Summary
Adjunctive intravenous immunoglobulin (IVIG) was infrequently used in patients with necrotizing fasciitis and shock at US academic hospitals. IVIG had no significant impact on mortality, even when administered within 2 days of hospitalization or specifically to patients with streptococcal or staphylococcal infections.
Keywords: intravenous immunoglobulin, toxic shock, necrotizing fasciitis.
Abstract
Background.
Shock frequently complicates necrotizing fasciitis (NF) caused by group A Streptococcus (GAS) or Staphylococcus aureus. Intravenous immunoglobulin (IVIG) is sometimes administered for presumptive toxic shock syndrome (TSS), but its frequency of use and efficacy are unclear.
Methods.
Adult patients with NF and vasopressor-dependent shock undergoing surgical debridement from 2010 to 2014 were identified at 130 US hospitals. IVIG cases were propensity-matched and risk-adjusted. The primary outcome was in-hospital mortality and the secondary outcome was median length of stay (LOS).
Results.
Of 4127 cases of debrided NF with shock at 121 centers, only 164 patients (4%) at 61 centers received IVIG. IVIG subjects were younger with lower comorbidity indices, but higher illness severity. Clindamycin and vasopressor intensity were higher among IVIG cases, as was coding for TSS and GAS. In-hospital mortality did not differ between matched IVIG and non-IVIG groups (crude mortality, 27.3% vs 23.6%; adjusted odds ratio, 1.00 [95% confidence interval, .55–1.83]; P = .99). Early IVIG (≤2 days) did not alter this effect (P = .99). Among patients coded for TSS, GAS, and/or S. aureus, IVIG use was still unusual (59/868 [6.8%]) and lacked benefit (P = .63). Median LOS was similar between IVIG and non-IVIG groups (26 [13–49] vs 26 [11–43]; P = .84). Positive predictive values for identifying true NF and debridement among IVIG cases using our algorithms were 97% and 89%, respectively, based on records review at 4 hospitals.
Conclusions.
Adjunctive IVIG was administered infrequently in NF with shock and had no apparent impact on mortality or hospital LOS beyond that achieved with debridement and antibiotics.
Necrotizing fasciitis (NF) is a deep bacterial infection involving fascia and muscle with rapidly progressive tissue destruction. Group A Streptococcus (GAS) remains the most common cause of monomicrobial NF, but Staphylococcus aureus (SA) is an emerging etiology [1, 2], and both of these have been associated with epidemiologically distinct toxic shock syndromes (TSSs). While streptococcal TSS always occurs in the context of severe, invasive infections such as NF, infection is often unapparent [3, 4] and survival is generally better [5] in staphylococcal TSS. In both forms of TSS, bacterial exotoxins act as superantigens to trigger polyclonal T-cell activation, cytokine cascade, and refractory shock [4]. Although most clinical isolates of S. aureus produce 1 or more superantigens [6], vasopressor-dependent hypotension in invasive S. aureus infections is typically classified as septic shock. In contrast, shock and organ failure in NF secondary to GAS are defined as TSS [7]. However, for either streptococcal or staphylococcal NF with shock, classic features of TSS, such as desquamation, are not invariably present and can occur weeks after the acute illness. Therefore, the clinical diagnosis of TSS as a surrogate for superantigen-induced shock is imperfect and the importance of this mechanism in NF with shock secondary to S. aureus or even GAS is not entirely clear. Notably, other necrotizing soft tissue infections (eg, due to Vibrio, Clostridia, or polymicrobial infections) may also present with shock but have not been associated with superantigen-mediated TSS.
Mortality in NF ranges from as low as 5% [8] to >50% in the presence of shock and organ failure [9]. Survival requires prompt, appropriate antibiotics and aggressive surgical control. The Infectious Diseases Society of America (IDSA) also recommends clindamycin for NF due to GAS to block exotoxin production and to overcome high bacterial inocula [10]. While intravenous immunoglobulin (IVIG) contains antibodies that neutralize exotoxin superantigens, support for use in critically ill patients with NF caused by GAS or SA remains controversial [11]. IVIG has been studied as an adjunctive treatment in TSS [12, 13], but the only multicenter, randomized, double-blinded, placebo-controlled trial (RCT) of IVIG in TSS was terminated due to the lack of recruitment after enrolling only 21 patients [14]. In contrast to an earlier small observational cohort in Canada [15], a larger observational study of streptococcal TSS in children [16] was unable to confirm benefit from adjunctive IVIG. Two recent comparative observational studies of prospectively identified cases of GAS TSS from Sweden (N = 67; 27% with NF) [17] and invasive GAS disease from Australia (N = 84; 79% with shock and 35% with NF) [18] concluded that IVIG either improved or may have improved survival, respectively. Unlike its strong recommendation for clindamycin, the IDSA determined that existing evidence is insufficient to recommend IVIG for NF-TSS and emphasized the need for further studies [10].
Given the cost of IVIG, interruptions in supply, unclear efficacy, and potential adverse effects, a reappraisal of its role in NF with shock is desirable. However, due to low incidence and controversy regarding equipoise, a sufficiently powered RCT has been difficult to operationalize [14]. Therefore, a large observational study may be the only realistic, near-term approach to inform the balance between benefits and risks of adjunctive IVIG [19]. Furthermore, available epidemiologic data on IVIG use in NF with shock in North America is outdated and varies considerably [20]. An adequately large, observational cohort study is necessary to determine the prevalence of this practice and better assess the current feasibility of conducting an RCT. Here, we used an enhanced administrative database with date-stamped medication administrations from US academic medical centers to examine utilization patterns of IVIG among hospitalized patients with NF and vasopressor-dependent shock (NF-shock) and evaluate the impact of adjunctive IVIG on survival.
METHODS
A retrospective cohort study was performed using the Clinical Database/Resource Manager (CDB/RM) of Vizient, formerly University Health-Systems Consortium (UHC; Chicago, Illinois; see Supplementary Data for details) [21]. The effectiveness of IVIG was evaluated in patients with NF and vasopressor-dependent shock who underwent surgical debridement. The primary outcome was an odds ratio (OR) of in-hospital mortality. The secondary outcome was median length of stay (LOS).
NF-Shock Case-Selection Algorithm
Case selection is presented in Figure 1. The CDB/RM was queried for adult inpatients between October 2010 and June 2014 with an International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis of NF (728.86). Patients coded for Fournier gangrene (608.83) or gas gangrene (040.0) but not NF were excluded, as IVIG has no defined role in these conditions [11]. Also excluded were patients with non-TSS indications for IVIG (Supplementary Table E2). The IDSA and Surgical Infection Society guidelines consider surgical intervention to be the primary therapeutic modality for NF and it is standard practice to have patients return to the operating room within 24–36 hours of the index procedure [10, 22]. Although the initial debridement is usually extensive, the extent of tissue necrosis is often underappreciated at that time. An isolated trip to the operating room is likely to represent cases where NF was ruled out by local exploration [23]. Consequently, our debridement algorithm was restricted to patients who underwent debridement ≥2 times and/or amputations (Supplementary Table E1) or who died following a single surgical procedure within 3 calendar days. Date-stamped charges identified vasopressor use to eliminate patients with no clear indication for IVIG.
Figure 1.
Flowchart for the selection of necrotizing fasciitis (NF) shock cases. Inpatients discharged between October 2010 and June 2014 from 130 US academic medical centers and affiliates in the Vizient Clinical Database/Resource Manager (CDB-RM) were selected based on International Classification of Diseases, Ninth Revision (ICD-9) coding for NF, adult status, any surgical debridement, debridement consistent with the appropriate surgical management of true NF, vasopressor use, and absence of non–toxic shock syndrome (TSS) indications for intravenous immunoglobulin (IVIG). Numbers in brackets indicate the number of centers.
Analysis
IVIG patients were matched to non-IVIG patients via propensity score matching. The matching algorithm controlled immortal time bias as well as balanced the covariates of Table 1. Multivariable logistic regression was performed on matched pairs relating the binary outcome of in-hospital mortality to IVIG group status and all the matching variables for the matched cohort. Further details on the primary and various sensitivity analyses are available in the Supplementary Data. Algorithms applied to administrative data were validated by chart review at 4 centers in 3 geographic regions with disparate NF-shock case volume and proportions of associated IVIG use (Supplementary Data).
Table 1.
Baseline Characteristics of the Necrotizing Fasciitis Shocka Cohort Before and After Propensity Matching
| Variable | IVIG Group (n = 164) |
Unmatched Non-IVIG Group (n = 3963) | P Value | Matched IVIG Group (n = 161) |
Matched Non-IVIG Group (n = 161) | P Value |
|---|---|---|---|---|---|---|
| Age group, y | .002 | .951 | ||||
| 18–44 | 60 (36.6) | 949 (23.9) | 59 (36.6) | 55 (34.2) | ||
| 45–59 | 63 (38.4) | 1677 (42.3) | 63 (39.1) | 68 (42.4) | ||
| 60–74 | 34 (20.7) | 1075 (27.1) | 32 (19.9) | 31(19.3) | ||
| ≥75 | 7 (4.27) | 262 (6.61) | 7 (4.35) | 7 (4.35) | ||
| Male sex | 91 (55.5) | 2270 (57.3) | .708 | 89 (55.3) | 98 (60.9) | .366 |
| Charlson Comorbidity Index score | .052 | .951 | ||||
| 0–3 | 142 (86.6) | 3129 (79.0) | 139 (86.3) | 139 (86.3) | ||
| 4–5 | 15 (9.15) | 505 (12.7) | 15 (9.32) | 14 (8.70) | ||
| ≥6 | 7 (4.27) | 329 (8.30) | 7 (4.35) | 8 (4.97) | ||
| 3M APR DRG severity of illness assignment | <.001 | .827 | ||||
| 1–2 (mild and moderate) | 1 (0.61) | 61 (1.54) | 1 (0.62) | 2 (1.24) | ||
| 3 (major) | 7 (4.27) | 775 (19.6) | 7 (4.35) | 5 (3.11) | ||
| 4 (extreme) | 156 (95.1) | 3127 (78.9) | 153 (95.0) | 154 (95.7) | ||
| 3M APR DRG risk of mortality assignment | <.001 | .808 | ||||
| 1–2 (mild and moderate) | 6 (3.66) | 622 (15.7) | 6 (3.73) | 5 (3.11) | ||
| 3 (major) | 5 (3.05) | 756 (19.1) | 5 (3.11) | 7 (4.35) | ||
| 4 (extreme) | 153 (93.3) | 2585 (65.2) | 150 (93.2) | 149 (92.5) | ||
| Relative expected mortalityb | .06 | .835 | ||||
| 1–2 (anything below observed) | 53 (32.3) | 1585 (40.0) | 52 (32.3) | 57 (35.4) | ||
| 3–4 (0–25% above observed) | 71 (43.3) | 1379 (34.8) | 69 (42.9) | 65 (40.4) | ||
| 5 (>25% above observed) | 40 (24.4) | 999 (25.2) | 40 (24.8) | 39 (24.2) | ||
| ICD-9 diagnosis code | ||||||
| TSSc | 34 (20.7) | 55 (1.39) | <.001 | 31 (19.3) | 31 (19.3) | 1.000 |
| GAS/Staphylococcus aureusd | 43 (26.2) | 774 (19.5) | .045 | 40 (24.8) | 40 (24.8) | 1.000 |
| Any S. aureus (without GAS) | 14 (8.5) | 575 (14.5) | .03 | 13 (8.07) | 17 (10.6) | .57 |
| Any GAS | 29 (17.7) | 199 (5.02) | <.001 | 27 (16.8) | 23 (14.3) | .64 |
| Clindamycin use | 156 (95.1) | 2828 (71.4) | <.001 | 156 (95.0) | 156 (95.0) | 1.000 |
| Clindamycin timing relative to debridement windowe | <.001 | .694 | ||||
| Before | 26 (15.9) | 417 (10.5) | 26 (16.1) | 34 (21.1) | ||
| During | 127 (77.4) | 2284 (57.6) | 125 (77.6) | 117 (72.7) | ||
| After | 3 (1.83) | 127 (3.2) | 2 (1.24) | 2 (1.24) | ||
| NA | 8 (4.88) | 1135 (28.6) | 8 (4.97) | 8 (4.97) | ||
| No. of vasopressorsf | <.001 | .159 | ||||
| 1 | 50 (30.5) | 2485 (62.7) | 49 (30.4) | 62 (38.5) | ||
| ≥2 | 114 (69.5) | 1478 (37.3) | 112 (69.6) | 99 (61.5) | ||
| Median No. of debridement proceduresg | 5.0 (3.0–8.25) | 4.0 (3.0–7.0) | .017 | 5.0 (3.0–9.0) | 5.0 (3.0–9.0) | .718 |
| Day of first IVIG dose/vital status | 3.57 (4.00) | 3.60 (4.03) | .929 | 3.59 (4.03) | 3.59 (4.03) | 1.000 |
| Transfer from an acute care hospital | 83 (50.6) | 1919 (48.4) | .639 | 82 (50.9) | 86 (53.4) | .738 |
| Restrictedh IVIG dispensing | 45 (27.4) | 1131 (28.5) | .828 | 43 (26.7) | 43 (26.7) | 1.000 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: APR DRG, All Patients Refined Diagnosis Related Groups; GAS, group A Streptococcus; IVIG, intravenous immunoglobulin; ICD-9, International Classification of Diseases, Ninth Revision; NA, Not applicable; TSS, toxic shock syndrome.
aPatients aged ≥18 y with ICD-9 diagnosis code for necrotizing fasciitis who have undergone at least 1 surgical debridement procedure and received at least 1 vasopressor during inpatient encounter.
bVizient’s mapping of individual patient mortality predictions to categories based on the ratio of the patient’s expected mortality to the patient’s model cohort population mortality.
c ICD-9 code for toxic shock syndrome: 040.82.
d ICD-9 code for GAS infection: 04101; S. aureus infection: 03.811, 03.812, 04.111, 04.112.
eBetween and including 1 day prior to and 2 days after day of initial debridement procedure.
fMaximum count on any day during the debridement window. Initial vasopressor could be norepinephrine, dopamine, phenylephrine, or epinephrine. Additional vasopressor could include any of these as well as vasopressin.
gList of ICD-9 debridement procedure codes can be found in the Supplementary Data.
hRequiring prior approval of infectious disease physician or pharmacy leadership.
RESULTS
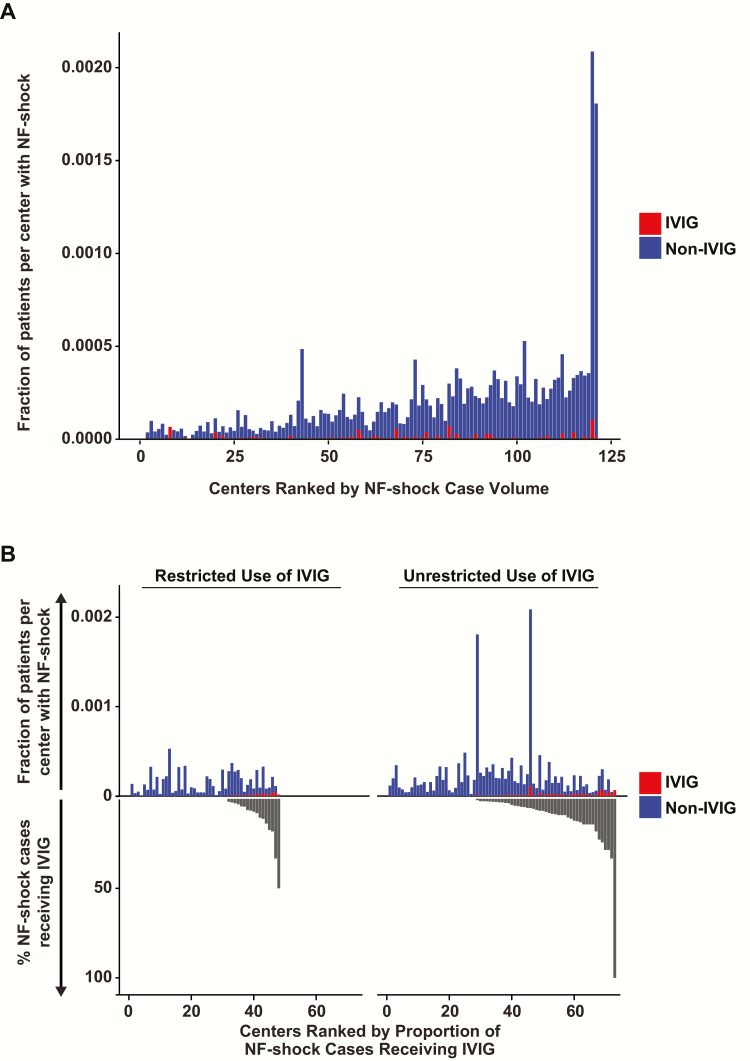
There were 11776 unique adult inpatient encounters with the NF diagnosis code at 130 hospitals. Of these, 10006 (85%) patients were coded for ≥1 surgical debridement, but only 5655 (48%) met the debridement algorithm. Nearly three-quarters of these (n = 4154) received ≥1 vasopressor charge(s), and this group comprised the NF-shock cohort (Figure 1). Excluding 27 patients with other indications for IVIG yielded 4127 cases, of which 164 received IVIG. Baseline characteristics of overall and matched cohorts are presented in Table 1. Patients given IVIG were younger (mean age, 48.8 ± 15.0 vs 54.0 ± 14.1 years) and had fewer comorbid conditions (median Charlson Comorbidity Index, 1.00 [0.00–2.25] vs 2.00 [1.00–3.00]). However, IVIG cases were more acutely ill, as indicated by a higher proportion that (1) were in extreme categories of the 3M All Patients Refined Diagnosis Related Groups Severity of Illness and Risk of Mortality scales; (2) received >1 vasopressor on the same day (69.5% vs 37.3%); and (3) had a greater number of debridement procedures (median, 5.0 [3.0–8.25] vs 4.0 [3.0–7.0]). Nearly half in both the IVIG and non-IVIG groups were transferred from another acute care hospital. Less than 30% of cases in both groups were at hospitals practicing unrestricted dispensing of IVIG for off-label indications such as NF and TSS. Considerable center-level variation was seen in the distribution of NF-shock case-volume and proportional IVIG administration (Figure 2). The mean NF-shock case density was 9.94 per 100000 admissions (range, 0–197.97 per 100000 admissions). The mean proportion of NF-shock cases that received adjunctive IVIG remained low in the overall cohort at 4%, as well as among the 61 hospitals reporting any IVIG use at 5.8% (range, 0–100%). Even among patients specifically coded for TSS, GAS, and/or SA, the use of IVIG remained infrequent at only 6.8%. The initial IVIG dose was administered within the first 2 days of hospitalization in 92 of 164 (56%) of the cases (Supplementary Figure E1). The frequency of IVIG use was significantly lower in hospitals following a restrictive policy for dispensing IVIG in NF or TSS (P = .015).
Figure 2.
Variation in volume of necrotizing fasciitis (NF) shock cases, proportion of admissions classified as NF-shock, and distributions of intravenous immunoglobulin (IVIG) use and associated dispensing restrictions across the US academic medical centers and affiliates in the Vizient Clinical Database/Resource Manager. A, Centers with at least 1 NF-shock case between October 2010 and June 2014 are ranked in ascending order of total case volume. Non-IVIG and IVIG cases are shown in blue and red, respectively. B, NF-shock cases are dichotomized based on whether IVIG dispensing is restricted at the treating center. The dispensing policy for IVIG was classified as restricted if prior approval was required from infectious diseases or pharmacy. The positive y-axis depicts NF-shock case density (non IVIG cases shown in blue; IVIG cases shown in red) for each hospital, ordered by increasing proportion of IVIG use in NF-shock, which is represented by the negative y-axis (black). Test of IVIG proportion medians between restricted and unrestricted centers by the Wilcoxon rank-sum test with continuity correction yielded a P value of .015, suggesting that restriction significantly reduced IVIG use for this indication.
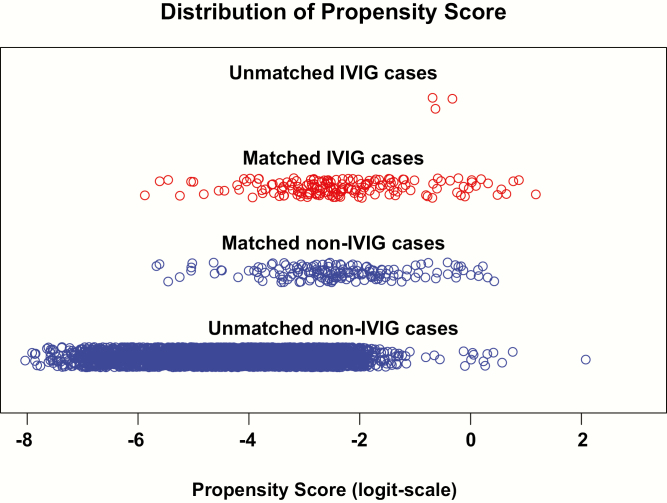
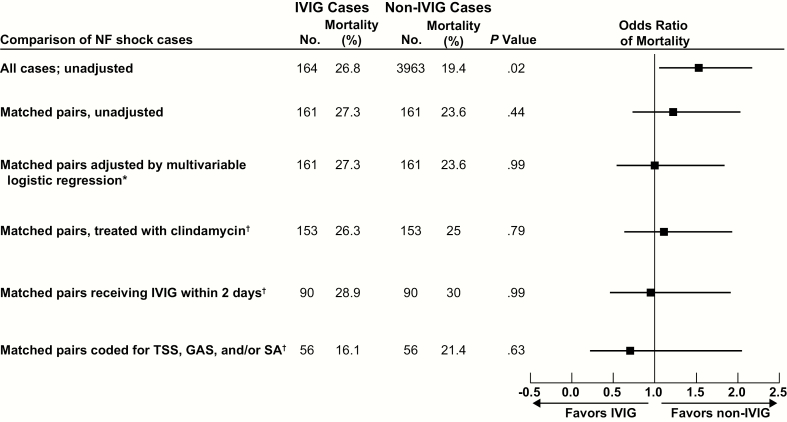
Of the 164 IVIG recipients, 161 were propensity-matched to 161 non-IVIG cases (Figure 3), producing remarkably well-balanced groups (Table 1) for exploring the impact of IVIG on outcome. Within the combined GAS/SA category, separate coding for GAS and SA was equivalent across the 2 matched groups. IVIG had no impact on in-hospital mortality, the primary outcome (crude mortality, 27.3% vs 23.6%; adjusted OR, 1.00 [95% confidence interval {CI}, .55–1.83]; P = .99) (Figure 4). For matched pairs that received clindamycin, mortality did not differ (IVIG group 26.8% vs non-IVIG group 24.8%; n = 153; P = .79). Mortality also did not differ in sensitivity analyses where the IVIG subject received IVIG within 2 days (28.9% vs 30.0%; OR, 0.95 [95% CI, .47–1.90]; P = .99) or where matched pairs were coded for TSS, GAS, and/or SA (IVIG group 16.1% vs matched non-IVIG group 21.4%; n = 56; P = .63). Importantly, the discharge destinations of survivors (home, institution, hospice) did not differ between the groups (P = .57). Median LOS was 26 (13–49) days in the IVIG and 26 (11–43) days in the matched non-IVIG group (P = .84).
Figure 3.
Distribution of propensity scores. Propensity scores are calculated from a logistic regression relating intravenous immunoglobulin (IVIG) group status as a binary outcome to the matching variables as predictors for 4127 individuals. Table 1 lists all the matching variables as well as their levels and categories. From the model, a fitted probability (propensity score) for each subject was calculated for how likely they are to be in the IVIG group based on their covariate profile of matching variable values. These 4127 propensity scores are visualized on the logit-scale in the figure for 4 groups, from top to bottom: unmatched IVIG (n = 3), matched IVIG (n = 161), matched non-IVIG (n = 161), unmatched non-IVIG (n = 3966). The 161 matched non-IVIG subjects are selected from all non-IVIG propensity scores so that the matched IVIG partner has a similar propensity score and with perfect balance on the presence of toxic shock syndrome, group A Streptococcus and/or Staphylococcus aureus codes, clindamycin use, and the value of first day of IVIG dose/vital status in the analysis. Note how similar the 161 propensity scores for the matched IVIG (n = 161) and matched non-IVIG (n = 161) groups are as opposed to how dissimilar the distributions are for matched IVIG (n = 161) and unmatched non-IVIG (n = 3966). The matched pairs are then put in a logistic regression model relating dichotomous variable in-hospital mortality to the matching variables and IVIG status as predictors. The exponentiated coefficient of the IVIG covariate in this subsequent logistic regression gives the adjusted odds ratio of mortality.
Figure 4.
In-hospital mortality across all analyses of intravenous immunoglobulin (IVIG) use in necrotizing fasciitis (NF) shock. The figure reports the odds ratios (ORs) of in-hospital mortality and 95% confidence intervals in the unmatched and unadjusted analysis, matched and unadjusted analysis, and primary analysis of all propensity-matched pairs adjusted by logistic regression, as well as sensitivity analyses on (1) propensity-matched pairs treated with clindamycin; (2) propensity-matched pairs where first administration of IVIG occurred within 2 days of hospitalization; and (3) propensity-matched pairs with coding for toxic shock syndrome (TSS), group A Streptococcus (GAS), and/or Staphylococcus aureus (SA) infection. There was no statistically significant difference in the ORs for in-hospital mortality between IVIG and propensity-matched non-IVIG cases in the primary analysis (*) as well as all sensitivity (†) analyses.
At the 4 hospitals prospectively selected for chart review, the NF-shock case-definition algorithm identified 126 cases (including 29 IVIG cases; 18% of the total NF-shock cohort that received IVIG), which were all manually reviewed. Median initial IVIG dose was 1 g/kg (0.66–1) and median number of doses was 1 [1, 2]. Of the 29 patients treated with IVIG, 28 (97%) met the NF case definition. For these 28 cases, the microbiologic etiology was monomicrobial in 19 (15 with GAS, 1 each with Enterobacter, Pseudomonas, Candida, and Apophysomyces species), polymicrobial in 8 (including 5 with SA and 1 with both GAS and SA), and culture negative in 2 cases (Supplementary Table E3). GAS was found in surgical cultures in 57.1% of IVIG cases and 22.5% of non-IVIG cases (P < .001) and SA in 17.9% of IVIG and 62.5% of non-IVIG cases (P < .001), respectively (Table 2). Therefore, among confirmed NF-shock cases by chart review, 75% of those both treated and not treated with IVIG were culture positive for GAS and/or SA, but in roughly reverse proportions for the 2 organisms. This result is perhaps expected because GAS is a recognized etiologic agent of TSS in the setting of NF, and SA is not. The positive predictive value of the debridement algorithm in capturing true surgical debridement for NF was equivalent in reviewed IVIG and non-IVIG cases (89% vs 88%). Additional comparisons of severity of acute illness markers and algorithm performance between reviewed IVIG and non-IVIG cases are presented in Table 2.
Table 2.
Comparison of Characteristics Between Manually Reviewed Casesa of Necrotizing Fasciitis Shock With and Without Intravenous Immunoglobulin Use at 4 US Academic Medical Centersb
| Variable | All IVIG Cases (n = 29) | All Non-IVIG Cases (n = 94c) |
P Value |
|---|---|---|---|
| NF diagnosis by case definitiond | 28 | 80 | |
| NF with any GAS in surgical cultures (total) | 16 (57.1) | 18 (22.5) | <.001 |
| NF with any Staphylococcus aureus in surgical cultures (total) | 5 (17.9) | 50 (62.5) | <.001 |
| Markers of severity of acute illness | |||
| Norepinephrine equivalente (µg/min) between days –1 and +2 from index debridement, mean ± SD |
29.6 ± 27.8 | 12 ± 21.3 | .003 |
| Admission SOFA score, mean ± SD | 11.1 ± 5.4 | 6.6 ± 4.6f | <.001 |
| Median hours from arrival to: | |||
| Clindamycin administration | 1.6 | 5.3 | .27 |
| First debridement | 3.4 | 6.6 | .14 |
| Median No. of debridement proceduresg | 3 | 3 | .43 |
| Positive predictive value | |||
| NF-shock algorithm for NF by case definitiond | 97% | 85% | |
| NF-shock algorithm for TSS by CDC case definitionh | 72% | 43% | |
| GAS/S. aureus ICD-9 codesg for true infection | 100% | 91% | |
| Debridement algorithmi for true surgical therapy for NF | 89% | 88% | |
| IVIG charges for actual administration | 97% | NA | |
| Negative predictive value | |||
| IVIG charges for actual administration | NA | 98% |
Data are presented as No. (%) unless otherwise indicated. One case in the Harborview Medical Center cohort was enrolled in a randomized controlled trial investigating the role of AB-103 (synthetic CD28 mimetic octapeptide that inhibits binding of exotoxin superantigen to the CD28 co-stimulatory receptor on TH1 lymphocytes) in NF, but was randomized to the placebo arm (Bulger EM JAMA Surg 2014; 149:528–36).
Abbreviations: CDC, Centers for Disease Control and Prevention; GAS, group A Streptococcus; ICD-9, International Classification of Diseases, Ninth Revision; IVIG, intravenous immunoglobulin; NF, necrotizing fasciitis; SD, standard deviation; SOFA, Sequential (Sepsis-Related) Organ Failure Assessment; TSS, toxic shock syndrome.
aAll cases identified as being from these 4 centers in the University Health-Systems Consortium database.
bHarborview Medical Center (Seattle, Washington); Barnes Jewish Hospital (St Louis, Missouri); and Brigham and Women’s Hospital and Massachusetts General Hospital (Boston, Massachusetts).
cData inaccessible for review in 3 of 97 non-IVIG cases.
dNecrosis of soft tissues with involvement of the fascia PLUS 1 or more of the following: (1) death; (2) shock (systolic blood pressure <90 mm Hg; (3) disseminated intravascular coagulopathy; (4) failure of organ systems: respiratory and/or renal and/or hepatic PLUS isolation or visualization on Gram stain of suspected pathogenic organism(s) from a normally sterile body site or necrotic tissue (based on the case definition for NF secondary to GAS: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2626872/pdf/8903167.pdf). See Supplementary Data for distribution of causative organisms among reviewed cases.
eNorepinephrine equivalent (µg/min) = [norepinephrine (μg/min)] + [dopamine (μg/kg/min) / 2] + [epinephrine (μg/min)] + [phenylephrine (μg/min) / 10] [29].
fTwelve percent missingness of respiratory and 16% missingness of hepatic system variables for corresponding SOFA components.
gSee Supplementary Data for list of ICD-9 diagnosis and/or procedure codes.
hBased on clinical and laboratory criteria provided by the CDC for diagnosing streptococcal TSS (https://wwwn.cdc.gov/nndss/conditions/streptococcal-toxic-shock-syndrome/case-definition/2010/) and nonstreptococcal TSS (https://wwwn.cdc.gov/nndss/conditions/toxic-shock-syndrome-other-than-streptococcal/case-definition/2011/).
iConsidered positive in the presence of at least 2 ICD-9 debridement procedure codes (or 1 debridement procedure code and death) dated within 4 days of each other, where day 1 is the day of initial debridement.
DISCUSSION
We found that IVIG use in NF-shock is infrequent, sporadic, and highly variable across academic centers and affiliates in the United States. Adjunctive IVIG was not associated with a survival benefit in NF-shock patients who received aggressive surgical management and antibiotics, even when IVIG was initiated within the first 2 days of hospital admission. Likewise, specific ICD-9 coding for TSS, GAS, and/or SA or clindamycin use did not identify a NF-shock subgroup for which IVIG significantly improved outcome. Consistent with this lack of a survival benefit, IVIG use for NF-shock did not affect LOS. This multicenter retrospective observational study comprises the largest cohort of patients with NF-shock for whom the clinical effectiveness of IVIG has been evaluated to date, and is the first report examining timing of IVIG administration. Records review enabled validation of assumptions made in the analysis of administrative data. Like previous studies on IVIG efficacy, propensity-score matching was employed to minimize confounding by indication [16]. Our propensity score model included several covariates that are likely to influence the decision to use adjunctive IVIG. Although matching at the primary analysis level was not center specific, IVIG restriction status was used to mitigate the potential for selection bias. Date stamps provided temporal associations between debridement and the administration of vasopressors, clindamycin, and IVIG, which further enhanced matching and risk adjustment.
The population incidence of invasive GAS disease is estimated at 30–39 cases per million [24–26] with NF occurring in 2.55 cases [26] and NF-TSS in 1.47 cases per million [24]. The rarity of TSS, with or without NF, has hampered the ability to conduct clinical trials. The only RCT [14] to date investigating the role of IVIG was terminated due to low accrual. Even assuming an optimistic 24.8% reduction below the control group mortality of 21.4% (seen in our subgroup of matched pairs coded for TSS, GAS, and/or SA), a future RCT would require 888 NF-shock patients in each arm in order to attain 80% power to detect a survival benefit from IVIG (see Supplementary Data for sample size calculations and an overview of ongoing RCTs). Furthermore, ambiguity remains around IVIG dosages and duration. Thus, this reappraisal here of existing use patterns and clinical effectiveness of IVIG in NF-shock in the real-world setting is an important first step for designing any future RCT.
Despite sharing an immunological mechanism, staphylococcal and streptococcal TSSs are clinically and epidemiologically distinct diseases [7, 9, 27]. Shock occurring during NF due to GAS is by definition TSS, while a similar case due to SA is typically classified as septic shock, a less specific entity. Nonetheless, SA clinical isolates commonly produce a wide variety of exotoxins, including TSST-1 and various staphylococcal enterotoxins that have superantigen activity and the potential to cause TSS [6]. We grouped together ICD-9 coding for TSS, GAS, and SA because of this ambiguity. However, and as expected, NF due to SA represented only a small minority of IVIG cases. Conversely, non-IVIG cases were significantly enriched for SA infections, suggesting that clinicians are following Centers for Disease Control and Prevention case definitions for TSS and avoiding IVIG use in NF-shock caused by SA. Although higher doses of IVIG may be needed to neutralize SA compared to GAS exotoxins in vitro, the relative clinical efficacy of IVIG across these 2 types of TSS is not known [28].
NF and TSS secondary to GAS are associated with high case-fatality rates (29% and 38%, respectively) [26], and GAS is the only etiology of NF for which IVIG has a specific recommendation. Accordingly, NF-shock recipients of IVIG were 2.5 times more likely to be culture positive for GAS. In our study, IVIG was administered more frequently to younger patients with lower comorbidity burden; this pattern has been previously described and suggests a more aggressive approach in this population [17]. Records review revealed significantly higher norepinephrine equivalents [29] and Sequential (Sepsis-Related) Organ Failure Assessment (SOFA) scores [30] among overall IVIG cases compared with non-IVIG cases of NF-shock. These findings indicate that IVIG recipients were more acutely ill, underscoring the importance of highly effective matching. Ninety-seven percent of IVIG cases and 85% of non-IVIG cases had true NF upon records review (Table 2), indicating that our case-selection algorithm performed well. Two or more operating room visits are generally required for obtaining complete surgical control [23]. Eighty-nine percent of reviewed debridement algorithm–positive cases correctly represented debridement for NF. The prevalence of true NF cases that might have been managed with only beside examinations after initial OR debridement could not be discerned, but bedside exploration is generally poorly tolerated by patients and at variance with standard surgical practice for NF. We also did not study the role of IVIG as definitive therapy in the absence of surgical debridement as this practice, although reported, is not considered standard of care [31].
Our study has several important limitations. First, while propensity score matching can minimize confounding by indication, its success is contingent upon the quality and granularity of available variables. Differences in baseline severity of illness, extent of tissue involvement, and causative pathogen between matched pairs may still be contributing to residual confounding. However, we were able to perfectly balance matched pairs for GAS or SA diagnosis codes, as well as the TSS code and clindamycin use. The inclusion of clindamycin and number of debridements as a matching variable precluded independent evaluation of its efficacy in our study. However, clindamycin selected for non-IVIG cases with clear indications for IVIG use, such as coding for TSS and GAS, and therefore greatly enhanced match balance. Importantly, evidence supporting a survival benefit for clindamycin in NF caused by GAS and expert opinion has resulted in a strong recommendation in IDSA guidelines [10]. Second, duration of stay and treatments administered (including IVIG) during previous hospitalizations were not available for transfers from other centers. Survival bias is an inherent limitation associated with transferred patients. However, we matched on transfer status to mitigate these effects. Third, excluding the single debridement cases could have eliminated some cases successfully treated with IVIG and a single debridement. Fourth, we were unable to match on use of hyperbaric oxygen, a treatment with unclear benefit in NF, as only 3 (1.8%) of the IVIG cases received this therapy. Fifth, we were unable to evaluate the impact of dose and duration of IVIG, although the median initial dose on chart review equaled the initial dose of the 3-day course administered in the Scandinavian clinical trial (day 1: 1 g/kg intravenously, days 2 and 3: 0.5 g/kg intravenously); there are currently no guidelines on recommended dosing [14]. Sixth, our study was not designed to determine cost effectiveness as information on center-specific costs is unavailable in the database. Vizient, the largest member-owned healthcare services company in the United States, priced the daily cost of IVIG in 2016 at $73 per gram. Seventh, sucrose content (associated with osmotic nephrosis and renal failure) and the specificity of antibody content differ between preparations of IVIG, which were not discernible in the database. These differences in IVIG preparations could potentially affect outcome.
CONCLUSIONS
Routine use of IVIG in NF with vasopressor-dependent shock provided no benefit when added to aggressive surgical debridement, appropriate antibiotics, and supportive care. This lack of benefit persisted upon limiting the analysis to patients who received IVIG early and to those who had coding for GAS and/or SA, as well as to those who received clindamycin. Intravenous immunoglobulin is a costly treatment with potential harms that is currently used sparingly across US academic medical centers. Additional investigations using clinical- and microbiology-enriched data sources may further clarify the impact of IVIG in this rare but serious condition. Our results underscore the challenges of conducting a sufficiently powered trial of IVIG in NF-shock. These data can also help inform the design and conduct of trials investigating newer, more specific agents targeting the superantigen mechanism of excessive immune activation.
Supplementary Material
Notes
Acknowledgments. The authors thank informationist Judith Welsh for conducting the literature search and Kelly Byrne for assisting with formatting manuscript text, tables, and figures.
Disclaimer. The opinions expressed in this article are those of the authors and do not represent any position or policy of the National Institutes of Health, the US Department of Health and Human Services, or the US government.
Financial support. This work was supported by the National Institutes of Health Intramural Research Program.
Potential conflicts of interest. All authors: No potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 2005; 352:1445–53. [DOI] [PubMed] [Google Scholar]
- 2. McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg 1995; 221:558–63; discussion 63–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray RJ. Recognition and management of Staphylococcus aureus toxin-mediated disease. Intern Med J 2005; 35:S106–19. [DOI] [PubMed] [Google Scholar]
- 4. Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis 2009; 9:281–90. [DOI] [PubMed] [Google Scholar]
- 5. McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol 2001; 55:77–104. [DOI] [PubMed] [Google Scholar]
- 6. Bohach GA, Fast DJ, Nelson RD, Schlievert PM. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol 1990; 17:251–72. [DOI] [PubMed] [Google Scholar]
- 7. Stevens DL. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis 1995; 1:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaul R, McGeer A, Low DE, Green K, Schwartz B. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med 1997; 103:18–24. [DOI] [PubMed] [Google Scholar]
- 9. Low DE. Toxic shock syndrome: major advances in pathogenesis, but not treatment. Crit Care Clin 2013; 29:651–75. [DOI] [PubMed] [Google Scholar]
- 10. Stevens DL, Bisno AL, Chambers HF, et al. ; Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–52. [DOI] [PubMed] [Google Scholar]
- 11. Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg 2014; 51:344–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Norrby-Teglund A, Kaul R, Low DE, et al. Plasma from patients with severe invasive group A streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T cell proliferation and cytokine production. J Immunol 1996; 156:3057–64. [PubMed] [Google Scholar]
- 13. Bergdoll MS, Crass BA, Reiser RF, et al. An enterotoxin-like protein in Staphylococcus aureus strains from patients with toxic shock syndrome. Ann Intern Med 1982; 96:969–71. [DOI] [PubMed] [Google Scholar]
- 14. Darenberg J, Ihendyane N, Sjölin J, et al. ; StreptIg Study Group Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003; 37:333–40. [DOI] [PubMed] [Google Scholar]
- 15. Kaul R, McGeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis 1999; 28:800–7. [DOI] [PubMed] [Google Scholar]
- 16. Shah SS, Hall M, Srivastava R, Subramony A, Levin JE. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis 2009; 49:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linnér A, Darenberg J, Sjölin J, Henriques-Normark B, Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis 2014; 59:851–7. [DOI] [PubMed] [Google Scholar]
- 18. Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis 2014; 59:358–65. [DOI] [PubMed] [Google Scholar]
- 19. Arends JE, Harkisoen S. Efficacy of polyspecific intravenous immunoglobulin therapy in streptococcal toxic shock syndrome. Clin Infect Dis 2015; 60:324. [DOI] [PubMed] [Google Scholar]
- 20. Chen C, Danekas LH, Ratko TA, Vlasses PH, Matuszewski KA. A multicenter drug use surveillance of intravenous immunoglobulin utilization in US academic health centers. Ann Pharmacother 2000; 34:295–9. [DOI] [PubMed] [Google Scholar]
- 21. Kadri SS, Hohmann SF, Orav EJ, et al. Tracking colistin-treated patients to monitor the incidence and outcome of carbapenem-resistant gram-negative infections. Clin Infect Dis 2015; 60:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. May AK. Skin and soft tissue infections: the new surgical infection society guidelines. Surg Infect (Larchmt) 2011; 12:179–84. [DOI] [PubMed] [Google Scholar]
- 23. Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg 2009; 208:279–88. [DOI] [PubMed] [Google Scholar]
- 24. Darenberg J, Luca-Harari B, Jasir A, et al. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis 2007; 45:450–8. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs) Report, Emerging Infections Program Network: group A Streptococcus. Atlanta, GA: CDC, 2013. [Google Scholar]
- 26. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis 2016; 63:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention. Toxic shock syndrome (other than streptococcal) (TSS) 2011 case definition. Atlanta, GA: CDC, 2011. [Google Scholar]
- 28. Darenberg J, Söderquist B, Normark BH, Norrby-Teglund A. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: implications for therapy of toxic shock syndrome. Clin Infect Dis 2004; 38:836–42. [DOI] [PubMed] [Google Scholar]
- 29. Patel BM, Chittock DR, Russell JA, Walley KR. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology 2002; 96:576–82. [DOI] [PubMed] [Google Scholar]
- 30. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–10. [DOI] [PubMed] [Google Scholar]
- 31. Norrby-Teglund A, Muller MP, Mcgeer A, et al. Successful management of severe group A streptococcal soft tissue infections using an aggressive medical regimen including intravenous polyspecific immunoglobulin together with a conservative surgical approach. Scand J Infect Dis 2005; 37:166–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.