In children with Staphylococcus aureus skin infection and colonization, systemic antibiotics in conjunction with incision and drainage reduced the likelihood of longitudinal colonization and recurrent infection. Clindamycin was superior to trimethoprim-sulfamethoxazole in eradicating S. aureus colonization and preventing recurrent infection.
Keywords: Staphylococcus aureus, systemic antibiotics, colonization, SSTI
Abstract
Background
Staphylococcus aureus colonization poses risk for subsequent skin and soft tissue infection (SSTI). We hypothesized that including systemic antibiotics in the management of S. aureus SSTI, in conjunction with incision and drainage, would reduce S. aureus colonization and incidence of recurrent infection.
Methods
We prospectively evaluated 383 children with S. aureus SSTI requiring incision and drainage and S. aureus colonization in the anterior nares, axillae, or inguinal folds at baseline screening. Systemic antibiotic prescribing at the point of care was recorded. Repeat colonization sampling was performed within 3 months (median, 38 days; interquartile range, 22–50 days) in 357 participants. Incidence of recurrent infection was ascertained for up to 1 year.
Results
Participants prescribed guideline-recommended empiric antibiotics for purulent SSTI were less likely to remain colonized at follow-up sampling (adjusted hazard ratio [aHR], 0.49; 95% confidence interval [CI], .30–.79) and less likely to have recurrent SSTI (aHR, 0.57; 95% CI, .34–.94) than those not receiving guideline-recommended empiric antibiotics for their SSTI. Additionally, participants remaining colonized at repeat sampling were more likely to report a recurrent infection over 12 months (aHR, 2.37; 95% CI, 1.69–3.31). Clindamycin was more effective than trimethoprim-sulfamethoxazole (TMP-SMX) in eradicating S. aureus colonization (44% vs 57% remained colonized, P = .03) and preventing recurrent SSTI (31% vs 47% experienced recurrence, P = .008).
Conclusions
Systemic antibiotics, as part of acute SSTI management, impact S. aureus colonization, contributing to a decreased incidence of recurrent SSTI. The mechanism by which clindamycin differentially affects colonization and recurrent SSTI compared to TMP-SMX warrants further study.
Skin and soft tissue infections (SSTI) rank as one of the top causes of pediatric hospitalization and account for >14 million outpatient visits each year in the United States [1–3]. Rates of pediatric hospitalization for SSTI increased by 36% between 2000 and 2012, more than for any other diagnosis [2]. Staphylococcus aureus is the most common cause of SSTI [4, 5]. The incidence of S. aureus SSTI has increased substantially since the late 1990s, largely driven by the emergence of the USA300 clone [6, 7]. Recurrence of S. aureus SSTI is common and may occur in >50% of pediatric patients [8–11]. Staphylococcus aureus nasal colonization is a predisposing factor for SSTI development [12–14]. Additionally, colonization with S. aureus at multiple sites (ie, higher burden of colonization) has been associated with higher recurrence rates [15].
Currently, there is equipoise surrounding the utilization of antibiotics in management of S. aureus skin abscesses. Incision and drainage (I&D) has been considered the mainstay of treatment for uncomplicated S. aureus skin abscesses, consistent with guidelines from the Infectious Diseases Society of America (IDSA) [16]. For patients with purulent cellulitis and skin abscesses for whom antibiotics are prescribed in the outpatient setting, the guidelines recommend empiric treatment with non-β-lactam antibiotics, including clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), a tetracycline, or linezolid [17]. Several recent studies investigating the effects of antibiotic administration on SSTI clinical cure rates have demonstrated both positive [18, 19] and negligible [8, 20, 21] effects. Other recent studies have demonstrated that patients who do not receive systemic antibiotics for SSTI, or those who do not receive an antibiotic active against the infecting organism (eg, methicillin-resistant S. aureus [MRSA]), are more likely to suffer recurrent infections [8, 21]. A recent trial of 786 patients with skin abscess randomized participants to receive clindamycin, TMP-SMX, or placebo after I&D for 10 days [22]. Those receiving antibiotics (either clindamycin or TMP-SMX) had a higher cure rate and reduced skin infections at new sites 1 month following treatment.
It is unknown whether systemic antibiotics decolonize the nares and skin. Oral antibiotic agents generally do not achieve suitable concentrations in the nares to effectively decolonize this site [23]; the impact of systemic antibiotics on S. aureus at other common sites of colonization, such as the axillae or inguinal folds, is largely undefined. However, multiple studies have evaluated oral antibiotic regimens as part of multipronged MRSA eradication strategies. In both Greece and the Netherlands, MRSA SSTI management algorithms that include systemic antibiotics have been successful in reducing carriage of MRSA [24, 25]. In hospitalized patients in Canada, a decolonization treatment bundle including chlorhexidine body washes, intranasal mupirocin, and oral rifampin and doxycycline, compared with no treatment, significantly reduced MRSA colonization at 3 and 8 months following treatment [26]. Other studies, however, have shown antibiotics to be variably effective decolonizing agents and linked with increased antimicrobial resistance [27–29]; thus, prescription of oral antibiotics as part of decolonization efforts (in the absence of acute SSTI) has not been encouraged by the IDSA [17].
In the present study, we sought to illuminate the link between oral antibiotic therapy and reduction in recurrent SSTI. We tested the hypothesis that inclusion of guideline-recommended oral antibiotics in the management of S. aureus skin abscesses in addition to I&D would result in eradication of S. aureus colonization as well as a decreased incidence of recurrent SSTI.
METHODS
Study Design and Participant Recruitment
The cohort for this study is an aggregate of participants <21 years old with community-onset S. aureus SSTI and S. aureus colonization whose data were collected prospectively from 2008–2016 during one of several studies [10, 11, 22, 30], in addition to nascent participants recruited for this study. Participants were recruited from St Louis, Missouri (n = 376; St Louis Children’s Hospital and pediatric practices affiliated with the Washington University Pediatric and Adolescent Ambulatory Research Consortium) and Springfield, Illinois (n = 7; St John’s Children’s Hospital, Memorial Medical Center, and Southern Illinois University pediatric clinics). Across study populations, patient characteristics were similar. All participants presented with acute, community-onset SSTI for which an I&D procedure was performed. For participants receiving oral antibiotics, the class, dose, and duration of antibiotic therapy varied. Children with immunodeficiency, those hospitalized within the previous 14 days, or those who performed decolonization measures (with mupirocin ointment, chlorhexidine gluconate, or bleach baths) in the prior month were excluded. This study was approved by the institutional review boards at Washington University and Southern Illinois University. Informed consent was obtained for each participant.
Baseline Screening and Longitudinal Data and Sample Collection
Across all study populations, patients with SSTI were swabbed by study personnel for detection of S. aureus colonization in the anterior nares, axillae, and inguinal folds at time of presentation and I&D procedure (baseline screening). Those with S. aureus SSTI and S. aureus colonization (n = 383) were then enrolled in a longitudinal study. Three-hundred fifty-seven of these participants underwent a repeat colonization swab at a follow-up visit (median, 38 days; interquartile range, 22–50 days). Each set of colonization swabs was collected by study team personnel during in-person longitudinal study visits. At the time of collection of each set of colonization swabs, a detailed questionnaire was administered and medical records were reviewed to ascertain antibiotic use (class and duration) and decolonization with topical antimicrobials. Most participants were prescribed decolonization measures (eg, intranasal mupirocin, chlorhexidine body washes, and/or dilute bleach water baths; Table 1), as previously described [10, 11]. Subsequent follow-up surveys were completed in person and by mail or telephone (depending on the study) up to 5 times over 12 months to ascertain incidence of recurrent SSTI. Infections were confirmed by review of medical records when available.
Table 1.
Participant Characteristics (N = 383)
| Characteristic | No. (%) |
|---|---|
| Age, y, median (range) | 3.0 (0.5–20.3) |
| Female sex | 216 (56) |
| Race | |
| White | 143 (37) |
| African American or biracial | 237 (62) |
| Asian | 3 (1) |
| Baseline infecting organism | |
| MRSA | 309 (81) |
| MSSA | 74 (19) |
| Baseline colonization status | |
| MRSA | 241 (63) |
| MSSA | 105 (27) |
| MRSA and MSSAa | 37 (10) |
| Anatomic site of baseline S. aureus colonization | |
| Nares only | 80 (21) |
| Axillae only | 16 (4) |
| Inguinal folds only | 108 (28) |
| Nares and axillae | 21 (5) |
| Nares and inguinal folds | 74 (19) |
| Axillae and inguinal folds | 19 (5) |
| Nares, axillae, and inguinal folds | 65 (17) |
| Decolonization measures prescribed over the longitudinal study periodb | |
| None | 80 (21) |
| Intranasal mupirocin only | 40 (10) |
| Chlorhexidine body washes only | 1 (0.3) |
| Dilute bleach water baths only | 4 (1) |
| Intranasal mupirocin and chlorhexidine body washes | 223 (58) |
| Intranasal mupirocin and dilute bleach water baths | 35 (9) |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
aAt different anatomic sites.
bOne hundred thirteen of 357 (32%) participants with follow-up colonization swabs were prescribed decolonization measures between samplings.
Microbiology
Antibiotic susceptibility data for each infecting isolate was obtained from the medical record. Colonization swabs were inoculated to tryptic soy broth with 6.5% sodium chloride and incubated overnight at 35°C. Broth was plated to trypticase soy agar with 5% sheep’s blood and incubated for 24–48 hours. Confirmatory testing for S. aureus (by colony morphology, catalase activity, latex agglutination, and Gram stain) and antibiotic susceptibility testing (by Kirby-Bauer disk diffusion, including testing for inducible clindamycin resistance) were performed in accordance with published guidelines [31].
Statistical Analysis
Data were analyzed with IBM SPSS for Windows version 23. The χ2 test was performed to compare the proportion of participants colonized with S. aureus or reporting a recurrent infection at follow-up between those receiving antibiotics recommended for purulent SSTI at the point of care (ie, clindamycin, TMP-SMX, a tetracycline, or linezolid, per IDSA guidelines for empiric therapy of purulent SSTI [17]) and those not prescribed guideline-recommended antibiotics. Incidence of recurrent SSTI was also compared between participants remaining colonized at repeat sampling and those not remaining colonized. Cox proportional hazards regression analyses were used to determine whether receipt of guideline-recommended antibiotics at the time of index SSTI was independently associated with (1) remaining colonized with S. aureus at follow-up sampling and (2) incidence of recurrent SSTI for up to 1 year. Other covariates included in the regression analyses were age, race, methicillin susceptibility of the SSTI isolate (MRSA vs methicillin-susceptible S. aureus), prescription of decolonization measures for baseline SSTI, and burden (ie, number of anatomical sites) of S. aureus colonization at baseline. Staphylococcus aureus colonization status at follow-up sampling was also included in the recurrent infection regression analysis. All tests of significance were 2-tailed, and a P value of <.05 was considered significant.
RESULTS
The 383 participants with S. aureus SSTI and S. aureus colonization had a median age of 3.0 years (range, 0.5–20.3 years), and 56% were female. A majority (62% [n = 237]) of participants were African American or biracial, while 37% (n = 143) were white and 1% (n = 3) were Asian; 2% (n = 8) were of Hispanic or Latino origin. Of 383 participants colonized with S. aureus at the time of presentation with SSTI requiring I&D (ie, at baseline screening), 204 (53%) were colonized at 1 anatomic site, 114 (30%) at 2 sites, and 65 (17%) at all 3 sites (Table 1).
Of 383 participants with an S. aureus SSTI requiring I&D and S. aureus colonization, 355 (93%) were empirically prescribed a guideline-recommended antibiotic. Most participants received clindamycin (n = 220 [57%]) or TMP-SMX (n = 199 [52%]); 19 (5%) received vancomycin, 12 (3%) received a β-lactam (penicillin, amoxicillin, amoxicillin-clavulanate, piperacillin-tazobactam, ceftriaxone, cephalexin, or cefadroxil), and 27 (7%) received no systemic antibiotics. For 81 (21%) participants, the SSTI treatment course included >1 class of antibiotics (median, 1; range, 0–4). The antibiogram for all recovered S. aureus isolates is included in Table 2.
Table 2.
Antimicrobial Susceptibility Profiles of Staphylococcus aureus Isolates
| Type of S. aureus | No. of Staphylococcus aureus Isolates | % Susceptible | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| METa | CLIb | ERY | TMP-SMX | RIF | TET | CIP | LZD | CPT | MUP | VAN | ||
| Overall S. aureusc | 1301 | 29 | 85 | 17 | 100 | 100 | 97 | 55 | 100 | 100 | 100 | 100 |
| Infecting | 383 | 19 | 90 | 9 | 100 | 100 | 96 | 42 | 100 | 100 | 100 | 100 |
| Colonizing | 918 | 33 | 83 | 21 | 99 | 100 | 97 | 56 | 100 | 100 | 100 | 100 |
| MRSA | 925 | 0 | 85 | 6 | 99 | 100 | 97 | 44 | 100 | 100 | 100 | 100 |
| Infecting | 309 | 0 | 90 | 5 | 100 | 100 | 97 | 35 | 100 | 100 | 100 | 100 |
| Colonizing | 616 | 0 | 83 | 6 | 99 | 100 | 97 | 45 | 100 | 100 | 100 | 100 |
| MSSA | 376 | 100 | 85 | 46 | 100 | 100 | 97 | 79 | 100 | 100 | 100 | 100 |
| Infecting | 74 | 100 | 88 | 27 | 100 | 100 | 95 | 80 | 100 | 100 | 100 | 100 |
| Colonizing | 302 | 100 | 84 | 51 | 100 | 100 | 97 | 79 | 100 | 100 | 100 | 100 |
Data may represent multiple S. aureus isolates recovered from the same participant.
Abbreviations: CIP, ciprofloxacin; CLI, clindamycin; CPT, ceftaroline; ERY, erythromycin; LZD, linezolid; MET, methicillin; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; MUP, mupirocin; RIF, rifampin; TET, tetracycline; TMP-SMX, trimethoprim-sulfamethoxazole; VAN, vancomycin.
aAs predicted by cefoxitin or oxacillin testing.
bClindamycin-susceptible isolates exhibiting inducible clindamycin resistance (ie, D-test positive) were considered clindamycin resistant.
cIsolates underwent susceptibility testing to varying antimicrobials; therefore, % susceptible is out of <1301 for the following: CLI (n = 1283), ERY (n = 1282), TMP-SMX (n = 1246), RIF (n = 991), TET (n = 1006), CIP (n = 931), LZD (n = 947), CPT (n = 90), MUP (n = 64), VAN (n = 1158).
Of 383 participants with S. aureus SSTI and S. aureus colonization, follow-up colonization sampling was performed in 357; of these, 178 (50%) remained colonized at repeat sampling. Within this cohort, among 331 participants prescribed guideline-recommended empiric antibiotics at the point of care, 48% (n = 158) remained colonized with S. aureus, while 77% (n = 20) of 26 participants not prescribed guideline-recommended empiric antibiotics remained colonized (P = .004) (Figure 1). Of the 357 participants, 269 were prescribed either clindamycin (n = 142) or TMP-SMX (n = 127). Whereas 44% (n = 62) of those receiving clindamycin for their SSTI remained colonized with S. aureus at follow-up, 57% (n = 72) of those receiving TMP-SMX remained colonized (P = .03).
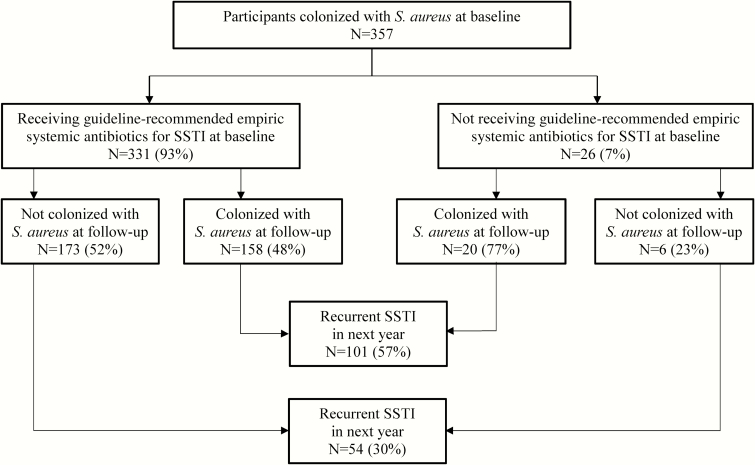
Figure 1.
Participants with Staphylococcus aureus skin and soft tissue infection (SSTI) and colonization receiving and not receiving guideline-recommended empiric systemic antibiotics for SSTI in conjunction with incision and drainage. Participants receiving guideline-recommended empiric systemic antibiotics for SSTI were less likely to remain colonized with S. aureus (P = .004 by χ2 testing) than those not prescribed guideline-recommended antibiotics. Participants remaining colonized with S. aureus at the follow-up sampling were more likely to report a recurrent SSTI (P < .001 by χ2 testing) than those not colonized at follow-up.
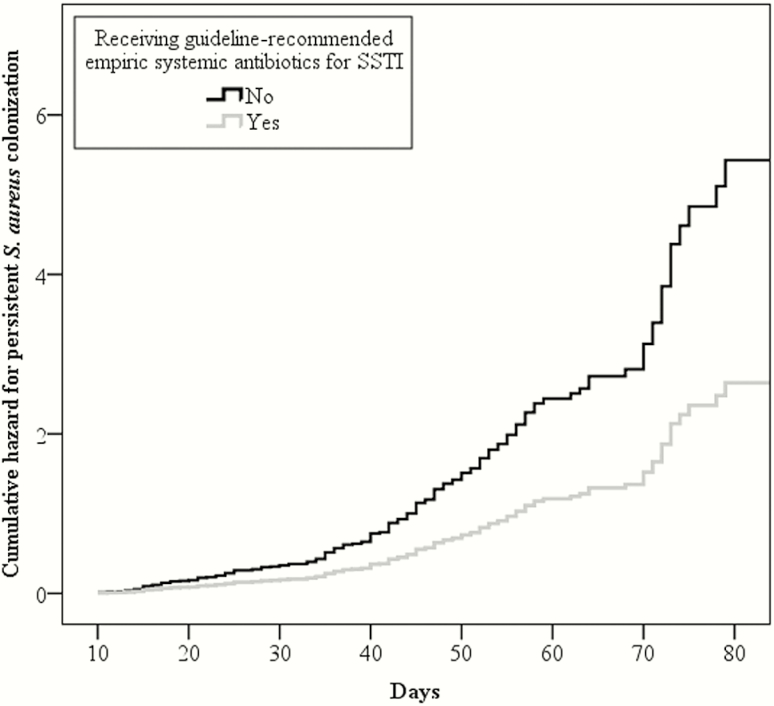
In a Cox proportional hazards regression analysis adjusting for age, race, methicillin susceptibility of the SSTI isolate, prescription of decolonization measures, and number of anatomic sites of baseline S. aureus colonization (ie, burden of colonization), participants prescribed guideline-recommended antibiotics for SSTI at the point of care were less likely to remain colonized at their second sampling (adjusted hazard ratio [aHR], 0.49; 95% CI, .30–.79) (Figure 2). Additionally, decolonization measures prescribed for baseline SSTI were independently associated with reduced prevalence of colonization at follow-up (aHR, 0.29; 95% CI, .20–.40). Older children were also less likely to remain colonized at their second sampling (each additional year of age associated with aHR of 0.97; 95% CI, .95–.99).
Figure 2.
Cox proportional hazards regression analysis for remaining colonized with Staphylococcus aureus at follow-up sampling between participants receiving and not receiving guideline-recommended empiric systemic antibiotics for skin and soft tissue infection (SSTI) in conjunction with incision and drainage. Colonized participants receiving guideline-recommended empiric systemic antibiotics for SSTI were less likely to remain colonized with S. aureus than those not prescribed guideline-recommended antibiotics (adjusted hazard ratio, 0.49; 95% confidence interval, .30–.79).
All 383 participants with S. aureus SSTI and S. aureus colonization were followed longitudinally (for up to 12 months) to ascertain incidence of recurrent SSTI. Of these, 161 (42%) reported a recurrent infection: 40% (143/355) of those prescribed guideline-recommended antibiotics for SSTI at the point of care reported a recurrent SSTI, whereas 64% (18/28) of those not prescribed guideline-recommended antibiotics reported a recurrence (P = .01). Of the 383 participants, 291 were prescribed either clindamycin (n = 156) or TMP-SMX (n = 135). While 31% (n = 49) of those prescribed clindamycin reported a recurrent SSTI, 47% (n = 63) of those prescribed TMP-SMX reported a recurrence (P = .008).
We next tested a direct link between the effects of antibiotics (given at the time of SSTI) on subsequent S. aureus colonization and on recurrent SSTI. Of 357 children who had both repeat colonization sampling and longitudinal follow-up to ascertain recurrent SSTI, those remaining colonized with S. aureus at follow-up sampling were more likely to report a recurrent SSTI (101/178 [57%]) than those who were not colonized (54/179 [30%], P < .001) (Figure 1).
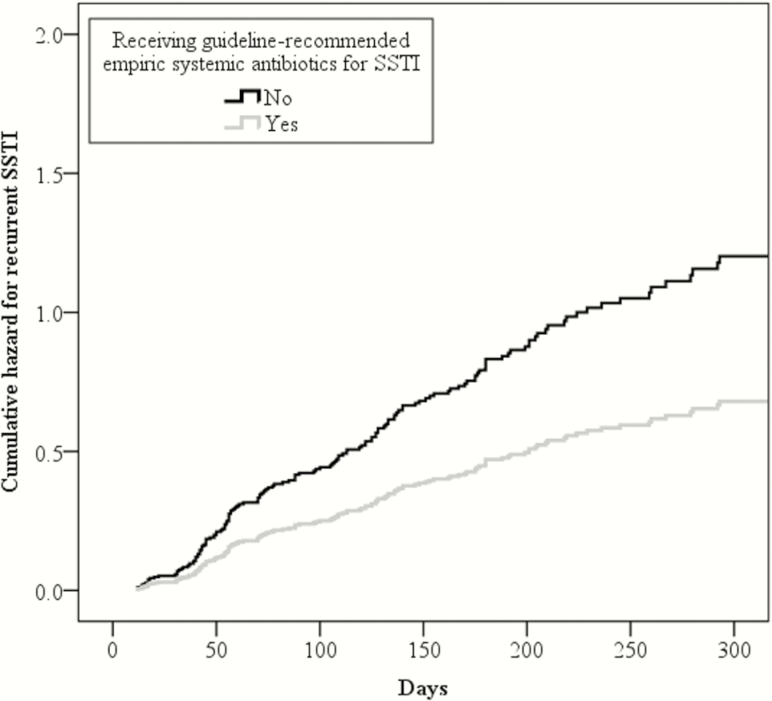
In a Cox proportional hazards regression analysis adjusting for age, race, methicillin susceptibility of the SSTI isolate, prescription of decolonization measures, burden of baseline S. aureus colonization, and colonization status at repeat sampling, participants prescribed guideline-recommended antibiotics for SSTI at the point of care were less likely to have a recurrent SSTI in the year following enrollment (aHR, 0.57; 95% CI, .34–.94) (Figure 3). Additionally, participants remaining colonized with S. aureus at repeat sampling were more likely to report a recurrent infection over 12 months (aHR, 2.37; 95% CI, 1.69–3.31) than those not colonized at follow-up sampling.
Figure 3.
Cox proportional hazards regression analysis for recurrent skin and soft tissue infection (SSTI) for up to 1 year between participants receiving and not receiving guideline-recommended empiric systemic antibiotics for SSTI in conjunction with incision and drainage. Participants receiving guideline-recommended empiric systemic antibiotics for SSTI were less likely to report a recurrent SSTI than those not prescribed guideline-recommended antibiotics (adjusted hazard ratio, 0.57; 95% confidence interval, .34–.94).
DISCUSSION
Prior studies have demonstrated a significant reduction in recurrent SSTI after antibiotic utilization for the index SSTI [8, 18, 21]. However, the mechanism driving this phenomenon is unknown. We tested the hypothesis that this observation may be a result of the impact of systemic antibiotics on the burden of S. aureus colonization, which is a known risk factor for subsequent SSTI. In this study, we demonstrated a reduction in the prevalence of S. aureus colonization longitudinally, and confirmed a reduced incidence of recurrent infection, in children who received guideline-recommended empiric antibiotics in conjunction with I&D for their baseline S. aureus SSTI compared to those who did not receive guideline-recommended antibiotics. In looking for a link between these associations, we showed that children who remained colonized with S. aureus were more likely to report a recurrent infection than those in whom S. aureus colonization was eradicated. Hence, we have demonstrated that antibiotic administration at the time of acute SSTI affects S. aureus colonization, and that persistent colonization is associated with recurrent infection.
Children who were colonized with S. aureus at baseline screening and prescribed guideline-recommended antibiotics for their SSTI were less likely to remain colonized at their subsequent sampling (median of 38 days later) than children not prescribed antibiotics, suggesting that systemic antibiotics impact skin and nasal S. aureus colonization. Similar to the present study, in 144 children and adults with uncomplicated SSTI in Chicago, the prevalence of S. aureus colonization decreased significantly from baseline to 40-day follow-up after completion of a 10-day course of clindamycin or TMP-SMX [32]. Though not recommended for routine use as a decolonizing agent by the IDSA [17], systemic antibiotic administration for SSTI has benefits beyond resolution of the acute infection.
Topical decolonizing agents were prescribed for many of the participants in this study. To ensure that use of topical antimicrobials was not confounding the relationship between systemic antibiotics and S. aureus decolonization, use of such agents was included in the Cox proportional hazards regression analyses. Systemic antibiotics and topical antimicrobials were each independently associated with reduced S. aureus colonization at follow-up sampling. A randomized controlled trial of 146 MRSA-colonized hospitalized patients in Ontario, comparing a 7-day decolonization treatment consisting of chlorhexidine body washes, intranasal mupirocin, and oral rifampin and doxycycline vs no treatment, found this regimen to be efficacious in eradicating MRSA carriage 3 and 8 months following treatment [26]. This study, however, did not elucidate whether the topical or systemic components of the decolonization regimen were most effective in decolonizing patients of MRSA.
While the impact of systemic antibiotics on clinical cure of SSTI has varied among studies, several studies have demonstrated that patients receiving antibiotics experience significantly fewer recurrent SSTIs [8, 18, 21, 33]. In the present longitudinal study, risk of recurrent infection was decreased by half when antibiotics were prescribed after I&D for SSTI management, consistent with the results of several placebo-controlled trials. Among 1247 patients ≥12 years of age enrolled at 5 US emergency departments, TMP-SMX was superior to placebo in preventing subsequent I&D, skin infections at new sites, and skin infections in household members 14 days posttreatment [18]. In patients recruited from a pediatric emergency department in St Louis, TMP-SMX was superior to placebo in preventing new lesions at 10-day follow-up in 149 children with SSTI and I&D [8]. Additionally, among 190 adult patients with uncomplicated SSTI at 4 military emergency departments, fewer recurrent SSTIs were reported over 1 month following treatment in patients receiving TMP-SMX compared to placebo [21]. Length of therapy also impacts the incidence of recurrent infection. Of 249 children presenting to an emergency department in Buffalo with MRSA SSTI, recurrent infection was significantly higher 1 month following treatment among patients randomized to receive a 3-day course of TMP-SMX compared to those receiving a 10-day course of TMP-SMX [33].
Choice of antibiotic (eg, clindamycin vs TMP-SMX) may also be important in the management of SSTI. In the present study, within the subgroup of subjects receiving guideline-recommended antibiotics, clindamycin was superior to TMP-SMX in lowering the prevalence of persistent S. aureus colonization and in reducing the incidence of recurrent SSTI. There are conflicting reports in the literature regarding the superiority of clindamycin in the treatment of SSTI. Two studies of SSTI in Texas found no differences between clindamycin and TMP-SMX in terms of treatment failure or recurrence of infection [34, 35]. However, a retrospective comparative effectiveness study of nearly 50000 children with SSTI in Tennessee revealed an increased risk of treatment failure and recurrent infection in those prescribed TMP-SMX compared to clindamycin in both patients with and without a drainage procedure [36]. Additionally, 3 randomized clinical trials of patients prescribed clindamycin or TMP-SMX found that clindamycin recipients were less likely to experience recurrent infection than TMP-SMX recipients [22, 37, 38]. Last, a Pennsylvania study of serial MRSA colonization cultures in patients with MRSA SSTI found that those receiving clindamycin for their SSTI showed earlier clearance of colonization [39] and were less likely to have recurrence of MRSA colonization after clearance than those prescribed other antibiotics [40]. While some have proposed a differential impact on the skin microbiome, the mechanism driving these observed effects requires further investigation [34, 39].
The present study has several limitations that should be noted. Participants were drawn from multiple S. aureus research studies, thus introducing some variation in both the interval between baseline and follow-up colonization swabs and longitudinal documentation of recurrent SSTI; however, this limitation was in part mitigated by use of Cox proportional hazards regression models. Additionally, antibiotic prescription and decolonization regimens varied. As most participants were seen as outpatients, antibiotic use was recorded as prescribed; proof of consumption was not feasible. Possibly reflecting current clinical practice and despite IDSA guidelines (based largely on expert opinion) suggesting that I&D alone is likely adequate for management of uncomplicated SSTI [17], the cohort of patients that did not receive antibiotics as part of treatment for their SSTI was small. Finally, all study participants presenting with S. aureus SSTI included in this cohort were also colonized with S. aureus; thus, the results of this study and the longitudinal impact of systemic antibiotics may not be generalizable to patients presenting with S. aureus SSTI who are not also colonized with S. aureus.
In summary, we have demonstrated that systemic antibiotics prescribed at the time of I&D for acute SSTI reduces S. aureus colonization, which is protective against recurrent infection, establishing benefit of systemic antibiotics beyond the resolution of acute infection. Clindamycin was more effective than TMP-SMX at eradicating S. aureus colonization and preventing recurrent SSTI. These data will inform continuing conversations about optimal clinical management of SSTI in the contemporary era.
Notes
Acknowledgments. We thank Carol Peterson and Myto Duong, MD, MB, BCh, BAO, for their work on patient recruitment, participant follow-up visits, and phone calls; the St Louis Children’s Hospital microbiology laboratory for their assistance in collecting specimens; Henry Chambers, MD, principal investigator of National Institutes of Health (NIH) grant number U01-HHSN272200700031C; and Jason Newland, MD, MEd, and David Hunstad, MD, for their thoughtful reviews of this manuscript.
Disclaimer. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the NIH or the Agency for Healthcare Research and Quality (AHRQ).
Financial support. This work was supported by the IDSA/National Foundation for Infectious Diseases Pfizer Fellowship in Clinical Disease; NIH (grant numbers UL1-RR024992, UL1-TR000448, KL2-RR024994, K23-AI091690, and U01-HHSN272200700031C); AHRQ (grant numbers R01-HS021736 and R01-HS024269); Children’s Discovery Institute of Washington University and St Louis Children’s Hospital; Memorial Medical Center Foundation; and Friends of St John’s Hospital.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–91. [DOI] [PubMed] [Google Scholar]
- 2. Witt WP, Weiss AJ, Elixhauser A.. Overview of hospital stays for children in the United States, 2012. Healthcare Cost and Utilization Project (HCUP) statistical brief No. 187. Rockville, MD:Agency for Healthcare Research and Quality, 2014. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.pdf. Accessed 14 April 2017. [PubMed] [Google Scholar]
- 3. Miller LG, Eisenberg DF, Liu H et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis 2015; 15:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaplan SL. Community-acquired methicillin-resistant Staphylococcus aureus infections in children. Semin Pediatr Infect Dis 2006; 17:113–9. [DOI] [PubMed] [Google Scholar]
- 5. Creech CB, Al-Zubeidi DN, Fritz SA. Prevention of recurrent staphylococcal skin infections. Infect Dis Clin North Am 2015; 29:429–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bocchini CE, Mason EO, Hulten KG, Hammerman WA, Kaplan SL. Recurrent community-associated Staphylococcus aureus infections in children presenting to Texas Children’s Hospital in Houston, Texas. Pediatr Infect Dis J 2013; 32:1189–93. [DOI] [PubMed] [Google Scholar]
- 7. King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med 2006; 144:309–17. [DOI] [PubMed] [Google Scholar]
- 8. Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med 2010; 55:401–7. [DOI] [PubMed] [Google Scholar]
- 9. Miller LG, Eells SJ, David MZ et al. Staphylococcus aureus skin infection recurrences among household members: an examination of host, behavioral, and pathogen-level predictors. Clin Infect Dis 2015; 60:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fritz SA, Camins BC, Eisenstein KA et al. Effectiveness of measures to eradicate Staphylococcus aureus carriage in patients with community-associated skin and soft-tissue infections: a randomized trial. Infect Control Hosp Epidemiol 2011; 32:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fritz SA, Hogan PG, Hayek G et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis 2012; 54:743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004; 39:971–9. [DOI] [PubMed] [Google Scholar]
- 13. Fritz SA, Epplin EK, Garbutt J, Storch GA. Skin infection in children colonized with community-associated methicillin-resistant Staphylococcus aureus. J Infect 2009; 59:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toshkova K, Annemüller C, Akineden O, Lämmler C. The significance of nasal carriage of Staphylococcus aureus as risk factor for human skin infections. FEMS Microbiol Lett 2001; 202:17–24. [DOI] [PubMed] [Google Scholar]
- 15. Kaplan SL, Forbes A, Hammerman WA et al. Randomized trial of “bleach baths” plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin Infect Dis 2014; 58:679–82. [DOI] [PubMed] [Google Scholar]
- 16. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med 1985; 14:15–9. [DOI] [PubMed] [Google Scholar]
- 17. Liu C, Bayer A, Cosgrove SE et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52:285–92. [DOI] [PubMed] [Google Scholar]
- 18. Talan DA, Mower WR, Krishnadasan A et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med 2016; 374:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruhe JJ, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis 2007; 44:777–84. [DOI] [PubMed] [Google Scholar]
- 20. Chen AE, Carroll KC, Diener-West M et al. Randomized controlled trial of cephalexin versus clindamycin for uncomplicated pediatric skin infections. Pediatrics 2011; 127:e573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmitz GR, Bruner D, Pitotti R et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med 2010; 56:283–7. [DOI] [PubMed] [Google Scholar]
- 22. Daum RS, Miller LG, Immergluck L et al. DMID 07-0051 Team A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med 2017; 376:2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McConeghy KW, Mikolich DJ, LaPlante KL. Agents for the decolonization of methicillin-resistant Staphylococcus aureus. Pharmacotherapy 2009; 29:263–80. [DOI] [PubMed] [Google Scholar]
- 24. Ammerlaan HS, Kluytmans JA, Berkhout H et al. MRSA Eradication Study Group Eradication of carriage with methicillin-resistant Staphylococcus aureus: effectiveness of a national guideline. J Antimicrob Chemother 2011; 66:2409–17. [DOI] [PubMed] [Google Scholar]
- 25. Tzermpos F, Kanni T, Tzanetakou V et al. An algorithm for the management of Staphylococcus aureus carriage within patients with recurrent staphylococcal skin infections. J Infect Chemother 2013; 19:806–11. [DOI] [PubMed] [Google Scholar]
- 26. Simor AE, Phillips E, McGeer A et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis 2007; 44:178–85. [DOI] [PubMed] [Google Scholar]
- 27. Peterson LR, Quick JN, Jensen B et al. Emergence of ciprofloxacin resistance in nosocomial methicillin-resistant Staphylococcus aureus isolates. Resistance during ciprofloxacin plus rifampin therapy for methicillin-resistant S aureus colonization. Arch Intern Med 1990; 150:2151–5. [PubMed] [Google Scholar]
- 28. Strausbaugh LJ, Jacobson C, Sewell DL, Potter S, Ward TT. Antimicrobial therapy for methicillin-resistant Staphylococcus aureus colonization in residents and staff of a Veterans Affairs nursing home care unit. Infect Control Hosp Epidemiol 1992; 13:151–9. [DOI] [PubMed] [Google Scholar]
- 29. Falagas ME, Bliziotis IA, Fragoulis KN. Oral rifampin for eradication of Staphylococcus aureus carriage from healthy and sick populations: a systematic review of the evidence from comparative trials. Am J Infect Control 2007; 35:106–14. [DOI] [PubMed] [Google Scholar]
- 30. Fritz SA, Tiemann KM, Hogan PG et al. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013; 56:1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. CLSI supplement M100S; 26th ed. Wayne, PA, 2016. [Google Scholar]
- 32. Kumar N, David MZ, Boyle-Vavra S, Sieth J, Daum RS. High Staphylococcus aureus colonization prevalence among patients with skin and soft tissue infections and controls in an urban emergency department. J Clin Microbiol 2015; 53:810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holmes L, Ma C, Qiao H et al. Trimethoprim-sulfamethoxazole therapy reduces failure and recurrence in methicillin-resistant Staphylococcus aureus skin abscesses after surgical drainage. J Pediatr 2016; 169:128–34.e1. [DOI] [PubMed] [Google Scholar]
- 34. Frei CR, Miller ML, Lewis JS 2nd et al. Trimethoprim-sulfamethoxazole or clindamycin for community-associated MRSA (CA-MRSA) skin infections. J Am Board Fam Med 2010; 23:714–9. [DOI] [PubMed] [Google Scholar]
- 35. Hyun DY, Mason EO, Forbes A, Kaplan SL. Trimethoprim-sulfamethoxazole or clindamycin for treatment of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J 2009; 28:57–9. [DOI] [PubMed] [Google Scholar]
- 36. Williams DJ, Cooper WO, Kaltenbach LA et al. Comparative effectiveness of antibiotic treatment strategies for pediatric skin and soft-tissue infections. Pediatrics 2011; 128:e479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller LG, Daum RS, Creech CB et al. DMID 07-0051 Team Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl J Med 2015; 372:1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Talan DA, Lovecchio F, Abrahamian FM et al. A randomized trial of clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated wound infection. Clin Infect Dis 2016; 62:1505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cluzet VC, Gerber JS, Nachamkin I et al. Duration of colonization and determinants of earlier clearance of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2015; 60:1489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cluzet VC, Gerber JS, Nachamkin I et al. Risk factors for recurrent colonization with methicillin-resistant Staphylococcus aureus in community-dwelling adults and children. Infect Control Hosp Epidemiol 2015; 36:786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]