for all patients. The inclusion and exclusion criteria were described in an earlier report on the interim population PK analysis of the data for the first 105 patients enrolled in the study (16 were receiving RRT) [4]. The current report includes data from an additional 110 patients for a total of 215 patients (29 receiving RRT on the PK study day). Demographic characteristics are provided in Table 1 and RRT conditions in Supplementary Data Table S1.
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Age, median (range), y | 66 (19–101) |
| Weight median (range), kg | 61 (30–122) |
| Height median (range), cm | 165 (140–193) |
| APACHE II score, median (range) | 21 (4–43) |
| Creatinine clearance, median (range), mL/min | 39.8 (0–314) |
| Sex, No. (%) | |
| Male | 138 (64.2) |
| Female | 77 (35.8) |
| Comorbid condition, No. (%) | |
| Malignancy | 36 (16.7) |
| Hepatic failure | 20 (9.3) |
| Diabetes mellitus | 58 (27.0) |
| Immunosuppression | 31 (14.4) |
| RRT on study day, No. (%) | |
| Intermittent hemodialysis | 16 (7.4) |
| Sustained low-efficiency dialysis | 4 (1.9) |
| CRRTa | 9 (4.2) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CRRT, continuous renal replacement therapy; RRT, renal replacement therapy.
aContinuous venovenous hemodialysis (n = 7) or hemofiltration (n = 2).
Colistin Administration, Sample Collection, and Analysis
Colistimethate was administered intravenously for the treatment of bloodstream infections or pneumonia caused by gram-negative bacteria [4]. The dosage regimen was at the discretion of the physician caring for the patient; the median daily dose of colistin base activity (CBA) was 200 mg (range, 75–600 mg); 33 mg of CBA is approximately 1 million IU. Blood samples were collected across a dosage interval on days 3–5 of the regimen to quantify steady-state plasma concentrations of colistimethate and colistin; patients receiving intermittent dialysis may also have had blood samples collected on another day if dialysis was not conducted on the day of the above-mentioned sampling [4]. The unbound fraction of colistin in plasma was determined by ultracentrifugation [6] in samples collected from 66 of the patients; samples were from near the end of the dosage interval to minimize the concentration of colistimethate present and were supplemented with colistin (2 mg/L).
Population PK Analysis
Nonlinear mixed-effects modeling (S-ADAPT MCPEM algorithm) was used to analyze the plasma concentration-time data for both colistimethate and colistin in all 215 patients [4, 10]. The structural model from the interim analysis [4] was used as the starting point, and all relevant covariates were retested. These included body size (actual and ideal body weight, body surface area [BSA], body mass index), sex, age, creatinine clearance [11], and Acute Physiology and Chronic Health Evaluation (APACHE) II score on clearance of colistimethate and colistin; and body size (actual and ideal body weight, BSA, body mass index) on volume of distribution.
Development of an Algorithm for Colistimethate Daily Dose in Patients Not Receiving RRT
The model-derived post hoc parameter estimates from the population PK modeling were used to compute the area under the plasma colistin concentration-time curve across 24 hours at steady state (AUCss,0–24) for each patient. Division of each AUCss,0–24 value by 24 hours generated the corresponding Css,avg. This concentration and the physician-selected dose administered, along with Cockcroft and Gault estimated creatinine clearance [12], were used to derive the dosing algorithm. The algorithm was based on the relationship between the dose of colistimethate (as CBA) that would be needed to achieve each 1 mg/L of Css,avg and creatinine clearance. As noted below, the best regression fit between these variables was observed when colistimethate dose was in the logarithmic scale. The equation of the regression line for the log-linear relationship was used as a starting point to establish the dosing algorithm. Given that the line of best fit goes through the middle of the data, if one used the actual regression equation only approximately 50% of patients to whom the algorithm was applied would achieve the desired exposure. Thus, the intercept of the regression line for the log-linear relationship between colistimethate dose for each 1 mg/L Css,avg and creatinine clearance was adjusted such that, when it was applied back to the patients in this study, overall >80% of the patients would achieve a Css,avg of the quantum value in the algorithm (ie, 1 mg/L), and >90% would achieve plasma exposure of ≥75% of the quantum value (ie, 0.75 mg/L). In adjusting the intercept, consideration was also given to the very narrow therapeutic window of colistin [7–10], such that <30% of the patients would achieve plasma exposure of greater than twice the quantum value (ie, 2 mg/L).
The intercept-adjusted regression equation (“the algorithm”) for the relationship between the log10 dose of CBA needed to achieve each 1 mg/L (“the quantum”) of colistin Css,avg and creatinine clearance was used to compute the dose needed for each patient to achieve a plasma colistin Css,avg of twice the quantum (ie, 2 mg/L). This concentration is a reasonable target for therapy with intravenous colistimethate [10]. From the algorithm, the dose needed to achieve a Css,avg of ≥2 mg/L was determined at the midpoint of 10-mL/min increments of creatinine clearance. A maximum daily dose of 360 mg CBA (~11 million IU) was set [10, 13]. The computed dose for the midpoint of each 10-mL/min creatinine clearance window was then applied back to each patient within that narrow renal function window; if the computed CBA dose for a patient exceeded 360 mg/d, the latter value was applied. From knowledge of the plasma colistin Css,avg actually achieved from the physician-selected daily dose of colistimethate in each patient, the anticipated Css,avg for each patient if the algorithm-predicted daily dose or the capped daily dose had been administered was computed as described elsewhere [10].
Target attainment rates for plasma colistin Css,avg of ≥2 mg/L with the computed dose or the capped dose were determined for clusters of creatinine clearance (<30, 30 to <50 mL/min, 50 to <80, and ≥80 mL/min), as as described previously [10]. In addition, using the same computed or capped dose the attainment rates for plasma colistin Css,avg of ≥0.5 mg/L, ≥1 mg/L, ≥1.5 mg/L, and ≥4 mg/L were determined.
Determination of Colistimethate Daily Dose in Patients Receiving RRT
For patients receiving intermittent dialysis, the colistimethate daily dose on a nondialysis day was from the algorithm above for a patient with a creatinine clearance of 0 mL/min. The increase in the apparent clearance of colistin when patients were receiving dialysis was determined from the population PK analysis and used to guide the magnitude of supplemental dose needed. For patients receiving continuous RRT (CRRT), the suggested colistimethate daily dose was calculated from the apparent colistin clearance determined in the population PK analysis.
RESULTS
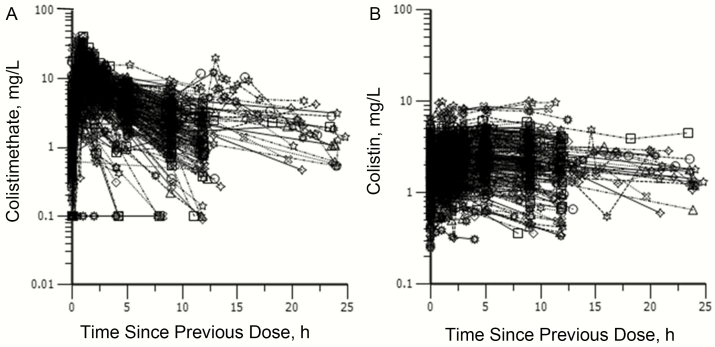
The plasma concentration-time profiles for all patients are presented in Figure 1. The median plasma colistin Css,avg was 2.35 mg/L, but individual values varied widely (0.24–9.92 mg/L) with the physician-selected doses. The mean (SD) unbound fraction of colistin in plasma was 0.49 (0.11).
Figure 1.
Steady-state plasma concentrations of colistimethate (A) and formed colistin (B) across a dosage interval in 215 critically ill patients. Patients were receiving colistimethate either every 8, 12, or 24 hours.
In the population PK analysis, the final model remained very similar to the model reported by Garonzik et al [4]; the only significant covariates were creatinine clearance for clearance of colistimethate and colistin, and body weight for volume of the central and peripheral compartments of colistimethate. The only difference is that, in the previously reported model, body weight was found to be a covariate only for the central compartment volume of colistimethate [4]. Details of the goodness of fit of the final population PK model to the data are in Supplementary Data (Figures S1 and S2). All model-fitted parameters were estimated with good to excellent precision (standard errors, 2.94%–27.7%), with moderate to high interindividual variability for this very diverse patient population (Table S2, Supplementary Data). Parameter estimates were similar to those from the interim analysis [4]. For 1 patient (37-year old man; body weight, 110 kg; APACHE II score, 11; creatinine clearance, 314 mL/min), all plasma colistimethate concentration data were below the limit of quantification of the assay, probably resulting in a poor clearance estimate that led to a substantial misfit (residual error, >3x relative standard error) in the algorithm plot; the data for this patient were excluded, and the creatinine clearance range for the remaining 214 patients was 0–236 mL/min.
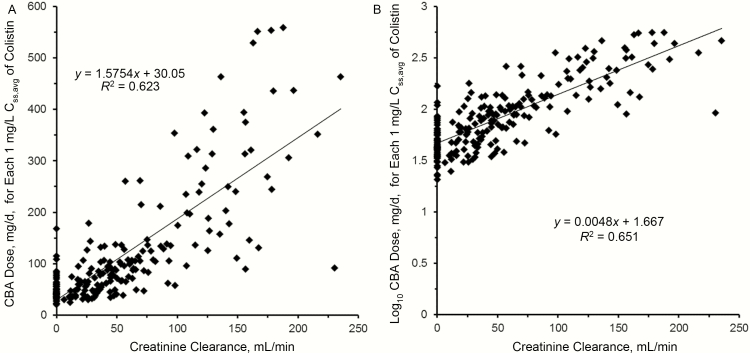
In regard to the renally based daily dosing algorithm, the relationship between the daily dose of CBA needed for each 1 mg/L of plasma colistin Css,avg and creatinine clearance is shown in Figure 2; the log-linear version provided the best regression fit. The final step in the development of the dosing algorithm was to adjust the intercept of the regression line in Figure 2B from 1.667 to 1.825. The algorithm is Equation 2 (Table 2). When this relationship was applied back to each of the 214 patients for a Css,avg of 1 mg/L (the quantum), 81.3% of patients achieved this concentration, 93.5% achieved 75% of the quantum, and 20.6% achieved a concentration twice that of the quantum. This performance satisfied the criteria set before the algorithm was developed (see Methods).
Figure 2.
Linear-linear (A) and log-linear (B) plots of the relationship between the daily dose of colistin base activity (CBA) needed for each 1 mg/L of the average steady-state plasma concentration of colistin (Css,avg) and creatinine clearance. The regression equation in panel B with the intercept adjusted from 1.667 to 1.825 is the renally based dosing algorithm (Equation 2 in Table 2).
Table 2.
Suggested Loading and Daily Doses of Colistimethate for a Desired Target colistin Css,avg of 2 mg/L in Various Categories of Critically ill Patients
| Dose | Category of Critically Ill Patient | Dosing Suggestionsa |
|---|---|---|
| Loading dose | All patient categories | Equation 1: Loading dose of CBA (mg) = Css,avg target (mg/L) × 2.0 × ideal body weight (kg) To achieve a Css,avg of 2 mg/L in a patient with an ideal body weight of 75 kg, the loading dose would be 300 mg CBA (9 million IU), the suggested maximum loading dose. The 1st regular daily dose should be administered 12 h later. |
| Daily doseb | Not receiving RRT | Equation 2c: Daily dose of CBA (mg) = Css,avg target (mg/L) × 10(0.0048 × CrCl + 1.825) See Table 3 (“look-up” table) for the daily dose to target a plasma colistin Css,avg of 2 mg/L, depending on the patient’s creatinine clearance. |
| Receiving RRT | The baseline daily dose of colistimethate for a Css,avg of 2 mg/L in a patient with creatinine clearance of 0 mL/min is 130 mg/d of CBA (3.95 million IU/d) (see Table 3)d; the supplement to the baseline daily dose needed during receipt of RRT is 10% of the baseline dose per 1 h of RRT. | |
| Intermittent hemodialysis | Nondialysis day: CBA dose of 130 mg/d (3.95 million IU/d), ie, baseline dosing for a Css,avg of 2 mg/L; dialysis day supplement: add 30% or 40% to baseline daily dose after a 3- or 4-h session, respectively.e The dialysis session should occur toward the end of a colistimethate dosing interval, and the supplement to the baseline (nondialysis) daily dose should be administered with next regular dose, after the dialysis session has ended. | |
| SLED | During SLED: add 10% per 1 h of SLED replacement to baseline daily dose for a Css,avg of 2 mg/Lf; for a patient receiving a 10-h nocturnal SLED session each day and receiving colistimethate every 12 h, the dose would be (baseline CBA dose of 130 mg/d for a patient with creatinine clearance of 0 mL/min + supplemental dose comprising 10% of the baseline dose per h × 10 h); ie, for this case the CBA dose would be 260 mg/d (7.9 million IU/d). It is suggested that the SLED session begin 1–2 h after the afternoon/evening dose; in such a case, it may be most convenient and safe to administer 130 mg CBA (3.95 million IU) every 12 h. | |
| CRRT | During CRRT: add 10% per 1 h of CRRT to the baseline daily dose for a Css,avg of 2 mg/Lg; the suggested CBA dose is 440 mg/d (~13 million IU/d). |
Abbreviations: CBA, colistin base activity; CrCl, creatinine clearance (mL/min); CRRT, continuous renal replacement therapy; Css,avg, average steady-state plasma concentration of colistin; RRT, renal replacement therapy; SLED, sustained low-efficiency dialysis.
aSuggested doses are based on the population pharmacokinetic (PK) analysis for 214 critically ill patients. Doses are expressed primarily as milligrams of CBA for various categories of critically ill patients; doses are also provided in millions of international units.
bDaily dose administered in 2 divided doses 12 h apart.
cEquation 2 is based on the regression equation in Figure 2B, with the intercept adjusted from 1.667 to 1.825 and with creatinine clearance (CrCl) expressed in mL/min.
dBased on use of Equation 2 and setting CrCl to 0.
eSupplemental dose of colistimethate to achieve a similar Css,avg on a dialysis day as occurs on a nondialysis day, based on the population PK analysis for 16 critically ill patients in whom intermittent hemodialysis dialysate clearance of colistimethate and colistin was determined.
fBased on the population PK analysis for 4 critically ill patients in whom SLED dialysate clearance of colistimethate and colistin was determined.
gBased on the population PK analysis for 9 critically ill patients in whom CRRT dialysate clearance of colistimethate and colistin was determined.
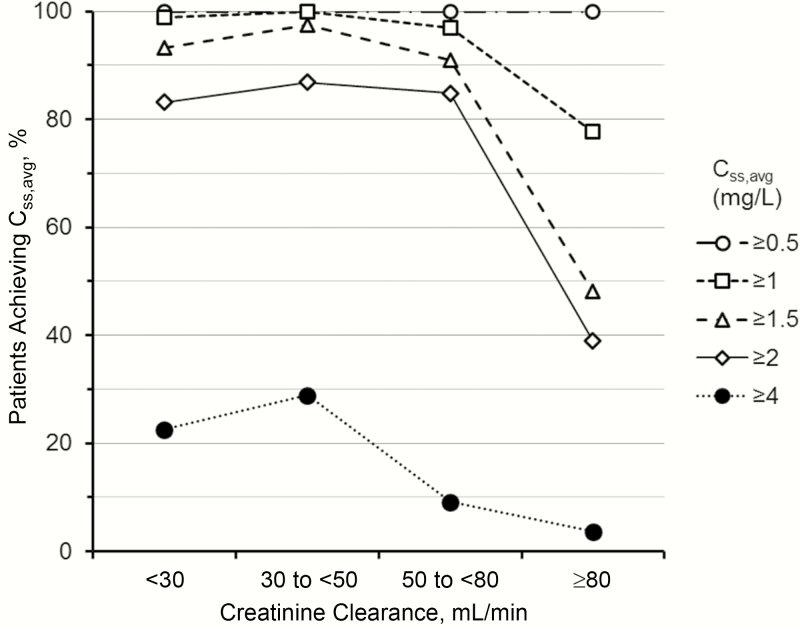
The daily dosing algorithm (Equation 2, Table 2) was then used to determine the dose needed to achieve a clinically reasonable target plasma colistin Css,avg of 2 mg/L [10] for hypothetical patients at the midpoint of 10-mL/min increments of creatinine clearance. This yielded discrete daily doses for these narrow renal function windows (Table 3). When the renally based doses in Table 3 were applied back to individual patients not receiving RRT (including those prescribed intermittent dialysis who were on a nondialysis day), >80% and >90% of patients in each of the 3 lowest creatinine clearance clusters achieved a plasma colistin Css,avg of ≥2 and ≥1.5 mg/L, respectively (Figure 3), in accordance with the criteria set in development of the algorithm. As creatinine clearance increased above 80 mL/min, the suggested daily dose soon reached the maximum (360 mg CBA; Table 3) and consequently <40% and <50% of patients attained a plasma colistin Css,avg of ≥2 and ≥1.5 mg/L, respectively (Figure 3). With the suggested doses in Table 3, <30% of patients in all 4 creatinine clearance clusters achieved a plasma colistin Css,avg ≥4 mg/L.
Table 3.
“Look-up” Table of Daily Doses of Colistimethate for a Desired Target colistin Css,avg of 2 mg/L for Narrow Windows of Creatinine Clearance
| Creatinine clearance, mL/min |
Dose of Colistimethate for Css,avg of 2 mg/La | |
|---|---|---|
| CBA, mg/d | Million IU/d | |
| 0 | 130 | 3.95 |
| 5 to <10 | 145 | 4.40 |
| 10 to <20 | 160 | 4.85 |
| 20 to <30 | 175 | 5.30 |
| 30 to <40 | 195 | 5.90 |
| 40 to <50 | 220 | 6.65 |
| 50 to <60 | 245 | 7.40 |
| 60 to <70 | 275 | 8.35 |
| 70 to <80 | 300 | 9.00 |
| 80 to <90 | 340 | 10.3 |
| ≥90 | 360 | 10.9 |
Abbreviations: CBA, colistin base activity; Css,avg , average steady-state plasma concentrations of colistin.
aDaily doses were calculated using Equation 2 in Table 2 and the midpoint of the creatinine clearance bands in the first column in the present table. Daily dose administered in 2 divided doses 12 h apart.
Figure 3.
Percentage of patients in each creatinine clearance cluster achieving average steady-state plasma concentrations of colistin (Css,avg) of ≥0.5, ≥1, ≥1.5, ≥2, and ≥4 mg/L using the daily dose of colistimethate in Table 3 relevant to the actual creatinine clearance of each patient.
For a plasma colistin Css,avg of 2 mg/L, patients prescribed intermittent dialysis would receive 130 mg CBA daily (baseline dosing, Table 2) when not receving dialysis (assuming creatinine clearance of 0 mL/min). This dosage regimen when applied back to these patients achieved attainment rates for plasma colistin Css,avg ≥1.5, ≥2, and ≥4 mg/L of 95%, 85%, and 25%, respectively. The dialysis clearance of colistimethate and colistin was similar for the various types of RRT (Table S2, Supplementary Data). The supplemental dosing required to compensate the loss via dialysis was determined to be 10% of the baseline dose per hour of dialysis (Table 2). For patients receiving CRRT, when we applied back to each of the 9 patients receiving this modality a daily colistimethate dose of 440 mg of CBA for a target plasma colistin Css,avg of 2 mg/L (Table 2), 8 patients (89%) attained 1.5 and 2 mg/L, and only 1 patient had a concentration >4 mg/L.
DISCUSSION
The influence of renal function on colistin clearance and hence on the daily dose of CBA required to achieve each 1 mg/L of plasma colistin Css,avg is discernible from the relationships in Figure 2. Of note is the very wide interpatient variability; even at a given creatinine clearance there was up to an approximately 10-fold range (Figure 2A), corresponding to approximately 1-log10 range (Figure 2B), in the daily dose of CBA. This variability is the result of a similarly wide range in the apparent clearance of colistin at a given creatinine clearance [4, 5, 14]. This probably arises from interpatient differences in the fraction of each colistimethate dose converted to colistin, because the clearance of polymyxin B, which is not administered as a prodrug, shows only approximately 3–4-fold variability across a very wide range of renal functions [15, 16]. Whatever the cause of the variability with colistimethate, it complicates the selection of dosing regimens, especially given the very narrow therapeutic window of colistin.
The algorithms for loading and ongoing daily doses (Table 2) can be used to determine doses of colistimethate for a desired Css,avg. Based on PK/pharmacodynamic data from the murine thigh infection model [6] together with plasma protein binding in patients reported here, and clinical PK/toxicodynamic data indicating that risk of nephrotoxicity increases as plasma colistin exposure exceeds approximately 2.5 mg/L [7–9], a plasma colistin Css,avg of 2 mg/L may be a suitable target concentration at initiation of intravenous treatment of bloodstream and some other infections when the colistin MIC is ≤2 mg/L [10]. Murine lung infection was more refractory to parenteral therapy [6], and therefore a plasma colistin Css,avg of 2 mg/L with intravenous colistimethate may be adequate for treatment of respiratory infections if the colistin MIC is <1 mg/L [10]. The choice of a plasma colistin Css,avg of 2 mg/L is driven by risk of nephrotoxicity and the fact that the MIC of the causative organism may not be known when colistimethate is initiated [10]; the actual MIC should be obtained urgently. It is important to recognize that the target plasma colistin Css,avg of 2 mg/L is not appropriate for infections in which the MIC is >2 mg/L, and it may be insufficient for pulmonary infections even with MICs <1 mg/L unless other approaches are undertaken (eg, combination with other antibiotics, nebulization of colistimethate) [6, 10].
If the plasma colistin Css,avg target is 2 mg/L, as proposed above, a loading dose of 300 mg CBA (~9 million IU) is suggested (Table 2), as supported by findings in other studies involving smaller patient numbers [4, 5, 14]. For ongoing daily dosing targeting a plasma colistin Css,avg of 2 mg/L, a clinician-friendly “look-up” table (Table 3) lists suggested colistimethate doses, expressed as both milligrams per day of CBA and international units per day. For patients with creatinine clearance <80 mL/min receiving the suggested renally adjusted doses (Table 3), attainment rates of 80%–90% for a plasma colistin Css,avg ≥2 mg/L were achieved (Figure 3). Attainment rates of >90% would be desirable. This could be achieved by more aggressive dosing, but that would result in higher plasma colistin Css,avg in all patients and, in the 2 lowest creatinine clearance clusters, >30% of patients attaining a Css,avg ≥4 mg/L (Figure 3).
The suggested doses (Table 3) represent a compromise approach and are designed to achieve acceptably high attainment rates (ie, >80%) for 2 mg/L in patients in whom this is possible with submaximal daily doses of colistimethate, while minimizing the risk of nephrotoxicity. For patients with creatinine clearance ≥80 mL/min, a very large increase in the dose above the maximum of 360 mg/d of CBA used here would be required to push the attainment rate from approximately 40% (Figure 3) to >80%. However, this would be expected to increase the risk of colistin-associated nephrotoxicity [7–9, 17]. Combination therapy should be strongly considered for patients with creatinine clearance ≥80 mL/min, especially if the patient has a respiratory tract infection and/or the MIC of the infecting organism is ≥1 mg/L [4, 6, 10]. The attainment rates for a plasma colistin Css,avg ≥2 mg/L in patients receiving intermittent dialysis or CRRT receiving the doses proposed in Table 2 were within the 80%–90% range.
Compared with the interim analysis [4], the final analysis reported here comprised a larger number of patients with low creatinine clearances (including those prescribed either intermittent or continuous renal support), which has improved the ability to predict doses for these patients. For patients with creatinine clearance >30 mL/min, the daily doses for a plasma colistin Css,avg of 2 mg/L (Tables 2 and 3) are very similar to those proposed from the interim analysis (assuming a BSA of 1.73 m2) [4]. However, the doses proposed here for patients with creatinine clearance <30 mL/min (including patients prescribed intermittent dialysis who are on a nondialysis day) are up to approximately 100% higher than those from the interim analysis [4] and those currently approved by the Food and Drug Administration [18]. The Food and Drug Administration–approved daily dose for patients with creatinine clearance <30 mL/min results in an attainment rate of <40% for a plasma colistin Css,avg of 2 mg/L [10]. For patients in this renal function category, the doses in Tables 2 and 3 are similar to those approved recently by the European Medicines Agency for patients not prescribed intermittent dialysis [13]. For patients prescribed intermittent dialysis, the doses in Tables 2 and 3 for a patient when not receiving dialysis are higher than the European Medicines Agency–approved dose.
This final analysis incorporating a larger number of patients receiving RRT than in the interim analysis has confirmed previous findings that colistimethate and colistin undergo efficient extracorporeal clearance [4, 19–29]. Consistent with the earlier recommendation by Garonzik et al [4], intermittent dialysis should occur toward the end of a colistimethate dose interval, and a supplement to the baseline dose should be administered (Table 2). Patients receiving CRRT should receive a daily dose similar to, or higher than, what would be used in a patient with normal renal function (Table 2), confirming the previous suggestion [4] and supported by other studies [19, 28, 29].
In conclusion, the influence of renal function on daily dose requirements and the extensive interpatient variability even at a given creatinine clearance, together with the very narrow therapeutic window for colistin, highlight the need for dosing guidance. The dosing suggestions for intravenous colistimethate in critically ill patients reported herein are based on the results of the largest population PK study conducted to date. To assist clinicians in various parts of the world, doses are provided as both milligrams of CBA and international units, and a convenient look-up table is provided for suggested daily doses for various creatinine clearance values. The study highlights circumstances in which monotherapy with intravenous colistimethate may be suboptimal.
Supplementary Material
Notes
Acknowledgments. We are grateful to the patients and their families, and we gratefully acknowledge the dedication of the research support staff at the various study sites, in particular Diana Lynn Pakstis, Mary Ellen Carey, Peerawong Werarak, Sunee Thanakhumtorn, Laksame Wattanamongkonsil, Heidi Yu, Anima Poudyal, and Jovan Jacobs.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID) or the National Institutes of Health.
Financial support. This work was supported by the NIAID (grant R01 AI070896 to R. L. N., A. F., D. L. P., J. L., and F. P. S.) and the Australian National Health and Medical Research Council (senior research fellowship to J. L.).
Potential conflicts of interest. D. L. P. has received honoraria for speaking engagements and advisory board membership from Merck, AstraZeneca, Cubist, Pfizer, GlaxoSmithKline, and Shionogi. All other authors report no potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis 2006; 6:589–601. [DOI] [PubMed] [Google Scholar]
- 2. Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2006; 50:1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnett M, Bushby SR, Wilkinson S. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol Chemother 1964; 23:552–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karaiskos I, Friberg LE, Pontikis K, et al. Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrob Agents Chemother 2015; 59:7240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 2015; 70:3291–7. [DOI] [PubMed] [Google Scholar]
- 7. Sorli L, Luque S, Grau S, et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis 2013; 13:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forrest A, Silveira FP, Thamlikitkul V, et al. Toxicodynamics for colistin-associated changes in creatinine clearance. Presented at: Interscience Conference on Antimicrobial Agents and Chemotherapy; 5–9 September 2014; Washington, DC. [Google Scholar]
- 9. Horcajada JP, Sorli L, Luque S, et al. Validation of a colistin plasma concentration breakpoint as a predictor of nephrotoxicity in patients treated with colistin methanesulfonate. Int J Antimicrob Agents 2016; 48:725–7. [DOI] [PubMed] [Google Scholar]
- 10. Nation RL, Garonzik SM, Li J, et al. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 2016; 62:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jelliffe R. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am J Nephrol 2002; 22:320–4. [DOI] [PubMed] [Google Scholar]
- 12. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency. Annex III: amendments to relevant sections of the summary of product characteristics and the package leaflets. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Polymyxin_31/WC500176332.pdf Accessed 11 January 2017. [Google Scholar]
- 14. Gregoire N, Mimoz O, Megarbane B, et al. New colistin population pharmacokinetic data in critically ill patients suggesting an alternative loading dose rational. Antimicrob Agents Chemother 2014; 58:7324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandri AM, Landersdorfer CB, Jacob J, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 2013; 57:524–31. [DOI] [PubMed] [Google Scholar]
- 16. Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 2014; 59:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–84. [DOI] [PubMed] [Google Scholar]
- 18. Food and Drug Administration. Coly-Mycin M Parenteral (Colistimethate for Injection, USP) . Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050108s030lbl.pdf Last accessed 11 January 2017. [Google Scholar]
- 19. Karvanen M, Plachouras D, Friberg LE, et al. Colistin methanesulfonate and colistin pharmacokinetics in critically ill patients receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 2013; 57:668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leporati M, Bua RO, Mariano F, et al. Determination by LC-MS/MS of colistins A and B in plasma and ultrafiltrate from critically ill patients undergoing continuous venovenous hemodiafiltration. Ther Drug Monit 2014; 36:182–91. [DOI] [PubMed] [Google Scholar]
- 21. Li J, Rayner CR, Nation RL, et al. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 2005; 49:4814–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marchand S, Frat JP, Petitpas F, et al. Removal of colistin during intermittent haemodialysis in two critically ill patients. J Antimicrob Chemother 2010; 65:1836–7. [DOI] [PubMed] [Google Scholar]
- 23. Luque S, Sorli L, Li J, et al. Effective removal of colistin methanesulphonate and formed colistin during intermittent haemodialysis in a patient infected by polymyxin-only-susceptible Pseudomonas aeruginosa. J Chemother 2014; 26:122–4. [DOI] [PubMed] [Google Scholar]
- 24. Strunk AK, Schmidt JJ, Baroke E, et al. Single- and multiple-dose pharmacokinetics and total removal of colistin in a patient with acute kidney injury undergoing extended daily dialysis. J Antimicrob Chemother 2014; 69:2008–10. [DOI] [PubMed] [Google Scholar]
- 25. Jitmuang A, Nation RL, Koomanachai P, et al. Extracorporeal clearance of colistin methanesulphonate and formed colistin in end-stage renal disease patients receiving intermittent haemodialysis: implications for dosing. J Antimicrob Chemother 2015; 70:1804–11. [DOI] [PubMed] [Google Scholar]
- 26. Markou N, Fousteri M, Markantonis SL, et al. Colistin pharmacokinetics in intensive care unit patients on continuous venovenous haemodiafiltration: an observational study. J Antimicrob Chemother 2012; 67:2459–62. [DOI] [PubMed] [Google Scholar]
- 27. Jacobs M, Gregoire N, Megarbane B, et al. Population pharmacokinetics of colistin methanesulphonate (CMS) and colistin in critically ill patients with acute renal failure requiring intermittent haemodialysis. Antimicrob Agents Chemother 2016; 60:1788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mariano F, Leporati M, Carignano P, Stella M, Vincenti M, Biancone L. Efficient removal of colistin A and B in critically ill patients undergoing CVVHDF and sorbent technologies. J Nephrol 2015; 28:623–31. [DOI] [PubMed] [Google Scholar]
- 29. Karaiskos I, Friberg LE, Galani L, et al. Challenge for higher colistin dosage in critically ill patients receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents 2016; 48:337–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.