Summary
HIV/hepatitis C virus coinfection and elevated IL-18 levels are both associated with inflammatory disease progression. IL-18 levels are significantly elevated in coinfected individuals, correlated inversely with CD4 count, and increased with incident HIV infection, potentially explaining enhanced inflammatory disease progression in coinfection.
Keywords: HIV, HCV, coinfection, inflammation, cytokines.
Abstract
Background.
Human immunodeficiency virus (HIV)/hepatitis C virus (HCV) coinfection and elevated interleukin (IL)-18 levels are both associated with enhanced progression of hepatic inflammation and increased risk of diabetes, kidney disease, and cardiovascular disease. IL-18 is a proinflammatory cytokine made upon activation of the inflammasome, an innate sensing system. We assessed whether increased IL-18 could explain the increased incidence and progression of inflammatory conditions seen with HIV/HCV coinfection.
Methods.
Serum samples from 559 subjects with HIV monoinfection, HCV monoinfection, HIV/HCV coinfection, or people who inject drugs with neither infection were tested for IL-18 by enzyme-linked immunosorbent assay and for 16 other analytes by electrochemiluminescence immunoassay. IL-18 levels were measured in 14 additional chronically HCV-infected subjects who developed incident HIV infection to determine if IL-18 increases with coinfection.
Results.
IL-18 was significantly elevated in coinfected individuals vs both monoinfections (P < .0001) independent of age, sex, and race. IL-18 levels were significantly higher in HIV monoinfection than in HCV monoinfection. High IL-18 levels were correlated with detectable HIV viremia and inversely with CD4 cell count (P < .0001), consistent with HIV activation of the inflammasome resulting in CD4 T-cell depletion. Incident HIV infection of chronically HCV-infected subjects resulted in increased IL-18 (P < .001), while HIV suppression was associated with normal IL-18 levels. Four additional analytes (IP-10, IL-12/23p40, IFN-γ, IL-15) were found to be elevated in HIV/HCV coinfection when compared to both monoinfections.
Conclusions.
HIV/HCV coinfection results in significantly elevated serum IL-18. The elevated levels of this proinflammatory cytokine may explain the increased incidence and progression of inflammatory illnesses seen in coinfected individuals.
Antiretroviral therapy (ART) has reduced the incidence of human immunodeficiency virus (HIV)–related morbidity and mortality. However, increasing evidence suggests that chronic inflammatory medical illnesses, including cardiovascular disease, diabetes mellitus, chronic kidney and liver disease, osteoporosis, and cancer, occur more frequently and/or at earlier ages in HIV-infected individuals [1–7]. Hepatitis C virus (HCV) persists in approximately 75% of infected individuals, and chronic infection is similarly associated with liver inflammation and increased risk of diabetes mellitus, kidney disease, and liver cancer [8–10]. Coinfection with HIV and HCV is associated with enhanced progression of hepatic inflammation–linked pathology, increased HIV-related kidney disease and renal failure, cardiovascular disease, and higher risk of diabetes relative to monoinfections [3, 11]. The pathogenesis of HIV- and HCV-related chronic inflammation is complex and not completely understood.
One of the important pathways of inflammation associated with viral infection involves activation of inflammasomes. Inflammasome assembly results in recruitment and activation of caspase-1 and cleavage of the pro-forms of interleukin (IL)-1β and IL-18 into active, mature cytokines that are secreted. IL-1β and IL-18 are proinflammatory cytokines known to mediate inflammation. Elevated IL-18 is associated with a variety of inflammatory conditions, including diabetes, atherosclerosis, and kidney and liver disease. Elevated levels of IL-18 in human serum are associated with a significantly increased risk of type 2 diabetes, and systemic administration of IL-18 promotes diabetes development in young nonobese diabetic mice [12, 13]. IL-18 promotes atherosclerotic plaque growth and vulnerability, and high levels of circulating IL-18 precede the onset of coronary events in healthy men and in simian immunodeficiency virus–infected and –uninfected macaques [14, 15]. IL-18 plays an integral role in the development of renal dysfunction during a variety of inflammatory disease processes and in endotoxin-induced liver inflammation and sepsis [16, 17].
Existing studies suggest a role for inflammasome activation in HIV and HCV infection. Previous studies have shown that viremic HIV-infected individuals have higher plasma levels of IL-18 compared with uninfected and ART-treated individuals [18, 19]. Our group showed previously that IL-18 plasma levels are low prior to HCV infection, increase significantly with acute HCV infection, then decrease to intermediate levels with progression to chronic infection [20]. Polymorphisms in the IL-18 promoter that lead to increased IL-18 and IL-18 binding protein production increase the odds of spontaneous HCV control [21]. We demonstrated previously that endocytosis of HIV or HCV virions activates the inflammasome in monocytes and macrophages and induces production of IL-18 and other proinflammatory cytokines [22]. Finally, a recent study demonstrated that HIV-induced CD4 T-cell depletion is driven primarily by inflammasome-mediated cell death (pyroptosis) through abortive HIV infection of CD4 T cells in lymphoid tissues [23]. In this study, we assessed IL-18 levels in HCV monoinfection, HIV monoinfection, and HIV/HCV coinfection, which might explain pathogenesis seen in HIV/HCV coinfection.
METHODS
Study Participants
Serum samples from HCV-monoinfected, HIV-monoinfected, and HIV/HCV-coinfected subjects were obtained from the AIDS Linked to the IntraVenous Experience (ALIVE) study, an ongoing, prospective, community-recruited, observational cohort study of people who inject drugs (PWID) in Baltimore, Maryland. Participants in this cohort provided written informed consent and received counseling to reduce drug use. A detailed study protocol has been previously described [24]. The ALIVE cohort enrolled HIV-uninfected and HIV-infected PWID, 89% of whom were HCV seropositive at baseline, and collected semiannual blood samples during follow-up. This study conformed to all relevant ethical guidelines, and the institutional review board of the Johns Hopkins University approved this study protocol. Samples were selected from 440 individuals chronically infected with one or both infections and divided into three categories: HIV-monoinfected (HIV antibody [Ab] positive, HCV-RNA negative), HCV-monoinfected (HIV-Ab negative, HCV-Ab positive, HCV-RNA positive at ≥1 visit in the chronic infection phase), and HIV/HCV-coinfected (HCV-Ab and HIV-Ab positive, and HCV-RNA positive at ≥1 visit in the chronic infection phase). An additional 32 samples were included from the John Hopkins Adult HIV clinic to increase the number of white subjects studied because the ALIVE cohort is primarily African American [25]. Both of these samples derived from the same underlying Baltimore population and approximately 40% of HIV-infected ALIVE participants receive their HIV care from the Johns Hopkins HIV clinic. None of the 472 subjects tested self-reported being on ART, but 25 subjects had HIV-RNA levels <500 copies/mL.
HIV/HCV-antibody seronegative subjects were defined as uninfected and were obtained from the Baltimore Before and After Acute Study of Hepatitis (BBAASH), an ongoing prospective, community-recruited, observational cohort study of PWID in Baltimore, Maryland [26]. Participants in this cohort provided written informed consent and received counseling to reduce drug use. A detailed study protocol has been previously described [26]. Uninfected donors remained HCV-RNA negative and HCV-Ab negative for 24 consecutive monthly visits at the time of release from the study.
To assess the effect of incident HIV, an additional 14 subjects with chronic HCV infection who acquired incident HIV infection were also selected from the ALIVE cohort. In addition, to assess the effects of ART on IL-18 levels, we obtained 55 HIV/HCV-coinfected and 21 HIV-monoinfected additional subjects from ALIVE on ART with undetectable HIV-RNA.
IL-18 Measurement
Serum IL-18 was measured using the human IL-18 enzyme-linked immunosorbent assay kit (MBL International, Woburn, MA). The assay was performed per the manufacturer’s recommendations. In brief, all samples were diluted 1:5 and run in duplicate. Average IL-18 values were reported as picograms per milliliter (pg/mL). Data were acquired using a SpectaMax M5 (Molecular Devices, Sunnyvale, CA).
Cytokine Measurement
The Meso Scale Discovery (MSD; Rockville, Maryland) multiplex cytokine, proinflammatory, and chemokine electrochemiluminescence immunoassays were used to assess 16 additional analytes (see Results). The assays were performed in accordance with the manufacturer’s recommendations. Data were acquired on a SECTOR Imager SI2400. Results were analyzed using MSD Workbench software.
Statistical Analysis
Before analysis, IL-18 values were normalized by log10 transformation. To compare whether IL-18 levels differed across groups, we used 1-way analysis of variance (ANOVA) and subsequent t tests for pairwise comparisons. Univariable and multivariable linear regression models were run to identify independent associations between HIV/HCV status, demographic characteristics, and clinical factors independently associated with IL-18 and other cytokine and chemokine levels. Finally, paired t tests were used for intraindividual comparisons of IL-18 levels prior to and after HIV infection.
RESULTS
Study Subjects
IL-18 and MSD multiplex testing was completed on 559 subjects. Of the 559 subjects, 86 were HIV monoinfected, 198 were HCV monoinfected, 188 were HIV/HCV coinfected, and 87 were infected with neither virus. To confirm that we were not observing confounding effects from drug use, the 87 HIV-negative and HCV-negative subjects used as the uninfected control group were active PWID. Demographic characteristics of the infected subjects are shown in Table 1. The demographic characteristics of the HIV/HCV-negative subjects were unavailable. Of the remaining infected subjects, 71.8% of subjects were male and 81.4% were African American, and the median age was 46 years (range, 21–70 years).
Table 1.
Demographic and Clinical Characteristics of Study Participants (N = 559)
| Characteristic | No. (%) |
|---|---|
| Infection status | |
| HIV/HCV negative | 87 (15.6) |
| HIV monoinfection | 86 (15.6) |
| HCV monoinfection | 198(35.4) |
| HIV/HCV coinfection | 188 (33.4) |
| Sex (infected subjectsa) (n = 472) | |
| Male | 339 (71.8) |
| Female | 133 (28.2) |
| Race (infected subjectsa) | |
| African American | 384 (81.4) |
| Not African American | 88 (18.6) |
| Age, y, median (range) | 46 (21–70) |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Sex and race information was not available for all HIV/HCV-uninfected subjects and is therefore shown only for the remainder of the subjects. Interleukin 18 levels were very low in nearly all uninfected subjects and not expected to differ by race, age, or sex.
Elevated Levels of the Proinflammatory Cytokine IL-18 Are Observed in Untreated HIV/HCV Coinfection
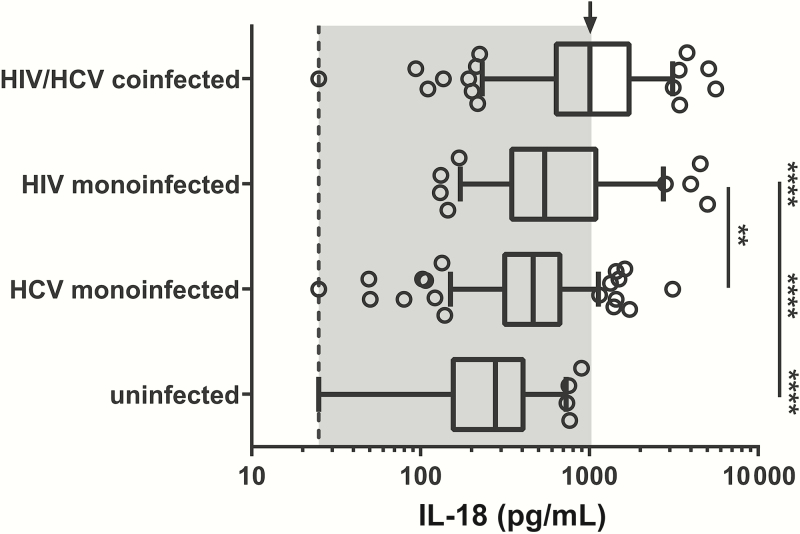
We initially evaluated IL-18 levels in subjects who had not been on ART and in whom HCV had not been treated. The median values of IL-18 seen in each group were as follows: HIV/HCV uninfected, 278 pg/mL (interquartile range [IQR], 156–406 pg/mL); HCV monoinfected, 466 pg/mL (IQR, 316–669 pg/mL); HIV monoinfected, 544 pg/mL (IQR, 347–1095 pg/mL); and HIV/HCV coinfected, 1009 pg/mL (IQR, 637–1722 pg/mL). Serum IL-18 levels were significantly higher in HIV/HCV-coinfected and HCV- and HIV-monoinfected subjects compared with uninfected active PWID subjects (P < .0001; Figure 1). Additionally, HIV/HCV-coinfected subjects had significantly higher IL-18 levels than either HCV- or HIV-monoinfected subjects (P < .0001; Table 1 and Supplementary Table 1). Last, HIV-monoinfected subjects had significantly higher IL-18 levels than HCV-monoinfected subjects (P < .001; Figure 1). The inflammasome-associated cytokine IL-1β did not differ significantly between groups. However, serum levels were very low in all groups. Of the 559 subjects tested for IL-1β, 13% had undetectable levels, and 86% of those detectable were not quantifiable (Supplementary Figure 3).
Figure 1.
Serum interleukin-18 (IL-18) concentrations are significantly higher in untreated human immunodeficiency virus (HIV)/hepatitis C virus (HCV)–coinfected subjects. Serum levels of IL-18 in untreated HIV/HCV-uninfected, HCV-monoinfected, HIV-monoinfected, and HIV/HCV-coinfected subjects. Dashed line: lower limit of quantitation. Arrow: median of HIV/HCV coinfection group. IL-18 levels in uninfected, HCV-monoinfected, and HIV-monoinfected subjects are statistically different from those in HIV/HCV-coinfected subjects (****P < .0001). IL-18 levels were significantly higher in HIV-monoinfected subjects than in HCV-monoinfected subjects (**P < .001). Groups were compared using a 1-way analysis of variance and adjusted for multiple comparisons.
IL-18 Levels Are Associated With Detectable HIV-RNA, CD4 Count, and Race
Univariable linear regression was performed to evaluate what factors other than infection status were associated with serum IL-18 levels (Table 2). In this model, detectable HIV-RNA was strongly associated with higher IL-18 levels, as were lower CD4 counts. We could not assess the relationship between HCV-RNA levels and IL-18 levels because multiple methods were used to detect HCV-RNA in the ALIVE cohort. However, we have previously shown no correlation between the level of HCV-RNA and IL-18 [20]. No association was found between IL-18 and sex. However, there was an association between older age and lower IL-18. African American subjects had higher IL-18, a finding of borderline statistical significance in univariable analysis. In the multivariable analysis, race became more significantly associated with IL-18 levels (Table 2). However, controlling for sex, race, and age did not affect the associations of HIV/HCV coinfection vs either monoinfection (Table 2, Supplementary Table 1).
Table 2.
Variables Associated With Serum Interleukin-18 Levels
| Characteristic | Univariate Estimate (SE) | P Value | Multivariate Estimate (SE) | P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | −0.026 (0.039) | .478 | -0.005 (0.03) | .879 |
| Age | ||||
| 5-y increments | −0.005 (0.002) | .0003 | -0.002 (0.001) | .119 |
| Race | ||||
| African American | 0.074 (0.04) | .075 | 0.077 (0.04) | .049 |
| Infection status | ||||
| HIV–/HCV+ | Reference | Reference | ||
| HIV+/HCV– | 0.145 (0.042) | .0006 | 0.128 (0.044) | .0033 |
| HIV+/HCV+ | 0.347 (0.033) | < .0001 | 0.332 (0.035) | < .0001 |
| HIV VLa | ||||
| HIV uninfected | Reference | Reference | ||
| VL not detectable | 0.179 (0.07) | .01 | 0.172 (0.07) | .015 |
| VL detectable | 0.230 (0.03) | < .0001 | 0.232 (0.03) | < .0001 |
| CD4 count, cells/μL | ||||
| HIV uninfected | Reference | Reference | ||
| >500 | 0.103 (0.05) | .048 | 0.087 (0.05) | .103 |
| 200–499 | 0.175 (0.043) | < .0001 | 0.171 (0.045) | .0002 |
| <200 | 0.407 (0.035) | < .0001 | 0.392 (0.037) | < .0001 |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; SE, standard error; VL, viral load.
Lower limit of detection is 500 copies/μL.
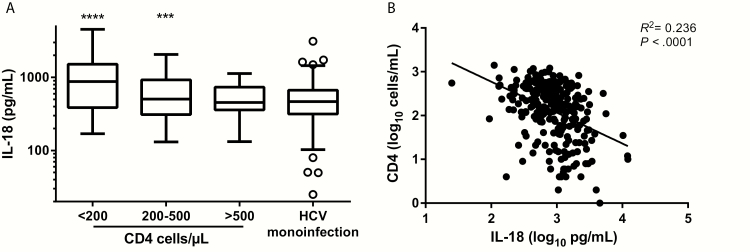
Detectable HIV-RNA remained strongly associated with IL-18 in multivariable analysis (Table 2), as did CD4 level [18, 22]. To determine if the significantly higher IL-18 levels in HIV monoinfection compared with HCV monoinfection observed in Figure 1 held true across all CD4 counts, IL-18 levels were stratified by CD4 count (<200, 200–500, and >500 cells/μL). Serum IL-18 levels in HIV-monoinfected subjects with <200 CD4 cells/μL and with 200–500 CD4 cells/μL were significantly higher than levels in HCV-monoinfected subjects (P < .0001 and P = .0002, respectively; Figure 2A). In contrast, serum IL-18 levels in subjects with CD4 counts >500 cells/μL were not statistically different from levels in HCV-monoinfected subjects (Figure 2A). However, serum IL-18 levels in HIV-monoinfected subjects with CD4 counts >500 CD4 cells/μL remained statistically higher than the levels in HIV- and HCV-uninfected PWID (P = .0002, data not shown). In combined analysis of HIV-monoinfected and HIV/HCV-coinfected subjects, CD4 count was inversely correlated with IL-18 levels (R2 = 0.236; Figure 2B).
Figure 2.
CD4 counts in human immunodeficiency virus (HIV)–infected subjects are inversely associated with interleukin-18 (IL-18) levels and may explain the increased IL-18 seen in HIV monoinfection compared with hepatitis C virus (HCV) monoinfection. A, Serum IL-18 levels grouped by absolute CD4 counts <200, 200–500, and >500 cells/μL in HIV-monoinfected subjects and compared to levels in HCV-monoinfected subjects. Serum IL-18 levels in subjects with <200 and 200–500 CD4 cells/μL were significantly higher than in HIV-uninfected HCV-infected subjects (****P < .0001 and ***P = .0002, respectively). Serum IL-18 levels in subjects with >500 CD4 cells/μL were not statistically different from levels in HCV-infected subjects without HIV. B, Linear regression of log10 IL-18 vs log10 CD4 counts; negative correlation R2 = 0.236. Statistical analysis was performed using univariable and multivariable linear regression models.
Infection With HIV and ART Suppression of HIV Alter IL-18 Production
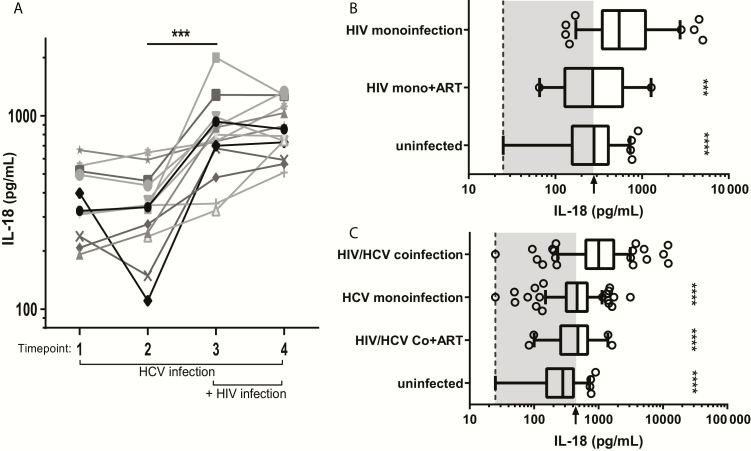
To test whether IL-18 induction is additive in HIV/HCV coinfection, we obtained an additional 14 subjects from the ALIVE cohort who were chronically HCV infected and subsequently acquired incident HIV infection. Two time points during chronic HCV/pre-HIV infection and two time points after HIV acquisition were tested. The first HIV/HCV-coinfected time point tested for all subjects was the first visit at which HIV-Ab became detectable. IL-18 levels in the same subject did not significantly differ between the two pre-HIV infection specimens or between the two post-HIV infection specimens (Figure 3A). In contrast, comparison of IL-18 levels pre- and post- HIV infection revealed a significant increase in IL-18 levels upon incident HIV infection in the setting of chronic HCV infection (Figure 3A). To further assess IL-18 variability over time in the absence of incident HIV infection, 10 chronically HCV-monoinfected subjects from the ALIVE cohort were examined at two time points approximately 10 years apart. In contrast to changes seen with incident HIV infection, we saw no significant difference in IL-18 levels in chronic HCV infection over time (Supplementary Figure 1).
Figure 3.
Infection with human immunodeficiency virus (HIV) increases serum interleukin-18 (IL-18) whereas antiretroviral therapy (ART) suppression of HIV is associated with lower IL-18 production. A, Serum IL-18 levels were measured in 14 subjects with chronic hepatitis C virus (HCV) infection at two time points prior to and two time points after incident HIV infection. IL-18 levels were significantly different upon incident HIV infection (between the last preinfection and first postinfection time point; ***P < .001, paired Student t test), but not between the two baseline preinfection visits or between the paired post-HIV infection visits. B, Serum IL-18 levels were compared between HIV-monoinfected, HIV-monoinfected on ART, and HIV/HCV-uninfected subjects. Dashed line: lower limit of quantitation (LLOQ). Arrow: HIV/HCV-uninfected subject median. HIV-monoinfected on ART and the HIV/HCV-uninfected groups were both statistically different from the HIV-monoinfected group (***P < .001 and ****P < .0001, respectively), but not from each other. C, HIV/HCV-coinfected, HCV-monoinfected, HIV/HCV-coinfected on ART, and HIV/HCV-uninfected subjects. Dashed line: LLOQ. Arrow: HCV monoinfection median. All groups were statistically different from HIV/HCV coinfection (P < .0001). Both B and C were compared using a 1-way analysis of variance and adjusted for multiple comparisons.
To test whether HIV treatment is associated with normalization of IL-18 levels, we obtained an additional 55 HIV/HCV-coinfected and 21 HIV-monoinfected subjects from ALIVE on ART with undetectable HIV-RNA. In HIV-monoinfected subjects, we saw that HIV suppression by ART was associated with significantly lower IL-18 levels vs HIV-monoinfected subjects not on ART (P < .001). In fact, HIV suppression by ART was associated with IL-18 levels in HIV-monoinfected subjects comparable to IL-18 levels in uninfected subjects (Figure 3B). In coinfected subjects, we saw that HIV suppression by ART was associated with lower IL-18 levels vs HIV/HCV-coinfected subjects not on ART (P < .0001; Figure 3C). However, IL-18 levels remained significantly higher than in uninfected subjects and were comparable to IL-18 levels measured in HCV monoinfection. This suggests that the elevated IL-18 levels observed in HIV/HCV coinfection can be reduced by ART but that HCV infection remains a cause of IL-18 elevation in coinfected patients treated with ART.
Other Serum Cytokines and Chemokines Are Also Associated With HIV/HCV Coinfection
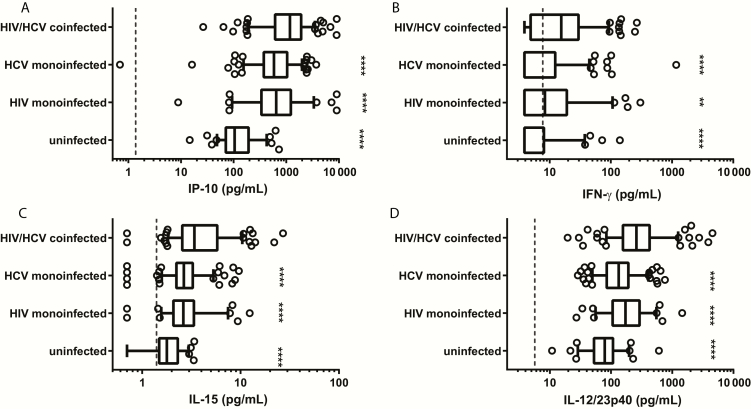
In addition to increased IL-18, HIV/HCV coinfection also led to increased levels of IFN-gamma-inducible protein 10 (IP)-10, IL12/23p40, IL-15, and interferon gamma (IFN-γ) vs either monoinfection (Figure 4). Additionally, HCV- or HIV-monoinfected subjects had significantly higher levels of these analytes than did uninfected subjects (Figure 4). Controlling for age, sex, and race in a multivariable analysis showed that IP-10, IL12/23p40, IL-15, and IFN-γ were independently associated with HIV/HCV coinfection when compared to either monoinfection (Supplementary Table 2).
Figure 4.
Serum interferon-gamma (IFN-γ)-inducible protein 10 (IP-10), IFN-γ, interleukin (IL) 15, and IL12/23p40 are increased in human immunodeficiency virus (HIV)/hepatitis C virus (HCV)–coinfected subjects. Serum IP-10, IFN-γ, IL-15, and IL12/23p40 levels were significantly increased in coinfection compared with either monoinfection and uninfected groups. Dashed line: lower limit of quantitation for each analyte. ****P < .0001; **P < .01. Groups were compared using a 1-way analysis of variance and adjusted for multiple comparisons.
Serum levels of 10 additional analytes (tumor necrosis factor–α, Macrophage inflammatory protein (MIP)-1β, IL-7, IL-16, IL-6, IL-10, IL-8, eotaxin, MIP-1α, and IL-2) were assessed in the 4 groups. No significant differences in the levels of those analytes were found between HIV/HCV coinfection and either monoinfection (Supplementary Figures 2 and 3). Median values, IQRs, and multivariate estimates for these analytes are listed in Supplementary Tables 2 and 3.
DISCUSSION
This study provides a detailed assessment of serum IL-18 levels in HIV and HCV monoinfections and in HIV/HCV coinfection, controlling for the first time for demographic factors and comparing to both monoinfections. One previously published study demonstrated higher IL-18 levels in HIV/HCV coinfection than in HIV monoinfection, but there was no assessment of IL-18 levels in HCV monoinfection and the total number of subjects in all categories combined was 29, preventing control for other variables that could affect IL-18 levels, including race, sex, or age [27]. We have shown that IL-18 is higher in coinfected than in monoinfected or uninfected subjects (Figure 1A, Table 2). Higher IL-18 in HIV and HCV monoinfection compared with uninfected individuals has been shown previously. Thus, we chose to focus on comparing IL-18 levels among infections. The observation of increased IL-18 in HIV/HCV coinfection vs both monoinfections is novel and may explain the advanced disease progression seen in coinfected individuals [11]. Interestingly, when comparing HIV monoinfection to HCV monoinfection, we saw that IL-18 levels were significantly higher in HIV monoinfection, suggesting that HIV more robustly induces inflammasome activation (Figure 1A, Table 2).
To further investigate the role HIV and HCV play in coinfection pathogenesis, we assessed the relationship between IL-18 and characteristics of viral infection. We found an association between IL-18 levels and detectable HIV-RNA (Table 2). Our group has previously shown no correlation between IL-18 and HCV-RNA levels except that detectable HCV-RNA is associated with increased IL-18 [20]. Additionally, we found that lower CD4 cell counts correlated with higher IL-18 levels (Figure 2B). The association between CD4 depletion and serum IL-18 in vivo supports a prior study showing that CD4 depletion results from abortive HIV infection and activation of the inflammasome, resulting in pyroptosis and release of IL-18 and IL-1β [23]. Unfortunately, we were unable to assess production of IL-1β because the cytokine is undetectable in serum (Supplementary Figure 3). However, we would hypothesize that IL-1β follows a similar trend within the tissue.
Incident HIV infection in subjects chronically infected with HCV resulted in a significant increase in IL-18 (Figure 3A). Conversely, ART was associated with significantly lower IL-18 levels in both HIV/HCV-coinfected and HIV-monoinfected subjects compared with subjects not on ART (Figure 3B and 3C). These findings support that sensing of virus drives IL-18 production, consistent with previous in vitro data [21, 28, 29]. We were unable to assess how acquisition or resolution of HCV infection affected IL-18 levels in HIV-infected subjects, as HCV is almost universally acquired first in PWID and spontaneous HCV resolution in the setting of HIV infection is rare. However, we have previously shown that spontaneous control of HCV monoinfection results in return of IL-18 levels to baseline levels measured prior to infection [20].
Finally, we found 3 additional cytokines (IL-12/23p40, IL-15, and IFN-γ) and 1 chemokine (IP-10) to be significantly elevated in HIV/HCV coinfection compared with both monoinfections (Figure 4). IP-10 is thought to be a cross-talk cytokine in HIV/HCV coinfection and has been previously reported as increased in this setting [30, 31]. IL-15 elevation, also previously reported, has been suggested to play a role in inflammation, liver damage, and advanced fibrosis seen in coinfected subjects [32, 33]. Increased IL12/23p40 subunit and IFN-γ have not been previously reported to be elevated in coinfection. IL-12 and IL-23 can regulate both IFN-γ and IL-18 responses to viral infections. Additionally, IL-18 is known to induce T cells to make IFN-γ, particularly in the presence of IL-12 [34–36]. Detection of elevated levels of these cytokines that are coordinately regulated with or by IL-18 supports our finding of elevated IL-18 in HIV/HCV coinfection.
In summary, we have shown that IL-18 is increased in HIV/HCV coinfection when compared with monoinfection and that this increase is likely due to additive sensing of both viruses. Incident HIV increases IL-18 in HCV-infected subjects, and ART is associated with reduced IL-18 levels in both HIV-monoinfected and coinfected subjects. Given the association with increased IL-18 and inflammatory conditions seen more commonly in HIV and HCV infection and coinfection, increased IL-18 levels may explain the enhanced disease progression observed in coinfected individuals. ART has been shown in some studies to reduce the risk of inflammatory complications in coinfected individuals over those with HCV alone, but does not eliminate all risk associated with HIV [37]. The time of ART initiation is variable and damage from unchecked HIV viremia may occur prior to ART administration. Thus, ART-associated reductions in IL-18 might not completely mitigate the increased risk of coinfection. ART use in coinfected subjects was associated with reduced IL-18 levels, but only to HCV monoinfection levels, not to the levels seen in uninfected controls. IL-18 production returns to baseline with spontaneous HCV clearance, and we hypothesize that successful HCV treatment would mimic spontaneous clearance [20]. These data provide additional support for early treatment of HIV and, by analogy, HCV. There is a range of IL-18 levels in coinfection, potentially permitting an assessment of the relationship between elevated serum IL-18 and increased incidence and progression of inflammatory conditions in coinfection. If IL-18 is implicated, this will enhance our understanding of coinfection pathogenesis and direct potential therapeutic interventions.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Supplementary Material
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant numbers U19 AI088791 and R01 AI108403) and the National Institute on Drug Abuse (grant numbers R01 DA12568, U01 DA036297, and U01 DA036935).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 2012; 308:387–402. [DOI] [PubMed] [Google Scholar]
- 2. Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis 2012; 205:S383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sulkowski MS, Mehta SH, Torbenson MS, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS 2007; 21:2209–16. [DOI] [PubMed] [Google Scholar]
- 4. Wyatt CM. The kidney in HIV infection: beyond HIV-associated nephropathy. Top Antivir Med 2012; 20:106–10. [PMC free article] [PubMed] [Google Scholar]
- 5. Walker Harris V, Brown TT. Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. J Infect Dis 2012; 205:S391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 8. Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003; 38:50–6. [DOI] [PubMed] [Google Scholar]
- 9. Johnson RJ, Gretch DR, Yamabe H, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 1993; 328:465–70. [DOI] [PubMed] [Google Scholar]
- 10. Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology 2004; 127:1372–80. [DOI] [PubMed] [Google Scholar]
- 11. Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep 2011; 8:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thorand B, Kolb H, Baumert J, et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes 2005; 54:2932–8. [DOI] [PubMed] [Google Scholar]
- 13. Oikawa Y, Shimada A, Kasuga A, et al. Systemic administration of IL-18 promotes diabetes development in young nonobese diabetic mice. J Immunol 2003; 171:5865–75. [DOI] [PubMed] [Google Scholar]
- 14. Blankenberg S, Luc G, Ducimetière P, et al. ; PRIME Study Group Interleukin-18 and the risk of coronary heart disease in European men: the prospective epidemiological study of myocardial infarction (PRIME). Circulation 2003; 108:2453–9. [DOI] [PubMed] [Google Scholar]
- 15. Yearley JH, Xia D, Pearson CB, Carville A, Shannon RP, Mansfield KG. Interleukin-18 predicts atherosclerosis progression in SIV-infected and uninfected rhesus monkeys (Macaca mulatta) on a high-fat/high-cholesterol diet. Lab Invest 2009; 89:657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leslie JA, Meldrum KK. The role of interleukin-18 in renal injury. J Surg Res 2008; 145:170–5. [DOI] [PubMed] [Google Scholar]
- 17. Tsutsui H, Matsui K, Okamura H, Nakanishi K. Pathophysiological roles of interleukin-18 in inflammatory liver diseases. Immunol Rev 2000; 174:192–209. [DOI] [PubMed] [Google Scholar]
- 18. Iannello A, Samarani S, Debbeche O, et al. Potential role of IL-18 in the immunopathogenesis of AIDS, HIV-associated lipodystrophy and related clinical conditions. Curr HIV Res 2010; 8:147–64. [DOI] [PubMed] [Google Scholar]
- 19. Watanabe D, Uehira T, Yonemoto H, et al. Sustained high levels of serum interferon-γ during HIV-1 infection: a specific trend different from other cytokines. Viral Immunol 2010; 23:619–25. [DOI] [PubMed] [Google Scholar]
- 20. Chattergoon MA, Levine JS, Latanich R, Osburn WO, Thomas DL, Cox AL. High plasma interleukin-18 levels mark the acute phase of hepatitis C virus infection. J Infect Dis 2011; 204:1730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. An P, Thio CL, Kirk GD, Donfield S, Goedert JJ, Winkler CA. Regulatory polymorphisms in the interleukin-18 promoter are associated with hepatitis C virus clearance. J Infect Dis 2008; 198:1159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chattergoon MA, Latanich R, Quinn J, et al. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog 2014; 10:e1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doitsh G, Galloway NL, Geng X, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 2014; 505:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vlahov D, Anthony JC, Munoz A, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr 1991; 109:75–100. [PubMed] [Google Scholar]
- 25. Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 17:S38–41. [DOI] [PubMed] [Google Scholar]
- 26. Cox AL, Netski DM, Mosbruger T, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis 2005; 40:951–8. [DOI] [PubMed] [Google Scholar]
- 27. Tornero C, Alberola J, Tamarit A, Navarro D. Effect of highly active anti-retroviral therapy and hepatitis C virus co-infection on serum levels of pro-inflammatory and immunoregulatory cytokines in human immunodeficiency virus-1-infected individuals. Clin Microbiol Infect 2006; 12:555–60. [DOI] [PubMed] [Google Scholar]
- 28. Negash AA, Ramos HJ, Crochet N, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog 2013; 9:e1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shrivastava S, Mukherjee A, Ray R, Ray RB. Hepatitis C virus induces interleukin-1β (IL-1β)/IL-18 in circulatory and resident liver macrophages. J Virol 2013; 87:12284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rider PJ, Liu F. Crosstalk between HIV and hepatitis C virus during co-infection. BMC Med 2012; 10:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roe B, Coughlan S, Hassan J, et al. Elevated serum levels of interferon- gamma–inducible protein-10 in patients coinfected with hepatitis C virus and HIV. J Infect Dis 2007; 196:1053–7. [DOI] [PubMed] [Google Scholar]
- 32. Jiménez-Sousa MA, Berenguer J, Rallón N, et al. IL15 polymorphism is associated with advanced fibrosis, inflammation-related biomarkers and virological response in human immunodeficiency virus/hepatitis C virus coinfection. Liver Int 2016; 36:1258–66. [DOI] [PubMed] [Google Scholar]
- 33. Allison RD, Katsounas A, Koziol DE, et al. Association of interleukin-15-induced peripheral immune activation with hepatic stellate cell activation in persons coinfected with hepatitis C virus and HIV. J Infect Dis 2009; 200:619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pirhonen J, Matikainen S, Julkunen I. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J Immunol 2002; 169:5673–8. [DOI] [PubMed] [Google Scholar]
- 35. Kohno K, Kataoka J, Ohtsuki T, et al. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol 1997; 158:1541–50. [PubMed] [Google Scholar]
- 36. Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995; 378:88–91. [DOI] [PubMed] [Google Scholar]
- 37. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.