Fibrosis progression begins to accelerate during perimenopause in women coinfected with human immunodeficiency virus and hepatitis C virus. Although prior data show higher-stage fibrosis among postmenopausal women, the current study reveals earlier onset of accelerated scaring associated with reproductive aging.”.
Keywords: menopause, hormones, fibrosis markers, anti-müllerian hormone, hepatic scarring
Abstract
Background
Severity of hepatic fibrosis is greater in postmenopausal than in premenopausal women, perhaps owing to protective effects of estrogens. However, prior studies of estrogen and liver fibrosis lack serial fibrosis measures, adjustment for age, or longitudinal observations in coinfected populations.
Methods
In a longitudinal cohort of women coinfected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV), we assessed fibrosis progression across reproductive age, using validated serum fibrosis markers, aminotransferase platelet ratio index (APRI) and fibrosis 4 (FIB-4). Fibrosis rate was evaluated within each woman as she transitioned from pre- to postmenopause, defined by a biomarker of ovarian function.
Results
The median follow-up (n = 405) was 9.1 years (interquartile range, 5.0–15.2 years), with a median menopausal age of 49 years (47–52 years). When fully controlled for chronologic aging, the fibrosis progression rate was accelerated during perimenopause, as shown using FIB-4 (0.12 units per year faster than during premenopause; 95% confidence interval [CI], .02–.21; P = .01) and APRI (0.05 units per year faster; −.002 to .09; P = .06). Accelerated fibrosis was also observed during postmenopause compared with premenopause, for FIB-4 (0.14 units per year faster; 95% CI, −.01 to .29; P = .07) and APRI (0.07 units per year faster; −.003 to .15; P = .06). Accelerated fibrosis in perimenopause persisted after adjustment for Hispanic ethnicity, antiretroviral use, and alcohol (0.10 FIB-4 units per year faster than during premenopause; 95% CI, .008–.20; P = .03).
Conclusions
In HIV/HCV-coinfected women, hepatic fibrosis accelerates with reproductive aging. Accelerated fibrosis begins in perimenopause, highlighting a previously unrecognized group of women at increased risk for advanced fibrosis and associated complications. Longitudinal analyses of fibrosis rates across reproductive age should be conducted in non–HCV-related liver diseases, given potential implications in a broader spectrum of women.
In the United States, approximately 2.3 million persons are chronically infected with hepatitis C virus (HCV) [1]. Because both prevalent and incident HCV infection are more common in HIV, the burden of HCV remains quite high, with 10%–15% of HIV-infected individuals having HCV coinfection [2], compared with <5% in most HIV-uninfected populations [3]. Liver-related death remains a leading cause of death in HIV-infected individuals [4]. HIV infection affects the natural history of HCV, resulting in accelerated hepatic fibrosis progression compared with HCV infection alone [5, 6].
Notable sex differences in rates of HCV-related fibrosis have been described, although prior studies derive from HCV-monoinfected cohorts. HCV-infected men have more severe fibrosis than women, perhaps owing to the protective effects of estrogen. Cross-sectional data demonstrate higher stages of fibrosis in postmenopausal than in premenopausal women [7–9], a difference that may be due to loss of estrogen or to general aging. Prior cross-sectional data demonstrate higher stages of fibrosis in postmenopausal than in premenopausal women [7–9], supporting the protective effects of estrogens against hepatic scarring in humans. However, these data have been limited by inadequate adjustment for chronologic aging, have included only 1 or 2 time points of fibrosis assessment, and assume that progression between fibrosis stages is a linear process [10]. These studies also relied on self-report of menopausal status and lacked serial fibrosis measurements as women transition through menopause. Finally, the relation of estrogen with fibrosis progression has not been studied in women coinfected with HIV.
To address these knowledge gaps, we conducted a longitudinal investigation of fibrosis progression using serial noninvasive serum markers of hepatic fibrosis across reproductive age in HIV/HCV-coinfected women followed up in the prospective Women’s Interagency HIV Study (WIHS). Using serial measures of anti-müllerian hormone (AMH), a hormonal measure of ovarian reserve [11], we compared rates of fibrosis in each woman during peri- and postmenopause, compared with premenopause. We hypothesized that reproductive aging in HIV/HCV-coinfected women would be associated with accelerated fibrosis progression, independent of chronologic aging. If identified, these findings would support the urgency of HCV treatment in HIV-infected menopausal women and might potentially highlight an unrecognized high-risk group of women that includes those of perimenopausal age.
METHODS
Study Population
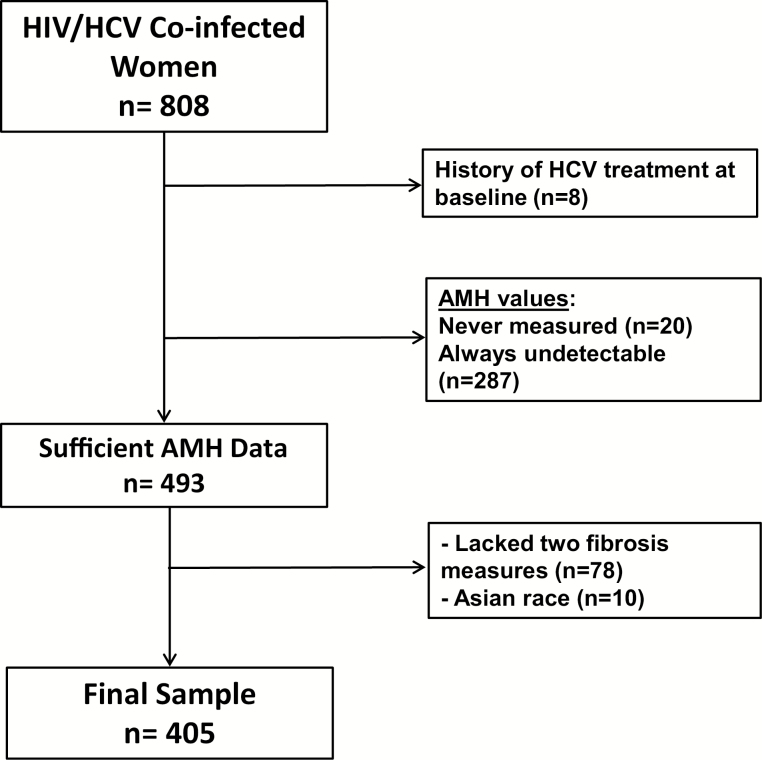
We conducted a nested cohort study of women participating in the WIHS. The WIHS is a multicenter, longitudinal observational cohort of women at risk for, or with a current diagnosis of, HIV infection. Enrollment in WIHS took place in 3 waves, in1994–1995, 2001–2002, and 2011–2012. The current study uses the first 2 waves conducted at 6 clinical sites across the United States [12, 13]. Women are seen twice yearly and undergo detailed histories, physical examinations, structured interviews, and laboratory testing. The current study was approved by the WIHS Executive Committee and the institutional review boards at the 6 participating WIHS sites. Study eligibility included HIV/HCV coinfection at WIHS entry (within first 3 visits), as defined by detectable HCV RNA, HCV antibody, and positive HIV Western blot (Figure 1). Eligible participants had detectable AMH consistent with premenopausal status and subsequent AMH levels measured throughout follow-up. Women reporting Asian race were excluded because there were too few Asian women for meaningful racial comparisons.
Figure 1.
Cohort of women in the Women’s Interagency HIV Study coinfected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) and meeting inclusion or exclusion criteria. AMH, anti-müllerian hormone.
Predictor and Outcome Measures
Our primary predictor was reproductive stage, which was characterized as pre-, peri-, or postmenopausal. AMH was used to define reproductive stage as this hormone is produced by ovarian follicles and reflects ovarian reserve [11, 14]. Premenopause was defined by detectable AMH, perimenopause as within 5 years of when AMH became undetectable (hereafter referred to as “AMH loss”), and postmenopause as >5 years after AMH loss [15].
The primary outcome was rate of hepatic fibrosis progression, defined by change in aspartate aminotransferase platelet ratio index (APRI) and fibrosis 4 (FIB-4) units per year. The fibrosis rate was evaluated for each woman as she transitioned across reproductive age, with fibrosis estimates in peri- and postmenopause compared with premenopause as the reference. FIB-4 was calculated as follows: age [years] × AST [U/L])/((platelet count [109/L]) × (ALT [U/L])1/2) [16]. APRI reflects the ratio of AST to platelet count [17–19]. FIB-4 and APRI values were not calculated if AST or ALT was >10 times the upper limit of normal or if platelet counts were <25000 × 106 cells/L, because these extreme values are unlikely to be due to chronic liver fibrosis. FIB-4 and APRI have been validated to stage fibrosis in patients with HIV/HCV coinfection including participants of the WIHS [16, 20, 21]. Bridging fibrosis/cirrhosis were defined as FIB-4 values >3.25 and APRI values >1.5 [17, 22].
Patient Characteristics
Demographics included age and race/ethnicity. Alcohol use was defined by self-report of weekly consumption. HIV-related factors included HIV RNA levels, CD4 cell count, and antiretroviral therapy (ART) use; liver-related factors included HCV RNA levels (measured at enrollment), HCV genotype (available in subset of participants), HCV treatment history and chronic hepatitis B virus infection; metabolic factors included diabetes (defined as any fasting glucose measurement of >126 mg/dL, hemoglobin A1c measured at ≥6.5%, or self-report of taking antidiabetic medication), dyslipidemia, body mass index, and waist circumference; and medication history included self- reported use of exogenous hormone therapy. Homeostatic model assessment of insulin resistance was calculated using the following equation: [fasting glucose (mmol/L) × fasting insulin (mU/L)]/22.5. Metabolic syndrome was defined as having ≥3 of the following: fasting glucose ≥110 mg/dL, fasting triglycerides ≥150 mg/dL, fasting high-density lipoprotein cholesterol <50 mg/dL, systolic blood pressure >130 mm Hg and/or diastolic blood pressure ≥85 mm Hg, or waist circumference >88 cm. Alcohol intake was ascertained semiannually as the average number of drinks per week during the preceding 6-month interval. Our recent data in WIHS have found no association of 7–14 drinks per week with fibrosis progression as assessed by APRI or FIB-4 [23]; therefore, heavy use in this cohort was defined as >14 drinks per week.
Plasma HIV RNA levels were measured using the NASBA/NuciSens HIV RNA assay (BioMerieux), in laboratories certified by the National Institute of Allergy and Infectious Diseases Virology Quality Assurance Certification Program, National Institutes of Health (NIH). HCV and hepatitis B virus serologic markers were measured using standard commercial assays; these markers included hepatitis C antibody (EIA 3.0; Ortho-Clinical Diagnostics) and hepatitis B surface antigen (Abbott Laboratories). HCV RNA levels were measured using the COBAS Amplicor Monitor 2.0 assay (Roche Diagnostics) with a linear range of 600–700 000 IU/mL, or COBAS TaqMan (Roche Diagnostics), with a linear range of 10–2.0 × 108 IU/mL. AMH levels were determined using a commercially available enzyme-linked immunosorbent assay (Gen II; Beckman Coulter). Plasma samples were frozen at −80°C and not thawed before testing. Interassay coefficients of variations have been reported elsewhere as 8.2% at 2.8 ng/mL and 9.4% at 8.5 ng/mL [24]. The lower limit of AMH detection was 0.08 ng/mL.
Statistical Analysis
The fibrosis progression rate was evaluated using mixed-effect linear regression models with random intercepts and slopes. Random intercepts accounted for between-woman differences in baseline fibrosis levels while random slopes allowed for heterogeneous rates of fibrosis progression over time. Menopausal status was modeled as the cumulative years at each visit that each woman had been exposed to perimenopause (possible maximum of 5 years for each women) and postmenopause since WIHS enrollment, such that the regression coefficients for peri- and postmenopause reflect the increase in the rate of change in APRI and FIB-4 levels when exposed to that menopausal status, as compared with premenopause.
Similarly, other covariates were modeled as cumulative years of exposure at each WIHS visit since enrollment; heavy alcohol use reflects years of exposure to >14 drinks per week; black race and Hispanic ethnicity reflect years of study follow-up for black and Hispanic women, respectively; obesity reflects years of exposure to body mass index ≥30; and no ART, diabetes, and metabolic syndrome each reflect years of exposure to that given condition. These covariates were defined to model effects on the rates of change in the outcomes, that is, their estimated coefficients reflect acceleration or deceleration of FIB-4 and APRI progression rates. Chronologic aging was modeled by flexible linear splines to allow average rates of fibrosis progression per year to differ within different age ranges: <40, 40–45, 45–50, 50–55, and >55 years. We used very flexible modeling of age, employing 5 parameters, to ensure that we fully controlled for chronologic age, given its inherent association with reproductive aging. HIV load was modeled as the cumulative area under the curve for log10(viral load) by time and above log10(80). CD4 cell count was modeled as the cumulative area over the curve for CD4 cell count by time and ≤250.
Forward stepwise selection (P < .05 for model inclusion) was used to build the multivariable models, with covariates of interest (menopausal status) and biologic plausibility (heavy alcohol use) included in the final model regardless of statistical significance. The base model included time spent in peri- and postmenopausal phases, adjusted for age. We added each candidate variable to the model as a single addition. The explanatory variable with the lowest P value was retained in the model. Remaining candidate variables were tested again, retaining the covariate with the lowest P value until no additional variables achieved P < .05 when added to the model. In the case of missing data, the last observation was carried forward until a new observation was available or for 1 year, whichever occurred first. If a new measurement was not available within 1 year, subsequent measurements were considered missing. Data were excluded at and after the time of HCV treatment initiation or first undetectable HCV RNA. Analyses were performed using SAS software, version 9.4 (SAS Institute).
RESULTS
We identified 405 HIV/HCV-coinfected women who were premenopausal at baseline as confirmed by detectable AMH levels (Figure 1). Women were followed up for a median of 9.1 years (interquartile range [IQR], 5.0–15.2 years). Their median age at study entry was 37 years (IQR, 34–41 years), with a median age at menopause of 49 years (47–52 years). The majority of women were non-Hispanic black (57.8%), followed by Hispanic (21.7%) and non-Hispanic white (20.5%). At study entry, just under 6% of women had fibrosis of stage 3 or higher, which increased to 32.4% and 19.8% based on FIB-4 and APRI, respectively at last available follow-up. Heavy alcohol use during WIHS follow-up was uncommon (6.2%). Most women received ART (88.2%), whereas less than a quarter (22.9%) received any form of HCV treatment, including 2% who received direct-acting antivirals. Regarding metabolic risk factors approximately 11% ever had metabolic syndrome diagnosed, and just over a quarter had history of diabetes. Just under 20% of women reported using some form of hormonal replacement therapy during follow-up (Table 1).
Table 1.
Cohort Characteristics (n = 405)
| Characteristic | Value |
|---|---|
| Age at entry, median (IQR), y | 37 (34–41) |
| Age at menopause, median (IQR), y | 49 (47–52) |
| Duration of follow-up, median (IQR), y | 12.3 (7.1–15.2) |
| Race/ethnicity, % | |
| Non-Hispanic white | 20.5 |
| Non-Hispanic black | 57.8 |
| Hispanic | 21.7 |
| FIB-4, median (IQR) | |
| Entry | 1.3 (0.9–1.9) |
| Last available measure | 2.2 (1.4–4.2) |
| Fibrosis of stage 3 or higher by FIB-4, %a | |
| Entry | 5.9 |
| Last available measure | 32.4 |
| APRI, median (IQR) | |
| Entry | 0.55 (0.38–0.92) |
| Last available measure | 0.68 (0.37–1.56) |
| Fibrosis of stage 3 or higher by APRI, %b | |
| Entry | 5.7 |
| Last available measure | 19.8 |
| Heavy alcohol use, % | |
| Entry | 12.5 |
| Last available measure | 6.2 |
| Marijuana use, % | |
| Entry | 27.6 |
| Last available measure | 16.5 |
| CD4 cell count, median (IQR), cells/μL | |
| Entry | 381 (226–567) |
| Last available measure | 311 (108–574) |
| HIV RNA, IU/mL, median (IQR) | |
| Entry | 15000 (1606–67000) |
| Last available measure | 280 (20–45000) |
| Undetectable HIV RNA at last available measure, % | 25.9 |
| ART use during study, % | 88.2 |
| HCV RNA, IU/mL, median (IQR) | |
| Entry | 2.2 million (580 000–4.0 million) |
| Last available measure | 2.4 million (745 000–5.4 million) |
| HCV treatment history (any type), % | 22.9 |
| Direct-acting antiviral HCV treatment, % | 2.0 |
| HCV genotype, %c | |
| 1 | 88.1 |
| 2 | 2.7 |
| 3 | 7.1 |
| Positive hepatitis B surface antigen, % | 2.5 |
| History of diabetes mellitus, % | 27.2 |
| Metabolic syndrome, % | |
| Entry | 0.7 |
| Last available measure | 10.6 |
| HOMA-IR, median (IQR) | |
| Entry | 2.93 (1.60–5.11) |
| Last available measure | 3.31 (1.82–5.71) |
| Fasting glucose ever ≥110 mg/dL, % | 23.0 |
| Fasting triglycerides ever ≥150 mg/dL, % | 43.4 |
| Waist circumference ever >88 cm, % | 58.3 |
| HDL ever <50 mg/dL, % | 53.3 |
| Body mass index, kg/m2, median (IQR) | 25.2 (22.3–28.7) |
| Entry | |
| Last available measure | 24.7 (21.0–29.3) |
| Hormone replacement therapy, % | 17.9 |
Abbreviations: APRI, aspartate aminotransferase platelet ratio index; ART, antiretroviral therapy; FIB-4, fibrosis 4; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HOMA-IR, homeostasis model assessment-estimated insulin resistance; IQR, interquartile range.
aDefined as FIB-4 >3.25.
bDefined as APRI >1.5.
cHCV genotype was missing in 30% of the cohort
After adjustment for the effect of chronologic aging, the rate of fibrosis in units per year was greater during peri- and postmenopause than during premenopause, as estimated based on either FIB-4 or APRI (Figure 2). Figure 2 reflects person-years of observation as follows: 405 women in premenopause, contributing a median of 5.0 years (IQR, 2.5–18.5 years) for a total of 2138 person-years of follow-up; 239 women in perimenopause, contributing a median of 5.0 years (3.5–5.0 years) for a total of 986 person-years of follow-up; and 141 women in postmenopause, contributing a median of 5.0 years (2.5–8.0 years) for total of 784 person-years of follow-up.
Figure 2.
Age-adjusted rate of liver fibrosis progression across reproductive age as determined using fibrosis 4 (FIB-4) levels (A) and aminotransferase platelet ratio index (APRI; B). Progression rates shown reflect estimates within the 45–50-year age range. Although rates differ by age, estimated differences between the 3 statuses remain constant. Analyses were performed using mixed-effect linear regression models with random intercepts and slopes.
Table 2 shows the age-adjusted regression analyses for factors associated with fibrosis progression. Using premenopausal status as the reference, the change in FIB-4 was 0.12 units per year faster during perimenopause and 0.14 units faster during postmenopause, though the latter estimate did not reach statistical significance. Likewise, change in APRI was 0.05 units per year faster during perimenopause and 0.07 units faster during postmenopause, though P values for these estimates were both .06. Other factors associated with accelerated fibrosis progression at age-adjusted analyses included Hispanic ethnicity and HIV load, with a suggestive P value for heavy alcohol use. Low CD4 cell count and lack of ART were associated with fibrosis progression but were not statistically significant for APRI (Table 2).
Table 2.
Age-Adjusted Hepatic Fibrosis Estimates Using FIB-4 and APRIa
| Variable | FIB-4 | APRI | ||
|---|---|---|---|---|
| Increase in Progression Rate (95% CI) | P Value | Increase in Progression Rate (95% CI) | P Value | |
| Age group, y | ||||
| <40 | 0.152 (.099–.205) | <.001 | 0.060 (.018–.101) | .005 |
| 40–45 | 0.250 (.187–.312) | <.001 | 0.078 (.040–.116) | <.001 |
| 45–50 | 0.232 (.162–.302) | <.001 | 0.062 (.025–.098) | <.001 |
| 50–55 | 0.255 (.168–.342) | <.001 | 0.081 (.034–.128) | <.001 |
| >55 | 0.205 (.071–.340) | .003 | 0.030 (−.048 to .108) | .45 |
| Black vs white race | −0.041 (−.133 to .052) | .39 | −0.020 (−.071 to .031) | .44 |
| Hispanic ethnicity | 0.189 (.058–.320) | .005 | 0.100 (.029–.171) | .006 |
| Heavy alcohol use | 0.294 (−.013 to .602) | .06 | 0.120 (−.009 to .250) | .07 |
| CD4 cell count ≤250 cells/μL | 0.062 (.001–.124) | .047 | 0.023 (−.009 to .055) | .159 |
| Log HIV load, IU/mL | 0.072 (.037–.108) | <.001 | 0.027 (.009–.045) | .004 |
| No ART | 0.109 (.022–.196) | .01 | 0.031 (−.015 to .078) | .19 |
| Diabetes | −0.035 (−.148 to .078) | .54 | −0.016 (−.070 to .039) | .58 |
| Metabolic syndrome | −0.014 (−.218 to .190) | .89 | 0.003 (−.104 to .110) | .96 |
| Obesity | −0.027 (−.123 to .069) | .58 | −0.020 (−.102 to .061) | .62 |
| Perimenopause vs premenopause | 0.119 (.024–.214) | .01 | 0.045 (−.002 to .093) | .06 |
| Postmenopause vs premenopause | 0.140 (−.013 to .294) | .07 | 0.071 (−.003 to .146) | .06 |
Abbreviations: APRI, aspartate aminotransferase platelet ratio index; ART, antiretroviral therapy; CI, confidence interval; FIB-4, fibrosis 4; HIV, human immunodeficiency virus.
aEstimates were obtained using mixed-effect linear regression models with random intercepts and slopes and reflect fitted rate of progression per year within each age range. This was modeled in 5-year ranges, without regard to statistical significance, to reduce the possibility that age could still confound the estimates of reproductive aging effects.
With further adjustment for ART use, Hispanic ethnicity, and heavy alcohol use, the peri- and postmenopausal estimates for fibrosis progression remained relatively consistent for APRI, though no longer statistically significant (Table 3). In the fully adjusted models, estimates of the peri- and postmenopause effects were 14%–24% smaller than the age-adjusted estimates, all remaining in the direction of accelerated progression. The estimated effect of perimenopause on FIB-4 progression remained statistically significant (0.10; 95% confidence interval, .007–.20; P = .04). We evaluated each remaining unselected predictor as a single addition to the primary multivariate models shown in Table 3. These additions changed the peri- and postmenopausal estimates by a maximum of 14% for APRI and 27% for FIB-4.
Table 3.
Association of Reproductive Age With Fibrosis Progression: Adjusted Analysesa
| Adjustment | FIB-4 | APRI | ||
|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | |
| Age-adjusted (n = 405) | ||||
| Perimenopause | 0.119 (.024–.214) | .01 | 0.045 (−.002 to .093) | .06 |
| Postmenopause | 0.140 (−.013 to .294) | .07 | 0.071 (−.003 to .146) | .06 |
| Adjusted for age and ART (n = 399) | ||||
| Perimenopause | 0.117 (.023 to .211) | .02 | 0.041 (−.005 to .086) | .08 |
| Postmenopause | 0.147 (−.003 to .297) | .055 | 0.069 (−.004 to .140) | .06 |
| Adjusted for age, ART, and Hispanic ethnicity (n = 399) | ||||
| Perimenopause | 0.115 (.021–.209) | .02 | 0.040 (−.006 to .086) | .09 |
| Postmenopause | 0.141 (−.009 to .291) | .07 | 0.066 (−.007 to .140) | .08 |
| Adjusted for age, ART, Hispanic ethnicity, and heavy alcohol use (n = 388) | ||||
| Perimenopause | 0.103 (.008–.197) | .03 | 0.038 (−.009 to .085) | .11 |
| Postmenopause | 0.109 (−.043 to .262) | .16 | 0.058 (−.020 to .137) | .15 |
Abbreviations: APRI, aspartate aminotransferase platelet ratio index; ART, antiretroviral therapy. CI, confidence interval ; FIB-4, fibrosis 4.
aEstimates were obtained using mixed-effect linear regression models with random intercepts and slopes and reflect fitted rate of progression per year within each age range. This was modeled in 5-year ranges, without regard to statistical significance, to reduce the possibility that age could still confound the estimates of reproductive aging effects. The reference group for all analyses is premenopause.
DISCUSSION
In this longitudinal prospective cohort of HIV/HCV-coinfected women, we found that liver fibrosis rates, as assessed using serial noninvasive serum measures, increased as women transitioned through menopause. Importantly, we employed a robust statistical approach to account for potential confounding effects of chronologic aging, and we evaluated reproductive stages through hormonal confirmation of ovarian reserve. Acceleration of hepatic fibrosis also began during perimenopause, suggesting that women may be at increased risk of liver scarring earlier than suggested by prior data relying on self-reported menopausal age.
The protective effects of estrogens against hepatic fibrosis were initially demonstrated in cross-sectional studies reporting higher stages of hepatic fibrosis in postmenopausal than in premenopausal women, and less scarring in postmenopausal women receiving hormone replacement therapy [7, 8]. These clinical observations are consistent with in vitro animal data that demonstrate dose-dependent reductions in collagen production by hepatic stellate cells incubated with estradiol [25]. Cross-sectional data in nonalcoholic fatty liver disease have also noted lower stages of hepatic fibrosis in premenopausal than in postmenopausal women [26], with similar severity of scarring in postmenopausal women compared with men [26, 27]. Earlier age at self-reported menopause has also been associated with more severe hepatic fibrosis, suggesting that longer duration of relative estrogen deficiency may increase fibrosis progression [28].
The current findings represent an important advance in our understanding of the effects of reproductive aging on liver fibrosis by highlighting the accelerated fibrosis that begins as early as during perimenopause. Using AMH as a reference standard measurement of ovarian reserve [14], we were able to evaluate each woman’s fibrosis rate as she transitioned across reproductive stages. This approach avoids the misclassification of menopausal status that may result from relying on menstrual cycle pattern and self-reported menopause, particularly given potential effects of HIV infection on menstrual irregularity [29].
Although direct-acting antiviral agents have improved tolerability and response to HCV treatment, HCV infection in HIV-infected populations remains an important public health consideration. Both prevalent and incident HCV infection are more common in HIV, so the burden of HCV remains quite high, with 10%–15% of HIV-infected individuals having HCV coinfection [2], compared with <5% in most HIV-uninfected populations [3]. HIV also enhances HCV replication and results in accelerated fibrosis compared with HCV monoinfection [5, 6]. Access to HCV treatment remains limited in underserved populations [30–32], and drug-drug interactions and potential cross-resistance between antiretroviral and direct-acting antiviral agents are important considerations in coinfected patients [33]. Therefore, identifying individuals with more urgent need for therapy to reduce incident cirrhosis and its complications remains a public health priority. Women during peri- and postmenopause indeed represent such at-risk groups, given their accelerated hepatic fibrosis.
The current study has notable limitations. Only 141 women reached postmenopause, contributing a median of 8 (IQR, 4–15) fibrosis measures per patient during postmenopause, which caused confidence intervals to be wide for the estimated postmenopausal effects. These were larger than the estimated perimenopausal effects but had larger P values because of their wide uncertainty. However, the consistency of postmenopausal estimates in age-adjusted and fully adjusted models lends confidence to our findings. We acknowledge that age is a component of the FIB-4 equation, and even if other components of FIB-4 remained constant the average FIB-4 value would increase owing to the chronologic age component. However, our model included a linear component of age (and departures from linearity), which modeled such an increase. With years in the study included as a random effect, the model also accounted for variation in this “by-definition” age effect between women. Because we were careful to minimize possible confounding with chronologic aging, we also minimized the effects of including chronologic age in the FIB-4 definition. We also lacked serial liver biopsy specimens for confirmation of stage of fibrosis, though liver biopsies are invasive, subject to inter- and intraobserver variability in fibrosis staging, and may underestimate fibrosis stage, particularly given the variable adequacy of tissue length [34, 35].
An important strength of this study was the use of AMH as a measure of reproductive stage. AMH production in women is specific to preantral ovarian follicles, and it strongly correlates with ovarian reserve, or total follicular pool [36, 37]. An added strength of AMH includes the stability of levels throughout the menstrual cycle, unlike other biomarkers of ovarian reserve [38]. AMH does have limitations, including potentially lower levels in women with tobacco [24] or oral contraceptive [39] use, although data on the association of these exposures with AMH are conflicting [40–42]. Because neither tobacco nor oral contraceptives have been shown to affect hepatic fibrosis progression, these factors were not considered as potential confounders in the current study.
Using a robust statistical approach and serial noninvasive serum markers, we were able to capture granular changes in fibrosis estimates over time, while avoiding many difficulties that afflict analyses of biopsy-determined fibrosis stages [43] Notably, we focus on progression only while women were under observation, with unexplained variation in starting levels of fibrosis accounted for by random intercepts, and unexplained variation in rates of fibrosis progression during the study accounted for by random slopes. Although age at HCV infection was not available, any effect of this is reflected by the random effects and unlikely to be strongly associated with age at AMH loss. In addition, the widely accepted association of older age at infection with more rapid progression may be spurious, because this can arise from bias caused by imperfect knowledge of age at infection [44]. A previous study that rigorously avoided such bias found that current age was more likely than age at infection to influence the rate of progression [43].
In summary, in this analysis of HIV/HCV-coinfected women, hepatic fibrosis progression was found to accelerate across reproductive age, independent of chronologic aging. Accelerated fibrosis began during perimenopause, highlighting a previously unrecognized group of women at increased risk for progressive fibrosis and associated complications. Similar analyses using serial measures of fibrosis should be conducted in non–HCV-related liver diseases, including in women without HIV infection, given the potential implications of ovarian reserve on fibrosis progression in women with a broad spectrum of liver diseases.
Notes
Acknowledgments. We thank Geralyn-Lambert Messerlian, PhD, whose laboratory performed anti-müllerian hormone (AMH) measurements for this study, as well as the University of California, San Francisco, Liver Center (P30 DK026743). Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS).
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases, by site as follows: University of Alabama, Birmingham and University of Mississippi Medical Center (UAB-UMMC) WIHS (principal investigators, Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker; grant U01-AI-103401; Atlanta WIHS (I. O. and Gina Wingood; grant U01-AI-103408; Bronx WIHS (Kathryn Anastos; grant U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson; grant U01-AI-031834; Chicago WIHS (Mardge Cohen and A. L. F.; grant U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye; grant U01-AI-034994; Miami WIHS (M. F. and Lisa Metsch; grant U01-AI-103397; UNC WIHS (Adaora Adimora; grant U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (R. M. G., Bradley Aouizerat, and Phyllis Tien; grant U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub; grant U01-AI-042590; and Southern California WIHS (Joel Milam; grant U01-HD-032632 (WIHS I–WIHS IV). The WIHS receives additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects is provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders, and the NIH Office of Research on Women’s Health. WIHS data collection is supported by the University of California, San Francisco, Clinical and Translational Research Awards (CTRA) (grant UL1-TR000004) and Atlanta CTRA (grant UL1-TR000454). This work is also supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K23 grant DK111944 to M. S.) and NIDDK (R21 grant A1088351 to M. P.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ditah I, Ditah F, Devaki P et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol 2014; 60:691–8. [DOI] [PubMed] [Google Scholar]
- 2. Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44:S6–9. [DOI] [PubMed] [Google Scholar]
- 3. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–42. [DOI] [PubMed] [Google Scholar]
- 4. Sherman KE, Thomas DL, Chung RT. Human immunodeficiency virus and liver disease forum 2010: conference proceedings. Hepatology 2011; 54:2245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konerman MA, Mehta SH, Sutcliffe CG et al. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology 2014; 59:767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leite AG, Duarte MI, Mendes-Correa MC. Fibrosis progression in paired liver biopsies from HIV/HCV-coinfected patients without prior treatment of hepatitis C. J Int Assoc Provid AIDS Care 2015; 14:463–8. [DOI] [PubMed] [Google Scholar]
- 7. Codes L, Asselah T, Cazals-Hatem D et al. Liver fibrosis in women with chronic hepatitis C: evidence for the negative role of the menopause and steatosis and the potential benefit of hormone replacement therapy. Gut 2007; 56:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Martino V, Lebray P, Myers RP et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology 2004; 40:1426–33. [DOI] [PubMed] [Google Scholar]
- 9. Villa E, Vukotic R, Cammà C et al. Reproductive status is associated with the severity of fibrosis in women with hepatitis C. PLoS One 2012; 7:e44624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48:418–31. [DOI] [PubMed] [Google Scholar]
- 11. Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014; 20:688–701. [DOI] [PubMed] [Google Scholar]
- 12. Bacon MC, von Wyl V, Alden C et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barkan SE, Melnick SL, Preston-Martin S et al. The women’s interagency HIV study. WIHS Collaborative Study Group. Epidemiology 1998; 9:117–25. [PubMed] [Google Scholar]
- 14. Amanvermez R, Tosun M. An update on ovarian aging and ovarian reserve tests. Int J Fertil Steril 2016; 9:411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sowers MR, Eyvazzadeh AD, McConnell D et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 2008; 93:3478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterling RK, Lissen E, Clumeck N et al. ; APRICOT Clinical Investigators Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25. [DOI] [PubMed] [Google Scholar]
- 17. Wai CT, Greenson JK, Fontana RJ et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–26. [DOI] [PubMed] [Google Scholar]
- 18. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med 2013; 158:807–20. [DOI] [PubMed] [Google Scholar]
- 19. Lin ZH, Xin YN, Dong QJ et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011; 53:726–36. [DOI] [PubMed] [Google Scholar]
- 20. Bambha K, Pierce C, Cox C et al. Assessing mortality in women with hepatitis C virus and HIV using indirect markers of fibrosis. AIDS 2012; 26:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Mohri H, Cooper C, Murphy T, Klein MB. Validation of a simple model for predicting liver fibrosis in HIV/hepatitis C virus-coinfected patients. HIV Med 2005; 6:375–8. [DOI] [PubMed] [Google Scholar]
- 22. Vallet-Pichard A, Mallet V, Nalpas B et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–6. [DOI] [PubMed] [Google Scholar]
- 23. Kelly E, Dodge J, Bacchetti P et al. Moderate alcohol use is not associated with fibrosis progression in HIV/HCV co-infected women: a prospective cohort study. Clin Infect Dis 2016; 63:512–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scherzer R, Greenblatt RM, Merhi ZO et al. Use of antimüllerian hormone to predict the menopausal transition in HIV-infected women. Am J Obstet Gynecol 2017; 216:46.e1–46.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology 1999; 29:719–27. [DOI] [PubMed] [Google Scholar]
- 26. Yang JD, Abdelmalek MF, Pang H et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014; 59:1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang JD, Abdelmalek MF, Guy CD et al. Patient sex, reproductive status, and synthetic hormone use associate with histologic severity of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2017;15:127–131 e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klair JS, Yang JD, Abdelmalek MF et al. ; Nonalcoholic Steatohepatitis Clinical Research Network A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016; 64:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ezechi OC, Jogo A, Gab-Okafor C et al. Effect of HIV-1 infection and increasing immunosuppression on menstrual function. J Obstet Gynaecol Res 2010; 36:1053–8. [DOI] [PubMed] [Google Scholar]
- 30. Stepanova M, Younossi ZM. Interferon-free regimens for chronic hepatitis C: barriers due to treatment candidacy and insurance coverage. Dig Dis Sci 2015; 60:3248–51. [DOI] [PubMed] [Google Scholar]
- 31. Lynch SM, Wu GY. Hepatitis C virus: a review of treatment guidelines, cost-effectiveness, and access to therapy. J Clin Transl Hepatol 2016; 4:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Canary LA, Klevens RM, Holmberg SD. Limited access to new hepatitis C virus treatment under state Medicaid programs. Ann Intern Med 2015; 163:226–8. [DOI] [PubMed] [Google Scholar]
- 33. Sulkowski MS. HCV-HIV co-infected patients: no longer a ‘special’ population? Liver Int 2016; 36suppl 1:43–6. [DOI] [PubMed] [Google Scholar]
- 34. Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 2003; 39:239–44. [DOI] [PubMed] [Google Scholar]
- 35. Regev A, Berho M, Jeffers LJ et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97:2614–8. [DOI] [PubMed] [Google Scholar]
- 36. van Rooij IA, Broekmans FJ, te Velde ER et al. Serum anti-müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod 2002; 17:3065–71. [DOI] [PubMed] [Google Scholar]
- 37. Gruijters MJ, Visser JA, Durlinger AL, Themmen AP. Anti-müllerian hormone and its role in ovarian function. Mol Cell Endocrinol 2003; 211:85–90. [DOI] [PubMed] [Google Scholar]
- 38. Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy Ch, Englert Y. Stable serum levels of anti-müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod 2007; 22:1837–40. [DOI] [PubMed] [Google Scholar]
- 39. Jung S, Allen N, Arslan AA et al. Demographic, lifestyle, and other factors in relation to antimüllerian hormone levels in mostly late premenopausal women. Fertil Steril 2017; 107:1012–1022.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kucera R, Ulcova-Gallova Z, Topolcan O. Effect of long-term using of hormonal contraception on anti-müllerian hormone secretion. Gynecol Endocrinol 2016; 32:383–5. [DOI] [PubMed] [Google Scholar]
- 41. Hawkins Bressler L, Bernardi LA, De Chavez PJ, Baird DD, Carnethon MR, Marsh EE. Alcohol, cigarette smoking, and ovarian reserve in reproductive-age African-American women. Am J Obstet Gynecol 2016; 215:758.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kline J, Tang A, Levin B. Smoking, alcohol and caffeine in relation to two hormonal indicators of ovarian age during the reproductive years. Maturitas 2016; 92:115–22. [DOI] [PubMed] [Google Scholar]
- 43. Bacchetti P, Boylan R, Astemborski J et al. Progression of biopsy-measured liver fibrosis in untreated patients with hepatitis C infection: non-Markov multistate model analysis. PLoS One 2011; 6:e20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bacchetti P, Tien PC, Seaberg EC et al. Estimating past hepatitis C infection risk from reported risk factor histories: implications for imputing age of infection and modeling fibrosis progression. BMC Infect Dis 2007; 7:145. [DOI] [PMC free article] [PubMed] [Google Scholar]