In a retrospective cohort study of 170 heart, liver, and kidney transplant recipients, secondary prophylaxis with valganciclovir following treatment of cytomegalovirus disease was not shown to have any sustained benefit on the likelihood of relapse.
Keywords: cytomegalovirus, transplant, relapse, valganciclovir, secondary prophylaxis
Abstract
Background
Cytomegalovirus (CMV) is a major contributor to morbidity and mortality in solid organ transplant recipients (SOTRs). Ganciclovir and valganciclovir are highly effective antiviral drugs with a well-established role in primary prophylaxis and treatment of CMV disease. Our objective in this study was to examine the effect of secondary prophylaxis (SP) on the risk of relapse in SOTRs following an episode of CMV disease.
Methods
We performed a retrospective cohort study of SOTRs from 1995 to 2015 and used propensity score–based inverse probability of treatment weighting methodology to control for confounding by indication. A weighted Cox model was created to determine the effect of SP on time to relapse within 1 year of treatment completion.
Results
Fifty-two heart, 34 liver, 79 kidney, and 5 liver–kidney transplant recipients who completed treatment for an episode of CMV infection/disease were included. A total of 120 (70.6%) received SP (median duration, 61 days; range, 5–365) and 39 (23%) relapsed. SP was protective against relapse from 0 to 6 weeks following treatment completion (hazard ratio [HR], 0.19; 95% confidence interval [CI], 0.05–0.69). However, after 6 weeks, risk of relapse did not significantly differ between the 2 groups (HR, 1.18; 95% CI, 0.46–2.99).
Conclusions
Our findings demonstrate that use of SP following treatment of CMV disease did not confer long-term protection against relapse, although it did delay relapse while patients were receiving antivirals. This suggests that SP has limited clinical utility in the overall prevention of recurrent CMV disease.
Reactivation of latent cytomegalovirus (CMV) infection is a major cause of morbidity, graft loss, and mortality in solid organ transplant recipients (SOTRs). It can result in a diverse array of clinical manifestations that range from a nonspecific febrile illness to severe end organ disease with syndromes such as colitis, pneumonitis, and hepatitis [1]. Treatment protocols are well established and involve a reduction of immunosuppression, where possible, as well as several weeks of intravenous ganciclovir or oral valganciclovir. CMV immune globulin (CMVIG) is occasionally used as adjunctive therapy. The total duration of antiviral therapy is now typically determined by clinical response and resolution of DNAemia [2–4]. Following treatment, relapse occurs in 20%–30%. Several risk factors for relapse that relate to the extent of initial disease and level of immunosuppression have been identified [4]. These include primary CMV infection, high initial viral load, prolonged DNAemia, persistent DNAemia at treatment completion, multiorgan disease, CMV pneumonitis, treatment for rejection, cadaveric kidney/kidney–pancreas/thoracic organ transplantation, and “extensive disease” in gastrointestinal CMV [2, 5–11].
A key strategy used by many clinicians in the hope of reducing the risk of relapse is secondary prophylaxis (SP) with valganciclovir, where a prophylactic dose is continued following successful treatment [12]. This practice has become widespread with valganciclovir, and is based primarily on extrapolation of prevention of relapse of treated CMV retinitis in patients with AIDS and the success of primary prophylaxis in SOTRs [13–18]. In fact, in the VICTOR trial, all patients received maintenance-dose valganciclovir following treatment [3]. There are no randomized trials that demonstrate efficacy of SP, and most published observational studies show no clear benefit. However, most of these studies had a small sample size, lacked a comparator group, or did not adequately control for potential confounders [6, 9, 19–22]. A recent well-performed retrospective study examined 282 SOTRs with CMV infection (48% with asymptomatic DNAemia), 80% of whom received SP for a median of 50.5 days [22]. Relapse occurred in 32% of patients who received SP vs 23% of those who did not. However, after adjusting for confounders using multivariate logistic regression, there was no significant difference between the groups (P = .15). There was a trend toward a delay in relapse, with a longer time to recurrence from initial viral clearance in those receiving SP (median 69 days vs 44 days; P = .01). However, the authors did not control for all potential confounders such as severity of CMV disease, peak viral load, and use of antilymphocyte therapy. Concern for confounding in these studies is particularly relevant as SP is likely to be targeted to those perceived to be at highest risk of relapse, given its cost and potential for bone marrow toxicity [23, 24].
To determine if there is any sustained long-term benefit of SP, we performed a retrospective cohort study of SOTRs who successfully completed treatment for an episode of CMV disease. We compared the time to relapse between those who received SP and those who did not while controlling for confounding by indication using a propensity score–based inverse probability weighting approach.
METHODS
Study Design and Data Collection
The study population included heart, liver, kidney, heart–kidney, and liver–kidney transplant recipients who developed an episode of CMV infection at Tufts Medical Center between 1995 and 2015. Cases were identified by searching electronic medical records for positive test results for CMV, including viral cultures, histopathology, and viral load testing. Patients were excluded if they did not complete a course of antiviral therapy; died, were lost to follow-up, or experienced graft failure during treatment or within 2 weeks following treatment completion; or did not have sufficient clinical information available in the medical records. The Tufts Medical Center Institutional Review Board approved the study. Informed consent was not required given the minimal risk and retrospective nature of the study.
Clinical data were collected through review of hospital medical records. A panel of 3 transplant infectious disease physicians assessed all cases individually to determine if they met our predetermined inclusion and exclusion criteria and whether they received SP. Potential relapses (up to 1 year from the completion of treatment) were evaluated independently, with reviewers blinded to SP status to determine whether they met the definition for relapse.
Immunosuppression, Rejection, and Primary Cytomegalovirus Prophylaxis Protocols
Standard maintenance immunosuppression across all organ types consisted of a calcineurin inhibitor, an antimetabolite, and prednisone. There was a shift from cyclosporine/azathioprine without induction immunosuppression to tacrolimus/mycophenolate with induction using an antilymphocyte agent, routinely in kidney transplant recipients and for calcineurin inhibitor sparing in heart/liver transplant recipients with renal impairment (glomerular filtration rate <30 mL/min, calculated using the Chronic Kidney Disease Epidemiology Collaboration, equation [25]) from approximately year 2000. Antilymphocyte agents included antithymocyte globulin, OKT3, daclizumab, and basiliximab [26–30].
Primary CMV prophylaxis was routinely used (except in donor and recipient seronegative patients) after it became available around 1995. This consisted of the administration of intravenous (IV) ganciclovir 5 mg/kg once daily, oral ganciclovir 1000 mg 3 times daily, or oral valganciclovir 900 mg once daily, with doses adjusted for renal impairment according to the package insert [23, 24]. Between 1997 and 2002, patients received 3 months of oral ganciclovir; those who were donor seropositive, recipient seronegative (D+/R–) received 7 doses of CMVIG. After 2002, most patients received 3–6 months (longer in high-risk patients) of oral valganciclovir without routine CMVIG (except for D+/R– heart transplant recipients, who continued to receive CMVIG until 2014). There was no routine viral load monitoring after primary prophylaxis, although some patients received this at the discretion of their treating physicians.
All rejection was biopsy proven; if moderate or severe, it was treated with intravenous methylprednisolone 1 g daily for 3–5 days. If there was no initial response, lymphocyte-depleting anti–T-cell therapy (most commonly antithymocyte globulin) was used for cell-mediated rejection or anti–B-cell therapy (rituximab, plasmapheresis, intravenous immunoglobulin ± bortezomib) was used for antibody-mediated rejection. A small number of patients underwent photopheresis for rejection refractory to standard therapy. Patients were defined as having received steroids for rejection if they received IV methylprednisolone or if their oral prednisone dose was increased by ≥3 fold.
Cytomegalovirus Diagnosis and Treatment
CMV infection was defined as a positive test for CMV at any body site. Viral load, viral culture, and histopathologic testing were performed using standard techniques. Over the 20-year study period, the following 3 viral load assays were used: (1) the Hybrid Capture CMV DNA assay (version 2.0), Digene Corp (now Qiagen), Silver Spring, Maryland, a whole blood assay with a detection range of 2.1 to >830 pg/mL (1997–2008); (2) a whole blood assay performed by Quest Diagnostics (Chantilly, Virginia) with a range of detection of 200 to >200000 copies/mL (2008–2011); or (3) a plasma viral load assay (SimplexaTM CMV Assay, Focus Diagnostics, Cypress, California) with a range of detection of 1000 (values below 1000 can be detected but not quantified) to 500000 copies/mL (2011–present). Invasive procedures (eg, gastroscopy, colonoscopy, bronchoscopy) were performed as clinically indicated to obtain tissue samples for additional testing. Tissue or cytology specimens were examined for the presence of characteristic CMV inclusion bodies with hematoxylin and eosin staining. Immunohistochemical stains using CMV-specific mouse monoclonal antibodies (Cell Marque, Sigma-Aldrich, Rocklin, California) were performed on request or clinical suspicion. Blood buffy coat, tissue, urine, and bronchoalveolar lavage samples were cultured using the rapid shell-vial technique and conventional viral culture using human fibroblast cell lines, with CMV detection by direct immunofluorescence using conjugated monoclonal antibodies against CMV immediate early antigen 1 and 2 (Light Diagnostics, EMD Millipore Corporation, Temecula, California).
CMV end organ disease required laboratory confirmation of CMV plus clinical evidence of organ dysfunction, categorized as proven (positive pathology or cultures at a nonblood site with attributable symptoms), probable (DNAemia plus attributable symptoms), or possible (DNAemia with clinical symptoms suggestive of end organ involvement but a potential alternative diagnosis present). Patients with CMV syndrome had detection of CMV in blood plus 2 or more of the following: fever for ≥2 days, new or increased malaise, neutropenia (<1.5 × 103/μL) or thrombocytopenia (<150 × 103/μL), or elevated aspartate aminotransferase (AST)/alanine aminotransferase (ALT) (>2× upper limit of normal, non-liver recipients only).
Standard treatment of CMV infection was with intravenous ganciclovir 5 mg/kg twice daily or oral valganciclovir 900 mg twice daily, with doses adjusted for renal impairment according to the package insert [23, 24]. Duration of therapy was individualized and determined by clinical and virologic endpoints but was generally at least 2 or 3 weeks. If ganciclovir resistance was suspected, alternative antiviral agents were considered and genotypic testing was performed. The treating clinician determined the frequency of viral load monitoring following treatment completion.
Secondary Prophylaxis
SP was defined as the continuation of IV/oral ganciclovir or oral valganciclovir at the prophylactic dose following treatment completion. The decision to use SP and the duration was made by treating clinicians on an individualized basis.
Relapse
The primary outcome was time to relapse of CMV infection, defined as CMV infection or disease that occurred after the completion of successful treatment of an initial episode of CMV disease. Relapse could occur from any time following treatment completion up to 1 year, including during SP. Patients were considered to have relapsed if they (1) became symptomatic with any positive test for CMV, (2) were asymptomatic but had a single positive viral load test above the lower limit of detection of the assay, or (3) were asymptomatic but had 2 or more consecutively positive tests <1000 copies/mL (assay 3). Asymptomatic patients with a single positive but unquantifiable viral load were not counted as relapses. This definition was adapted from previous published studies and modified to suit our patient population and local diagnostic testing [7, 21, 26, 30, 31]. Censoring occurred at the time of relapse, death, graft loss (return to dialysis in kidney transplant recipients or retransplantation), loss to follow-up, or 1 year following treatment completion, whichever came first.
Statistical Analyses
Basic descriptive statistics were calculated. Categorical data were reported as percentages; continuous data were reported as means ± standard deviations if normally distributed and as medians with ranges if nonnormally distributed. Missing data for the variables of interest were negligible.
To adjust for potential nonrandom use of SP in our cohort, we developed a propensity score and applied it using inverse probability of treatment weighting methodology [32]. Balance between the groups was checked by calculating standardized mean differences (SMDs) across all covariates included in the propensity score (see Supplementary Material). After applying the weights, we performed a Cox proportional hazards analysis to estimate the hazard ratio (HR) associated with SP for the primary outcome. While developing the Cox models, a violation of the proportional hazards assumption was identified, indicating that the effect of SP varied over time. Because more than one-third of patients initially assigned to receive SP had stopped it by 6 weeks, we addressed this by calculating separate HRs for 0 to 6 weeks and 6 weeks to 1 year. Choosing these time periods minimized misclassification but also allowed sufficient time for some events to occur. We also performed a sensitivity analysis, treating SP as a time-dependent variable in a Cox model without propensity score weighting. All analyses were performed with the R statistical software platform, version 3.2.4 (RStudio version 0.99.903).
RESULTS
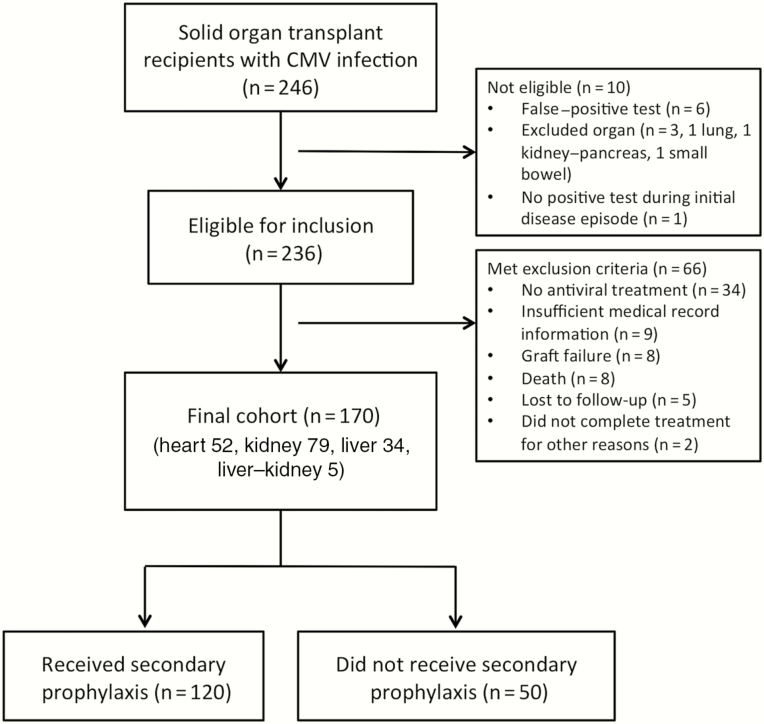
A total of 246 patients were initially identified from the laboratory database search. However, after review, 10 patients were not eligible for the study and 66 met our exclusion criteria, leaving 170 patients in our final cohort (Figure 1). The average age at CMV onset was 52.5 ± 13.6 years; there was a male predominance, and three-quarters were white (Table 1). Median time to CMV onset was 7 months. There were 79 (46.5%) kidney, 52 (30.6%) heart, 34 (20%) liver, and 5 (2.9%) combined liver–kidney transplant recipients. A total of 120 patients (70.6%) had evidence of end organ disease, most commonly in the gastrointestinal tract. As defined above, 31 had a proven disease site, 74 a probable site, and 15 a possible site. The majority of patients commenced or completed therapy with oral valganciclovir, though one-fifth received IV ganciclovir alone. Median treatment duration was 28.5 days. Five patients had clinically suspected ganciclovir resistance, 4 of whom received SP. One had a confirmed UL97 mutation but responded to high-dose ganciclovir, and another was treated empirically with foscarnet.
Figure 1.
Number and flow of study participants. Abbreviation: CMV, cytomegalovirus.
Table 1.
Baseline Characteristics of the Overall Cohort of Solid Organ Transplant Recipients With Cytomegalovirus Disease
| Characteristic | Number (%) n = 170 |
|---|---|
| Age at CMV onset (y), mean ± SD | 52.5 ± 13.6 |
| Male sex | 111 (65.2) |
| White race (vs nonwhite) | 127 (74.7) |
| Year of CMV disease, median, range | 2007, 1995–2016 |
| Previous transplanta | 16 (9.4) |
| Transplanted organ | |
| Heart | 52 (30.6) |
| Liver | 34 (20) |
| Liver–kidney | 5 (2.9) |
| Kidney (living related donor) | 15 (8.8) |
| Kidney (living unrelated donor) | 16 (9.4) |
| Kidney (deceased donor) | 48 (28.2) |
| Receiving hemodialysis during CMV treatment | 5 (2.9) |
| CMV serostatus at time of transplant | |
| Donor positive, recipient negative | 85 (50.0) |
| Donor positive, recipient positive | 53 (31.2) |
| Donor negative, recipient negative | 6 (3.5) |
| Donor negative, recipient positive | 24 (14.1) |
| Donor unknown, recipient positive | 2 (1.2) |
| Induction immunosuppression with antilymphocyte agent | 81 (47.6) |
| Number of immunosuppressive drugs at treatment completion | |
| 1 | 13 (7.6) |
| 2 | 85 (50) |
| 3 | 72 (42.4) |
| Antilymphocyte agent within 1 year prior to treatment completion | 61 (35.9) |
| Steroid-treated rejection within 1 year prior to treatment completion | 38 (22.4) |
| Steroid-treated rejection after CMV (to 1 year or relapse) | 15 (8.8) |
| Any antilymphocyte agent after CMV (to 1 year or relapse) | 1 (0.6) |
| Days from transplant to CMV onset, median, range | 214.5, 25–7918 |
| Days of symptoms prior to proven onset, median, range | 11.5, 0–365 |
| Site of disease | |
| Asymptomatic | 6 (3.5) |
| CMV syndrome | 44 (25.9) |
| Disseminated | 2 (1.2) |
| Gastrointestinal | 111 (65.3) |
| Lung | 7 (4.1) |
| Type of end organ disease episode | |
| None | 50 (29.4) |
| Possible | 15 (8.8) |
| Probable | 74 (43.5) |
| Proven | 31 (18.2) |
| Peak viral load, median, rangeb | |
| Assay 1 (pg/mL, n = 88) | 35, 0–831 |
| Assay 2 (copies/mL, n = 23) | 5101, 0–200 001 |
| Assay 3 (copies/mL, n = 46) | 1343, 0–500 001 |
| Days from diagnosis to start of treatment, median, range | 3, –11 to 72 |
| Admitted for CMV | 134 (78.8) |
| Length of stay (days, n = 134), median, range | 6, 1–46 |
| Seen in infectious disease outpatient clinic | 133 (78.2) |
| Treatment duration (days), median, range | 28.5, 3–201 |
| Treatment type | |
| PO only | 72 (42.4) |
| IV and PO | 64 (37.6) |
| IV only | 34 (20) |
| CMV immune globulin used as part of initial treatment | 17 (10) |
| Laboratory results at treatment completion, mean ± SD | |
| Total white blood cell count (×103/μL, n = 140) | 4.6 ± 2.6 |
| Absolute lymphocyte count (×103/μL, n = 133) | 1.01 ± 0.66 |
| Absolute neutrophil count (×103/μL, n = 133) | 3.01 ± 1.9 |
| Chronic kidney disease epidemiology collaboration estimated glomerular filtration rate (mL/min/1.73m2, n = 140) | 56.9 ± 25.2 |
| Received SP | 120 (70.6) |
| Duration of SP (days), median, range | 60.5, 5-365c |
| Drug received for SP | |
| IV ganciclovir | 2 (1.7) |
| PO ganciclovir | 16 (13.3) |
| Valganciclovir | 102 (85) |
Abbreviations: CMV, cytomegalovirus; IV, intravenous; PO, oral; SD, standard deviation; SP, secondary prophylaxis.
aPatients who received a transplanted organ prior to the index transplant procedure associated with the episode of CMV.
bRefer to descriptions of assays in the Methods section.
cThree patients received secondary prophylaxis for more than 1 year but were truncated at 365 days for the purposes of analysis.
Almost three-quarters of the study cohort received SP; however, there was wide variation in duration, with a mean of 77 ± 70 days (median, 60.5; range, 5–365). One-third of patients had stopped by 6 weeks, half had stopped by 2 months, and three-quarters had stopped by 3 months. Three patients who received SP for more than 1 year were truncated at 365 days for analysis, and none of them relapsed within the year. Patients who received SP were more likely to be white, had their CMV infection more recently, received antilymphocyte therapy within 1 year, commenced antiviral therapy intravenously, and were less likely to have undergone transplantation more than once. These differences are summarized in Table 2. After applying the weights, the SMDs of these variables all decreased, and most other variables had an SMD <0.1.
Table 2.
Comparison of Weighted and Unweighted Patient Groups, Stratified by Use of Secondary Prophylaxis, Showing Variables Included in Propensity Score Model
| Characteristic | Unweighted Sample | Weighted Sample | ||||
|---|---|---|---|---|---|---|
| Did Not Receive SP (n = 50) | Received SP (n = 120) | SMD | Did Not Receive SP (n = 44.37) | Received SP (n = 117.44) | SMD | |
| Age at CMV onset, y, mean ± SD | 52 ± 12 | 53 ± 14 | 0.009 | 53 ± 15 | 53 ± 14 | 0.02 |
| Male sex, no. (%) | 33 (66.0) | 78 (65.0) | 0.021 | 29.3 (66.0) | 77.2 (65.7) | 0.005 |
| White race (vs nonwhite), no. (%) | 34 (68.0) | 93 (77.5) | 0.215 | 29.2 (65.9) | 84.6 (72.1) | 0.133 |
| Previous transplant, no. (%) | 7 (14.0) | 9 (7.5) | 0.211 | 6.0 (13.4) | 12.2 (10.4) | 0.095 |
| Year of CMV disease, mean ± SD | 2005.64 ± 6.53 | 2007.37 ± 4.69 | 0.304 | 2007.78 ± 5.70 | 2007.77 ± 5.06 | 0.001 |
| Transplanted organ, no. (%) | ||||||
| Heart | 17 (34.0) | 35 (29.2) | 0.115 | 13.8 (31.1) | 35.3 (30.1) | 0.175 |
| Liver/liver–kidney | 18 (36.0) | 45 (37.5) | 19.9 (44.8) | 45.2 (38.5) | ||
| Kidney (living unrelated) | 4 (8.0) | 12 (10.0) | 3.4 (7.7) | 11.0 (9.4) | ||
| Kidney (deceased/LR) | 11 (22.0) | 28 (23.3) | 7.3 (16.4) | 26.0 (22.1) | ||
| Receiving hemodialysis during CMV treatment, no. (%) | 2 (4.0) | 3 (2.5) | 0.085 | 1.0 (2.3) | 2.8 (2.4) | 0.009 |
| Recipient CMV seronegative, no. (%) | 27 (54.0) | 64 (53.3) | 0.013 | 20.5 (46.3) | 58.5 (49.8) | 0.071 |
| Antilymphocyte therapy within 1 year of treatment completion, no. (%) | 13 (26.0) | 48 (40.0) | 0.301 | 18.3 (41.2) | 45.5 (38.7) | 0.05 |
| Number of immunosuppressive drugs at treatment completion, mean ± SD | 2.34 (0.66) | 2.35 (0.60) | 0.016 | 2.34 (0.68) | 2.32 (0.60) | 0.04 |
| Steroid-treated rejection within 1 year of treatment completion, no. (%) | 14 (28.0) | 24 (20.0) | 0.188 | 11.0 (24.7) | 24.6 (20.9) | 0.09 |
| Days from transplant to CMV onset, mean ± SD | 777 ± 1462 | 582 ± 1050 | 0.153 | 572 ± 1071 | 599 ± 1056 | 0.025 |
| End organ disease, no. (%) | 34 (68.0) | 86 (71.7) | 0.08 | 30.8 (69.4) | 80.8 (68.8) | 0.012 |
| Peak viral load, mean ± SD | ||||||
| Assay 1, number | 23 (46.0) | 65 (54.2) | 0.164 | 18.4 (41.5) | 59.1 (50.3) | 0.178 |
| Assay 1, value (pg/mL) | 73 ± 181 | 81 ± 189 | 0.047 | 105 ± 235 | 82 ± 195 | 0.106 |
| Assay 2, number | 3 (6.0) | 20 (16.7) | 0.341 | 5.1 (11.4) | 16.2 (13.8) | 0.07 |
| Assay 2, value (copies/mL) | 3069 ± 19852 | 6621 ± 32999 | 0.13 | 2676 ± 15779 | 5631 ± 29796 | 0.124 |
| Assay 3, number | 15 (30.0) | 31 (25.8) | 0.093 | 17.6 (39.7) | 37.5 (31.9) | 0.164 |
| Assay 3, value (copies/mL) | 22544 ± 96136 | 31604 ± 105979 | 0.09 | 21363 ± 78095 | 26970 ± 98092 | 0.063 |
| Treatment duration, days, mean ± SD | 43 ± 36 | 37 ± 28 | 0.179 | 42 ± 30 | 39 ± 29 | 0.086 |
| Treatment type | ||||||
| PO only | 25 (50.0) | 47 (39.2) | 0.353 | 20.8 (47.0) | 53.9 (45.9) | 0.025 |
| IV and PO | 13 (26.0) | 51 (42.5) | 16.6 (37.4) | 44.3 (37.7) | ||
| IV only | 12 (24.0) | 22 (18.3) | 6.9 (15.6) | 19.2 (16.4) | ||
| CMV immune globulin used as part of initial treatment, no. (%) | 6 (12.0) | 11 (9.2) | 0.092 | 2.4 (5.5) | 8.7 (7.4) | 0.078 |
| Chronic kidney disease epidemiology collaboration estimated glomerular filtration rate, mL/min/1.73m2, mean ± SD | 57.1 ± 28.2 | 56.8 ± 24.5 | 0.01 | 58.6 ± 28.1 | 56.6 ± 25.9 | 0.074 |
Abbreviations: CMV, cytomegalovirus; IV, intravenous; LR, living related; PO, oral; SD, standard deviation; SMD, standardized mean difference.
Thirty-nine patients (23%) relapsed within the 1-year follow-up period, with 26/120 (21.7%) patients who received SP experiencing relapse compared to 13/50 (26%) of those who did not receive SP. Four patients relapsed while taking SP; none developed confirmed ganciclovir resistance. Seven patients had asymptomatic DNAemia, 17 had CMV syndrome, and 15 had evidence of end organ disease. Twenty-one patients (12.4%) were censored before 1 year, with 12 deaths, 1 graft failure, 3 lost to follow-up, 4 followed for less than 1 year at the time of analysis, and 1 who restarted SP after initially stopping it. Median time to relapse was 79.5 days (range, 28–314) in those who received SP and 35 days (14–357) in those who did not receive SP.
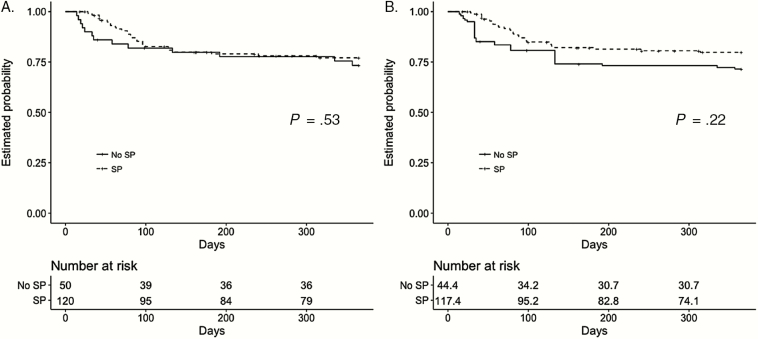
Figure 2 shows Kaplan-Meier plots of the probability of relapse-free survival in patients who did and did not receive SP, unweighted and after adjustment by weighting. In both groups, most relapses occurred within 100 days. While there appears to be a trend toward decreased probability of relapse among those patients who received SP, these differences were not statistically significant (P = .22 in weighted sample).
Figure 2.
Kaplan-Meier plots showing the estimated probability of relapse-free survival in patients who did and did not receive secondary prophylaxis before (A) and after (B) adjustment for confounders by propensity score–based inverse probability weighting. P values refer to log-rank test results. Abbreviation: SP, secondary prophylaxis.
Results of the weighted and unweighted Cox models are shown in Table 3. Applying the weights had only a modest effect on the HRs for SP, decreasing them slightly. The weighted HR for the effect of SP on relapse was 0.19 (95% confidence interval [CI], 0.05–0.69) for 0 to 6 weeks and 1.18 (95% CI, 0.0.46–2.99) for 6 weeks to 1 year after treatment completion. This indicates a statistically significant protective effect of SP during the early period (P = .01) but no significant difference in likelihood of relapse between those who received and those who did not receive SP during the later period.
Table 3.
Unweighted and Weighted Hazard Ratios Showing the Effect of Secondary Prophylaxis in the Early and Late Time Periods
| Time period | Unweighted | Weighted | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Early (0–6 weeks) | 0.22 (0.06–0.76) | .02 | 0.19 (0.05–0.69) | .01 |
| Late (6–52 weeks) | 1.51 (0.61–3.72) | .37 | 1.18 (0.46–2.99) | .73 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Our sensitivity analysis of SP as a time-dependent variable without propensity score weighting showed a strong protective effect while patients remained on therapy (HR, 0.17; 95% CI, 0.06–0.51; P < .01).
DISCUSSION
Our findings suggest that the use of SP with valganciclovir following the successful treatment of an episode of CMV disease in SOTRs is associated with reduced risk of early relapse but that this benefit is not sustained long term. Given the risk of long-term toxicities and costs of valganciclovir, our work represents an important contribution to the understanding of the potential benefits of SP. Patients who received SP appeared to have a prolonged time to relapse. However, even after we adjusted for confounding by indication using our statistical approach, we could not detect a significant difference in the overall likelihood of relapse occurring within 1 year. Given that the median duration of SP was only 2 months, this suggests that relapse is unlikely while patients are actively taking SP, but there is limited residual protective effect after cessation. These results are consistent with prior studies of SP, which have generally shown no overall long-term benefit [6, 9, 19, 21, 22].
A major strength of this study was the use of propensity score methodology to control for confounding by indication, which encompassed the measurable clinical factors associated with both use of SP and relapse, which were included in our propensity score model [33]. Failure to control for these confounders has been a limitation of previous studies. In the absence of data from randomized clinical trials, which are unlikely to be forthcoming due to feasibility constraints, applying propensity scores through inverse probability weighting enables the balancing of measured covariates between treatment groups, allowing for a more accurate estimation of true treatment effect, while preserving the original sample size. This methodology is being used in diverse areas of medicine to more closely emulate a randomized clinical trial when only retrospective observational data are available. It offers an advantage over conventional regression models when the number of confounding variables is large relative to the number of outcomes, as they were in this study [34–36].
Our study has some limitations that should be considered when interpreting our findings. While this was a large study, considering the rarity of the disease, statistical power was limited by the sample size of 170 patients, only 50 of whom did not receive SP. Given the retrospective nature of our study, it is possible that there may have been unmeasured confounders that could have influenced treatment allocation that were not accounted for in our propensity score model. There were changes in CMV diagnostics, treatment, and immunosuppression protocols over the 20-year study period; changes in viral load testing were particularly challenging to incorporate in a standardized fashion into our models. Patients did not have routine virologic surveillance following primary prophylaxis or treatment completion, so it is possible some cases of asymptomatic viremia were missed. Finally, while we accounted for the time-varying nature of SP, there was wide variation in duration of SP that individual patients received, complicating our analysis.
In conclusion, the results of our study suggest that SP can be used to delay early relapse following CMV infection. However, the effect only persists while patients are maintained on antiviral therapy, which corresponded to a time-limited benefit of approximately 6 weeks in our cohort. While these findings do not support the routine use of short-term SP for all patients, some high-risk patients may benefit from a prolonged course of SP. Future studies to identify predictors of relapse are needed in order to individualize therapy and target those most likely to benefit.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We acknowledge the assistance of Emily Miller, Nitin Jethmalani, and Kevin Lindell for help with data collection; Alejandro Moreno-Koehler, Robin Ruthazer, and Angie Rodday for statistical and data management support; and Robert Goldberg for assistance with manuscript editing.
Financial support. This work was supported by the Tufts Medical Center Division of Infectious Disease and Geographic Medicine Francis P. Tally MD Fellowship, the National Institutes of Health Clinical and Translational Science Award (grant number UL1TR001064), and the Royal Australasian College of Physicians Richard Kemp Memorial Fellowship.
Potential conflicts of interest. D. R. S. is on advisory boards for Merck, Shire, Summit, Chimerix, and Seres Therapeutics and has received grants from Merck, Summit, Actelion, Tetraphase, and Seres Therapeutics. All remaining authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Razonable RR, Humar A; AST Infectious Diseases Community of Practice Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13 Suppl 4:93–106. [DOI] [PubMed] [Google Scholar]
- 2. Asberg A, Humar A, Jardine AG et al. ; VICTOR Study Group Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant 2009; 9:1205–13. [DOI] [PubMed] [Google Scholar]
- 3. Asberg A, Humar A, Rollag H et al. ; VICTOR Study Group Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2007; 7:2106–13. [DOI] [PubMed] [Google Scholar]
- 4. Kotton CN, Kumar D, Caliendo AM et al. ; Transplantation Society International CMV Consensus Group Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013; 96:333–60. [DOI] [PubMed] [Google Scholar]
- 5. Asberg A, Jardine AG, Bignamini AA et al. ; VICTOR Study Group Effects of the intensity of immunosuppressive therapy on outcome of treatment for CMV disease in organ transplant recipients. Am J Transplant 2010; 10:1881–8. [DOI] [PubMed] [Google Scholar]
- 6. Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Clinical predictors of relapse after treatment of primary gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2010; 10:157–61. [DOI] [PubMed] [Google Scholar]
- 7. Falagas ME, Snydman DR, Griffith J, Werner BG, Freeman R, Rohrer R. Clinical and epidemiological predictors of recurrent cytomegalovirus disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG Study Group. Clin Infect Dis 1997; 25:314–7. [DOI] [PubMed] [Google Scholar]
- 8. Helanterä I, Lautenschlager I, Koskinen P. The risk of cytomegalovirus recurrence after kidney transplantation. Transpl Int 2011; 24:1170–8. [DOI] [PubMed] [Google Scholar]
- 9. Helanterä I, Schachtner T, Hinrichs C et al. Current characteristics and outcome of cytomegalovirus infections after kidney transplantation. Transpl Infect Dis 2014; 16:568–77. [DOI] [PubMed] [Google Scholar]
- 10. Humar A, Uknis M, Carlone-Jambor C, Gruessner RW, Dunn DL, Matas A. Cytomegalovirus disease recurrence after ganciclovir treatment in kidney and kidney-pancreas transplant recipients. Transplantation 1999; 67:94–7. [DOI] [PubMed] [Google Scholar]
- 11. Nafar M, Roshan A, Pour-Reza-Gholi F et al. Prevalence and risk factors of recurrent cytomegalovirus infection in kidney transplant recipients. Iran J Kidney Dis 2014; 8:231–5. [PubMed] [Google Scholar]
- 12. Le Page AK, Jager MM, Kotton CN, Simoons-Smit A, Rawlinson WD. International survey of cytomegalovirus management in solid organ transplantation after the publication of consensus guidelines. Transplantation 2013; 95:1455–60. [DOI] [PubMed] [Google Scholar]
- 13. Humar A, Limaye AP, Blumberg EA et al. Extended valganciclovir prophylaxis in D+/R– kidney transplant recipients is associated with long-term reduction in cytomegalovirus disease: two-year results of the IMPACT study. Transplantation 2010; 90:1427–31. [DOI] [PubMed] [Google Scholar]
- 14. Humar A, Lebranchu Y, Vincenti F et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant 2010; 10:1228–37. [DOI] [PubMed] [Google Scholar]
- 15. Montejo M, Montejo E, Gastaca M et al. Prophylactic therapy with valganciclovir in high-risk (cytomegalovirus D+/R–) liver transplant recipients: a single-center experience. Transplantat Proc 2009; 41:2189–91. [DOI] [PubMed] [Google Scholar]
- 16. Luan FL, Stuckey LJ, Park JM, Kaul D, Cibrik D, Ojo A. Six-month prophylaxis is cost effective in transplant patients at high risk for cytomegalovirus infection. J Am Soc Nephrol 2009; 20:2449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodson EM, Jones CA, Webster AC et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet 2005; 365:2105–15. [DOI] [PubMed] [Google Scholar]
- 18. Small LN, Lau J, Snydman DR. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: a meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis 2006; 43:869–80. [DOI] [PubMed] [Google Scholar]
- 19. Turgeon N, Fishman JA, Doran M et al. Prevention of recurrent cytomegalovirus disease in renal and liver transplant recipients: effect of oral ganciclovir. Transpl Infect Dis 2000; 2:2–10. [DOI] [PubMed] [Google Scholar]
- 20. Nankivell BJ, Malouf MA, Russ GR et al. Maintenance therapy with oral ganciclovir after treatment of cytomegalovirus infection. Clin Transplant 1998; 12:270–3. [PubMed] [Google Scholar]
- 21. Sullivan T, Brodginski A, Patel G, Huprikar S. The role of secondary cytomegalovirus prophylaxis for kidney and liver transplant recipients. Transplantation 2015; 99:855–9. [DOI] [PubMed] [Google Scholar]
- 22. Natori Y, Humar A, Husain S et al. Recurrence of CMV infection and the effect of prolonged antivirals in organ transplant recipients. Transplantation 2017; 101:1449–54. [DOI] [PubMed] [Google Scholar]
- 23. Genentech. Valcyte (valganciclovir), package insert. 2015. [Google Scholar]
- 24. Genentech. Cytovene (ganciclovir), package insert. 2016. [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH et al. ; Chronic Kidney Disease Epidemiology Collaboration A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34:1094–7. [DOI] [PubMed] [Google Scholar]
- 27. Munoz-Price LS, Slifkin M, Ruthazer R et al. The clinical impact of ganciclovir prophylaxis on the occurrence of bacteremia in orthotopic liver transplant recipients. Clin Infect Dis 2004; 39:1293–9. [DOI] [PubMed] [Google Scholar]
- 28. Nierenberg NE, Poutsiaka DD, Chow JK et al. Pretransplant lymphopenia is a novel prognostic factor in cytomegalovirus and noncytomegalovirus invasive infections after liver transplantation. Liver Transpl 2014; 20:1497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snydman DR, Werner BG, Dougherty NN et al. ; Boston Center for Liver Transplantation CMVIG Study Group Cytomegalovirus immune globulin prophylaxis in liver transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1993; 119:984–91. [DOI] [PubMed] [Google Scholar]
- 30. Ljungman P, Boeckh M, Hirsch HH et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 31. Falagas ME, Snydman DR. Recurrent cytomegalovirus disease in solid-organ transplant recipients. Transplantat Proc 1995; 27(5 Suppl 1):34–7. [PubMed] [Google Scholar]
- 32. Kent DM, Dahabreh IJ, Ruthazer R et al. Anticoagulant vs. antiplatelet therapy in patients with cryptogenic stroke and patent foramen ovale: an individual participant data meta-analysis. Eur Heart J 2015; 36:2381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA 2016; 316:1818–9. [DOI] [PubMed] [Google Scholar]
- 34. Freemantle N, Marston L, Walters K, Wood J, Reynolds MR, Petersen I. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ 2013; 347:f6409. [DOI] [PubMed] [Google Scholar]
- 35. Haukoos JS, Lewis RJ. The propensity score. JAMA 2015; 314:1637–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dahabreh IJ, Kent DM. Can the learning health care system be educated with observational data? JAMA 2014; 312:129–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.