Abstract
STUDY QUESTION
Is male factor infertility associated with an increased risk of developing diabetes?
SUMMARY ANSWER
The study provides evidence that male factor infertility may predict later occurrence of diabetes mellitus with the risk being related to the severity of the underlying fertility problem.
WHAT IS KNOWN ALREADY
Previous cross-sectional studies have shown an increased prevalence of comorbidities among infertile men when compared to controls.
STUDY DESIGN, SIZE, DURATION
In this prospective cohort study, 39 516 men who had since 1994 undergone fertility treatment with their female partner were identified from the Danish national IVF register, which includes data on assumed cause of couple infertility (male/female factor, mixed and unexplained infertility) and type of fertility treatment. With a median follow-up time of 5.6 years, each man was followed for diabetes occurrence from enrollment until 31 December 2012 using the National Diabetes Register (NDR). Men with a history of diabetes prior to their fertility diagnosis were excluded. Hazard ratios (HR) were estimated by Cox proportional hazard models with age as the underlying time scale. In addition to analyzing the data for the entire IVF registration period (1994–2012), separate analyses were performed for men identified from the first (1994–2005) and second (2006–2012) IVF registration period owing to heterogeneity in the reporting of male factor infertility in these two time periods, because the reason for male factor infertility was not available from the first register.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Male factor infertility was identified from the variable ‘yes’ or ‘no’ from the first IVF register and through a diagnosis code (e.g. oligospermia, azoospermia) from the second IVF register. The reference group was men with male factor infertility (=‘no’) and those with normal semen quality or sterilized men. Of the included men, 18 499 (46.8%) had male factor infertility and 21 017 (53.2%) made up the reference group.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 651 (1.6%) diabetes cases were identified during the follow-up period. The adjusted HR's for diabetes risk among men with male factor infertility when compared to the reference group were HR = 1.08 (95% CI: 0.89, 1.31) and HR = 1.45 (95% CI: 1.06, 1.97) for the first and second IVF registration period, respectively. When assessing the effects of individual causes of male factor infertility, the adjusted HR's for men with oligospermia, azoospermia and aspermia were HR = 1.44 (95% CI: 1.01, 2.06), HR = 2.10 (95% 1.25, 3.56) and HR = 3.20 (95% CI 1.00, 10.31), respectively.
LIMITATIONS, REASONS FOR CAUTION
We found no increased risk among men identified from the first IVF register, which may be related to exposure misclassification as the reason for male factor infertility was not available from this time period. The NDR does not distinguish between type 1 and type 2 diabetes.
WIDER IMPLICATIONS OF THE FINDINGS
These findings support previous studies that a man's reproductive and somatic health are closely intertwined and highlight the importance for further monitoring of these men. Further, implementation of diabetes screening may be especially relevant among aspermic and azoospermic men.
STUDY FUNDING/COMPETING INTERESTS
This article is part of the ReproUnion collaborative study, co-financed by the European Union, Intereg V Öresund-Kattegat-Skagerrak. None of the authors declare any conflict of interest.
TRIAL REGISTRATION NUMBER
None.
Keywords: male infertility, diabetes, oligospermia, azoospermia, register-based cohort study, comorbidity
Introduction
Diabetes mellitus (DM) is a complex metabolic disorder characterized by prolonged hyperglycemia (Zaccardi et al., 2016). In type 1 DM, which most commonly debuts in childhood, an autoimmune destruction of the pancreatic beta-cells ultimately leads to insulin destruction. Type 2 diabetes, which commonly debuts in middle age, is characterized by insulin resistance with risk factors mainly related to lifestyle although family history and genetic polymorphisms also play a role (Haffner, 1998). Insulin resistance, particularly in combination with obesity, has been linked to impaired spermatogenesis and increased sperm DNA damage (Kasturi et al., 2008). The prevalence of DM is rising and has been associated with overall risk of cardiovascular disease and mortality, if poorly regulated (Gu et al., 1998). Whether the occurrence of infertility has changed over time is uncertain, but the demand for ART has increased (Sallmen et al., 2005; Ferraretti et al., 2013). Nonetheless, both DM and infertility are common diseases which may compromise the well-being and have detrimental effects on the society (Monga et al., 2004; American Diabetes, 2013).
The association between male infertility and future health has been of interest in recent years (Ventimiglia et al., 2016). The main focus has been on the risk of testicular and prostate cancer with little focus on risk of non-malignant chronic diseases (Hotaling and Walsh, 2009; Walsh et al., 2010). Cross-sectional studies have found that young men, already at the time of an infertility diagnosis, are less healthy than their fertile peers suggesting that a man's reproductive and somatic health are closely associated, but whether these findings have a direct causation is difficult to determine (Salonia et al., 2009; Ventimiglia et al., 2015; Eisenberg et al., 2015a). Only one prospective study evaluated the risk of chronic non-malignant diseases among infertile men and found a 30% increased risk of diabetes (Eisenberg et al., 2015b). Importantly low levels of testosterone have been linked to both diabetes and mortality, suggesting common mechanistic pathways (Laughlin et al., 2008; Rao et al., 2013). In keeping with this, studies from Europe and the USA have found an association between poor semen quality and mortality (Groos et al., 2006; Jensen et al., 2009; Eisenberg et al., 2014).
As men are not subjects of regular health screening programmes as women are, the contact with the health care provider as part of a fertility evaluation is a unique opportunity to identify men who may be at increased risk of morbidity, in order to initiate proper preventive action. In this nationwide prospective cohort study we report on the risk of developing diabetes in a large population of men identified in the Danish National IVF register 1994–2012.
Materials and Methods
Setting
In Denmark, up to three reimbursed fresh IVF or ICSI cycles, and an unlimited number of frozen, thawed embryo transfer cycles and insemination cycles are offered in the public, tax-financed health care system to childless couples and childless single women younger than 40 years old. In the private health care system fertility treatment financed by the patient's themselves are offered up to the female age of 45 years. In 2015, 17 181 treatments were initiated with 9% of all Danish children being born after ART during that year (Danish Fertility Society Annual Report, 2015). The IVF clinics in Denmark perform a high volume of semen analyses used for fertility evaluations and sperm preparations for ART. Prior to the start of treatment, a semen sample was provided by masturbation into sterile containers as part of the male evaluation. If the first semen sample was not normal the men were generally asked to provide a second sample shortly after the first. Thus, any diagnosis of reduced semen quality (e.g. oligospermia and azoospermia) was generally based on two consecutive tests. The samples were generally analyzed according to the World Health Organization guidelines by trained personnel and the men were carefully instructed regarding abstinence time.
Study population
We created a historic cohort of all men whose partner had undergone fertility treatment between 1 January 1994 and 31 December 2012 with prospective follow-up in the Danish National Diabetes Register (NDR) through the use of unique personal identification numbers held by all Danish citizens, which enables individual linkage between all national registers (Pedersen, 2011). The identification numbers were further used to obtain information on date of death, emigration or disappearance of the cohort members. The men had to be born in Denmark, live in Denmark at the time of inclusion, and be without a prior diabetes diagnosis registered in the NDR.
The baseline examination included age and highest level of school attendance which can be related to socioeconomic status. The study was approved by Danish Data Protection Agency J. nr.: BFH-2015-091. According to the Danish legislation, register-based studies do not require ethical approval, as these studies do not involve direct contact with individuals.
Information on fertility status
The men were identified through the Danish National IVF register which records each time a woman undergoes a fertility treatment cycle (Andersen et al., 1999; Blenstrup and Knudsen, 2011). Mandatory by Danish law, all initiated treatments must be reported to the register. The register was established in 1994 and holds information on the reason for infertility (male/female factor, mixed or unexplained infertility) and of type of infertility treatment (IVF, ICSI, oocyte/sperm donation, frozen/fresh embryo transfer). In 2006 the register reporting went from manual to electronic with a more detailed documentation of the available variables from the latter. From the first IVF register (1994–2005) men were categorized as infertile, identified from the variable ‘male factor’ = ‘yes’ or ‘no’ with no missing values. No information regarding vasectomy or other reasons for male infertility was available in that registry. From the second IVF register (2006–present) the International Classification of Disease (ICD-10 codes) was used to create the variable for ‘male factor’ = ‘yes’ including the following ICD-10 codes: Aspermia (complete lack of ejaculate, N469A), azoospermia (N469B), oligospermia (N469C), oligo-teratozospermia (N469D), other reasons for male infertility (N469W), male infertility unspecified (N469X). If the female partner was diagnosed with ‘female infertility associated with male factors (N974)’ and the identified partner was not sterilized the men were categorized as ‘male fertility unspecified’. Sterilized men (Z302) or those with normal semen quality (EZDH01) were classified as ‘male factor = ‘no’ and used as the reference group. If no diagnosis code was available from the first visit, the diagnosis code of the second visit was used. Men with fictive national identification numbers (foreign citizens and sperm donators), a history of diabetes, from Greenland or those with an unknown fertility diagnosis (missing ICD-10 code) were excluded.
The Danish National Diabetes Register
The personal identification number was used to link the cohort members to the NDR to identify incident diabetes cases between baseline and either date of death, emigration, disappearance or end of follow-up (31 December 2012).
The NDR was established in 2006 to describe and monitor the occurrence of diabetes in Denmark and provide data for epidemiological research. The register has previously been described in detail (Carstensen et al., 2008, 2011). In brief, the NDR links three existing nationwide administrative records in the Danish Health system. Inclusion criteria include hospital discharge diagnoses of diabetes in the NDR since 1994; podiatry for diabetic patients, five blood glucose measurements within 1 year, or two blood glucose measurements per year for five consecutive years, as registered in the National Health Insurance Register (which contains all services provided by general and specialist practitioners since 1973); or two purchases of insulin or oral glucose-lowering drugs within 6 months, as registered in the Danish National Prescription Register since 1993 (Carstensen et al., 2008, 2011; Kildemoes et al., 2011).
Statistical analysis
First, we examined the cross-sectional association between the prevalence of male factor infertility and diabetes by logistic regression. Next, we analyzed the risk of new-onset diabetes according to male factor infertility and those with missing ICD-10 codes using the occurrence of diabetes in men from couples who had undergone fertility treatment. Hazard ratio (HR) of diabetes was calculated using Cox proportional hazards models with age as the underlying time scale ensuring that risk estimates were based on individuals at exactly the same age (Thiebaut and Benichou, 2004). We used left truncation at age of inclusion, so that the men were considered at risk from inclusion into the cohort, and right censoring at the age of diabetes (event), death, emigration, disappearance or end of follow-up on 31 December 2012, whichever came first.
Owing to the heterogeneity of the IVF registry data available from the two time periods, 1994–2005 and 2006–2012, we conducted separate analyses for assessing the effects of infertility on diabetes within these time periods in addition to an overall analysis of the entire population. Within each time period effects were evaluated in two steps: (1) adjusted only for age as part of the model (crude analysis) and (2) also including calendar year to adjust for time trends in fertility as well as diabetes incidence over follow-up time; and full adjustment for highest obtained education, which is an established proxy for socioeconomic status (Winkleby et al., 1992). From 2006 and onwards, we estimated the association between the subtypes of male factor infertility defined according to ICD-10 classifications (aspermia, azoospermia, oligospermia, oligo-teratozospermia and other/unspecified causes) and diabetes risk, with normal semen quality and sterilized as the reference groups. The results are expressed as HRs with two-sided 95% CIs on the basis of the Wald test statistic for regression parameters in SAS (version 9.2; SAS Institute, Cary, NC, USA).
Results
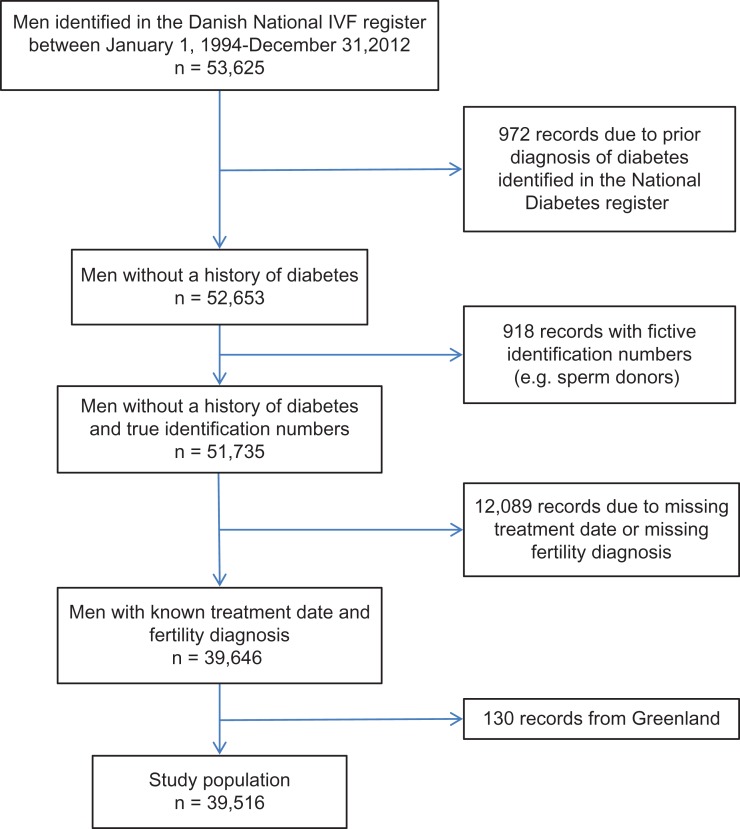
Among the 53 625 men identified in the Danish IVF register from 1 January 1994 to 31 December 2012, we excluded 972 men due to a diabetes record in the NDR before baseline; 918 with fictive identification numbers used for sperm donors and foreign citizens; 12 089 with missing fertility treatment date or a missing fertility diagnosis and 130 men living in Greenland (Fig. 1). The remaining 39 516 men were followed up for diabetes for an average of 5.6 years. At baseline, 1.9% had diabetes. We identified 651 (1.6%) cases of diabetes (prevalence rate 2.9 per 1000 person-years) during the follow-up years.
Figure 1.
Flow diagram of the cohort in a study of the risk of diabetes according to male factor infertility.
Overall, 18 499 (46.8%) men were diagnosed with male factor infertility for the entire IVF period (1994–2012). Men identified from the first IVF register (1994–2005) made up 36.1% of the cohort with male factor infertility identified in 39.5% of the cases. The second IVF register (2006–2012) made up the remaining 63.9% of the cohort with male factor infertility identified in 51% of the cases (Table I). The baseline characteristics across the different IVF registration periods were similar between those with male factor infertility and the reference group. The most common type of male factor infertility was oligospermia, with 8538 (33.8%) men, followed by other causes/unspecified male factor infertility 2907 (11.4%). The diabetes cases were unevenly distributed among those with male factor infertility and the reference group for the two IVF registration periods (Table II).
Table I.
Baseline characteristics of the cohort in a study of risk of diabetes according to male factor infertility.
| Male factor infertilitya | Reference groupb | |||
|---|---|---|---|---|
| N (%) | Median (fifth–95th percentile) | N (%) | Median (fifth–95th percentile) | |
| All men (1994–2012) (n = 39 516, 100%) | ||||
| N | 18 499 (46.8) | 21 017 (53.2) | ||
| Age at baseline (years) | 33.9 (26.4–45.8) | 33.8 (26.3–45.02) | ||
| Follow-up time (years) | 5.7 (0.65–14.9) | 5.3 (0.55–17.1) | ||
| Highest level of education | ||||
| Primary school | 2683 (14.9) | 3088 (15.2) | ||
| High school | 1367 (7.6) | 1460 (7.2) | ||
| Skilled trade | 8604 (47.8) | 9966 (48.9) | ||
| Bachelor degree | 2757 (15.3) | 3094 (15.2) | ||
| Higher University | 2594 (14.4) | 2756 (13.5) | ||
| Men included in IVF register (1994–2005) (n = 14 283, 36.1%)c | ||||
| N | 5642 (39.5) | 8641 (60.5) | ||
| Age at baseline (years) | 33.8 (26.7–45.4) | 33.8 (27.2–43.9) | ||
| Follow-up time (years) | 11.0 (7.2–16.9) | 12.4 (7.3–18.0) | ||
| Highest level of education | ||||
| Primary school | 922 (16.6) | 1435 (16.9) | ||
| High school | 390 (7.0) | 602 (7.1) | ||
| Skilled trade | 2833 (51.0) | 4225 (49.8) | ||
| Bachelor degree | 781 (14.1) | 1221 (14.4) | ||
| Higher University | 624 (11.2) | 995 (11.7) | ||
| Men included in IVF register (2006–2012) (n = 25 233, 63.9%)c | ||||
| N | 12 857 (50.9) | 12 376 (49.1) | ||
| Age at baseline (years) | 34.0 (26.2–46.0) | 33.7 (25.9–45.7) | ||
| Follow-up time (years) | 4.32 (0.40–4.3) | 2.73 (0.31–6.7) | ||
| ICD code | ||||
| Aspermia | 137 (0.5) | |||
| Azoospermia | 1275 (5.0) | |||
| Oligospermia | 8538 (33.8) | |||
| Other causes/unspecified | 2907 (11.4) | |||
| Highest level of education | ||||
| Primary school | 1761 (14.1) | 1653 (13.9) | ||
| High school | 977 (7.8) | 858 (7.2) | ||
| Skilled trade | 5771 (46.3) | 5741 (48.3) | ||
| Bachelor degree | 1976 (15.9) | 1873 (15.8) | ||
| Higher University | 1970 (15.8) | 1761 (14.8) | ||
aIncludes men with ‘male factor = yes’ identified from the first IVF register and men classified with male factor infertility based on following ICD-10 codes: N469A, N469B, N469C, N469D, N469W, N469X, N974 (if the man was not sterilized) from the second IVF register.
bIncludes men with ‘male factor = no’ identified from the first IVF register and men with either normal semen quality or those vasectomized identified from the second IVF register.
cRegistration in the Danish IVF registry was updated in 2006 from a manual to electronic system with a more detailed documentation in the recent years.
Table II.
Association between male factor infertility and risk of diabetes.
| Men at risk, n | Cases, n | Hazard ratios (95% CI) | Hazard ratios (95% CI) | |
|---|---|---|---|---|
| Crudea,b | Adjusted modela,b,c | |||
| First IVF period (1994–2005) | ||||
| Reference | 8641 | 286 | 1.00 | 1.00 |
| Male factor infertility | 5642 | 175 | 1.06 (0.87, 1.27) | 1.08 (0.89, 1.31) |
| Second IVF period (2006–2012) | ||||
| Reference | 12 376 | 61 | 1.00 | 1.00 |
| Male factor infertility | 12 857 | 129 | 1.42 (1.05, 1.93) | 1.45 (1.06, 1.97) |
| Unknownd | 10 078 | 74 | 0.99 (0.71, 1.39) | 1.01 (0.715, 1.43) |
| Fertile | 12 376 | 61 | 1.00 | 1.00 |
| Oligospermiae | 8538 | 66 | 1.29 (0.91, 1.83) | 1.44 (1.01, 2.063) |
| Other causes/unspecified | 2907 | 38 | 1.31 (0.80, 1.97) | 1.60 (1.04, 2.46) |
| Azoospermia | 1275 | 22 | 2.31 (1.42, 3.77) | 2.10 (1.25, 3.56) |
| Aspermia | 137 | 3 | 3.43 (1.07, 10.94) | 3.20 (1.00, 10.31) |
aAdjusted for age by using it as time scale in the Cox model.
bDue to exclusion of cohort members with a missing value for any covariate, the number of persons is identical in the crude and the adjusted analyses.
cAdjusted for birth calendar year and highest educational level (indicator: primary school/high school/skilled trade/bachelor/university).
dMen with unknown fertility status (missing ICD-10) ICD: International Classification of Disease.
eIncludes 30 men with oligoteratospermia.
At baseline, male factor infertility was associated with a higher prevalence of diabetes among men enrolled from both the first IVF register [odds ratio (OR) 1.57, 95% CI: 1.16, 2.11] and those from the second IVF register (OR 1.41, 95% CI: 1.20,1.66) after adjustment for age and education.
The adjusted HR for incident diabetes cases associated with male factor infertility for the first registration period was (HR = 1.08; 95% CI: 0.89, 1.31), whilst the adjusted HR for the second registration period was (HR = 1.45; 95% CI: 1.06, 1.97). There appeared to be an exposure-dependence over the different infertility types, with the adjusted HR for men with oligospermia, azoospermia and aspermia being HR = 1.44 (CI: 1.01, 2.06), HR = 2.10 (CI: 1.25, 2.56) and HR = 3.20 (CI: 1.0, 10.31), respectively (Table II). The adjusted HR comparing the men with unknown fertility status (missing ICD-10 codes) to the reference group was 1.01 (0.72,1.43).
Discussion
Main findings
Our prospective study of more than 39 000 men identified from the Danish National IVF register between 1994 and 2012 found that male factor infertility was associated with an increased risk of being diagnosed with diabetes among men identified from the second IVF register (2006–2012). When stratified by infertility type, we observed an increased risk across all subtypes with a particularly high risk among aspermic and azoospermic men. We did not observe an increased risk among men identified from the first IVF registration period (1994–2005), which may be due to exposure misclassification.
Thus there are important differences between the two IVF registration periods that warrant mention. In the first IVF register male factor was registered as either ‘yes’ or ‘no’ with no missing values, which may have the following consequences: first, that sterilized men are misclassified as ‘infertile’ (however, as less than 1% of the men are sterilized in the second register it is unlikely to be the sole explanation) and second, that men in a gray zone (e.g. subfertile men) may have been misclassified as ‘fertile’. Both scenarios would bias the results toward null and implicitly lead to an underestimation of the diabetes risk among the infertile. Further, this misclassification may explain the substantially higher percentage of male factor infertility cases from the second IVF register. Alternatively, limited statistical power owing to the smaller sample size of the first IVF register may also explain the differences in the risk estimates between the two registration periods although this is less likely as the study population still exceeded 14 000 men during the initial follow-up years. Overall, data captured from the first registration period appears less valid especially as the specific reason for male infertility was not available.
Prior literature and mechanisms
In our cohort, 1.9% of the men had diabetes at baseline. We estimated the percentage of Danish men with diabetes within the same age group based on data from NDR and Statistics Denmark and found 1.5% had diabetes (http://www.diabetes.dk/presse/diabetes-i-tal/det-nationale-diabetesregister.aspx ; http://statistikbanken.dk).With regards to the socioeconomic profile of the reference group, 15% had completed primary school and 13.5% had a higher university degree, which compares to the national averages of 19% and 11%, respectively (http://statistikbanken.dk). In line with other studies, our findings indicate that couples in ART treatment are more highly educated than the general population (Hotaling et al., 2012; Bay et al., 2013), which is perhaps expected as education level is important for both navigating health care options and the ability to understand treatment regimens (Maitra, 2010), which are both prerequisites for ART treatment.
Our results are comparable to a US study which reported a 30% increased risk of diabetes among infertile men (Eisenberg et al., 2015b). To our knowledge, no other prospective studies have examined this area, but several studies have explored the reverse association, with most studies suggesting a mild impact of diabetes on semen quality (Jangir and Jain, 2014). Further, diabetes may have its effects on male reproductive function by endocrine control of spermatogenesis, sperm maturation and impairment of ejaculation (Jangir and Jain, 2014). We found a particularly high diabetes risk among azoospermic and aspermic men. However, as 10–15% of azoospermic men display genetic abnormalities our findings are perhaps not surprising in this context (Krausz, 2011). Other reasons for azoospermia include hypogonadotrophic hypogonadism, urogenital infection or chemotherapy although these factors are more likely to have some impact on semen quality but not necessarily cause azoospermia (Krausz, 2011; Nordkap, 2016). Aspermia may be caused by androgen deficiency or retrograde ejaculation, which is a frequent condition among diabetic men (Fedder et al., 2013). Importantly, these findings indicate that some infertile men could have diabetic sequelae even before a diagnosis, as men with a history of diabetes were excluded from our cohort. Nonetheless, as we observed an increased risk across all subtypes of male factor infertility we also hypothesize that the link between male factor infertility and diabetes may be, in addition to a hormonal genesis, due to common health/lifestyle factors or shared genetic origins. Alternatively, some men may have a pre-diabetic condition that has not yet been diagnosed, which may explain the presence of aspermia and azoospermia prior to the debut of diabetes.
The association between low testosterone, a potential marker for male infertility, and diabetes risk has been investigated as testosterone plays a significant role in glucose and lipid metabolism (Kelly and Jones, 2013). Studies have shown that low levels of testosterone may predispose men to type 2 diabetes and metabolic syndrome (cluster of risk factors associated with an increased risk of heart disease or stroke) independently of BMI and other established risk factors for insulin resistance (Laaksonen et al., 2004; Li et al., 2010). Another recent study concluded testosterone to be a risk marker rather than a risk factor for subsequent type 2 diabetes as low levels of testosterone and sex hormone-binding globulin, but not LH, were associated with an increased risk of the disease (Holmboe et al., 2016). Testosterone, however, is a nonspecific marker for infertility/diabetes as low levels are associated with other conditions, such as cardiovascular disease, obesity and overall mortality, although conflicting results exist (Laughlin et al., 2008; Kelly and Jones, 2015).
Cross-sectional studies have shown that men with reduced fertility, already at the time of a fertility evaluation, present with more medical comorbidities than their fertile peers (Salonia et al., 2009; Ventimiglia et al., 2015; Eisenberg et al., 2015a). The observation of a higher baseline prevalence of diabetes among men with male factor infertility is concordant with this evidence. More specifically, one US study found that men with more semen abnormalities had higher rates of endocrine and circulatory system diseases (Eisenberg et al., 2015a) whereas an Italian study found higher rates of rates of oligospermia and azoospermia among men with poorer general health (Salonia et al., 2009). However, the relationship between male infertility and health is rather complex as several confounders may affect both. For example, as both age and smoking are known to affect semen quality (Kidd et al., 2001; Sharma et al., 2015) and diabetes risk (Biggs et al., 2010), these results must be interpreted with caution. A genetic link between male infertility and diabetes may exist as a recent study identified over 100 genes associated with both male infertility and several disease mechanisms, including metabolic disease pathways (Tarin et al., 2015). Further, as many genes are expressed during male germline cell differentiation, it seems plausible that possible mutations in this process could lead to both male infertility and risk of diabetes (Matzuk and Lamb, 2008).
Strengths and limitations
Our study had several advantages including the overall size and possibility for long-term follow-up through national health registers which means a minimal number of men were lost to follow-up. In addition, as the first three fresh in vitro treatments are free of charge in Denmark for childless couples/women younger than 40 years old, we were able to include men from different socioeconomic backgrounds in contrast to countries were treatments are expensive and paid for privately, which is of importance as previous studies have demonstrated a healthy patient effect among men identified from infertile couples (Jensen et al., 2009; Eisenberg et al., 2014). Our study also has limitations. First, we also relied on two important assumptions: first, that men with a diagnosis of male factor infertility had impaired semen parameters and second, that men with a diagnosis of normal semen quality had normal semen parameters, although we recognize that significant intra-variability in semen quality from the same men exist. This could lead to misclassification bias especially for the oligospermic men as they may be misclassified as ‘fertile’, which would lead our estimates towards null. In keeping with this, we also acknowledge that although our reference group consisted of men with normal semen quality or those who had been sterilized, they still belong to infertile couples which means their reproductive capacity still may be reduced in comparison to men from the general population. Further, far from all couples seek medical attention for infertility, which may limit generalizability. We also excluded men with a prior diagnosis of diabetes although it is possible that some men may have an undetected fertility problem, which actually precedes the diabetes diagnosis. We also lacked information regarding pre-hormonal IVF stimulation for male sterility. In Denmark, however, there is no tradition for administering FSH and/or clomiphene citrate to increase sperm counts. This applies to men with poor semen quality due to hypogonadotrophic hypogonadism, but they seldom undergo ART treatment as spontaneous pregnancy often is achieved. Among the limitations of NDR is the lack of information regarding whether the registered diabetes is type 1 or type 2. However, type 2 diabetes generally constitutes ~90% of all diabetes in this age group; and cohort participants with a diabetes diagnosis prior to enrollment were excluded. Further limitations include that the date of inclusion in the NDR register is only a proxy for the diagnosis, which was likely made some time prior to inclusion in the NDR (Glumer et al., 2003). Also the NDR likely underestimates the actual diabetes burden, as people without clinical diagnoses are not included. Lastly, as our study was register-based insufficient information regarding health behavioral factors (smoking, alcohol consumption and BMI) was available. However, by adjustment of socioeconomic factors we expect to have accounted for some of this possible confounding.
Conclusion
In our cohort of more than 39 000 men identified from the Danish National IVF register, male factor infertility was associated with a significantly higher risk of diabetes among men identified from the second IVF register. Furthermore, we observed a ‘dose–response’ relationship as the risk of diabetes appeared to increase with the severity of male factor infertility. We found no increased risk among men identified from the first IVF register which may be due to exposure misclassification as the reason for male factor infertility was not available from this time period. In line with other studies (Jensen et al., 2009; Salonia et al., 2009; Eisenberg et al., 2015a), these findings support an association between a man's reproductive and somatic health. Clinically, these findings can help to identify high risk populations where further monitoring may be necessary, which could include screening for diabetes among aspermic and azoospermic men.
Supplementary data
Supplementary data are available at Human Reproduction online.
Authors’ roles
J.P.B. and A.G. acquired funding for the study. C.H.G., E.V.B. and J.P.B. designed the study. C.H.G. and E.V.B. analyzed data and C.H.G. wrote the manuscript. All authors contributed to data analysis/interpretation, critical revision of the paper and final approval of the manuscript.
Funding
This article is part of the ReproUnion collaborative study, co-financed by the European Union, Intereg V Öresund-Kattegat-Skagerrak (NYPS 20200407).
Conflict of interest
None declared.
Supplementary Material
References
- American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AN, Westergaard HB, Olsen J. The Danish in vitro fertilisation (IVF) register. Dan Med Bull 1999;46:357–360. [PubMed] [Google Scholar]
- Bay B, Mortensen EL, Hvidtjorn D, Kesmodel US. Fertility treatment and risk of childhood and adolescent mental disorders: register based cohort study. BMJ 2013;347:f3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs ML, Mukamal KJ, Luchsinger JA, Ix JH, Carnethon MR, Newman AB, de Boer IH, Strotmeyer ES, Mozaffarian D, Siscovick DS. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA 2010;303:2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenstrup LT, Knudsen LB. Danish registers on aspects of reproduction. Scand J Public Health 2011;39:79–82. [DOI] [PubMed] [Google Scholar]
- Carstensen B, Kristensen JK, Marcussen MM, Borch-Johnsen K. The National Diabetes Register. Scand J Public Health 2011;39:58–61. [DOI] [PubMed] [Google Scholar]
- Carstensen B, Kristensen JK, Ottosen P, Borch-Johnsen K, Steering Group of the National Diabetes Register . The Danish National Diabetes Register: trends in incidence, prevalence and mortality. Diabetologia 2008;51:2187–2196. [DOI] [PubMed] [Google Scholar]
- Danish Fertility Society Annual Report 2015. http://www.fertilitetsselskab.dk/(2015).
- Eisenberg ML, Li S, Behr B, Cullen MR, Galusha D, Lamb DJ, Lipshultz LI. Semen quality, infertility and mortality in the USA. Hum Reprod 2014;29:1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertil Steril 2015. a;103:66–71. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Cullen MR, Baker LC. Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertil Steril 2015. b;105:629–636. [DOI] [PubMed] [Google Scholar]
- Fedder J, Kaspersen MD, Brandslund I, Hojgaard A. Retrograde ejaculation and sexual dysfunction in men with diabetes mellitus: a prospective, controlled study. Andrology 2013;1:602–606. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, de Mouzon J, Castilla JA, Erb K, Korsak V, Nyboe Andersen A, European IVF-Monitoring (EIM) Consortium for the European Society of Human Reproduction and Embryology (ESHRE) . Assisted reproductive technology in Europe, 2009: results generated from European registers by ESHRE. Hum Reprod 2013;28:2318–2331. [DOI] [PubMed] [Google Scholar]
- Glumer C, Jorgensen T, Borch-Johnsen K, Inter99 study . Prevalences of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care 2003;26:2335–2340. [DOI] [PubMed] [Google Scholar]
- Groos S, Krause W, Mueller UO. Men with subnormal sperm counts live shorter lives. Soc Biol 2006;53:46–60. [DOI] [PubMed] [Google Scholar]
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care 1998;21:1138–1145. [DOI] [PubMed] [Google Scholar]
- Haffner SM. Epidemiology of type 2 diabetes: risk factors. Diabetes Care 1998;21:C3–C6. [DOI] [PubMed] [Google Scholar]
- Holmboe SA, Jensen TK, Linneberg A, Scheike T, Thuesen BH, Skakkebaek NE, Juul A, Andersson AM. Low testosterone: a risk marker rather than a risk factor for Type 2 diabetes. J Clin Endocrinol Metab 2016;101:3180–3190. [DOI] [PubMed] [Google Scholar]
- Hotaling JM, Davenport MT, Eisenberg ML, VanDenEeden SK, Walsh TJ. Men who seek infertility care may not represent the general U.S. population: data from the National Survey of Family Growth. Urology 2012;79:123–127. [DOI] [PubMed] [Google Scholar]
- Hotaling JM, Walsh TJ. Male infertility: a risk factor for testicular cancer. Nat Rev Urol 2009;6:550–556. [DOI] [PubMed] [Google Scholar]
- Jangir RN, Jain GC. Diabetes mellitus induced impairment of male reproductive functions: a review. Curr Diabetes Rev 2014;10:147–157. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol 2009;170:559–565. [DOI] [PubMed] [Google Scholar]
- Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl 2008;29:251–259. [DOI] [PubMed] [Google Scholar]
- Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol 2013;217:R25–R45. [DOI] [PubMed] [Google Scholar]
- Kelly DM, Jones TH. Testosterone and obesity. Obes Rev 2015;16:581–606. [DOI] [PubMed] [Google Scholar]
- Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril 2001;75:237–248. [DOI] [PubMed] [Google Scholar]
- Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab 2011;25:271–285. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 2008;93:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ford ES, Li B, Giles WH, Liu S. Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care 2010;33:1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004;27:1036–1041. [DOI] [PubMed] [Google Scholar]
- Maitra S. Can patient self-management explain the health gradient? Goldman and Smith's ‘Can patient self-management help explain the SES health gradient?’ (2002) revisited. Soc Sci Med 2010;70:802–812. discussion 813-805. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med 2008;14:1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga M, Alexandrescu B, Katz SE, Stein M, Ganiats T. Impact of infertility on quality of life, marital adjustment, and sexual function. Urology 2004;63:126–130. [DOI] [PubMed] [Google Scholar]
- Nordkap L, Priskorn L, Bang AK, Nordström Joensen U, Fedder J, Carlsen E, Lundström P, Jørgensen N Nedsat sædkvalitet/mandlige fertilitetsproblemer 2016. www.andrology.dk.
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011;39:22–25. [DOI] [PubMed] [Google Scholar]
- Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol 2013;9:479–493. [DOI] [PubMed] [Google Scholar]
- Sallmen M, Weinberg CR, Baird DD, Lindbohm ML, Wilcox AJ. Has human fertility declined over time? Why we may never know. Epidemiology 2005;16:494–499. [DOI] [PubMed] [Google Scholar]
- Salonia A, Matloob R, Gallina A, Abdollah F, Sacca A, Briganti A, Suardi N, Colombo R, Rocchini L, Guazzoni G et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur Urol 2009;56:1025–1031. [DOI] [PubMed] [Google Scholar]
- Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol 2015;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin JJ, Garcia-Perez MA, Hamatani T, Cano A. Infertility etiologies are genetically and clinically linked with other diseases in single meta-diseases. Reprod Biol Endocrinol 2015;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut AC, Benichou J. Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Stat Med 2004;23:3803–3820. [DOI] [PubMed] [Google Scholar]
- Ventimiglia E, Capogrosso P, Boeri L, Serino A, Colicchia M, Ippolito S, Scano R, Papaleo E, Damiano R, Montorsi F et al. Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril 2015;104:48–55. [DOI] [PubMed] [Google Scholar]
- Ventimiglia E, Montorsi F, Salonia A. Comorbidities and male infertility: a worrisome picture. Curr Opin Urol 2016;26:146–151. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Schembri M, Turek PJ, Chan JM, Carroll PR, Smith JF, Eisenberg ML, Van Den Eeden SK, Croughan MS. Increased risk of high-grade prostate cancer among infertile men. Cancer 2010;116:2140–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health 1992;82:816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J 2016;92:63–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.