Summary
Klebsiella pneumoniae colonization is a significant risk factor for infection in ICU, with approximately half of K. pneumoniae infections resulting from patients’ own microbiota. Screening for colonization on admission could limit risk of infection in the colonized patient and others.
Keywords: Klebsiella pneumoniae, gastrointestinal colonization, genomic epidemiology, intensive care, hospital acquired infection.
Abstract
Background.
Klebsiella pneumoniae is an opportunistic pathogen and leading cause of hospital-associated infections. Intensive care unit (ICU) patients are particularly at risk. Klebsiella pneumoniae is part of the healthy human microbiome, providing a potential reservoir for infection. However, the frequency of gut colonization and its contribution to infections are not well characterized.
Methods.
We conducted a 1-year prospective cohort study in which 498 ICU patients were screened for rectal and throat carriage of K. pneumoniae shortly after admission. Klebsiella pneumoniae isolated from screening swabs and clinical diagnostic samples were characterized using whole genome sequencing and combined with epidemiological data to identify likely transmission events.
Results.
Klebsiella pneumoniae carriage frequencies were estimated at 6% (95% confidence interval [CI], 3%–8%) among ICU patients admitted direct from the community, and 19% (95% CI, 14%–51%) among those with recent healthcare contact. Gut colonization on admission was significantly associated with subsequent infection (infection risk 16% vs 3%, odds ratio [OR] = 6.9, P < .001), and genome data indicated matching carriage and infection isolates in 80% of isolate pairs. Five likely transmission chains were identified, responsible for 12% of K. pneumoniae infections in ICU. In sum, 49% of K. pneumoniae infections were caused by the patients’ own unique strain, and 48% of screened patients with infections were positive for prior colonization.
Conclusions.
These data confirm K. pneumoniae colonization is a significant risk factor for infection in ICU, and indicate ~50% of K. pneumoniae infections result from patients’ own microbiota. Screening for colonization on admission could limit risk of infection in the colonized patient and others.
The emergence of multidrug-resistant (MDR) Klebsiella pneumoniae has resulted in a dramatic increase in research into reservoirs and risk factors for healthcare-associated (HA) K. pneumoniae infections, largely focused on extended spectrum beta-lactamase (ESBL) or carbapenemase-producing (CP) bacteria isolated from infections and intrahospital outbreaks [1–3]. These studies have demonstrated transmission of ESBL or CP K. pneumoniae between patients and show that gastrointestinal (GI) tract colonization with ESBL or CP K. pneumoniae can be a risk factor for infection [3, 4]. Yet although the majority of K. pneumoniae HA infections are not ESBL or CP [5, 6], there are little data on the frequency and clinical relevance of colonization with K. pneumoniae more generally.
Klebsiella pneumoniae is known to asymptomatically colonize the skin, mouth, respiratory and GI tracts, although few studies address this specifically. Using culture-free methods, K. pneumoniae was detected in approximately 10% of Human Microbiome Project samples from the mouth, nares, and skin, and 3.8% of stool samples [7]. Using bacteriological culture, a 2010 study detected nasopharyngeal carriage in 15% of Indonesian adults and 7% of children [8], whereas a 2014 study detected nasopharyngeal carriage in 2.7% of Vietnamese adults and throat carriage in 14% [9].
Patients suffering from CP K. pneumoniae infections (typically ST258), or from pyogenic liver abscess caused by hypervirulent K. pneumoniae (ST23), have been shown to carry their infecting strain in their GI tract for between 30 days (≤74%) and 6 months (<30%) following discharge from hospital [10]. However, the question of whether K. pneumoniae colonization on admission to hospital poses a risk for subsequent infection is much less clear. A 1971 study found 18.5% of patients admitted to various wards in the Denver Veterans Administration Hospital were culture-positive for rectal carriage of K. pneumoniae, and carriage was significantly associated with risk of subsequent HA infection (45% vs 11%) [11]. A 2016 study at the University of Michigan Health System tertiary care hospital reported similar colonization rates (23%) and increased risk of infection following colonization (5.2% in colonized vs 1.3% in noncolonized) [12].
Here, we assessed the prevalence of K. pneumoniae colonization in an at-risk cohort in an intensive care unit (ICU) within a modern, well-equipped, and well-managed tertiary teaching hospital in Australia. Additionally, we investigated whether colonization on admission enhances risk of subsequent K. pneumoniae infection among ICU patients and the relative contribution of patients’ own gut microbiota and intra-hospital transmission to the burden of K. pneumoniae carriage and infection in the ICU.
METHODS
Ethics
Ethical approvals for these studies were granted by the Alfred Hospital Ethics Committee (project numbers 550/12 and 526/13).
Recruitment and Specimen and Data Collection
The Klebsiella Acquisition Surveillance Project at Alfred Health (KASPAH) was conducted from April 1, 2013, to March 31, 2014. Eligible patients (adults aged ≥18 years and expected to spend ≥3 days in ICU) were recruited as soon as possible after admission, and baseline rectal and throat screening swabs were collected. For the first 9 months verbal consent was required to participate. For the last 3 months a universal surveillance study for multidrug resistant organisms was conducted for which consent was waived; samples and data from this period were also included in KASPAH (see Supplementary Methods and Supplementary Figure 1). Follow-up swabs were repeated each 5–7 days after baseline for the duration of ICU stay and up to 4 days following transfer to another ward. Information on age, sex, dates of hospital and ICU admission/s, surgery in the last 30 days, and antibiotic treatment in the last 7 days were extracted from hospital records at the time each swab was taken. Dates of discharge and/or death were extracted from hospital records at the conclusion of the study. All clinical isolates recovered from ICU patients and identified as K. pneumoniae infections by the hospital diagnostic laboratory as part of routine care were included in the study. Full details are given in Supplementary Methods.
Community Associated vs Healthcare Associated Carriage
Individuals in the community-associated (CA) screening group include patients who were both (i) admitted to the Alfred Hospital ICU either directly (day 0) or via another ward on day 0, 1, or 2 of the original hospital admission; and (ii) first swabbed on day 0, 1, or 2 of that admission. Patients first swabbed on day 3 or later of their hospital admission are included in the HA/Day 3+ screening group. Individuals referred to the Alfred Hospital ICU by the trauma ward of another hospital were assumed to be emergency admissions from the community and were assigned to the CA/Day 0–2 or HA/Day 3+ screening groups according to the day of first swab relative to their Alfred Hospital admission. All other patients transferred from another hospital were included in the HA/D3+ screening group. As such, the CA/Day 0–2 screening groups represent individuals admitted directly to the hospital from the community, whereas the HA/D3+ group includes individuals with recent hospital exposure.
DNA Extraction and Sequencing
DNA was extracted from overnight cultures using a phenol:chloroform protocol and phase lock gel tubes and sequenced via Illumina HiSeq to generate 125 bp paired-end reads (see Supplementary Methods). Following quality control checks of the sequence data, 148 isolates from 106 patients were subjected to comparative genomic analysis (see Supplementary Table 1 for genome sequence accessions). A maximum likelihood phylogenetic tree was inferred from an alignment of all single-nucleotide polymorphisms (SNPs) identified within core K. pneumoniae genes using FastTree v2.1.8 [13, 14]. Lineages were defined based on this tree using RAMI [15] and multilocus sequence types were assigned using SRST2 [16]. Isolates falling within the same lineage were further investigated to identify pairwise SNPs via assembly and read mapping; full details of genomic analyses are given in Supplementary Methods.
Statistical Analysis
All statistical analyses were conducted using R (v3.3.1) (details in Supplementary Methods).
RESULTS
Klebsiella pneumoniae Carriage
A total of 498 patients expected to spend ≥3 days in ICU were recruited and screened for K. pneumoniae carriage. This represents 33% of eligible patients during the consent-based recruitment period and 75% in the universal surveillance period (18% of all ICU admissions in the study period). Fifty-four patients (10.8%) tested positive at baseline screening (50 GI carriage only, 2 throat carriage only, 2 both). Carriage was detected at the same frequency in males and females (11.0% vs 10.4%; P = .9), and the median age of carriers was moderately higher than that of carriage-negative participants (67 vs 58 years, Supplementary Figure 2; P = .06, Wilcoxon rank-sum test).
We estimated the rate of CA K. pneumoniae GI carriage, among patients recruited and swabbed in the ICU within 2 days of their first recorded admission to the Hospital (CA/D0-2 group), to be 5.9% (95% confidence interval [CI], 3% – 8%, Table 1, Supplementary Table 2). The HA GI carriage rate, among patients who were first swabbed in the ICU on or after the third day of admission to the Alfred hospital or following referral from another hospital (HA/D3+ group, Supplementary Figure 3), was significantly higher at 19% (95% CI, 13.6% – 25.7%, odds ratio [OR] = 3.75, P = .00001).
Table 1.
Klebsiella pneumoniae GI Carriage Detected at Baseline and Followup Screening of Alfred Hospital Patients
| Baseline Carriage Status | Number of Patients (%) | MDR Isolates (%) | Follow-up +ve (%) | Follow-up MDR (%) |
|---|---|---|---|---|
| CA/Day 0–2 | 324 | |||
| Baseline Kp +ve | 19 (5.9%) | 0 (0%) | 2/4 (50%) | 0 (0%) |
| Baseline Kp −ve | 305 (94.1%) | … | 14*/96 (14.6%) | 0 (0%) |
| HA/Day 3+ | 174 | |||
| Baseline Kp +ve | 33 (19%) | 6 (17.6%) | 5/13* (38.5%) | 2 (40%) |
| Baseline Kp −ve | 141 (81%) | … | 5/57 (8.8%) | 2 (40%) |
| Total baseline Kp +ve | 52 (10.4%) | 6 (11.3%) | 7/17 (41.2%) | 2 (28.6%) |
| Total baseline Kp −ve | 446 (89.6%) | … | 19/153 (12.4%) | 2 (10.5%) |
| Total | 498 | 6 (1.2%) | 26/170 (15.3%) | 4 (15.4%) |
Patient groups: ICU CA/Day 0–2, rectal screening swab obtained on day 0, 1, or 2 of admission to Alfred Hospital and not referred from another hospital (except from trauma unit); ICU HA/Day 3+, rectal screening swab obtained on day 3 or later of admission to Alfred Hospital or referred from another hospital. *These indicate 2 patients from whom swabs yielded isolates that were identified in the hospital laboratory as K. pneumoniae, but sequencing of subcultures identified substantial E. coli, indicating likely presence of both species.
Abbreviations: GI, gastrointestinal; ICU, intensive care unit; MDR, multidrug-resistant.
One third of the participants in the ICU screening study (n = 170) contributed 1 or more follow-up screening swabs (Table 1). The overall GI carriage rate at follow-up was 15.3% (n = 26/170), similar to the HA GI carriage rate of 19% (OR = 1.3; 95% CI, 0.71–2.38). Participants testing positive on follow-up rectal swabs included 19 who tested negative for K. pneumoniae on their baseline rectal swab, yielding a conversion rate of 12%.
None of the 19 CA baseline carriage isolates were MDR (Table 1). GI carriage of MDR strains was detected at similar rates among HA baseline isolates (18%, including 4 ESBL and 2 CP isolates, all in patients who had received antibiotics in the last 7 days) and follow-up screening isolates (16% of patients, including 4 with ESBL and 1 with CP isolates). A total of seven patients contributed both baseline and follow-up GI carriage isolates (Table 1). For 5/7 patients the resistance profiles for the follow-up isolate remained the same as the baseline isolate, and for 2/7 patients the follow-up isolate was more resistant (see Supplementary Tables 1 and 3).
GI Colonization Is a Source of Klebsiella Infection
A total of 49 patients (1.8% of all adult ICU admissions) who spent time in the ICU during their hospital stay were identified as having K. pneumoniae infections (11 ESBL, of which 3 were also CP). Most (n = 38) were in the ICU when the K. pneumoniae–positive diagnostic specimen was taken, and 10 were in another ward shortly after transfer from ICU. Pneumonia was the most frequent form of K. pneumoniae infection in ICU patients (60%), followed by wound infections (15%), nondisseminated urinary tract infection (UTI) (10%), and bacteremia with sepsis (8%). MDR was most common among wound and blood isolates (≥50%) (Table 2).
Table 2.
Patients With Infection(s) and Time in the ICU
| Pneumonia | UTI (Non-invasive) | Wound | Other | Bacteremia With Sepsis | Total | |
|---|---|---|---|---|---|---|
| Recipient in transmission chain | 2 | 1 | 2 | 0 | 1 | 6 |
| Donor in transmission chain | 3 | 0 | 1 | 0 | 0 | 4 |
| Prior GI colonization | 6 | 1 | 1 | 0 | 0 | 8 |
| Prior throat colonization | 1 | 0 | 0 | 0 | 0 | 1 |
| Unknown source (unique lineage) | 16 (12) | 3 (3) | 4 (1) | 3 (2) | 3 (1) | 29 |
| Total |
28
(5 MDR) |
5 |
8
(4 MDR) |
3 |
4
(2 MDR) |
48 |
Type of infection and source of infection outlined (position/presence in transmission chain, prior colonization, unknown). Note that 3 patients had UTI and bacteremia with sepsis; they are represented here in the bacteremia with sepsis column. “Unknown source” includes those infections for which there is no genetic or epidemiological evidence to indicate whether the infection has arisen from a patient’s own carriage strains or through transmission from another source; numbers in brackets indicate the number of such infections associated with a lineage that was unique to that patient. Note that “unknown source” also includes one patient who had a wound infection diagnosed as K. pneumoniae but genome sequencing found the subcultured isolate to be dominated by A. baumannii DNA; this is consistent with mixed infection or contamination, and prevents reliable comparative analysis with other K. pneumoniae strains.
Abbreviations: GI, gastrointestinal; ICU, intensive care unit; MDR, multidrug-resistant; UTI, urinary tract infection.
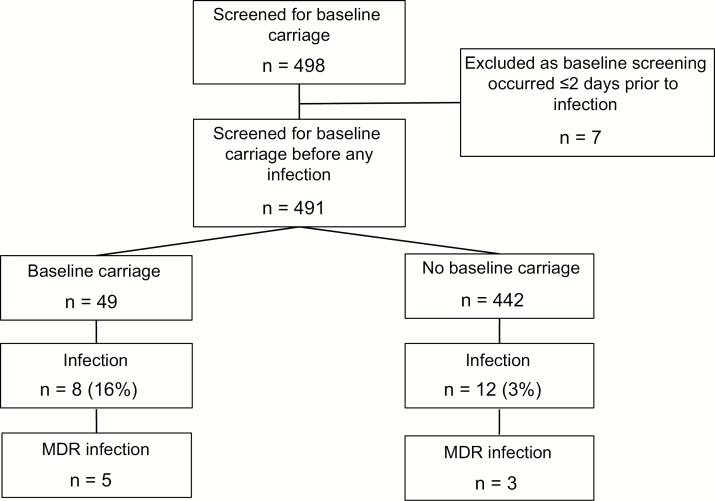
To assess whether K. pneumoniae GI carriage on admission to ICU was a risk factor for subsequent K. pneumoniae infection during hospital stay, we examined the subset of 491 individuals whose baseline screening swab was obtained at least 2 days prior to collection of any clinical specimen from which K. pneumoniae was isolated (Figure 1). The rate of K. pneumoniae infection was significantly higher among patients who were culture-positive for GI carriage at baseline compared to those who were culture-negative (16% vs 3%; OR = 6.9, 95% CI, 2.3 – 19.7, P < .001, see Figure 1). Of all ICU patients who developed K. pneumoniae infections and contributed baseline screening swabs, 48% (n = 13/27) tested positive for K. pneumoniae GI carriage at baseline (including 8 who were screened >2 days prior to developing the infection).
Figure 1.
Flowchart outlining number of patients included in each part of carriage rates analyses.
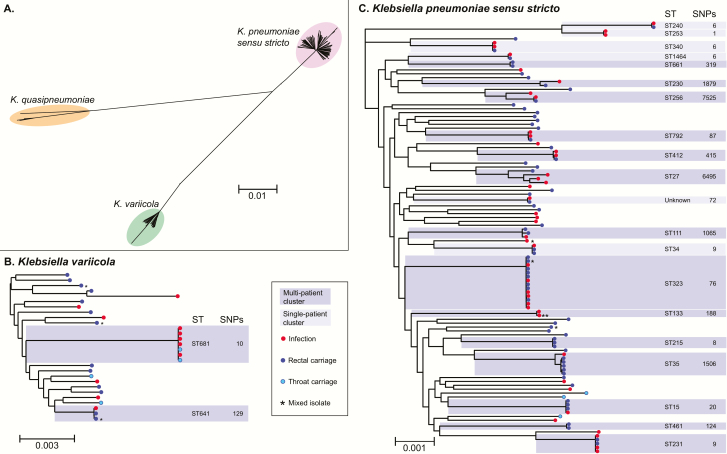
To determine whether infections were caused by patients’ own colonizing bacteria and to identify transmission between ICU patients, we sequenced the genomes of all K. pneumoniae isolated from patients who had spent any time in the ICU during their hospital stay(s). A total of 143 high-quality whole genome sequences were obtained from 106 patients, including 56 clinical, 80 GI carriage, and 7 throat carriage isolates. Core genome phylogenetic analysis (Figure 2) revealed the presence of 61 lineages of K. pneumoniae sensu stricto (n = 111 isolates) and 24 lineages of 2 closely related species: 20 K. variicola lineages (n = 28 isolates) and 4 K. quasipneumoniae lineages (n = 4 isolates). The average distance between lineages within each species was 0.5% nucleotide divergence, representing thousands of years of evolutionary separation based on molecular clock estimates for K. pneumoniae [17].
Figure 2.
Genome diversity of isolates from ICU patients identified as Klebsiella pneumoniae. All trees are maximum likelihood trees inferred from core genome SNP alignments. Scale bars indicate average number of substitutions per site across the genome. Tip colours indicate isolate source as per inset legend. *Possible mixed isolate (0.02–0.1 het/hom SNP ratio, excluded from pairwise SNP analysis in Figure 3). **Clinical isolate from sputum (KC0048), may not represent an infection. Phylogenetic lineages to which more than one ICU isolate belongs are highlighted and labeled with their corresponding multi-locus sequence type (ST) and the total number of SNPs identified between isolates in the lineage; darker shading indicates multiple patients contributed isolates in that cluster, as per inset legend. (A), Unrooted tree of all isolates, revealing three distinct species that are typically identified as K. pneumoniae in diagnostic laboratories. (B), Midpoint rooted species tree for K. variicola isolates. (C), Midpoint rooted species tree for K. pneumoniae sensu stricto isolates.
Abbreviations: ICU, intensive care unit; SNP, single-nucleotide polymorphism.
Most Klebsiella lineages (n = 69/85, 81%) were identified in just 1 patient, and 60% of patients (n = 64) had their own unique lineage not observed in any other patients (Figure 2, Supplementary Table 3). Half the infections (n = 24/48) were caused by a lineage unique to the patient. Fifteen patients had both GI carriage and infection isolates available for genome comparison; 12 of these pairs matched at the lineage level (including 6 patients whose carriage isolate was collected >2 days prior to the infection).
Klebsiella Transmission in the ICU
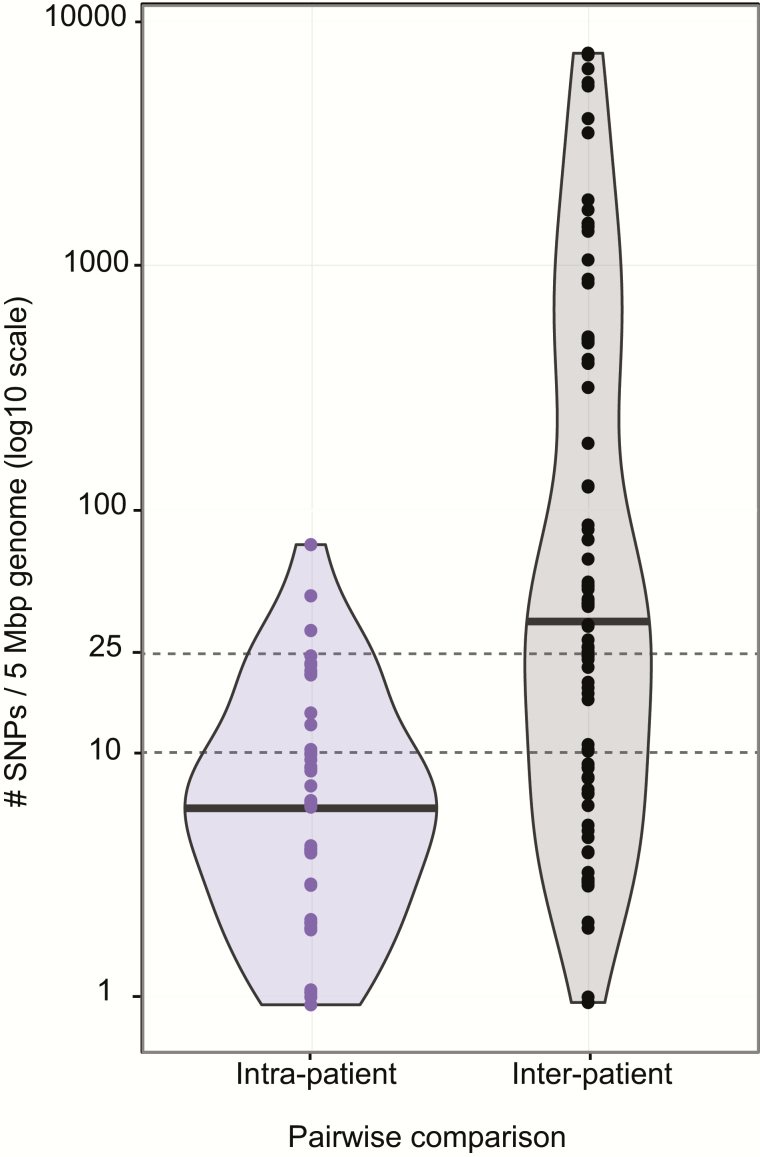
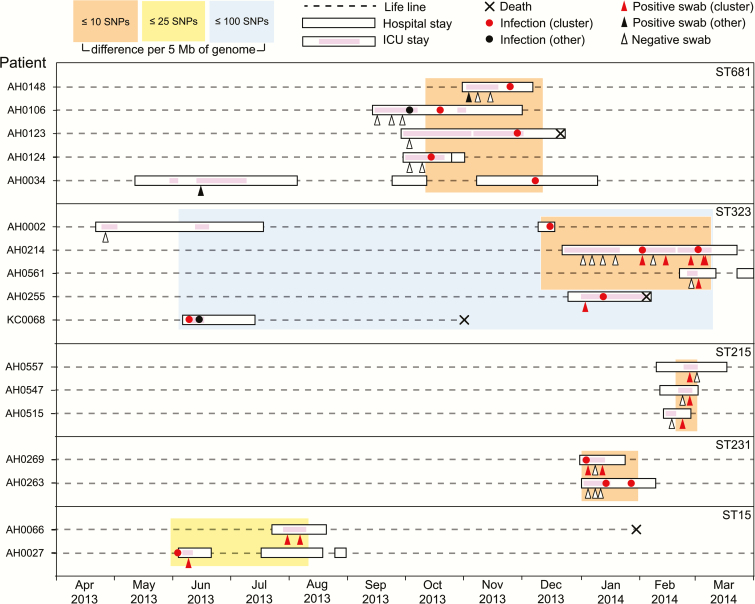
Sixteen Klebsiella lineages were detected in more than 1 patient (dark shading, Figure 2). Lineage sharing between patients could result from recent transmission of bacteria within the hospital (strain sharing) or by independent acquisition of a lineage that has been circulating in the community (lineage sharing). To distinguish these possibilities we compared intra-patient and inter-patient pairwise SNP distances (Figure 3). Intra-patient genetic distances were nearly all (97%) less than 25 SNPs per 5 Mbp, and most (82%) were less than 10 SNPs, whereas inter-patient genetic distances ranged from 0 to >5000 SNPs (Figure 3). Using 25 and 10 SNPs per 5 Mbp as cut-offs to indicate likely and very likely strain sharing between patients, we identified 5 groups of ICU patients that likely shared Klebsiella strains. Strikingly, each of these groups comprised patients with overlapping admissions, making them epidemiologically plausible intra-hospital transmission chains (Figure 4). For the other 11 lineages that were detected in more than 1 ICU patient, between-patient SNP distances exceeded 80 SNPs per 5 Mbp and ICU admissions were generally nonoverlapping (Supplementary Figure 4). Six infection episodes (n = 6/49, 12%) were attributable to these intra-hospital transmission chains (n = 4 ST681 [K. variicola], n = 1 ST323, n = 1 ST231; Figure 4). These included 2 episodes of pneumonia, 2 wound infections, and 2 UTIs, one of which disseminated to cause bacteremia with sepsis (Table 2, Supplementary Table 3). Of the 4 donors in the transmission chains, 3 had pneumonia and 1 had a wound infection. Most of the infections associated with transmission were MDR (3/4 donors and 3/6 recipients), yielding a strong association between MDR infections and transmission in the ICU (OR = 13.6, P = .002). In addition, we identified 4 patients whose K. pneumoniae carriage was attributable to intra-hospital transmission chains but did not result in any recorded K. pneumoniae infection during hospital stay (n = 1 ST323 [MDR], n = 2 ST215 [non-MDR], n = 1 ST15 [MDR]), representing 5% of all carriage-positive patients.
Figure 3.
Pairwise genetic distances between isolates belonging to the same lineage, expressed as SNPs per 5 Mbp of genome in order to normalise for differences in shared gene content between strain pairs. Violin plots showing distribution of pairwise genetic distances intra- and inter-patient; black bars indicate the median value. Note the log10 scale which excludes display of 1 strain pair that was separated by 0 SNPs.
Abbreviation: SNP, single-nucleotide polymorphism.
Figure 4.
Timelines for all lineages detected in multiple patients that show any inter-patient pairwise genetic distance between isolates of ≤25 SNPs per 5 Mbp. Lineages are boxed and labeled with their multi-locus sequence type (ST). Each horizontal dashed line indicates the time line for a patient, labelled to the left (crosses indicate date of death where applicable). Periods of Alfred Hospital admission are indicated as white boxes, periods in ICU as pink shading. Circles indicate K. pneumoniae infection isolates (red, belonging to the lineage; black, other lineage); triangles indicate rectal screening swabs (red, K. pneumoniae belonging to the lineage; black, K. pneumoniae of another lineage; unfilled, negative for K. pneumoniae). Orange boxes indicate groups of isolates for which all patients have at least one pairwise genetic distance of ≤10 SNPs per 5 Mbp with another in the group; similarly for yellow (≤25 SNPs) and blue (≤100 SNPs) boxes.
Abbreviations: ICU, intensive care unit; SNP, single-nucleotide polymorphism.
DISCUSSION
We estimated a 5.9% CA rate for culture-positive GI carriage of K. pneumoniae, similar to the 3.9% estimated among healthy individuals in the Human Microbiome Project based on 16S rRNA amplicon sequencing of stool samples [7]. HA carriage among ICU patients was estimated to be much higher at 19%, with 12% of patients converting from culture-negative at baseline to culture-positive on follow-up. This likely reflects acquisition of bacteria in the hospital (transmission of strains was directly observed in 5% of cases) and/or selection for growth of pre-existing K. pneumoniae in the GI microbiome during hospitalization (MDR rate was 18% in HA baseline carriage and 15% at follow-up, but MDR was not detected in CA baseline carriage). The HA rate estimated here is similar to the culture-positive GI carriage rates estimated in other hospital studies [11, 12].
Our data demonstrate that K. pneumoniae is a fairly common component of the human GI microbiome (5.9%) and of clinical significance in the ICU setting, as: (i) K. pneumoniae carriage on admission to ICU was significantly associated with subsequent K. pneumoniae infection (OR = 6.9, P = .0003), consistent with the results reported from the 1970s Denver study (OR = 4.0, P = .0009 [11]) and the 2016 Michigan study (OR = 4.1, P = .00002 [12]); and (ii) the WGS data confirmed a direct link between colonizing and infecting strains in 13 patients (80% of those with paired isolates available for testing), also consistent with the Michigan study [12]. We found strong evidence that a large proportion of ICU K. pneumoniae infections are attributable to patients’ own GI microbiota: (i) of all 49 K. pneumoniae infections diagnosed in ICU patients during the study period, 49% were associated with K. pneumoniae lineages unique to the patient; and (ii) of the 27 K. pneumoniae infections diagnosed in ICU patients from whom screening swabs were obtained, 48% occurred in patients who tested positive for prior GI colonization with K. pneumoniae. In contrast, only 12% of infections showed evidence of resulting from intra-hospital transmission in this setting. This suggests that although measures to reduce cross-contamination between patients are necessary, they are not sufficient to eliminate K. pneumoniae infections in hospitalized patients, and measures to minimize the risk of infection with the patients’ own microbiome deserves significant attention [21–23].
Key strengths of this study are the prospective cohort design and the use of WGS to confirm species identification and strain relatedness for all K. pneumoniae isolated from ICU patients, regardless of antimicrobial susceptibility. Most previous studies of K. pneumoniae colonization in hospitals have focused on ESBL and/or CP isolates only, which do not represent the major burden of K. pneumoniae infections. WGS demonstrated that some isolates identified as K. pneumoniae by MALDI-TOF actually belonged to closely related groups that have recently been described as separate species [24], K. quasipneumoniae and K. variicola (Figure 2). Because these species are very closely related to K. pneumoniae sensu stricto (~4% nucleotide divergence), are clinically indistinguishable, and are typically identified as K. pneumoniae in diagnostic laboratories, comparable studies reporting K. pneumoniae carriage encompass the entire K. pneumoniae complex. WGS also demonstrated that a small number of isolates identified as K. pneumoniae were contaminated to varying degrees with non-Klebsiella DNA (n = 6) or multiple Klebsiella strains (n = 8) (Supplementary Methods). These may reflect coinfection followed by selection for different subpopulations in the laboratory used for the different tests. These samples were therefore excluded from high-resolution genomic analysis for attribution purposes (Figure 1, Supplementary Table 1) but were included in calculation of overall rates of infection and carriage, which reflect solely the routine laboratory identification and are directly comparable with other reported results.
The main limitations of the study arise from the swabbing procedure to identify K. pneumoniae colonization on admission. Efforts were made to collect swabs as soon as possible following admission; however, delays due to obtaining consent and avoiding patient care disruption meant that it was often not possible to obtain screening swabs on the day of admission. Notably, similar culture-positive rates were observed for swabs collected on day 0, 1, or 2 of admission (Supplementary Table 2), so it is unlikely that this had a significant impact on CA colonization results. Although the recruitment rate was low (33%) during the consent-based recruitment period, the demographic features and K. pneumoniae colonization rate in this group were not significantly different from those recruited under the universal surveillance protocol (75% recruitment; Supplementary Figure 1); hence, there is no evidence of significant selection bias. We used rectal swab culture to determine K. pneumoniae GI colonization status; however, although this approach is standard for pathogen carriage screening [25–27], its sensitivity to detect K. pneumoniae is not well characterized and likely depends on the K. pneumoniae strain, GI microbiome composition, and recent antimicrobial exposures. There is likely a significant false negative rate. Hence our study probably underestimates the rate of colonization in the community and the contribution of colonisation to subsequent infection. Additionally, as only 1 isolate was stored from each K. pneumoniae positive infection or carriage specimen, it is possible that the proportion of matching infection/carriage pairs underestimates the contribution of carriage to infection and misses some instances of transmission.
Our conclusion that the GI microbiome is a source of K. pneumoniae infections in ICU patients echoes similar findings that colonizing strains of S. aureus, A. baumannii, Enterococcus, and Enterobacteriaceae are a common source of HA infections [12, 23–25]. Routine screening for nasal carriage of methicillin-resistant S. aureus or gut carriage of vancomycin-resistant Enterococcus or ESBL/CP Enterobacteriaceae has been introduced in various hospital settings [25–28]. A recent study of CP K. pneumoniae in Israel suggested screening and isolation of carriers could help end current outbreaks and prevent future ones [3]. A similar study introduced screening for ESBL K. pneumoniae in order to limit and prevent current and future outbreaks [29]. Although those studies focus on screening for CP or ESBL K. pneumoniae, our results indicate that routine screening for general K. pneumoniae carriage in the ICU could also be a valuable tool. Foreknowledge of the antimicrobial susceptibility profiles of K. pneumoniae, as well as other opportunistic pathogens resident in the microbiome, could guide the choice of prophylactic and therapeutic antimicrobial treatment [21, 22, 25].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors gratefully acknowledge the contribution and support of Janine Roney, Mellissa Bryant, Jennifer Williams, Iain Abbott and Noelene Browne at the Alfred Hospital, and the sequencing team at the Wellcome Trust Sanger Institute.
Funding. This work was supported by the National Health and Medical Research Council of Australia (Project 1043822 and Fellowship 1061409 to K. E. H.) and the Australian Government Research Training Program (Scholarship to C. L. G.).
Potential conflict of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centres for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013: Centres for Disease Control and Prevention, US Department of Health and Human Services, 2013. [Google Scholar]
- 2. Paterson DL, Ko WC, Von Gottberg A, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis 2004; 39:31–7. [DOI] [PubMed] [Google Scholar]
- 3. Wiener-Well Y, Rudensky B, Yinnon AM, et al. Carriage rate of carbapenem-resistant Klebsiella pneumoniae in hospitalised patients during a national outbreak. J Hosp Infect 2010; 74:344–9. [DOI] [PubMed] [Google Scholar]
- 4. Peña C, Pujol M, Ricart A, et al. Risk factors for faecal carriage of Klebsiella pneumoniae producing extended spectrum beta-lactamase (ESBL-KP) in the intensive care unit. J Hosp Infect 1997; 35:9–16. [DOI] [PubMed] [Google Scholar]
- 5. Weinstein RA, Gaynes R, Edwards JR; National Nosocomial Infections Surveillance System Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 2005; 41:848–54. [DOI] [PubMed] [Google Scholar]
- 6. Mathai D, Jones RN, Pfaller MA; SENTRY Participant Group North America Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1510 hospitalized patients: a report from the SENTRY Antimicrobial Surveillance Program (North America). Diagn Microbiol Infect Dis 2001; 40:129–36. [DOI] [PubMed] [Google Scholar]
- 7. Conlan S, Kong HH, Segre JA. Species-level analysis of DNA sequence data from the NIH Human Microbiome Project. PLoS One 2012; 7:e47075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farida H, Severin JA, Gasem MH, et al. Nasopharyngeal carriage of Klebsiella pneumoniae and other gram-negative bacilli in pneumonia-prone age groups in Semarang, Indonesia. J Clin Microbiol 2013; 51:1614–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dao TT, Liebenthal D, Tran TK, et al. Klebsiella pneumoniae oropharyngeal carriage in rural and urban Vietnam and the effect of alcohol consumption. PLoS One 2014; 9:e91999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feldman N, Adler A, Molshatzki N, et al. Gastrointestinal colonization by KPC-producing Klebsiella pneumoniae following hospital discharge: duration of carriage and risk factors for persistent carriage. Clin Microbiol Infect 2013; 19:E190–6. [DOI] [PubMed] [Google Scholar]
- 11. Selden R, Lee S, Wang WL, Bennett JV, Eickhoff TC. Nosocomial klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med 1971; 74:657–64. [DOI] [PubMed] [Google Scholar]
- 12. Martin RM, Cao J, Brisse S, et al. Molecular Epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere 2016; 1:e00261-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 2009; 26:1641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 2010; 5:e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pommier T, Canbäck B, Lundberg P, Hagström A, Tunlid A. RAMI: a tool for identification and characterization of phylogenetic clusters in microbial communities. Bioinformatics 2009; 25:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inouye M, Conway TC, Zobel J, Holt KE. Short read sequence typing (SRST): multi-locus sequence types from short reads. BMC Genomics 2012; 13:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duchêne S, Holt KE, Weill F-X, et al. Genome-scale rates of evolutionary change in bacteria. Microb Genom 2016; 2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larson E. Persistent carriage of gram negative bacteria on hands. Nurse Res 1982; 31:121. [DOI] [PubMed] [Google Scholar]
- 19. Krishna BV, Patil AB, Chandrasekhar MR. Extended spectrum beta lactamase producing Klebsiella pneumoniae in neonatal intensive care unit. Indian J Pediatr 2007; 74:627–30. [DOI] [PubMed] [Google Scholar]
- 20. Starlander G, Melhus Å. Minor outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in an intensive care unit due to a contaminated sink. J Hosp Infect 2012; 82:122–4. [DOI] [PubMed] [Google Scholar]
- 21. Hussein K, Raz-Pasteur A, Finkelstein R, et al. Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J Hosp Infect 2013; 83:307–13. [DOI] [PubMed] [Google Scholar]
- 22. Tosh PK, McDonald LC. Infection control in the multidrug-resistant era: tending the human microbiome. Clin Infect Dis 2011: cir899. [DOI] [PubMed] [Google Scholar]
- 23. Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holt KE, Wertheim H, Zadoks RN, et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A 2015; 112:E3574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho C, Lau A, Cimon K, Farrah K, Gardam M. Screening, isolation, and decolonization strategies for vancomycin-resistant Enterococci or extended spectrum beta-lactamase-producing organisms: a systematic review of the clinical evidence and health services impact. CADTH Technol Overv 2013; 3:e3102. [PMC free article] [PubMed] [Google Scholar]
- 26. Glick SB, Samson DJ, Huang ES, Vats V, Aronson N, Weber SG. Screening for methicillin-resistant Staphylococcus aureus: a comparative effectiveness review. Am J Infect Control 2014; 42:148–55. [DOI] [PubMed] [Google Scholar]
- 27. Faron ML, Ledeboer NA, Buchan BW. Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant enterococcus in the health care setting. J Clin Microbiol 2016; 54:2436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reyes K, Bardossy AC, Zervos M. Vancomycin-resistant Enterococci: epidemiology, infection prevention, and control. Infect Dis Clin North Am 2016; 30:953–65. [DOI] [PubMed] [Google Scholar]
- 29. Laurent C, Rodriguez-Villalobos H, Rost F, et al. Intensive care unit outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae controlled by cohorting patients and reinforcing infection control measures. Infect Control Hosp Epidemiol 2008; 29:517–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.