Summary
Testing for integrase inhibitor resistance at the time of HIV diagnosis, in addition to current standard-of-care genotypes, should not be recommended in treatment guidelines; our model-based analysis suggests that testing will likely result in worse clinical outcomes and increased costs.
Keywords: cost-effectiveness analysis, HIV, integrase resistance, ART-naive
Abstract
Background
Current guidelines recommend genotype resistance testing at diagnosis to guide initial selection of antiretroviral therapy (ART). Many standard resistance genotypes exclude testing for resistance to integrase inhibitors (“IR testing”), although this class of drugs is a component of most recommended first-line regimens.
Methods
We compared the 96-week clinical outcomes and cost-effectiveness of 2 strategies: no IR testing vs IR testing performed at human immunodeficiency virus (HIV) diagnosis. The base case prevalence of transmitted integrase strand transfer inhibitor (INSTI)–resistant (INSTI-R) virus is estimated at 0.1%. With no IR testing, all patients start dolutegravir (DTG)–based ART after genotype; 12-week suppression rates are 90% (INSTI-susceptible [INSTI-S] virus) and 35% (INSTI-R virus). Those not suppressed at 12 weeks undergo IR testing; if diagnosed with INSTI-R virus, they change to ritonavir-boosted darunavir (DRV/r)–based ART. With IR testing, all patients are diagnosed with INSTI-S/INSTI-R virus prior to ART initiation and start DTG- or DRV/r-based regimens, respectively. Costs include IR tests (175 US dollars [USD]) and ART (41100–44900 USD/year). We examined the impact of key parameters in sensitivity analyses.
Results
IR testing resulted in worse clinical outcomes compared to no IR testing and increased costs by 200 USD/person/year. Prevalence of transmitted INSTI-R virus did not affect the favored strategy. No IR testing remained clinically preferred unless DTG suppression of INSTI-R virus was <20% or 96-week DRV/r suppression was >92%. If quality of life was worse with DRV/r- than DTG-based ART, no IR testing was clinically preferred over an even broader range of parameters.
Conclusions
In patients with newly diagnosed HIV, IR testing is projected to result in worse outcomes and is not cost-effective. Pretreatment assessment for INSTI resistance should not be recommended in treatment guidelines.
The Panel on Antiretroviral Guidelines for Adults and Adolescents of the US Department of Health and Human Services (DHHS), the European AIDS Clinical Society (EACS), and the International AIDS Society–USA (IAS-USA) panel recommend genotype drug resistance testing for people newly diagnosed with human immunodeficiency virus (HIV) prior to antiretroviral therapy (ART) initiation [1–3]. The goal of this testing is to avoid the selection of therapies to which the patient is already resistant, thereby improving treatment outcomes. Most commercially available genotype tests detect mutations in the reverse transcriptase (RT) and protease (PR) genes. Four of the 5 first-line regimens recommended by DHHS include integrase strand transfer inhibitors (INSTIs) [1], yet standard genotypes often do not assess for INSTI resistance.
Transmitted INSTI-resistant (INSTI-R) virus among ART-naive patients was first reported in 2011 [4, 5]. Despite increased use of this drug class, cohort studies across the United States and Europe continue to demonstrate a low prevalence of transmitted INSTI-R virus (0–0.1%) [6–14]. Furthermore, although most cases reported have demonstrated resistance to the first-generation INSTIs, elvitegravir (EVG) and raltegravir (RAL), these viral isolates generally retain susceptibility to the second-generation INSTI, dolutegravir (DTG) [15].
Given the low prevalence of transmitted INSTI resistance and the susceptibility of some INSTI-R virus to DTG-based regimens, it is not clear if INSTI resistance testing before ART initiation provides additional value over standard genotypes. If an INSTI resistance test identifies resistance but DTG-based ART remains effective, INSTI resistance testing might lead to worse outcomes by leading physicians to initiate a less effective, more poorly tolerated, and more expensive non-INSTI-based regimen. We examined the conditions under which adding a test for INSTI resistance to standard RT and PR genotypes might improve clinical outcomes and be cost effective.
METHODS
Analytic Overview
We designed a decision tree model (TreeAge, Williamstown, MA, USA) to examine the clinical outcomes (quality-adjusted life years [QALYs]), costs, and cost-effectiveness of adding INSTI resistance testing to the baseline evaluation of newly diagnosed people with HIV in the United States. We compared 2 strategies of care prior to ART initiation, both in addition to standard genotype: (1) no INSTI resistance testing (“no IR testing”) and (2) testing for INSTI-R virus (“IR testing”). We assessed outcomes at 96 weeks, assuming equivalent clinical and economic outcomes thereafter. We used model output to calculate incremental cost-effectiveness ratios (ICERs or Δcosts/ΔQALYs) from the modified societal perspective and labeled a strategy as cost-effective, if the ICER were ≤100000 US dollars (USD)/QALY [16].
Model Structure
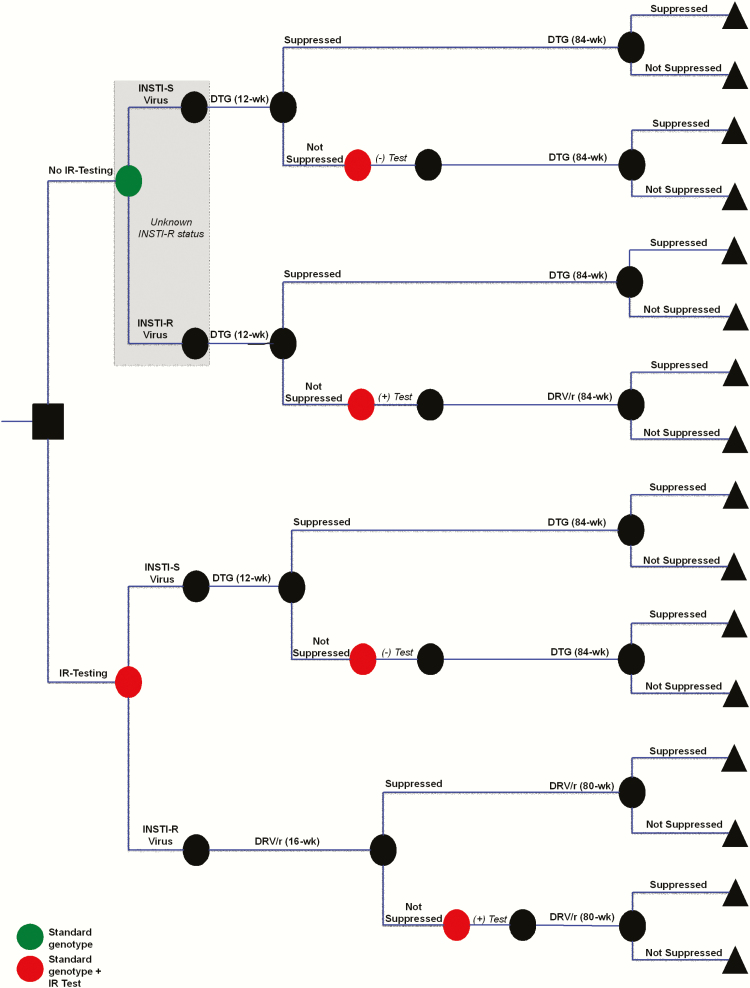
The model simulates a newly diagnosed HIV-infected, ART-naive patient presenting to clinic for baseline laboratory work, as per 2016 DHHS guidelines [1]. In the no IR testing strategy, standard genotype is performed as standard of care, and all are initiated on DTG-based ART with a nucleos(t)ide reverse transcriptase inhibitor (NRTI) pair chosen based on standard genotype results (Figure 1, top panel). Patients are reassessed at 12 weeks. Those who achieve virologic suppression remain on DTG-based ART. Those who are not virologically suppressed now undergo an IR test, as well as a repeat standard genotype.
Figure 1.
Decision tree to evaluate the clinical benefits and cost-effectiveness of integrase strand transfer inhibitor (INSTI) resistance testing (IR testing) at time of human immunodeficiency virus diagnosis. Simulated patients start at the square decision node (far left) where they receive either the standard genotype and no IR testing (top, green circle), or standard genotype and IR testing (bottom, red circle). In the no IR testing strategy, it is not known if patients have INSTI-resistant (INSTI-R) or INSTI-susceptible (INSTI-S) virus (gray box); all patients start dolutegravir (DTG)–based antiretroviral therapy (ART) and are assessed for virologic failure at 12 weeks. Those patients who are failing at 12 weeks then undergo repeat standard genotype and first-time IR testing (red circle). In the IR testing strategy, patients undergo both standard genotype and IR testing prior to ART initiation. If IR testing demonstrates INSTI-S virus, patients start DTG-based ART. If INSTI-R virus is diagnosed, patients start ritonavir-boosted darunavir (DRV/r)–based ART. Patients are assessed for suppression at 12 weeks if on DTG-based ART or at 16 weeks if on DRV/r-based ART; those not suppressed are tested with a repeat standard genotype and a repeat IR test. All patients receive a total of 96 weeks of ART and are followed to the end of the 96-week period (represented by triangles).
In the IR testing strategy (Figure 1, bottom panel), patients undergo both a standard genotype and an IR test at initial presentation, and these results guide ART regimen selection. Those with INSTI-susceptible (INSTI-S) virus start DTG-based ART. Those with diagnosed INSTI-R virus start ritonavir-boosted darunavir (DRV/r)–based ART. Patients are assessed for suppression at 12 weeks if on DTG-based ART or at 16 weeks if on DRV/r-based ART, given the slower decrease in viremia on protease inhibitor–based ART. Those not suppressed at 12 or 16 weeks are tested with both a repeat standard genotype and a repeat IR test.
A full tree with detailed inputs is available as Supplementary Figure 1.
Input Parameters
Cohort Characteristics
The cohort simulates a newly diagnosed individual with HIV in the United States. The median age is 43 (interquartile range [IQR], 34–50) years, and median CD4 count 317 (IQR, 135–517) cells/μL [17].
Baseline Prevalence of Transmitted Integrase Strand Transfer Inhibitor-Resistant Virus
Transmitted INSTI-R virus was defined by the Stanford University HIV Drug Resistance Database, the 2009 World Health Organization list, the French National Agency for Research on AIDS and Viral Hepatitis algorithm version 23, and the 2017 IAS-USA resistance mutations list. We pooled results of 14 published and presented studies, inclusive of US- and European-based case reports and cohort studies. Of the cohort studies, 3 reported a prevalence of primary INSTI resistance of 0.04%–0.1% [8, 13, 18], whereas 6 studies identified no INSTI resistance (primary or secondary) [6, 7, 10–12, 14]. Three of the studies reported a higher prevalence of secondary INSTI-R mutations (1.5%–5.9%), which data to date suggest do not increase the risk of INSTI failure [9, 12, 18] and are therefore considered polymorphisms and not evidence of transmitted INSTI-R. Based on this review, we conservatively chose the upper end of these results and assumed the prevalence of clinically important transmitted INSTI-R virus to be 0.1%.
Antiretroviral Therapy Efficacy
We defined ART efficacy as virologic suppression reported in prospective clinical trials. We included all contributing reasons for those who did not suppress, including virologic resistance, ART discontinuation due to adverse events, death, loss to follow-up, protocol deviation, withdrawal of consent, and missing data [19].
Among patients with INSTI-S virus, we estimated that 90% of patients achieve suppression with DTG-based ART at 12 weeks [20], and 80% have sustained suppression at 96 weeks [21] (Table 1). Because INSTI-R virus is so rarely transmitted, no specific data are published regarding suppression of INSTI-R virus with DTG-based ART in ART-naive patients. However, in ART-experienced patients with multidrug resistance including primary and secondary INSTI resistance, 69% of patients suppressed at 24 weeks when DTG 50 mg twice daily was included with an optimized background regimen [15]. Given the daily dosing of DTG in ART-naive patients and because phenotypic susceptibilities do not always correlate with clinical outcomes, we conservatively assumed that 35% of ART-naive patients with INSTI-R virus treated with DTG-based regimens would suppress at 12 and 96 weeks. For patients with either INSTI-S or INSTI-R virus, 65% are suppressed at 16 weeks when treated with DRV/r-based ART [21], and 71% suppressed at 96 weeks [21, 22].
Table 1.
Model Input Parameters for Analysis of Integrase Strand Transfer Inhibitor Resistance Testing Prior to Antiretroviral Therapy Initiation
| Parameters | Base Case | Range, (min-max) | Reference | |||
|---|---|---|---|---|---|---|
| Mean age, y | 43 | 34–50 | [17] | |||
| Median CD4 count, cells/μL | 317 | 135–517 | [17] | |||
| INSTI-R virus prevalence among ART-naïve | 0.1% | 0–100 | [6–14] | |||
| DTG-Based ART | DRV/r-Based ART | |||||
| Base Case | Range (min-max) | Reference | Base Case | Range (min-max) | Reference | |
| ART efficacy | ||||||
| INSTI-S virus | ||||||
| Suppression at 12 wk, % | 90 | … | [20] | … | … | … |
| Suppression at 16 wk, % | … | … | — | 65 | … | [21] |
| Suppression at 96 wk, % | 80 | 30–100 | [21] | 71 | 30–100 | [21, 22] |
| INSTI-R virus | ||||||
| Suppression at 96 wk, % | 35 | 30–100 | Adapted from [15] | 71 | 30–100 | [21, 22] |
| Base Case | Range (min-max) | Reference | ||||
| Quality of life | ||||||
| Virologically suppressed | 0.954 | … | [23, 24] | |||
| Viremia | 0.931 | 0.781–0.953 | ||||
| QoL for non-DTG-based ART (% compared to DTG-based ART) | 100 | 70–99 | Assumption | |||
| Cost, USD | ||||||
| Standard genotype cost | 351 | … | [25] | |||
| INSTI resistance test cost | 175 | 5–1500 | [25] | |||
| DTG-based ART, annual | 41100 | 12000–100000 | [26] | |||
| DRV/r-based ART, annual | 44900 | 12000–100000 | [26] | |||
Abbreviations: ART, antiretroviral therapy; DRV/r, ritonavir-boosted darunavir; DTG, dolutegravir; INSTI, integrase strand transfer inhibitor; INSTI-R, integrase strand transfer inhibitor resistant; INSTI-S, integrase strand transfer inhibitor susceptible; QoL, quality of life; USD, United States dollars.
Quality-Adjusted Life-Years
We stratified simulated patients into 1 of 2 health states: (1) viremia and (2) virologic suppression. We used health-related quality-of-life (QoL) values stratified by CD4 count and HIV RNA to characterize these health states. Because the median CD4 count at ART initiation is 317 (IQR, 135–517) cells/μL [17], we estimated the QoL for viremia (eg, pre-ART or failing ART) at 0.931 from AIDS Clinical Trial Group (ACTG) QoL data for CD4 count 301–500 cells/μL with HIV RNA >400 copies/mL [23, 24]. The median increase in CD4 count after 96 weeks of DTG-based ART is 260 (IQR, 185–400) cells/μL and 250 (IQR, 130–400) cells/μL with DRV/r-based ART [21]. Based on ACTG QoL data for CD4 count >500 cells/μL with HIV RNA <400 copies/mL [23, 24], we estimated QoL at 0.954 for patients who are virologically suppressed on ART.
Costs
A genotype test cost 351 USD, and an INSTI-R test cost 175 USD [25]. We estimated the annual costs for DTG-based ART (41100 USD) and DRV/r-based ART (44900 USD), both with either abacavir/lamivudine or emtricitabine (FTC)/tenofovir alafenamide (TAF) NRTI pair (Table 1) [26].
Sensitivity Analysis
We performed univariate sensitivity analyses on all parameters to assess the impact on projected clinical and economic outcomes, using ranges based on estimates of variance or by clinician-validated assumptions and including efficacy and costs of common first-line ART regimens (Table 1). Because INSTI-based ART is often the best tolerated of all ART regimens [21, 22, 27, 28], we assessed the impact of decreasing the QoL of non-DTG-based ART to 70%–99% of the QoL of people on DTG-based ART. Some regimens might lead to better adherence due to decreased pill burden. For instance, DTG is available as a fixed drug combination, in contrast to DRV/r (although a single pill formulation may soon be available as DRV/cobicistat/FTC/TAF). There could also be differences in long-term toxicity and durability of regimens (eg, tenofovir disoproxil fumarate [TDF] vs TAF). By simultaneously varying both suppression and QoL with different regimens, we examined the possible impact of pill burden or regimen durability. We performed multivariate sensitivity analyses on univariate parameters that most strongly influenced clinical outcomes, including prevalence of INSTI-R virus, DTG suppression of INSTI-R virus, DRV/r suppression, and QoL on non-DTG-based ART. We also performed probabilistic sensitivity analysis (Supplementary Appendix).
Scenario Analysis
We performed scenario analyses for other INSTI-based regimens in place of DTG-based ART, including EVG- or RAL-based ART (input data for these analyses provided in Supplementary Table 1). At 12 weeks, 85% are suppressed on EVG-based ART [29] and 80% on RAL-based ART [30]; at 96 weeks, 84% are suppressed on EVG-based ART [31] and 81% on RAL-based ART [32]. In contrast to DTG-based ART and to be conservative, we assumed that both EVG- and RAL-based ART would not suppress INSTI-R virus (0%). EVG (in the form of co-formulated EVG/cobicistat/FTC/TDF) cost 41600 USD/year. RAL combined with FTC/TAF cost 42600 USD/year [26].
Because some ART-naive patients are still initiated on nonnucleoside reverse transcriptase inhibitor (NNRTI)–based ART, we also performed a scenario analysis with efavirenz (EFV)–based ART in place of DRV/r-based ART. Virologic suppression using EFV-based regimen is 70% at 16 weeks [27] and 72% at 96 weeks [27]. The fixed-dose combination of EFV/FTC/TDF cost 36700 USD/year [26].
RESULTS
Base Case
When IR testing was compared to no IR testing in an ART-naive population treated with either DTG- or DRV/r-based ART, clinical outcomes were worse (by a small margin of 2.34 × 10–6 QALYs), and per-person costs increased by 200 USD (Table 2).
Table 2.
Base Case Results at 96 Weeks for Integrase Strand Transfer Inhibitor Resistance Testing Prior to Antiretroviral Therapy (ART) Initiation Among ART-Naive Patients
| Strategy | QALY | Costs (USD) | ICER (USD/QALY) |
|---|---|---|---|
| No IR testing | 1.754a | 76200 | … |
| IR testing | 1.754a | 76400 | Dominated |
Abbreviations: ICER, incremental cost-effectiveness ratio; IR, inhibitor resistance; QALY, quality-adjusted life-years; USD, United States dollars.
aThe no testing strategy resulted in 2.34 × 10–6 more QALYs than the testing strategy.
Univariate Sensitivity Analyses
No IR testing was clinically equal or preferred to IR testing over a wide range of the following parameters: prevalence of INSTI-R virus (0–100%, base case 0.1%); DTG suppression of INSTI-R virus (20%–100%, base case 35%); suppression at 96 weeks on DRV/r-based ART (30%–92%, base case 71%); QoL when viremic (0.781–0.955, base case 0.931); QoL on DRV/r-based ART (70%–100% of QoL on DTG-based ART, base case 100%). IR testing for INSTI-R virus was clinically preferred (ie, 2.46 × 10–7–3.24 × 10–6 more QALYs) only when suppression of patients with INSTI-R virus was <20% on DTG-based ART or when suppression with DRV/r-based ART was >92% at 96 weeks. Under the rare circumstances when IR testing resulted in better clinical outcomes, it was never cost-effective compared to the no IR testing strategy, even if IR testing cost only 5 USD.
Multivariate Sensitivity Analyses
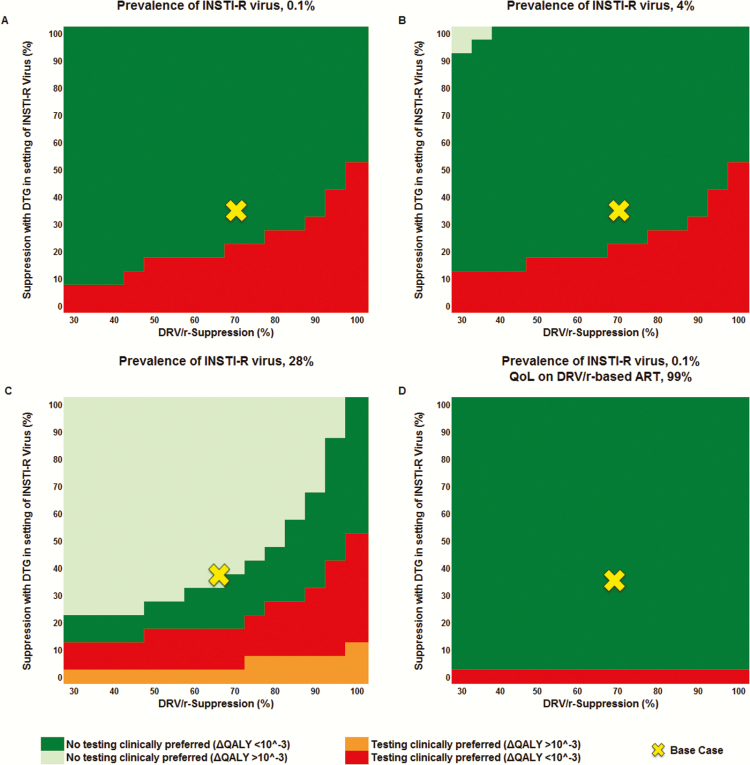
In multivariate sensitivity analyses, IR testing became clinically preferred as suppression on DRV/r-based ART improved (horizontal axis, Figure 2A), even at a greater probability of suppression with DTG-based ART for INSTI-R virus (vertical axis, Figure 2A). We assessed the impact of this relationship over a range of transmitted INSTI-R virus prevalence. Whereas the clinical preference for IR testing vs no IR testing remained unchanged, the clinical difference in QALYs between the 2 strategies increasingly favored no IR testing when transmitted INSTI-R virus was more prevalent (Figure 2B and 2C). The IR testing strategy was never cost-effective, even at INSTI-R virus prevalence of 80% (ICER >193300 USD/QALY; data not shown).
Figure 2.
Multivariate sensitivity analysis of the clinical impact (quality-adjusted life-years [QALYs]) of integrase strand transfer inhibitor (INSTI) resistance testing (IR testing) compared to no IR testing while varying the probability of dolutegravir (DTG) suppression of INSTI-resistant (INSTI-R) virus (vertical axis) and ritonavir-boosted darunavir (DRV/r) suppression (horizontal axis). Prevalence of transmitted INSTI-R virus is 0.1% in the base case (A). IR testing is clinically preferred (red and orange) when DTG suppression of INSTI-R virus is low (bottom) and suppression with DRV/r is high (right); no IR testing is clinically preferred (dark green and light green) when DTG suppression of INSTI-R virus is high (top) and virologic suppression with DRV/r is low (left). A–C, Quality of life (QoL) on DRV/r-based antiretroviral therapy (ART) is equivalent to DTG-based ART. D, QoL on DRV/r-based ART is reduced to 99% of that on DTG-based ART. Beginning at an INSTI-R prevalence of 4%, the no IR testing strategy showed a gain of ≥10–3 QALYs compared to the testing strategy (light green) (B); at an INSTI-R prevalence of 28%, the IR testing strategy resulted in a gain of ≥10–3 QALYs (orange) (C).
When the QoL for time spent on DRV/r-based ART was decreased to 99% of the QoL on DTG-based ART, no IR testing was clinically preferred over a much wider range of values (Figure 2D).
Probabilistic Sensitivity Analysis
In PSA, no IR testing was preferred >99.9% of the time at a willingness-to-pay threshold of 100000 USD/QALY compared to IR testing.
Scenario Analyses
When EVG- or RAL-based ART was substituted for DTG-based ART, IR testing resulted in small improvements in clinical outcomes (3.24 × 10–6 QALYs) and increased costs (200 USD) compared to no IR testing (Supplementary Figure 2A and 2C), but was not cost-effective (ICER >54 million USD/QALY). When INSTI-R virus was more prevalent, the magnitude of QALYs gained in the preferred strategy was greater; INSTI-R prevalence had to be ≥30% to reach 10–3 QALYs gained with IR testing (Supplementary Figure 2B and 2D). The IR testing strategy was never cost-effective.
When EFV-based ART was substituted for DRV/r-based ART, IR testing became economically attractive when INSTI-R prevalence was >5% and suppression with EFV-based ART was higher than with DTG-based ART (data not shown).
DISCUSSION
In this decision analysis model, testing for transmitted INSTI-R virus in patients newly diagnosed with HIV resulted in worse clinical outcomes compared to no testing and was never cost effective when DTG- and DRV/r-based ART were compared. These results remained unchanged regardless of the prevalence of transmitted INSTI-R virus. No IR testing was clinically preferred, as long as DTG-based ART achieved virologic suppression in at least 20% of patients with INSTI-R virus. If there was any decrease in QoL among patients treated with DRV/r-based ART compared to DTG-based ART, the no IR testing strategy was clinically preferred over an even wider range of conditions. In situations where EVG or RAL was the preferred INSTI and suppressed <21% of INSTI-R virus, IR testing resulted in minimally improved clinical outcomes but still was not cost-effective.
These results might appear counterintuitive given that IR testing leads to worse clinical outcomes. Without IR testing, more patients are initially exposed to empiric treatment with DTG-based ART; yet we conservatively estimated that 35% of patients with INSTI-R virus would still suppress on DTG-based ART, which is more potent, better tolerated, and less costly than DRV/r-based ART [21, 22, 26–28]. If INSTI resistance were detected by IR testing, physicians may shy away from choosing DTG-based ART, based on the results of the IR test. As such, the IR test could do harm by eliminating the option of DTG-based ART, when in fact a substantial minority of these patients would be successfully treated. The argument for no IR testing is further strengthened if bictegravir becomes the INSTI of choice, given its improved resistance profile and in vitro activity against some DTG-resistant virus [33, 34].
A rise in prevalence of transmitted INSTI-R virus did not affect which strategy was clinically preferred but did increase the clinical difference (ΔQALY) between the IR testing and no IR testing strategies. At higher prevalence of INSTI-R virus, a greater number of patients will achieve the clinical benefits of the preferred strategy, depending on suppression with DRV/r-based ART and suppression of INSTI-R virus achieved by DTG-based ART (Figure 2). Although adding IR testing to the baseline evaluation in clinical practice could detect an increase in INSTI resistance prevalence, carefully designed surveillance studies are better suited to this task.
In this analysis, the difference in outcomes and costs between the IR testing and no IR testing strategies was minute for 3 reasons. First, even when DTG-based ART failed due to undiagnosed INSTI resistance, routine virologic monitoring after 12 weeks of therapy identified this virologic failure, which was unlikely to have clinical significance over the lifetime of subsequent virologic suppression. Second, the difference in estimated QoL for the 2 health states was small (0.023), reflective of the presence or absence of viremia, which contributed to the small differences in clinical outcomes. Third, the relative cost of the IR test (175 USD) compared to overall costs of treatment was so low that IR testing could easily become cost-effective, provided even a small clinical benefit with IR testing. However, we projected worse clinical outcomes with IR testing as more patients would be placed on initial DRV/r-based therapy than necessary.
While this analysis was limited to the strategy of adding IR testing to the baseline evaluation, these results suggest that it may also be time to reconsider the role that standard RT and PR genotypes play today for newly diagnosed patients. Although a previous modeling analysis of baseline genotype testing found that this strategy was cost effective when first-line therapy was NNRTI based [35], it was conducted at a time when treatment options were more limited, less effective, and more expensive than they are today [35, 36]. Furthermore, the prevalence of clinically important transmitted NNRTI resistance was and remains substantially higher than other drug classes. These results also support initiation of ART before the results of baseline resistance testing become available. Exceptions might be made in the rare circumstance when an individual acquired HIV from a person known to be failing an INSTI-based regimen or if multiclass resistant virus is evident on standard genotype.
These results should be interpreted in the context of several limitations. First, we limited our time horizon to 96 weeks, assuming equivalent outcomes thereafter. This analysis would therefore not capture the impact if suppression with different ART regimens were substantially different at longer time horizons (eg, ART switches due to adverse effects; increased loss to follow-up due to poor tolerability of ART). The inclusion of longer time horizons would likely result in a stronger preference for the no IR testing strategy, given the increased cost of DRV/r-based ART and its poorer tolerability compared to DTG-based ART [21, 22, 26–28]. Second, we presumed patients would remain in care, such that routine virologic monitoring after 12 weeks of therapy would identify persistent viremia. Patients diagnosed with opportunistic infections or profound immunosuppression could be at risk for ongoing clinical decline during this early period of persistent viremia. However, empiric treatment with INSTI-based ART should be the best treatment option given the low prevalence of INSTI-R virus, the effectiveness and tolerability of INSTI regimens, and the ability of DTG to suppress at least a substantial minority of INSTI-R virus. Third, we did not model the impact of transmissions during viremia, in particular the 12 weeks in the no IR testing strategy when patients with INSTI-R virus would be treated empirically with DTG-based ART and could infect others despite being prescribed ART. Additionally, these results may not be generalizable to pregnant women, where 12 weeks of persistent viremia could theoretically lead to vertical transmission, given that the majority of in utero transmissions occur in the third trimester or at delivery [37–39].
In summary, testing for baseline INSTI resistance prior to ART initiation resulted in worse clinical outcomes and cost more than no IR testing, as DTG-based therapy may succeed despite transmitted INSTI resistance. Furthermore, even if virologic failure occurred, the duration of this viremia would be limited given routine viral load monitoring, and patients could be switched rapidly to an alternative suppressive regimen. These findings were even stronger when accounting for any decreased tolerability of DRV/r-based ART compared to DTG-based ART. Even when EVG- or RAL-based therapy was prescribed, IR testing was clinically preferred only when prevalence of transmitted INSTI resistance was implausibly high or virologic failure was universal with INSTI resistance. Based on these results, an assessment for transmitted INSTI resistance at the time of HIV diagnosis should not be recommended in treatment guidelines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the NIH (grant numbers R01 A142006 and K01 HL123349), and by Massachusetts General Hospital (the Steve and Deborah Gorlin MGH Research Scholars Award to R. P. W.).
Acknowledgments. We acknowledge Drs Pamela Pei and Robert Parker for their assistance in discussing distributions for the probabilistic sensitivity analysis.
Disclaimer. The content is solely the responsibility of the authors; the study’s findings and conclusions do not necessarily represent the official position of the National Institutes of Health (NIH).
Potential conflicts of interest. P. E. S. has served as a consultant or scientific advisory board member for AbbVie, BMS, Gilead, GSK/ViiV, Merck, and Janssen, and has received grant support to his institution from BMS, Gilead, and GSK/ViiV. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 29 March 2017.
- 2. European AIDS Clinical Society. Guidelines, version 8.2, 2017 Available at: http://www.eacsociety.org/files/guidelines_8.2-english.pdf. Accessed 29 March 2017.
- 3. Hirsch MS, Günthard HF, Schapiro JM et al. ; International AIDS Society-USA Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Top HIV Med 2008; 16:266–85. [PubMed] [Google Scholar]
- 4. Young B, Fransen S, Greenberg KS et al. Transmission of integrase strand-transfer inhibitor multidrug-resistant HIV-1: case report and response to raltegravir-containing antiretroviral therapy. Antivir Ther 2011; 16:253–6. [DOI] [PubMed] [Google Scholar]
- 5. Boyd SD, Maldarelli F, Sereti I et al. Transmitted raltegravir resistance in an HIV-1 CRF_AG-infected patient. Antivir Ther 2011; 16:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriquez C, Goujard C, Mercier-Darty M et al. Low frequency of resistance associated mutations by ultra-deep sequencing in HIV-1 primary infected patients [abstract P_47]. In: 14th European Meeting on HIV and Hepatitis, Rome, Italy Virology Education, 2015:33 Available at: http://regist2.virology-education.com/Abstractbook/2016_4.pdf. Accessed 29 March 2017. [Google Scholar]
- 7. Volpe JM, Yang O, Petropoulos CJ, Walworth CM.. Absence of integrase inhibitor resistant HIV-1 transmission in the California AIDS Healthcare Foundation Network. In: Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Diego, CA, 2015 Available at: http://www.natap.org/2015/ICAAC/ICAAC_08.htm. Accessed 29 March 2017. [Google Scholar]
- 8. Margot NA, Martin R, Miller MD, Callebaut C.. Drug resistance mutations in treatment-naive HIV-infected patients 2000–2013 [poster 578]. In: Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, 2014 Available at: http://www.croiconference.org/sites/all/abstracts/578.pdf. Accessed 29 March 2017. [Google Scholar]
- 9. Frange P, Assoumou L, Descamps D et al. ; French ANRS CO 6 PRIMO Cohort, the ANRS 147 OPTIPRIM Clinical Trial and the AC11 Resistance Study Groups HIV-1 subtype B-infected MSM may have driven the spread of transmitted resistant strains in France in 2007–12: impact on susceptibility to first-line strategies. J Antimicrob Chemother 2015; 70:2084–9. [DOI] [PubMed] [Google Scholar]
- 10. Doyle T, Dunn DT, Ceccherini-Silberstein F et al. ; CORONET Study Group Integrase inhibitor (INI) genotypic resistance in treatment-naive and raltegravir-experienced patients infected with diverse HIV-1 clades. J Antimicrob Chemother 2015; 70:3080–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stekler JD, McKernan J, Milne R et al. Lack of resistance to integrase inhibitors among antiretroviral-naive subjects with primary HIV-1 infection, 2007–2013. Antivir Ther 2015; 20:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tostevin A, White E, Dunn D et al. ; UK HIV Drug Resistance Database Recent trends and patterns in HIV-1 transmitted drug resistance in the United Kingdom. HIV Med 2017; 18:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hernandez AL, Banez Ocfemia MC, Oster AM et al. HIV integrase genotypic testing and resistance in the United States—9 jurisdictions [poster 478]. In: Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, 2017 Available at: http://www.croiconference.org/sites/default/files/posters-2017/478_Hernandez.pdf. Accessed 29 March 2017. [Google Scholar]
- 14. Sayan M, Gündüz A, Ersöz G et al. Integrase strand transfer inhibitors (INSTIs) resistance mutations in HIV-1 infected Turkish patients. HIV Clin Trials 2016; 17:109–13. [DOI] [PubMed] [Google Scholar]
- 15. Castagna A, Maggiolo F, Penco G et al. ; VIKING-3 Study Group Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis 2014; 210:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50000-per-QALY threshold. N Engl J Med 2014; 371:796–7. [DOI] [PubMed] [Google Scholar]
- 17. Althoff KN, Gange SJ, Klein MB et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis 2010; 50:1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scherrer AU, Yang WL, Kouyos RD et al. ; Swiss HIV Cohort Study Successful prevention of transmission of integrase resistance in the Swiss HIV Cohort Study. J Infect Dis 2016; 214:399–402. [DOI] [PubMed] [Google Scholar]
- 19. Center for Drug Evaluation and Research (CDER). Antiretroviral drugs using plasma HIV RNA measurements—clinical considerations for accelerated and traditional approval. Available at: https://www.fda.gov/OHRMS/DOCKETS/98fr/02d-0427-gdlooo1.doc. Accessed 3 April 2017. [Google Scholar]
- 20. Clotet B, Feinberg J, van Lunzen J et al. ; ING114915 Study Team Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
- 21. Molina JM, Clotet B, van Lunzen J et al. ; FLAMINGO Study Team Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV 2015; 2:e127–36. [DOI] [PubMed] [Google Scholar]
- 22. Lennox JL, Landovitz RJ, Ribaudo HJ et al. ; ACTG A5257 Team Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014; 161:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chaudhary MA, Moreno S, Kumar RN, Nocea G, Elbasha E. Cost-effectiveness analysis of raltegravir in treatment-experienced HIV type 1-infected patients in Spain. AIDS Res Hum Retroviruses 2009; 25:679–89. [DOI] [PubMed] [Google Scholar]
- 24. Simpson KN, Dietz B, Baran RW et al. Economic modeling of the combined effects of HIV-disease, cholesterol and lipoatrophy based on ACTG 5142 trial data. Cost Eff Resour Alloc 2011; 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Center for Medicare and Medicaid Services. Clinical laboratory fee schedule. Code 87901 (infectious agent genotype analysis by nucleic acid [DNA or RNA]) and code 87906 (infectious agent genotype analysis by nucleic acid [DNA or RNA]; HIV-1, other region [eg, integrase, fusion]). Available at: https://www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/17CLAB.zip. Accessed 29 March 2017. [Google Scholar]
- 26. Red Book Online. Micromedex solutions. Truven Health Analytics; Available at: http://www.micromedexsolutions.com. Accessed 29 March 2017. [Google Scholar]
- 27. Walmsley S, Baumgarten A, Berenguer J et al. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015; 70:515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Squires K, Kityo C, Hodder S et al. Integrase inhibitor versus protease inhibitor based regimen for HIV-1 infected women (WAVES): a randomised, controlled, double-blind, phase 3 study. Lancet HIV 2016; 3:e410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sax PE, DeJesus E, Mills A et al. ; GS-US-236-0102 Study Team Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. [DOI] [PubMed] [Google Scholar]
- 30. Lennox JL, DeJesus E, Lazzarin A et al. ; STARTMRK Investigators Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. [DOI] [PubMed] [Google Scholar]
- 31. Zolopa A, Sax PE, DeJesus E et al. ; GS-US-236-0102 Study Team A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr 2013; 63:96–100. [DOI] [PubMed] [Google Scholar]
- 32. Lennox JL, Dejesus E, Berger DS et al. ; STARTMRK Investigators Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010; 55:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sax PE, DeJesus E, Crofoot G et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV 2017; 4:e154–60. [DOI] [PubMed] [Google Scholar]
- 34. Tsiang M, Jones GS, Goldsmith J et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother 2016; 60:7086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sax PE, Islam R, Walensky RP et al. Should resistance testing be performed for treatment-naive HIV-infected patients? A cost-effectiveness analysis. Clin Infect Dis 2005; 41:1316–23. [DOI] [PubMed] [Google Scholar]
- 36. Fagard C, Colin C, Charpentier C et al. ; ANRS 139 TRIO Trial Group Long-term efficacy and safety of raltegravir, etravirine, and darunavir/ritonavir in treatment-experienced patients: week 96 results from the ANRS 139 TRIO trial. J Acquir Immune Defic Syndr 2012; 59:489–93. [DOI] [PubMed] [Google Scholar]
- 37. Lallemant M, Jourdain G, Le Coeur S et al. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. N Engl J Med 2000; 343:982–91. [DOI] [PubMed] [Google Scholar]
- 38. Ehrnst A, Lindgren S, Dictor M et al. HIV in pregnant women and their offspring: evidence for late transmission. Lancet 1991; 338:203–7. [DOI] [PubMed] [Google Scholar]
- 39. Rouzioux C, Costagliola D, Burgard M et al. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. The HIV Infection in Newborns French Collaborative Study Group. Am J Epidemiol 1995; 142:1330–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.