Six-month clinic return intervals were associated with decreased lateness, gaps in medication, and loss to follow-up in stable human immunodeficiency virus–infected patients in Lusaka, Zambia, when adjusting for patient characteristics and prior retention history compared to 3- and 1-month intervals.
Keywords: visit intervals, retention, appointment scheduling, HIV, Zambia
Abstract
Background
Extending appointment intervals for stable HIV–infected patients in sub-Saharan Africa can reduce patient opportunity costs and decongest overcrowded facilities.
Methods
We analyzed a cohort of stable HIV-infected adults (on treatment with CD4 >200 cells/μL for more than 6 months) who presented for clinic visits in Lusaka, Zambia. We used multilevel, mixed-effects logistic regression adjusting for patient characteristics, including prior retention, to assess the association between scheduled appointment intervals and subsequent missed visits (>14 days late to next visit), gaps in medication (>14 days late to next pharmacy refill), and loss to follow-up (LTFU; >90 days late to next visit).
Results
A total of 62084 patients (66.6% female, median age 38, median CD4 438 cells/μL) made 501281 visits while stable on antiretroviral therapy. Most visits were scheduled around 1-month (25.0% clinical, 44.4% pharmacy) or 3-month intervals (49.8% clinical, 35.2% pharmacy), with fewer patients scheduled at 6-month intervals (10.3% clinical, 0.4% pharmacy). After adjustment and compared to patients scheduled to return in 1 month, patients with six-month clinic return intervals were the least likely to miss visits (adjusted odds ratio [aOR], 0.20; 95% confidence interval [CI], 0.17–0.24); miss medication pickups (aOR, 0.47; 95% CI 0.39–0.57), and become LTFU prior to the next visit (aOR, 0.41; 95% CI, 0.31–0.54).
Conclusions
Six-month clinic return intervals were associated with decreased lateness, gaps in medication, and LTFU in stable HIV-infected patients and may represent a promising strategy to reduce patient burdens and decongest clinics.
Currently, there are 11.8 million HIV–infected people on antiretroviral therapy (ART) in sub-Saharan African, and this is expected to increase to 19.6 million by 2020 [1]. A successful public health response, therefore, depends on both expanding access to those yet unreached as well as retention in care and HIV RNA suppression in those already on treatment [2, 3]. Differentiated care—the idea that health systems should vary the frequency, location and nature of contact with patients—has been widely embraced as a strategy to achieve greater access, improve efficiency, unburden the health system and improve retention [4]. The community adherence group (CAG), first formed in Mozambique, is an archetypical model of differentiated care where patients form groups of 6 and take turns visiting the clinic each month to undergo clinical review while collecting medications for the others [5]. Other models include adherence clubs in South Africa and community drug distribution points in Uganda [5, 6].
Despite widespread adoption, the comparative effectiveness or implementability of these models has not been fully examined. One model urgently in need of further exploration is to simply extend intervals between clinic visits by dispensing a greater quantity of medications at each visit and minimizing clinical review for otherwise well patients. This strategy can similarly reduce patient opportunity costs and clinic congestion by minimizing visits, but without the organizational overhead, scale-up costs, or supervision needed for other models such as CAGs. Although the World Health Organization (WHO) and Zambian guidelines do recommend extending visit and pharmacy intervals from the typical 1 month to 3 to 6 months for stable patients, the supporting data are actually quite limited [7–11]. Most studies varied visit frequency in the context of more resource-intense interventions such as home visits and drug-delivery or adherence clubs, which did not directly address spacing intervals in routine clinic-based care models. Furthermore, even when longer spacing was evaluated, a majority of studies evaluated only 2- to 3-month intervals with highly selected patients, yielding limited data with uncertain generalizability on longer periods [11–18]. As the optimal interval in terms of safety, efficacy, and efficiency is yet to be defined, it is critical to have more robust information on the use of longer return intervals such as 6 months or even longer to inform differentiated models of care [11, 19]. To that end, we examined the association between assigned appointment interval and the ability of the patient to make the next visit in a network of facilities in Zambia.
METHODS
Patient Population
We analyzed a cohort of adult HIV-infected patients (aged >18 years) who were stable on ART and presented for routine HIV care visits at clinics in Lusaka Province, Zambia, from 1 January 2013 to 31 July 2015. We used adapted WHO criteria to define stable on ART as having been on ART with no changes and having a CD4 count >200 cells/μL for 6 months or more [7]. Patients with a diagnosis of tuberculosis were considered not stable for the duration of tuberculosis therapy. We examined patient encounters from 20 large (more than 1000 enrolled patients) Ministry of Health ART clinics in Lusaka Province supported by the Centre for Infectious Disease Research in Zambia, a Zambian nongovernmental organization that supports implementation of HIV care delivery and research across 4 of the 10 provinces in Zambia. We included all routine visits for clinical follow-up or pharmacy refills, excluding visits where patients presented solely for evaluation for tuberculosis or were recorded as being acutely ill, which may have mandated closer follow-up.
Measurements
All measurements were extracted from the national electronic medical record system used in routine HIV care in Zambia (SmartCare). To generate this database, providers manually fill out clinical forms during the patient encounter, and data clerks then enter this information into the electronic database. We extracted baseline patient characteristics (eg, age, sex, clinic site, date of ART initiation), clinical status (CD4 count, tuberculosis diagnoses), and clinical visit and pharmacy refill history (date, appointment type, next scheduled visit, medication dispensed). For a given visit, we identified patients’ next scheduled clinical follow-up, next pharmacy refill pickup, and also the earliest date they were scheduled to return to the clinic, regardless of whether the purpose was clinical follow-up or pharmacy pickup. Based on scheduled follow-up, we defined 3 measures of retention for patients deemed to be clinically significant due to their association with an increased risk of virologic failure and mortality [20–22]. We defined a missed visit as being >14 days late to a patient’s earliest scheduled return to clinic, gaps in medications as being >14 days late to the next scheduled pharmacy refill pickup, and loss to follow-up (LTFU) as being >90 days late to the earliest scheduled clinic return.
Analysis
First, we described the overall distribution of clinical follow-up, pharmacy refill, and earliest clinic return intervals, categorizing visit intervals into <3 weeks (<21 days), 1 month (21–45 days), 2 months (46–75 days), 3 months (76–105 days), 4–5 months (106–165 days), and ≥6 month (>165 days) for all analyses. We assessed concordance of clinical and pharmacy follow-up by assessing the percentage of visits in which time to clinical follow-up matched the duration of medications dispensed.
We used histograms of the distribution of visit intervals at individual clinics to graphically depict heterogeneity in appointment scheduling across the sites. To quantitatively estimate the impact of clinic site and ecologic health systems-levels factors on variability in appointment scheduling, we developed a multilevel mixed-effects linear regression model using time to the next assigned return to clinic at each visit as the outcome. Covariates were selected based on a priori hypotheses of causal relationships using directed acyclic graphs to identify confounders and exclude colliders. Patient-level characteristics (ie, sex, age, most recent CD4 count, years on ART, days late to the current visit, prior retention) were included as fixed-effect covariates, and clinic site and individual patients were included as random effects with random intercepts to account for the clustering of visits. We then calculated the intraclass correlation (ICC) to estimate the percent of variability in assigned clinic return intervals attributable to the clinic an individual attended apart from patient-level factors.
Third, we used a multilevel mixed-effects logistic regression to evaluate our hypothesis that shorter assigned return intervals lead to lapses of retention by estimating the association between length of scheduled return on 3 metrics of subsequent retention: missing the next visit, a gap in medication possession prior to the next visit, and becoming LTFU prior to the next visit. We sought to adjust for common causes of the assigned clinic return interval (the exposure) and subsequent retention (the outcome). Using directed acyclic graphs to identify covariates, we included sociodemographic characteristics (eg, sex, age), time-varying clinical characteristics (eg, most recent CD4 level, years on ART), and calendar time in our models. Importantly, we also adjusted for prior retention history summarized as prior medication possession ratio (MPR), visit adherence (ie, percent of visits missed), and percent of visits that led to LTFU. These measures of prior retention as well as calendar time were specified as restricted cubic splines to flexibly model any departures from linearity and obtain maximal control of confounding for these covariates. Clinic site and individuals were modeled as random effects to account for probable correlation in the outcome within these categories. All analyses were conducted with Stata version 14.2 (StataCorp LP, College Station, Texas).
The institutional review boards at the University of Zambia and University of California, San Francisco approved the study.
RESULTS
Patient Characteristics
From 1 January 2013 to 31 July 2015, 96179 patients made at least 1 visit to 1 of 20 clinics in Lusaka Province, totaling 979272 clinic visits. Of these patients, 62084 (64.6%) made at least 1 routine clinic visit while stable on ART (501281 total visits, 51.2% of all visits); 66.6% of patients who were stable for at least 1 visit were female with a median baseline age of 38 years (interquartile range [IQR], 32–44). Patients had been on ART for a median of 2.4 years (IQR, 0.9–4.9 years) with a median CD4 count of 438 cells/μL (IQR, 321–592; Table 1). Of all routine visits, 3.1% (15685) were for clinical follow-up only, 29.2% (149237) were for both clinical follow-up and pharmacy visits, and 64.3% (328340) were pharmacy-only visits.
Table 1.
Patient Characteristics, Appointment History, and Retention History, N = 62,084
| Baseline Characteristic | |
|---|---|
| Male sex, n (%) | 20748 (33.4) |
| Median age, y (IQR) | 38 (32–44) |
| Median time since ART initiation, y (IQR) | 2.4 (0.9–4.9) |
| Median CD4 count, cells/μL (IQR) | 438 (321–592) |
| ART regimen, n (%) | |
| First line | 60385 (97.4) |
| Second line | 1584 (2.6) |
| Prior history of tuberculosis, n (%) | 12033 (19.4) |
| Appointment History | |
| Median number of routine clinic visits, n (IQR) | 8 (4–11) |
| Median number of clinical follow-up visits, n (IQR) | 2 (1–4) |
| Median number of pharmacy refills, n (IQR) | 7 (4–11) |
| Retention History | |
| Median medication possession ratio, % (IQR) | 85.5 (75.2–94.0) |
| Median number of visits missed by >14 days, % (IQR) | 20 (7.1–36.8) |
| Patients with at least 1 episode of lost to follow-up, n (%) | 18946 (31.5) |
Abbreviations: ART antiretroviral therapy; IQR, interquartile range.
Lapses in retention were common. Overall, patients were on time or early for their next scheduled return to the clinic 57.3% of the time (early by more than 7 days 8.2% of the time), 1 to 14 days late 22.8% of the time, 15 to 90 days late 15.7% of the time, and >90 days late (or LTFU) 4.8% of the time. Median MPR was 85.5% (IQR, 75.2–94.0), and the median percent of visits missed per patient by >14 days was 20% (IQR, 7.1–36.8%). Overall, 76.5% of patients had missed at least 1 visit by >14 days, 72.1% had missed at least 1 pharmacy refill by 14 days, and 31.5% were considered LTFU at least once during our study period (Table 1).
Appointment Scheduling Patterns
The majority of appointment intervals were at 1 month (25.0% of clinical follow-up, 44.4% of pharmacy refills), 2 months (9.8% of clinical follow-up, 17.7% of pharmacy refills), and 3 months (49.8% of clinical follow-up, 35.2% of pharmacy refills). Also, 10.3% of all clinical follow-up was at 6-month intervals, though only 0.4% of pharmacy refills were that long. Few visits were scheduled in <3 weeks or at 4–5 months. Patients who had 1- and 3-month clinical follow-up intervals were also mostly given similarly long pharmacy refill intervals (83.1% and 85.1%, respectively). However, there was a lack of coordination between clinical follow-up and pharmacy refills at longer intervals, with only 4.6% of patients given 6-month clinical follow-up receiving a concordant pharmacy refill with enough medication to last until the next visit (73.7% received a 3-month refill; Table 2).
Table 2.
Coordination of Clinical Follow-up Intervals With Pharmacy Refill Intervals
| Pharmacy Refill Interval Received (%) | |||||||
|---|---|---|---|---|---|---|---|
| <3 weeks | 1 month | 2 months | 3 months | 4–5 months | 6 months | ||
| Clinical Follow-Up Interval | <3 weeks | 58.3 | 25.0 | 6.0 | 10.3 | 0.3 | 0.1 |
| 1 month | 1.9 | 83.1 | 7.3 | 7.5 | 0.2 | 0.1 | |
| 2 months | 1.2 | 14.2 | 74.6 | 9.6 | 0.3 | 0.1 | |
| 3 months | 0.6 | 7.8 | 5.6 | 85.1 | 0.5 | 0.4 | |
| 4–5 months | 0.4 | 7.6 | 6.0 | 41.4 | 44.2 | 0.5 | |
| 6 months | 0.1 | 7.4 | 13.5 | 73.7 | 0.7 | 4.6 | |
Values for which clinical and pharmacy follow-up are coordinated (i.e. same interval) are in bold.
Clinic-level Distribution of Appointment Intervals
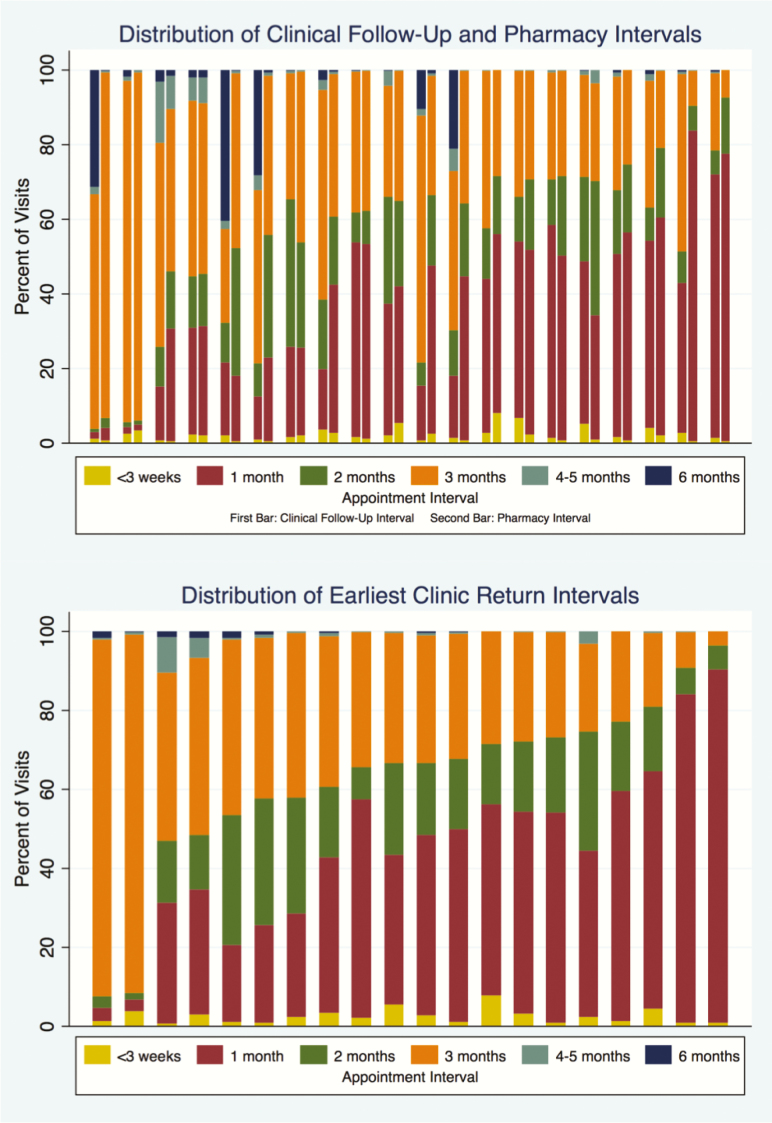
There was significant heterogeneity in clinical follow-up and pharmacy refill distribution between clinics (Table 3, Figure 1). For example, the frequency of 1-month clinical follow-up ranged from 1.8% to 70.6%, while 1-month pharmacy refills ranged from 1.5% to 83.3% across individual sites. The 6-month clinical follow-up ranged from <0.1% to 40.4%, but 6-month pharmacy refills ranged from <0.1% to 2.1%. Based on our mixed-effects linear regression model for scheduled clinic return intervals, we estimated the ICC for clinic site to be 21.7% (95% confidence interval [CI], 13.0%–34.1%), indicating that the clinic a patient attended accounted for 21.7% of all variability seen in the clinic return interval even when adjusting for patient-level covariates. There were no meaningful associations between patient-level characteristics (ie, sex, age, CD4 count) and the length of the scheduled appointment interval.
Table 3.
Clinic Characteristics, N = 20
| Facility Type, N, (%) | |
|---|---|
| Urban clinic | 17 (85) |
| Rural clinic | 2 (10) |
| District hospital | 1 (5) |
| Medan stable patient population, n (IQR) | 2556 (1868–4660) |
| Median number of routine clinic visits, n (IQR) | 19593 (12790–38100) |
| Median number of clinical follow-up visits, n (IQR) | 7252 (4705–10877) |
| Median number of pharmacy refills, n (IQR) | 18002 (12291–34905) |
| Clinic Averages | |
| Earliest scheduled return, days, median (IQR) | 58 (51–68) |
| Clinical follow-up interval, days, median (IQR) | 63 (55–90) |
| Pharmacy interval, days, median (IQR) | 61 (53–70) |
| Clinics with >20% of clinical follow-up at 6-month intervals, n (%) | 4 (20) |
| Clinics with >5% of pharmacy refills at 6-month intervals, n (%) | 0 (0) |
| Clinic Retention | |
| Median medication possession ratio, % (IQR) | 83.6 (78.7–85.1) |
| Median percent of visits missed by >14 days, % (IQR) | 21.9 (16.9–26.5) |
| Median percent of patients with at least 1 episode of lost to follow-up, % (IQR) | 31.0 (24.2–41.2) |
Abbreviation: IQR, interquartile range.
Figure 1.
Distribution of clinical follow-up and pharmacy refill intervals (top) and earliest clinic return intervals (bottom) across clinics. Each bar represents an individual clinic.
Estimate of Effect of Appointment Intervals on Subsequent Retention
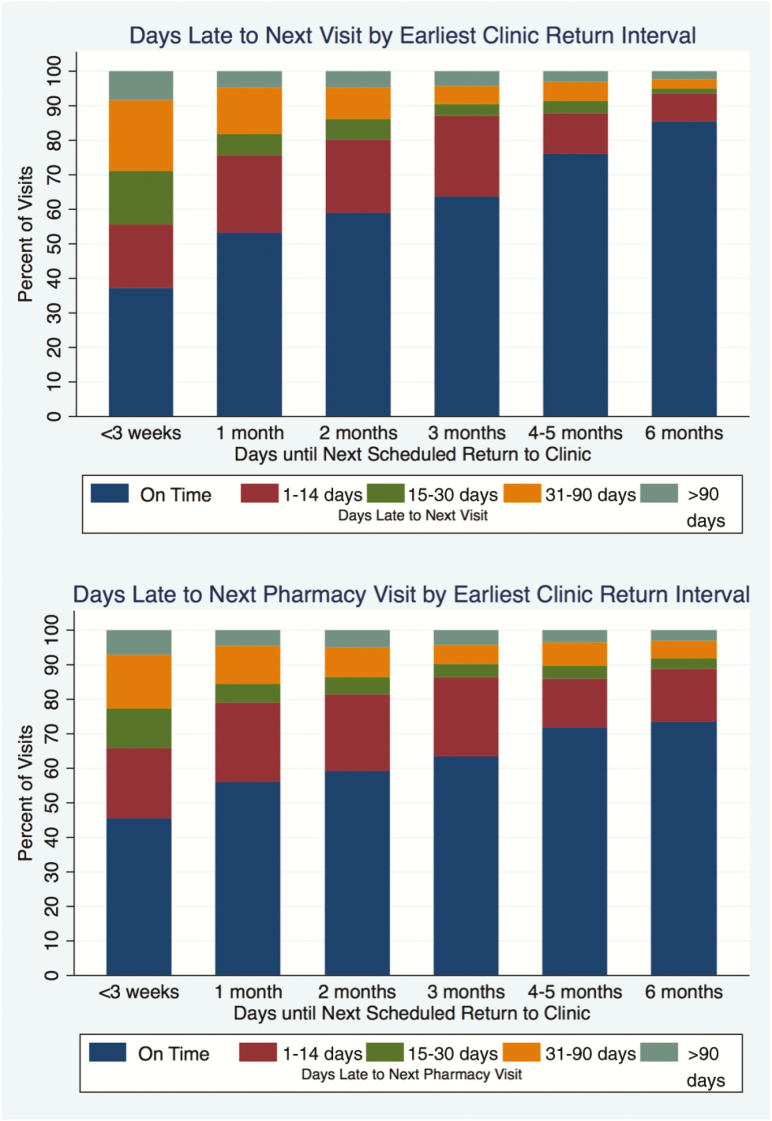
Overall, patients who had longer clinic return intervals were more likely to be on time to the following visit (Figure 2). In multilevel mixed-effects logistic regression using 1-month intervals as the reference, patients with longer clinic return intervals were less likely to miss their next visit by >14 days (6 months: adjusted odds ratio [aOR], 0.20; 95% CI, 0.17–0.24 and 3 months: aOR, 0.50; 95% CI, 0.49–0.52), have gaps in medication >14 days (6 months: aOR, 0.47; 95% CI, 0.39–0.57 and 3 months: aOR, 0.69; 95% CI, 0.67–0.70), and become LTFU prior to the next visit (6 months: aOR, 0.41; 95% CI, 0.31–0.54 and 3 months: aOR, 0.79; 95% CI, 0.76–0.82; Table 4). Patients receiving 6 month clinic return intervals fared the best overall, even when compared to those receiving 3 month intervals (8.0% decrease in missed visits [95% CI −9.1 to −7.0%], 4.2% decrease in missed pharmacy pickups [95% CI −6.0 to −2.3%], and 1.8% decrease in LTFU [95% CI −2.3 to −1.2%]). Additional risk factors for poor retention were being younger and being male (Table 4).
Figure 2.
Distribution of the days late to the subsequent appointment (top) and days late to next pharmacy refill by earliest clinic return interval (bottom).
Table 4.
Results of Multilevel Mixed-Effects Logistic Regression Evaluating the Effects of Clinic Return Intervals on Subsequent Missed Visits, Gaps in Medications, and Lost to Follow-up
| Missed Visit | Gap in Medication | Lost to Follow-up | ||||
|---|---|---|---|---|---|---|
| % | aOR (95% CI) |
% | aOR (95% CI) |
% | aOR (95% CI) |
|
| Appointment Interval | ||||||
| <3 weeks | 45.6 | 2.57 (2.46–2.69) |
35.3 | 1.94 (1.84–2.03) |
8.5 | 1.55 (1.43–1.67) |
| 1 month | 25.7 | 1.0 (reference) |
22.1 | 1.0 (reference) |
4.9 | 1.0 (reference) |
| 2 months | 20.7 | 0.75 (0.74–0.77) |
19.5 | 0.91 (0.89–0.93) |
4.9 | 0.87 (0.83–0.91) |
| 3 months | 14.5 | 0.50 (0.49–0.52) |
15.0 | 0.69 (0.67–0.70) |
4.3 | 0.79 (0.76–0.82) |
| 4–5 months | 12.6 | 0.40 (0.37–0.45) |
14.6 | 0.61 (0.55–0.68) |
3.2 | 0.52 (0.43–0.63) |
| 6 months | 7.2 | 0.20 (0.17–0.24) |
11.6 | 0.47 (0.39–0.57) |
2.6 | 0.41 (0.31–0.54) |
| Male sex | 1.10 (1.08–1.12) |
1.11 (1.09–1.13) |
1.2 (1.16–1.24) |
|||
| Age, per 10-year increase | 0.96 (0.95–0.97) |
0.94 (0.93–0.95) |
0.90 (0.88–0.91) |
|||
| Last CD4, per 50 cell/μL increase | 1.00 (1.00–1.00) |
1.00a (1.00–1.00) |
0.99b (0.99–1.0) |
|||
| Years since antiretroviral therapy initiation | 1.01 (1.00–1.01) |
1.01 (1.00–1.01) |
1.00c (0.99–1.01) |
|||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval
Restricted cubic splines of patients’ prior retention history (medication possession ratio, percentage of visits missed, and percentage of visits that led to loss to follow-up) and time (to account for secular trends) were also included as covariates in all models.
All P values are <.001, except aP = .012, bP = .001, cP = .999.
DISCUSSION
In this study, we describe the effects of appointment scheduling patterns on subsequent retention in care in a real-world public health setting in sub-Saharan Africa. First, we show that longer visit intervals are already being used in routine care. There was tremendous clinic-to-clinic variability, with the clinic an individual attended explaining 21.7% of the variability in appointment scheduling practices. Coordination of clinical follow-up and pharmacy refill intervals was poor, requiring patients to return to the clinic more frequently than is clinically necessary. Last, we found that longer appointment intervals were significantly associated with improved retention in dose–response fashion for stable HIV-infected patients, with 6-month intervals being best. Adjusting for patients’ prior retention history and compared to those who had a 1-month interval, patients who had 6-month clinic return intervals were less likely to miss their next visit by >14 days (aOR, 0.20), have a gap in medication of >14 days (aOR, 0.47), and become LTFU prior to their next visit (aOR, 0.41). Together, these findings suggest that extending clinic return intervals for stable patients could substantially reduce clinically significant lapses in retention in care and that clinic-level factors such as drug shortages, clinic management, and individual provider practices are important targets for intervention to address barriers to implementation [23–28].
These data add to the growing body of literature by offering evidence that the interval itself has an independent influence on making the next visit. Even though intervals beyond 3 months are endorsed by the WHO, there is currently only data from Médecins Sans Frontières from Malawi trialing a program of 6-month clinical appointments and 3-month pharmacy refills in stable patients [12, 13]. The researchers noted high retention (97% at 12 months) and decreased mortality for patients who participated in the program, but limitations to this study include stricter eligibility criteria with about one third of patients returning to routine care at some point (though some later reverted back to longer appointments), the inability to control for selection bias for those who chose to enroll in the program, and the fact that patients still need to return to the clinic for medication every 3 months [5, 12, 13]. Our study extends these data because it tests the hypothesis in a routine care environment outside of a specialized program, accounts for patients’ previous retention history, and, most importantly, assesses the effect of extending overall clinic return intervals up to 6 months (including pharmacy pickups). From our results, the effect of longer time between visits of up to 6 months was large in magnitude. The number needed to treat with a 6-month clinic return interval opposed to 3 months to prevent a missed visit, missed pharmacy pickup, or an episode of LTFU was 13, 24, and 57, respectively.
We believe these findings have important implications as countries rapidly incorporate visit spacing into their guidelines. First, addressing clinic-level factors by strengthening the supply chain to enable not just 3-month but 6-month supply of ART and targeting clinic management and healthcare worker behaviors to increase adoption of such practices offers a promising strategy to simultaneously enhance retention and decongest clinics [23–28]. Additional evidence is needed to affirm these findings, but this empiric observation comports with what is known about patient barriers to care in low-resource settings [29–32]. Patients face significant burdens and opportunity costs in attending clinic visits due to transportation costs, distance to the clinic, time away from work, and competing life or family priorities. Addressing systems-level barriers to reducing the frequency with which stable patients need to attend the clinic can allow them to more successfully balance their daily life needs with the benefits of remaining in HIV care and represents an important shift toward more patient-centered practices [33].
These findings also suggest that ongoing efforts to scale up differentiated care models should consider visit spacing as a first-line differentiated care model. Compared to other differentiated care models such as CAGs and adherence clubs, visit spacing requires fewer administrative activities once supply chain issues are addressed, such as additional staffing, training, and protocols to manage groups, and may therefore face fewer challenges with implementation and uptake at scale [34–38]. Furthermore, these models may not always be the most appropriate for patients. Qualitative analysis of CAGs from Mozambique indicates that concerns over stigma, strict rules, reliance on others, and group member conflicts cause some patients to return back to their standard of care [36]. Though some patients benefit from the social support and describe a positive impact on the community at large, the reduction in visit frequency is the primary benefit for many. Adaptive and patient-centered strategies may involve offering a choice between such groups and longer return intervals or reserving more resource-intense differentiated care models for those who first fail with extended visit intervals or who may benefit from additional social support.
There are several limitations of our study. First, we were unable to assess virologic suppression as viral load was not routinely collected in Zambia during our study period, though we did use outcomes associated with an increased risk of virologic failure and mortality. Recent results from the Zambia Population-Based HIV Impact Assessment do suggest that just <90% of patients in care on ART are virally suppressed [39]. Second, there is still the possibility of residual confounding. Nevertheless, in controlling for patients’ previous retention and adherence history, the consistency and dose-responsive nature of our results, and the fact that clinic site, as opposed to individual-level characteristics, was the strongest predictor of appointment scheduling, we think it is unlikely that a substantial degree of confounding remains. Hence, it is quite plausible that our results warrant a causal interpretation. Third, there are inherent limitations to our data source. We were unable to reliably exclude pregnant women in our definition of stable patients (though these women are routinely followed in the maternal and child health clinic rather than the ART clinic in Zambia), to identify the specific provider and their reasons for scheduling a particular visit interval for each encounter to better understand provider-specific practices, or to further breakdown LTFU into more detailed patient outcomes, such as out of care, silent transfers, and mortality [29, 40]. Still, there is substantial benefit of evaluating this intervention using programmatic data to understand its impact in real-world settings.
In conclusion, we found that 6-month clinic return intervals were associated with fewer missed visits, gaps in medications, and LTFU, providing important new data on extending visit intervals in sub-Saharan Africa in a routine clinical setting. Additionally, we note the significant heterogeneity in appointment scheduling practices between clinics, highlighting the need to understand and intervene on drivers of practice variation through additional implementation research. As we move into a new phase of ART treatment with “treat all,” adopting differentiated models of care that can simultaneously better address patient needs, foster sustained engagement, and accommodate the expected increase in patient populations will be crucial. Extending clinic follow-up and pharmacy refills up to 6 months, and potentially even longer, is a relatively straightforward solution that requires minimal additional infrastructure. Further study is needed on its impact on viral suppression, efficacy, and safety of even longer intervals (ie, 1 year), its role in an adaptive strategy combined with other models of differentiated care, and even in additional patient populations such those with poor retention due to structural barriers to care.
Notes
Acknowledgments. We thank Nancy L. Czaicki, PhD (in memorium), for her tremendous contribution to this work.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grants T32 AI060530 and K24 AI134413 to E. G.] and the Bill and Melinda Gates Foundation (grant OPP1105071).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. AIDS by the Numbers. UNAIDS, 2016. [Google Scholar]
- 2. Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med 2011; 8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: updates, goals, and recommendations for the future. AIDS Res Ther 2016; 13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Differentiated Care for HIV: A Decision Framework for Antiretroviral Therapy Delivery. International AIDS Society, 2016. [Google Scholar]
- 5. Bemelmans M, Baert S, Goemaere E et al. Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Trop Med Int Health 2014; 19:968–77. [DOI] [PubMed] [Google Scholar]
- 6. Okoboi S, Ding E, Persuad S et al. Community-based ART distribution system can effectively facilitate long-term program retention and low-rates of death and virologic failure in rural Uganda. AIDS Res Ther 2015; 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. World Health Organization, 2016. [PubMed] [Google Scholar]
- 8. Zambia Consolidated for Treatment and Prevention of HIV Infection. 2010. [Google Scholar]
- 9. Zambia Consolidated for Treatment and Prevention of HIV Infection. 2014. [Google Scholar]
- 10. Church K, Kiweewa F, Dasgupta A et al. A comparative analysis of national HIV policies in six African countries with generalized epidemics. Bull World Health Organ 2015; 93:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mutasa-Apollo T, Ford N, Wiens M et al. Effect of frequency of clinic visits and medication pick-up on antiretroviral treatment outcomes: a systematic literature review and meta-analysis. J Int AIDS Soc 2017; 20:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cawley C, Nicholas S, Szumilin E et al. Six-monthly appointments as a strategy for stable antiretroviral therapy patients: evidence of its effectiveness from seven years of experience in a Medecins Sans Frontieres supported programme in Chiradzulu district, Malawi. 21st International AIDS Conference Durban, South Africa, 2016. [Google Scholar]
- 13. McGuire M, Pedrono G, Mukhuna B et al. Optimizing patient monitoring after the first year of ART: three years of implementing 6-monthly clinical appointments in rural Malawi. 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention Rome, Italy, 2011. [Google Scholar]
- 14. Luque-Fernandez MA, Van Cutsem G, Goemaere E et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One 2013; 8:e56088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Selke HM, Kimaiyo S, Sidle JE et al. Task-shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community-based program in Kenya. J Acquir Immune Defic Syndr 2010; 55:483–90. [DOI] [PubMed] [Google Scholar]
- 16. Grimsrud A, Patten G, Sharp J, Myer L, Wilkinson L, Bekker LG. Extending dispensing intervals for stable patients on ART. J Acquir Immune Defic Syndr 2014; 66:e58–60. [DOI] [PubMed] [Google Scholar]
- 17. Jaffar S, Amuron B, Foster S et al. ; Jinja Trial Team Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet 2009; 374:2080–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kipp W, Konde-Lule J, Saunders LD et al. Antiretroviral treatment for HIV in rural Uganda: two-year treatment outcomes of a prospective health centre/community-based and hospital-based cohort. PLoS One 2012; 7:e40902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bigna JJ, Noubiap JJ, Plottel CS, Kouanfack C, Koulla-Shiro S. Factors associated with non-adherence to scheduled medical follow-up appointments among Cameroonian children requiring HIV care: a case-control analysis of the usual-care group in the MORE CARE trial. Infect Dis Poverty 2014; 3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blacher RJ, Muiruri P, Njobvu L et al. How late is too late? Timeliness to scheduled visits as an antiretroviral therapy adherence measure in Nairobi, Kenya and Lusaka, Zambia. AIDS Care 2010; 22:1323–31. [DOI] [PubMed] [Google Scholar]
- 21. Parienti JJ, Das-Douglas M, Massari V et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One 2008; 3:e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geng EH, Odeny TA, Lyamuya RE et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV 2015; 2:e107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brennan AT, Bor J, Davies MA et al. Tenofovir stock shortages have limited impact on clinic- and patient-level HIV treatment outcomes in public sector clinics in South Africa. Trop Med Int Health 2017; 22:241–51. [DOI] [PubMed] [Google Scholar]
- 24. Pasquet A, Messou E, Gabillard D et al. Impact of drug stock-outs on death and retention to care among HIV-infected patients on combination antiretroviral therapy in Abidjan, Côte d’Ivoire. PLoS One 2010; 5:e13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Empty Shelves, Come Back Tomorrow: ARV Stockouts Undermine Efforts to Fight HIV. Médecins Sans Frontières, 2015. [Google Scholar]
- 26. Rachlis B, Bakoyannis G, Easterbrook P et al. Facility-level factors influencing retention of patients in HIV care in East Africa. PLoS One 2016; 11:e0159994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maselle E, Muhanguzi A, Muhumuza S et al. Reducing turnaround time for laboratory test results does not improve retention of stable HIV-infected adults on POV program: experience from Uganda. J Int AIDS Soc 2014; 17:19607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCarthy EA, Subramaniam HL, Prust ML et al. Quality improvement intervention to increase adherence to ART prescription policy at HIV treatment clinics in Lusaka, Zambia: a cluster randomized trial. PLoS One 2017; 12:e0175534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geng EH, Odeny TA, Lyamuya R et al. Retention in care and patient-reported reasons for undocumented transfer or stopping care among HIV-infected patients on antiretroviral therapy in Eastern Africa: application of a sampling-based approach. Clin Infect Dis 2016; 62:935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shubber Z, Mills EJ, Nachega JB et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016; 13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav 2010; 14:778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav 2014; 18:1199–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grimsrud A, Bygrave H, Doherty M et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc 2016; 19:21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prust ML, Banda CK, Nyirenda R et al. Multi-month prescriptions, fast-track refills, and community ART groups: results from a process evaluation in Malawi on using differentiated models of care to achieve national HIV treatment goals. J Int AIDS Soc 2017; 20:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rasschaert F, Decroo T, Remartinez D et al. Sustainability of a community-based anti-retroviral care delivery model—a qualitative research study in Tete, Mozambique. J Int AIDS Soc 2014; 17:18910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rasschaert F, Telfer B, Lessitala F et al. A qualitative assessment of a community antiretroviral therapy group model in Tete, Mozambique. PLoS One 2014; 9:e91544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grimsrud A, Lesosky M, Kalombo C, Bekker LG, Myer L. Implementation and operational research: community-based adherence clubs for the management of stable antiretroviral therapy patients in Cape Town, South Africa: a cohort study. J Acquir Immune Defic Syndr 2016; 71:e16–23. [DOI] [PubMed] [Google Scholar]
- 38. Wilkinson L, Harley B, Sharp J et al. Expansion of the adherence club model for stable antiretroviral therapy patients in the Cape Metro, South Africa 2011–2015. Trop Med Int Health 2016; 21:743–9. [DOI] [PubMed] [Google Scholar]
- 39. Barradas D, Gupta S, Moyo C et al. Findings from the 2016 Zambia Population-based HIV Impact Assessment (ZAMPHIA): HIV prevalence, incidence and progress towards the 90-90-90 goals. 9th IAS Conference on HIV Science Paris, France, 2017. [Google Scholar]
- 40. Geng EH, Odeny TA, Lyamuya RE et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV 2015; 2:e107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]