Summary
In a Botswana national meningitis survey, we observed a high 2013–2014 incidence of cryptococcal meningitis, particularly in men in their fourth or fifth decade, highlighting the need to target populations at high risk for advanced HIV and adopt differentiated care models.
Keywords: cryptococcal meningitis, incidence, HIV, Botswana, sub-Saharan Africa
Abstract
Background
Botswana has a well-developed antiretroviral therapy (ART) program that serves as a regional model. With wide ART availability, the burden of advanced human immunodeficiency virus (HIV) and associated opportunistic infections would be expected to decline. We performed a nationwide surveillance study to determine the national incidence of cryptococcal meningitis (CM), and describe characteristics of cases during 2000–2014 and temporal trends at 2 national referral hospitals.
Methods
Cerebrospinal fluid data from all 37 laboratories performing meningitis diagnostics in Botswana were collected from the period 2000–2014 to identify cases of CM. Basic demographic and laboratory data were recorded. Complete national data from 2013–2014 were used to calculate national incidence using UNAIDS population estimates. Temporal trends in cases were derived from national referral centers in the period 2004–2014.
Results
A total of 5296 episodes of CM were observed in 4702 individuals; 60.6% were male, and median age was 36 years. Overall 2013–2014 incidence was 17.8 (95% confidence interval [CI], 16.6–19.2) cases per 100000 person-years. In the HIV-infected population, incidence was 96.8 (95% CI, 90.0–104.0) cases per 100000 person-years; male predominance was seen across CD4 strata. At national referral hospitals, cases decreased during 2007–2009 but stabilized during 2010–2014.
Conclusions
Despite excellent ART coverage in Botswana, there is still a substantial burden of advanced HIV, with 2013–2014 incidence of CM comparable to pre-ART era rates in South Africa. Our findings suggest that a key population of individuals, often men, is developing advanced disease and associated opportunistic infections due to a failure to effectively engage in care, highlighting the need for differentiated care models.
Botswana was the first country in Africa to establish a national antiretroviral therapy (ART) program and began providing treatment free of charge to its human immunodeficiency virus (HIV)–infected population in early 2002 [1]. National HIV care guidelines in 2012 recommended a CD4 T-cell threshold of <350 cells/μL for ART initiation, up from <200 cells/μL in 2008 [2, 3], and recent population-level data suggest that, in rural regions, the program is close to achieving the “90-90-90” targets set by the Joint United Nations Programme on HIV/AIDS (UNAIDS) [4]. Despite the success of the national ART program, Botswana, a country of approximately 2 million people, still has high incidence of new HIV infections in certain populations and an HIV prevalence among the highest in the world, with an estimated adult HIV prevalence of 25% in 2014 [5, 6].
In sub-Saharan Africa as a whole, as in Botswana, although millions now access life-saving ART, overall HIV prevalence is still not declining and ART is initiated at low CD4 T-cell counts in a majority of HIV-infected individuals [7–9], resulting in a large population at substantial risk of opportunistic disease and with high rates of mortality [10]. Care default and treatment failure further increase the size of this high-risk population, with pooled observational cohort data from African settings suggesting that only 65% of patients starting first-line ART remain in care at 36 months, and virological failure and acquired ART resistance are frequently observed [11–13]. Movement toward an HIV “test and treat” strategy in sub-Saharan Africa, which was adopted in Botswana in June 2016, will necessitate streamlining models of care to quickly expand treatment programs, which has the potential of leaving vulnerable patients further behind.
Cryptococcal meningitis (CM) is a severe fungal infection primarily seen in individuals with defective cell-mediated immunity [14], with the vast majority of cases occurring in the context of advanced HIV infection [15]. CM typically affects individuals with CD4 T-cell counts <100 cells/µL, and is now frequently described following late ART initiation and ART default, as well as in ART-naive individuals [16–18]. As countries move toward expanded HIV treatment programs, CM serves as an important indicator disease for national programs. An understanding of advanced HIV disease burden and temporal trends in subpopulations will guide program delivery and differentiated models of HIV care targeting groups most at risk for delayed HIV diagnosis, ART initiation, and care default, with the associated morbidity and mortality and high transmission risks.
We collected laboratory records from all facilities in Botswana that perform meningitis diagnostics and UNAIDS country-level HIV prevalence estimates to determine the 2013–2014 national incidence rate of CM, describe characteristics of CM cases over a 15-year period, and define temporal trends in cases diagnosed at the 2 national referral hospitals.
METHODS
Study Design
The Botswana National Meningitis Survey was a 15-year retrospective review of routine cerebrospinal fluid (CSF) laboratory records to determine the etiologies of meningitis in Botswana and temporal trends with maturation of the local HIV epidemic. The study was conducted in collaboration with the Botswana Ministry of Health and the National Health Laboratory. Institutional review board (IRB) approval was obtained from the University of Botswana, University of Pennsylvania, University of Washington, Health Research Development Council (Botswana Ministry of Health), and at hospitals with independent IRB committees (Letsholathebe Memorial Hospital, Mahalapye Hospital, Nyangabwe Referral Hospital, Princess Marina Hospital, Scottish Livingstone Hospital, and Sekgoma Memorial Hospital).
Participating Laboratories and Cerebrospinal Fluid Data Collection
Thirty-seven laboratories are registered by the Botswana National Health Laboratory to perform CSF testing for the diagnosis of CM by India ink stain, fungal culture, and/or cryptococcal antigen (CrAg) testing, including 2 national referral hospitals, 7 district hospitals (6 medical, 1 psychiatric), and 28 primary, private, mining, and military hospital-based, clinic-based, or stand-alone laboratories. Facilities were visited by the study team for collection of meningitis-related laboratory data. Most visits took place between February 2015 and May 2015. All available CSF records from 1 January 2000 through 1 January 2015 were located at facilities. Records were scanned on a password-protected study laptop, then numbered and entered into a REDCap database. To ensure data accuracy during transcription of paper-based records into the REDCap database, a minimum of 2 study team members reviewed each entry and discrepancies were adjudicated through consensus.
In addition to paper records, a majority of hospital-based laboratories maintain records on a government-financed electronic medical record (EMR), the Integrated Patient Management System (IPMS). At facilities with IPMS, clinical data are entered directly into IPMS with laboratory books used to keep records during Internet downtime or entered both in laboratory notebooks and IPMS. IPMS was queried centrally at the Ministry of Health to obtain all Botswana CSF-related electronic laboratory records and HIV-related data (CD4 T-cell count nearest to the date of CM diagnosis). Paper-based records from the REDCap database were merged with IPMS records using Stata software version 12 (StataCorp, College Station, Texas), matching by date, testing location, and patient identifiers (name, age, sex), with duplicate entries removed. Three hospitals used additional EMR platforms for limited periods during the study period. Electronic records from these EMR platforms were also queried using CSF-related search terms, and these data were entered into the REDCap database.
Population Denominator Data
The UNAIDS Spectrum model version 5.41 was used to determine population numbers [19], national HIV prevalence, new HIV infections, and the distribution of CD4 T-cell count. Two module sets were activated for this projection. The demographic set included DEMPROJ (to estimate the population by age and sex) and FAMPLAN (to estimate the impact of family planning services on population size). The HIV set included AIM (to estimate the impact of HIV), GOALS (to estimate the funding needed to achieve the national HIV response targets), and RNM (to estimate the costs of implementing the national HIV program). Model inputs were derived from published country reports, strategic plans, costing studies, programmatic data, and expert opinion [20].
Patients and Case Definition
Records were obtained of any patient who received a lumbar puncture (LP) for CSF analysis without an age cutoff. In Botswana, routine diagnostic CSF testing for CM includes India ink stain and fungal culture. CSF testing for CrAg was performed using latex agglutination tests by several centers and routinely at referral hospital laboratories. The cryptococcal lateral flow assay was not available in Botswana during the study period.
A “case” (or episode) of CM was defined as CSF with positive India ink stain, Cryptococcus culture, and/or positive CSF CrAg. As CSF analysis may have been repeated on patients receiving multiple LPs for lowering of intracranial pressure or other indications during an admission, all positive CSF samples for a unique patient within a single documented hospitalization (or in the absence of admission dates, within a ≤14-day window of any previous CSF sample) were considered part of the same “case.”
Data Analysis
Data were analyzed using Stata software version 12. Cases of CM in Botswana were enumerated using the case definition. The number of cases, recurrent cases, patient age and sex, and month and season of diagnosis were described using frequencies, percentage, or median and interquartile range (IQR), as appropriate. It was not possible to calculate national incidence rates over the full 15-year study period, as records prior to 2013 were partially complete. Complete CSF laboratory data from all laboratories were obtained during 2013 and 2014 calendar years. For these 2013–2014 national data, UNAIDS Spectrum model HIV estimates were used as a population denominator to determine the CM incidence rate in person-years of observation (PYO) with 95% confidence intervals (CIs) derived using a Poisson distribution. Incidence was estimated by sex and ordinal age categories as well as by CD4 T-cell count category stratified by sex in individuals ≥15 years of age.
The 2 national referral hospitals (Princess Marina Hospital and Nyangabwe Referral Hospital) adopted IPMS in 2004 and also maintained paper-based records, yielding a complete dataset for the 11-year period 2004–2014. At these 2 hospitals, annual cases diagnosed over this 11-year period were displayed graphically in relation to the “treatment gap” (UNAIDS-estimated HIV-infected population ≥15 years of age minus population receiving ART) and median CD4 T-cell count at ART initiation.
RESULTS
15-Year Cryptococcal Meningitis Data
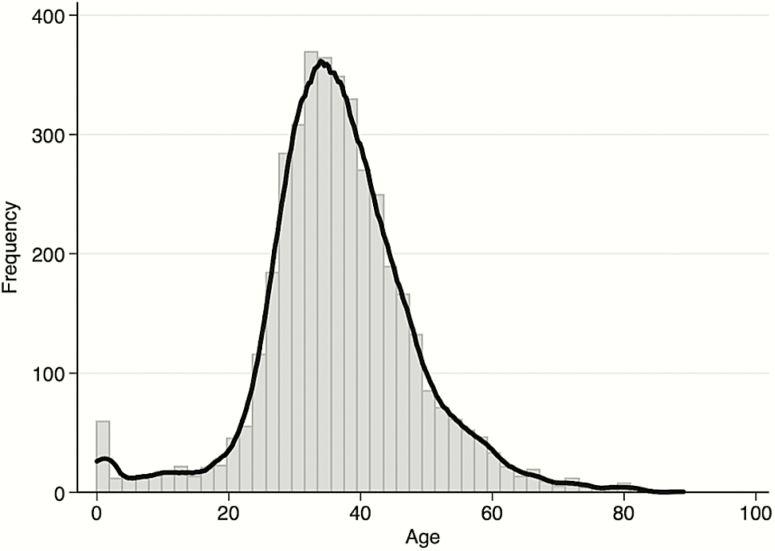
We identified 5296 unique cases of CM in 4702 individuals from the period 2000–2014 (Table 1). Of these individuals, 90.3% (4248/4702) experienced a single episode, 7.7% (360/4702) 2 episodes, 1.0% (48/4702) 3 episodes, and 1.0% (46/4702) 4 or more episodes of disease. Median age at diagnosis was 36 (IQR, 30–43) years (Figure 1) and more cases were diagnosed in males than females (60.6% males vs 39.4% females). There was no evidence of seasonality. Case numbers did not differ with respect to month or season at diagnosis.
Table 1.
Description of Cryptococcal Meningitis Cases in Botswana, 2000–2014
| Total CM episodes | 5296 | |||
| Total CM patients | 4702 | |||
| Single episode | 4248 | (90.3) | ||
| Two episodes | 360 | (7.7) | ||
| Three episodes | 48 | (1.0) | ||
| Four or more episodes | 46 | (1.0) | ||
| Variable | Value | No. With Data | No. (%)a | |
| Ageb | Median and IQR | 4056 | 36 years | 30–43 years |
| Sexb | Male | 4407 | 2670 | (60.6) |
| Female | 1737 | (39.4) | ||
| Sitec | Tertiary | 5296 | 3161 | (59.7) |
| Primary/Secondary | 2135 | (40.3) | ||
| Monthc | January | 5296 | 430 | (8.1) |
| February | 442 | (8.4) | ||
| March | 481 | (9.1) | ||
| April | 398 | (7.5) | ||
| May | 432 | (8.2) | ||
| June | 418 | (7.9) | ||
| July | 449 | (8.4) | ||
| August | 490 | (9.3) | ||
| September | 435 | (8.2) | ||
| October | 423 | (8.0) | ||
| November | 456 | (8.6) | ||
| December | 442 | (8.4) | ||
| Seasonc | Summer (Nov–Jan) | 5296 | 1328 | (25.1) |
| Autumn (Feb–Apr) | 1321 | (24.9) | ||
| Winter (May–Jul) | 1299 | (24.5) | ||
| Spring (Aug–Oct) | 1348 | (25.5) | ||
Abbreviations: CM, cryptococcal meningitis; IQR, interquartile range.
aUnless otherwise indicated.
bDe-duplicated to represent individual patients rather than CM episodes.
cData from all CM episodes including relapses.
Figure 1.
Age distribution of cryptococcal meningitis cases in Botswana, 2000–2014.
2013–2014 Incidence of Cryptococcal Meningitis
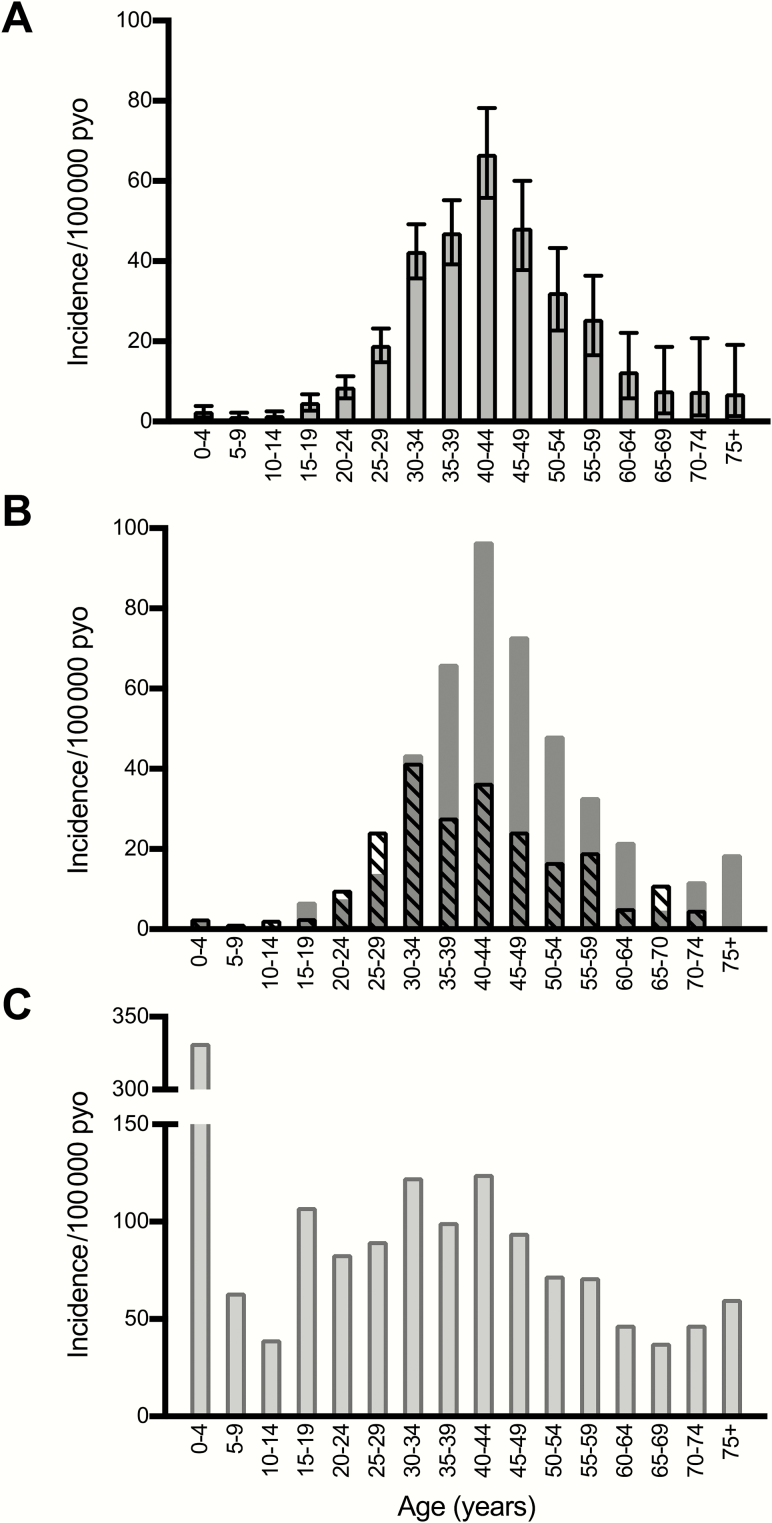
National incidence of diagnosed CM in 2013–2014 was 17.8 (95% CI, 16.6–19.2) cases/100000 PYO, with higher incidence observed in males than in females (22.0 [95% CI, 20.1–24.1] cases/100000 PYO in males vs 13.7 [95% CI, 12.1–15.3] cases/100000 PYO in females) (Table 2; Figure 2A and 2B). Peak incidence was observed in 40- to 44-year-olds, with an earlier peak among females (30–34 years) and a later peak among males (40–44 years), mirroring national age trends in HIV prevalence.
Table 2.
National Incidence of Cryptococcal Meningitis in Botswana, 2013–2014
| Strata | Category | No. of Cases | Person-years | Incidence (per 100000 PYO) | 95% CI (per 100000 PYO) |
|---|---|---|---|---|---|
| Overall incidence | |||||
| All | … | 755 | 4231095 | 17.8 | 16.6–19.2 |
| Sex | Male | 466 | 2115031 | 22.0 | 20.1–24.1 |
| Female | 289 | 2116063 | 13.7 | 12.1–15.3 | |
| Age, y | 0–4 | 10 | 475589 | 2.1 | 1.0–3.9 |
| 5–9 | 4 | 467021 | 0.9 | 0.2–2.2 | |
| 10–14 | 5 | 458710 | 1.1 | 0.4–2.5 | |
| 15–19 | 20 | 457334 | 4.4 | 2.7–6.8 | |
| 20–24 | 37 | 450494 | 8.2 | 5.8–11.3 | |
| 25–29 | 79 | 424031 | 18.6 | 14.8–23.2 | |
| 30–34 | 156 | 371214 | 42.0 | 35.7–49.2 | |
| 35–39 | 138 | 295495 | 46.7 | 39.2–55.2 | |
| 40–44 | 141 | 212707 | 66.3 | 55.8–78.2 | |
| 45–49 | 76 | 158636 | 47.9 | 37.8–60.0 | |
| 50–54 | 40 | 125781 | 31.8 | 22.7–43.3 | |
| 55–59 | 27 | 107792 | 25.1 | 16.5–36.4 | |
| 60–64 | 10 | 83350 | 12.0 | 5.8–22.1 | |
| 65–69 | 4 | 55035 | 7.3 | 2.0–18.6 | |
| 70–74 | 3 | 42068 | 7.1 | 1.5–20.8 | |
| ≥75 | 3 | 45839 | 6.5 | 1.4–19.1 | |
| HIV infected (any CD4 T-cell count) | |||||
| All | … | 755 | 779997 | 96.8 | 90.0–104.0 |
| Sex | Male | 466 | 344120 | 135.4 | 123.4–148.3 |
| Female | 289 | 435877 | 66.3 | 58.9–74.4 | |
| CD4 T-cell count <200 cells/µLa | |||||
| All | … | 690 | 241445 | 285.8 | 264.9–307.9 |
| Sex | Male | 432 | 119120 | 362.7 | 329.3–398.5 |
| Female | 259 | 122325 | 211.7 | 186.7–239.2 | |
| CD4 T-cell count <100 cells/µLa | |||||
| All | … | 590 | 74845 | 788.3 | 726.0–854.6 |
| Sex | Male | 370 | 39304 | 941.4 | 847.9–1042.4 |
| Female | 221 | 35541 | 621.8 | 542.5–709.4 | |
| CD4 T-cell count <50 cells/µLa | |||||
| All | … | 415 | 22376 | 1854.7 | 1680.5–2042.0 |
| Sex | Male | 260 | 11846 | 2194.8 | 1936.1–2478.5 |
| Female | 156 | 10530 | 1481.5 | 1258.1–1733.1 | |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; PYO, person-years of observation.
aRestricted to individuals aged ≥15 years.
Figure 2.
Incidence of cryptococcal meningitis in Botswana by age category, 2013–2014. A, Overall incidence and interquartile range. B, Incidence by sex (males, solid bars; females, striped bars). C, Incidence in human immunodeficiency virus–infected population. Abbreviation: PYO, person-years of observation.
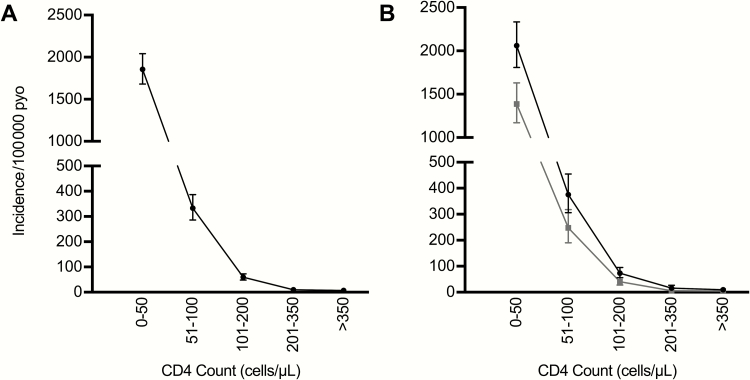
In the HIV-infected population, overall incidence was 96.8 (95% CI, 90.0–104.0) cases/100000 PYO, or 135.4 (95% CI, 123.4–148.3) cases/100000 PYO in males and 66.3 (95% CI, 58.9–74.4) cases/100000 PYO in females. Incidence rate was relatively uniform across adult age categories, peaking in the fourth through fifth decades of life (Figure 2C). Although the absolute number of CM cases in children was relatively low, incidence was comparatively high in the 2 youngest age categories (0–4 years and 5–9 years), declining in the 10- to 14-year age group, then increasing to adult levels in the adolescent category (15–19 years). Note that the high incidence in the 0–4 years age category may be spuriously high due to an underestimation of the denominator in the UNAIDS figures and low overall numbers in both the numerator and denominator categories. Incidence increased markedly with declining CD4 T-cell count strata. In those with CD4 T-cell counts <50 cells/μL, a nearly 2% annual incidence was observed, of 1854 (95% CI, 1680.5–2042.0) cases/100000 PYO, with higher incidence in males than females across CD4 T-cell count strata (Figure 3).
Figure 3.
Incidence of cryptococcal meningitis in Botswana in human immunodeficiency virus–infected population by CD4 strata, 2013–2014. A, Overall incidence. B, Incidence by sex (males, black lines; females, gray lines). Abbreviation: PYO, person-years of observation.
Referral Hospital Temporal Trends
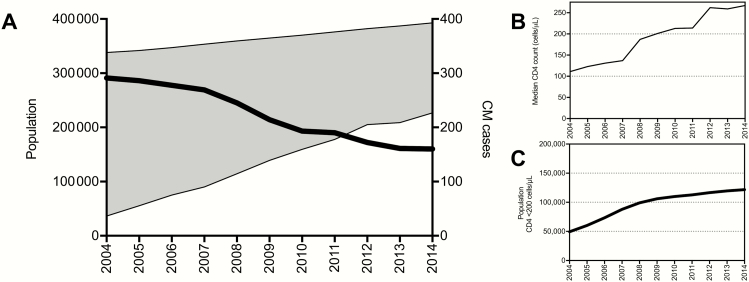
At the 2 national referral hospital laboratories, processing 60% of CSF samples, the number of cases of CM cases decreased between 2004 and 2014, with a marked decrease 2007–2009 but only a modest decline in 2010–2014, despite a shift in national ART guidelines recommending ART initiation at a higher CD4 T-cell count (<350 cells/μL in 2012 guidelines vs <200 cells/μL in 2008 guidelines) [2, 3]. Figure 4A shows cases of CM diagnosed at the referral hospitals, with total HIV-infected population in Botswana ≥15 years of age and population on ART over time. Over this period, the median CD4 T-cell count at ART initiation increased from 2007 to 2011 but remained stable during 2012–2014 (Figure 4B), with similar trends observed in the total population of HIV-infected individuals with CD4 T-cell counts <200 cells/μL (Figure 4C).
Figure 4.
Trends in diagnosed cases of cryptococcal meningitis (CM) at the 2 national referral hospitals in Botswana, 2004–2014. A, Cases diagnosed at referral hospitals (thick black line) and treatment gap in adults (≥15 years of age) (shaded gray area). B, Joint United Nations Programme on HIV/AIDS (UNAIDS) estimate of median CD4 T-cell count at antiretroviral therapy initiation in Botswana. C, UNAIDS estimate of total number of human immunodeficiency virus–infected individuals with CD4 T-cell counts <200 cells/μL.
DISCUSSION
These are the first robust national CM incidence estimates from a resource-limited country. Collection of complete records from all laboratories in Botswana performing CSF testing allowed us to accurately estimate 2013–2014 national incidence rates, revealing a high incidence of CM in Botswana in the context of the highest population ART coverage in Africa. The 2013–2014 Botswana national CM incidence is almost identical to the pre-ART era (2002–2004) laboratory surveillance–based incidence estimates from Gauteng Province, South Africa (17.8 vs 15.6 cases/100000 PYO, respectively), a period when estimated adult (15–49 years) HIV prevalence in South Africa was 21.5% [21, 22]. Limited to only HIV-infected populations, 2013–2014 Botswana incidence of 96.8 cases/100000 PYO is again comparable to South African incidence estimates from the pre-ART era (95 cases/100000 PYO), and >100-fold higher than the 2009 estimated United States HIV-associated CM rate of 7.7 cases/1000000 PYO [15].
The rate of decline in CM cases diagnosed at referral center laboratories has stagnated in recent years, despite national data showing high rates of HIV testing, ART uptake, and viral suppression [4]. These findings reflect a number of factors, including high ongoing national HIV prevalence; only modest improvements in median CD4 T-cell counts at ART initiation in recent years, from 191 (IQR, 115–239) cells/μL in 2010 to 258 (IQR, 147–337) cells/μL in 2013 despite a shift in national guidelines promoting earlier therapy [1–3, 23]; and high annual incidence of new HIV infection estimated at 1.5%–2.5% in adults [24]. Importantly, these findings suggest that, despite a decade and a half of free and widely available HIV testing and treatment services in Botswana, a population of vulnerable individuals is not being effectively reached, engaged, or retained by current testing and treatment services. This key population is now the main driver of HIV-related morbidity and mortality, and likely to be maintaining the high ongoing incidence of HIV acquisition in this setting.
With stabilizing or rising adult HIV prevalence in Botswana and the African region, eliminating the remaining treatment gap and advanced HIV disease will require innovative solutions to effectively reach key populations that are being missed by current testing and treatment models, to diagnose and treat early asymptomatic infection, and to promote lifelong engagement with treatment services. Updated 2016 guidelines in Botswana, in line with 2015 World Health Organization guidelines, now recommend the “test and treat” strategy of ART at any CD4 T-cell count for people living with HIV [25, 26]. This represents a critical step toward reducing advanced HIV disease and opportunistic infections such as CM and tuberculosis. However, the latest Botswana AIDS Indicator Survey data estimated 30% of the population aged 10–64 years had never undergone HIV testing in 2013 and ART coverage [24], at about 250000 HIV-infected individuals by 2015 [23], needs to expand by 100000, which will put a strain on existing services [27]. Similar scale-up will likely be even more disruptive throughout the rest of Africa, where the number of people receiving ART needs to double to cover all HIV-infected individuals [28]. To avoid leaving vulnerable individuals behind, differentiated care models should be considered to streamline care for populations with well-controlled disease and focus more intensive resources on those with higher need, who are now driving the epidemic.
Our study provides important insights into the characteristics of individuals presenting with advanced HIV and CM in Botswana. We found peak incidence of disease in adults in the fourth and fifth decades of life, suggesting a need to better engage young, working-age adults through work- or community-based care models that minimize lost opportunity costs. Strikingly, we observed a >2-fold higher incidence of disease in HIV-infected males than HIV-infected females. Although this did not completely correct when the analysis was stratified by CD4 T-cell count, perhaps in part reflecting a true sex-related biological predisposition to cryptococcal disease for which the pathogenesis is incompletely understood [29, 30], it highlights the high numbers of men presenting late to care or failing to engage with testing and treatment services. Young women may also have greater likelihood of HIV diagnosis and routine care with prenatal, postnatal, and contraceptive services.
This study had several important limitations. First, these estimates likely represent the lowest range of true CM incidence rates in Botswana due to the limitations of deriving incidence estimates from laboratory-based surveillance, including missing individuals who died before seeking medical care or who sought care but went undiagnosed due to misdiagnosis, stockout of LP equipment or laboratory reagents, or LP refusal [31]. Although we applied a rigorous approach to data collection and estimated incidence only for 2013–2014 when we believed laboratory data to be complete, missed case ascertainment may also have led to spuriously low incidence estimates. A small number of individuals may have sought care from neighboring countries, but this is not likely to have significantly impacted our findings. More than 85% of cases in the IPMS dataset had a Botswana national identification number, and the vast majority of the remainder could be identified as citizens using available identifying data. Uncertainty in UNAIDS Spectrum model denominator estimates could lead to either overestimation or underestimation, although figures are based on the most robust and contemporary data available and are validated against reliable national census and HIV population prevalence survey results [24]. Second, as a laboratory-based surveillance study, we did not have national data on ART treatment history and were unable to stratify incidence based on ART status. However, single-center data from Princess Marina Hospital indicate that 51% of CM patients are now presenting on ART [32], in keeping with other regional data [16, 17]. Third, we were unable to ascertain outcomes, although 2012–2014 data from Princess Marina Hospital showed a 10-week CM mortality of 50% and 1-year mortality of 65% [33]. The competing risk of high mortality could explain, in part, the relatively low observed relapse rate. Finally, we were unable to evaluate temporal trends in CM outside of the 2 referral hospitals due to incomplete records, which might differ from urban settings where referral centers are located. However, as almost two-thirds of cases were diagnosed at referral centers, this provides meaningful information regarding national trends.
In summary, we provide evidence for a high ongoing burden of advanced HIV disease in Botswana, a country with a mature ART program and the highest population ART coverage in Africa. Our findings highlight a need not only to adopt disease-specific measures to reduce CM, such as CrAg screening in patients with advanced disease [34–36], but the broader need to engage and link key populations to care who are being missed with current strategies. Adoption of HIV “test and treat” strategies could substantially reduce advanced HIV disease but must be coupled with paradigm shifts in testing, care linkage, and ART delivery such as differentiated care models and decentralized testing and HIV care delivery.
Notes
Acknowledgments. We thank the Afya Bora Consortium; Diakanyo Moalosi at the Botswana Ministry of Health for her assistance with querying IPMS records; Freedom Ernest at the Botswana National Health Laboratory for assistance with arranging laboratory visits; and multiple health facility and laboratory personnel at Athlone Hospital, Bobonong Primary Hospital, Deborah Retief Memorial Hospital, Ghanzi Primary Hospital, GoodHope Primary Hospital, Gumare Primary Hospital, Gweta Primary Hospital, Hukuntsi Primary Hospital, Kasane Primary Hospital, Lethlakane Primary Hospital, Lobatse Mental Hospital, Mahalapye Hospital, Masunga Primary Hospital, Letsholathebe Memorial Hospital, Mmadinare Primary Hospital, Nyangabwe Referral Hospital, Palapye Primary Hospital, Princess Marina Hospital, Rakops Primary Hospital, Scottish Livingstone Hospital, Sefhare Primary Hospital, Selibe-Phikwe Hospital, Sekgoma Memorial Hospital, Thamaga Primary Hospital, Tshabong Primary Hospital, Tutume Primary Hospital, Extension 2 Clinic, Charles Hill Clinic, Area W Clinic, Thebephatshwa Air Base, Bamalete Lutheran Hospital, Kanye Seventh-Day Adventist Hospital, Jwaneng Mine Hospital, Diagnofirm Medical Center, Gaborone Private Hospital Lancet Laboratory, Tati River Clinic Laboratory, and Bokamoso Hospital Laboratory for their assistance. Individually, we would like to thank Thato Mogapi, Bothwell Muviiwa, Kago Gofamodimo, Olefile Bickie Bagwasi, Tlotlo Bogatsu, David Lubasi, Nobley Chunda, H. Farrar Otieno, Felix C. Banda, Joy Mangilazi, Koabetswe Ramalepa, Modisaotsile Emdee Ramaologa, Caroline Kekana, Stephen Motlhagodi, Ephraim Dambe, Keoratile Ntshambiwa, George Simwanza, Phillip Joseph, Cynthia M. Kuate, Steven Tsima, Jenny Tommy, Patrick Rancholo, Finini Biyangidiki, Asini Mosango, Zein Sasikwa, Jacqueline Kasambala, Keontse Nyamadzabo, Keteng Mphoyakgosi, Olivier Betu, Frank Nyimbili, Moemedi Banda, Leabaneng Molomo, and Gabriel Malomo, among others who provided assistance to our study team during laboratory visits.
Financial support. This work was supported by the Penn Center for AIDS Research, a National Institutes of Health (NIH)–funded program (grant number P30 AI 045008 to J. N. J.) as well as the NIH Office of AIDS Research and Centers for Disease Control and Prevention/President’s Emergency Plan for AIDS Relief grant support (grant number U91HA06801B to M. W. T.). The REDCap platform is supported by grant UL1TR000423 from National Center for Research Resources/NIH.
Potential conflicts of interest. J. N. J. has received funding support from Gilead Sciences Europe. E. A. W. has received scholarship support from Bristol-Myers Squibb Pharmaceuticals. All other authors report no potential conflicts. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Farahani M, Vable A, Lebelonyane R et al. Outcomes of the Botswana national HIV/AIDS treatment programme from 2002 to 2010: a longitudinal analysis. Lancet Glob Health 2014; 2:e44–50. [DOI] [PubMed] [Google Scholar]
- 2. Botswana Ministry of Health. 2012 Botswana national HIV & AIDS treatment guidelines Available at: https://hivpolicywatch.org/duremaps/data/guidelines/BotswanaARTguidelines2012.pdf. Accessed 28 March 2017.
- 3. Botswana Ministry of Health. Botswana national HIV/AIDS treatment guidelines: 2008 version Available at: http://www.moh.gov.bw/Publications/HIVAIDS treatment guidelines.pdf. Accessed 28 March 2017.
- 4. Gaolathe T, Wirth KE, Holme MP et al. ; Botswana Combination Prevention Project Study Team Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV 2016; 3:e221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karim SA. Is the UNAIDS target sufficient for HIV control in Botswana? Lancet HIV 2016; 3:e195–6. [DOI] [PubMed] [Google Scholar]
- 6. Joint United Nations Programme on HIV/AIDS (UNAIDS). HIV and AIDS estimates—Botswana Available at: http://www.unaids.org/en/regionscountries/countries/botswana/. Accessed 28 March 2017.
- 7. Wang H, Wolock TM, Carter A et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV 2016; 3:e361–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis 2015; 60:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Avila D, Althoff KN, Mugglin C et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr 2014; 65:e8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brennan AT, Long L, Useem J, Garrison L, Fox MP. Mortality in the first 3 months on antiretroviral therapy among HIV-positive adults in low- and middle-income countries: a meta-analysis. J Acquir Immune Defic Syndr 2016; 73:1–10. [DOI] [PubMed] [Google Scholar]
- 11. Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008–2013. J Acquir Immune Defic Syndr 2015; 69:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boender TS, Hoenderboom BM, Sigaloff KC et al. Pretreatment HIV drug resistance increases regimen switches in sub-Saharan Africa. Clin Infect Dis 2015; 61:1749–58. [DOI] [PubMed] [Google Scholar]
- 13. TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis 2016; 16:565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS 2007; 21:2119–29. [DOI] [PubMed] [Google Scholar]
- 15. Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997-2009. PLoS One 2013; 8:e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scriven JE, Lalloo DG, Meintjes G. Changing epidemiology of HIV-associated cryptococcosis in sub-Saharan Africa. Lancet Infect Dis 2016; 16:891–2. [DOI] [PubMed] [Google Scholar]
- 17. Rhein J, Morawski BM, Hullsiek KH et al. ; ASTRO-CM Study Team Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jarvis JN, Harrison TS. Forgotten but not gone: HIV-associated cryptococcal meningitis. Lancet Infect Dis 2016; 16:756–8. [DOI] [PubMed] [Google Scholar]
- 19. Avenir Health. Spectrum Available at: http://www.avenirhealth.org/software- spectrum.php. Accessed 28 March 2017.
- 20. Avalos A, Phillips H, Jefferis K.. Botswana investment case: investment towards effective HIV prevention, health system strengthening and the end of AIDS. Presented at: Botswana Ministry of Health Gaborone, 2016. [Google Scholar]
- 21. McCarthy KM, Morgan J, Wannemuehler KA et al. Population-based surveillance for cryptococcosis in an antiretroviral-naive South African province with a high HIV seroprevalence. AIDS 2006; 20:2199–206. [DOI] [PubMed] [Google Scholar]
- 22. Joint United Nations Programme on HIV/AIDS (UNAIDS). 2004 report on the global AIDS epidemic Available at: http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2004/GAR2004_en.pdf. Accessed 28 March 2017.
- 23. Farahani M, Price N, El-Halabi S et al. Trends and determinants of survival for over 200 000 patients on antiretroviral treatment in the Botswana National Program: 2002-2013. AIDS 2016; 30:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Botswana Ministry of Health. Botswana AIDS impact survey IV 2013 Available at: http://botswana.microdatahub.com/index.php/catalog/14. Accessed 28 March 2017.
- 25. Botswana Ministry of Health. 2016 integrated HIV clinical care guidelines Available at: http://www.moh.gov.bw/Publications/Handbook_HIV_treatment_guidelines.pdf. Accessed 28 March 2017.
- 26. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. Accessed 28 March 2017. [PubMed]
- 27. McGovern S, Phillips H, Mosime W et al. Test results of testing cost-yield prioritization model for test and treat in Botswana. In: 21st International AIDS Conference, Durban, South Africa, 2016. [Google Scholar]
- 28. World Health Organization. Global health sector response to HIV, 2000–2015: Focus on innovations in Africa Available at: http://apps.who.int/iris/bitstream/10665/198065/1/9789241509824_eng.pdf. Accessed 28 March 2017.
- 29. McClelland EE, Hobbs LM, Rivera J et al. The role of host gender in the pathogenesis of Cryptococcus neoformans infections. PLoS One 2013; 8:e63632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitchell DH, Sorrell TC, Allworth AM et al. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis 1995; 20:611–6. [DOI] [PubMed] [Google Scholar]
- 31. Thakur KT, Mateyo K, Hachaambwa L et al. Lumbar puncture refusal in sub-Saharan Africa: a call for further understanding and intervention. Neurology 2015; 84:1988–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jarvis JN, Leeme T, Chofle AA. et al. High dose liposomal amphotericin for HIV-infected cryptococcal meningitis. In: Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, 2017. [Google Scholar]
- 33. Leeme TB, Patel RK, Azzo C et al. Mortality due to HIV-associated cryptococcal meningitis in Botswana in the ART era. In: Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, 2017. [Google Scholar]
- 34. Meya D, Rajasingham R, Nalintya E, Tenforde M, Jarvis JN. Preventing cryptococcosis—shifting the paradigm in the era of highly active antiretroviral therapy. Curr Trop Med Rep 2015; 2:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mfinanga S, Chanda D, Kivuyo SL et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 2015; 385:2173–82. [DOI] [PubMed] [Google Scholar]
- 36. Lechiile K, Mitchell HK, Mulenga F et al. Prevalence of advanced HIV disease and cryptococcal infection in Gaborone, Botswana. In: Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, 2017. [Google Scholar]