Human immunodeficiency virus (HIV)–related immunosuppression is a major risk factor for influenza illness and severity in Malawian adults. Household crowding, food insecurity, and poor sanitation are additional risk factors. Influenza preventive strategies should target HIV-infected adults in Africa.
Keywords: HIV, influenza, Malawi
Abstract
Background
The impact of human immunodeficiency virus (HIV) infection on influenza incidence and severity in adults in sub-Saharan Africa is unclear. Seasonal influenza vaccination is recommended for HIV-infected persons in developed settings but is rarely implemented in Africa.
Methods
We conducted a prospective cohort study to compare the incidence of laboratory-confirmed influenza illness between HIV-infected and HIV-uninfected adults in Blantyre, Malawi. In a parallel case-control study, we explored risk factors for severe influenza presentation of severe (hospitalized) lower respiratory tract infection, and mild influenza (influenza-like illness [ILI]).
Results
The cohort study enrolled 608 adults, of whom 360 (59%) were HIV infected. Between April 2013 and March 2015, 24 of 229 ILI episodes (10.5%) in HIV-infected and 5 of 119 (4.2%) in HIV-uninfected adults were positive for influenza by means of polymerase chain reaction (incidence rate, 46.0 vs 14.5 per 1000 person-years; incidence rate ratio, 2.75; 95% confidence interval, 1.02–7.44; P = .03; adjusted for age, sex, household crowding, and food security). In the case-control study, influenza was identified in 56 of 518 patients (10.8%) with hospitalized lower respiratory tract infection, and 88 or 642 (13.7%) with ILI. The HIV prevalence was 69.6% and 29.6%, respectively, among influenza-positive case patients and controls. HIV was a significant risk factor for severe influenza (odds ratio, 4.98; 95% confidence interval, 2.09–11.88; P < .001; population-attributable fraction, 57%; adjusted for season, sanitation facility, and food security).
Conclusions
HIV is an important risk factor for influenza-associated ILI and severe presentation in this high–HIV prevalence African setting. Targeted influenza vaccination of HIV-infected African adults should be reevaluated, and the optimal mechanism for vaccine introduction in overstretched health systems needs to be determined.
Influenza and its complications are leading causes of disease and death worldwide [1]. Vaccination of patient groups at increased risk of influenza-related complications is key to minimizing the impact of disease. Influenza vaccines are currently unavailable in the public sector in most sub-Saharan African countries [2]. The World Health Organization has therefore called for more data on influenza disease burden in the region to guide influenza prevention and control programs [3].
Persons with human immunodeficiency virus (HIV) infection are designated a priority for immunization in many well-resourced countries [4, 5], but data to support this recommendation are inconsistent. HIV does not significantly increase influenza burden or severity in developed settings [6, 7]. Conversely, in low-resource settings with high HIV prevalence there is a higher incidence of influenza illness [8–10] and a greater risk of hospitalization [11–13] and death among HIV-infected persons [9, 14, 15]. Current studies, however, are limited by incomplete ascertainment of HIV status, CD4+ cell counts, and antiretroviral treatment (ART), and few have studied the impact of environmental factors, such as household crowding and sanitation. We therefore aimed to determine the impact of HIV on the frequency and severity of adult laboratory-confirmed influenza illness in an urban, high–HIV prevalence African setting, and identify additional risk factors associated with influenza illness and severity.
METHODS
Study Setting and Design
Malawi is a low-income Southern African country, with an HIV prevalence of 10.6% [16]. Influenza predominantly circulates between January and April [17]. There is no national influenza immunization program. We performed 2 prospective observational studies at the Queen Elizabeth Central Hospital (QECH), the only inpatient facility providing free healthcare to 1.3 million residents in Blantyre district, and a primary care center adjacent to QECH.
We conducted a cohort study of HIV-infected and HIV-uninfected adults over 2 years; the primary end point was laboratory-confirmed influenza illness. We also performed a case-control study in adults presenting with mild and severe influenza, to establish risk factors for severe influenza (including HIV infection).
PROCEDURES
Cohort Study
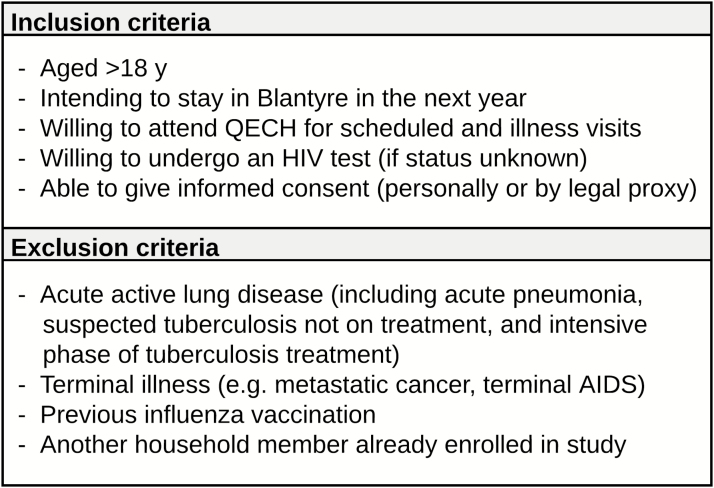
We enrolled adults (aged ≥18 years) from the ART and voluntary counseling and testing clinics at QECH beginning 1 April 2013 (eligibility criteria shown in Figure 1). Active follow-up comprised bimonthly routine clinic reviews. Participants were also instructed to attend the study clinic when they experience an influenza-like illness (ILI), defined as reported or documented fever (≥38°C) and ≥2 of the following symptoms: cough, rhinorrhea, sore throat, myalgia, headache, and vomiting/diarrhea. The study clinician assessed ill participants and instituted appropriate management. Paired nasopharyngeal and oropharyngeal swab samples (FLOQswabs; Copan Diagnostics) were obtained at routine and ILI visits [18].
Figure 1.
Eligibility criteria for cohort study. Abbreviations: HIV, human immunodeficiency virus; QECH, Queen Elizabeth Central Hospital.
We compared the incidence of laboratory-confirmed influenza-associated ILI between HIV-infected and HIV-uninfected participants. The at-risk period was calculated from enrollment to the study end date (31 March 2015), death, or loss to follow-up. For participants lost to follow-up, follow-up was censored on the date of relocation, withdrawal of consent, or the last recorded clinic visit.
Case-Control Study
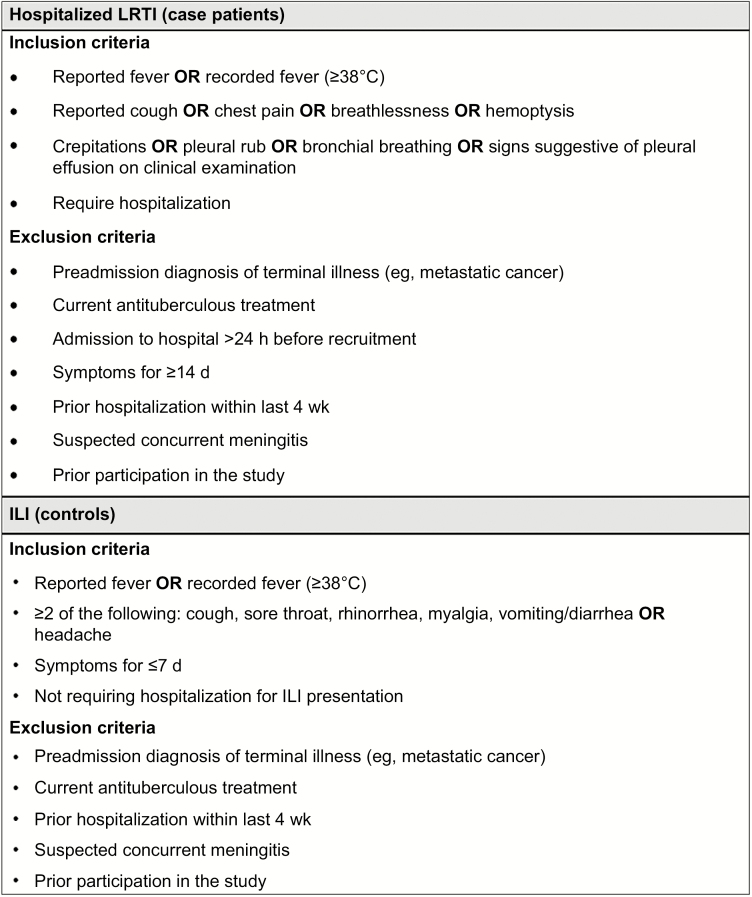
Between 15 May 2013 and 28 February 2015, we recruited adults admitted to QECH with acute lower respiratory tract infection (LRTI) (severe cases) and adults attending the primary care center with ILI (nonsevere disease) (eligibility criteria shown in Figure 2). Nasopharyngeal aspirate samples were obtained at enrollment. Participants with influenza-positive hospitalized LRTI and outpatient-managed ILI made up the case patients and controls, respectively.
Figure 2.
Eligibility criteria for case-control study. Abbreviations: ILI, influenzalike illness; LRTI, lower respiratory tract infection.
Laboratory Procedures
Laboratory testing was performed at the Malawi–Liverpool–Wellcome Trust Clinical Research Programme laboratory. HIV status was established by means of sequential rapid HIV tests (Alere Determine and Uni-Gold, Trinity Biotech) [19]. CD4+ cell counts were performed using a FACScount flow cytometer (Becton Dickinson; BD Biosciences). Nasopharyngeal specimens were tested for influenza A and B viruses using the Centers for Disease Control and Prevention human influenza quantitative reverse-transcription polymerase chain reaction (PCR) diagnostic panel and influenza A subtyping kit [20].
Statistical Analysis
Analysis was performed with Stata software (version 12.1). We tested differences in categorical variables using χ2 or Fisher exact test, and differences in continuous variables using t or Wilcoxon rank sum test, as appropriate. The cohort study was powered to detect an incidence rate ratio (IRR) of ≥3.0 (α = .05; 2-tailed β = .2), for influenza-associated ILI by HIV status, requiring 608 recruits and allowing for 20% loss-to follow-up, based on an estimated cumulative incidence of 40 per 1000 person-years [PY] in the HIV-uninfected cohort and a 60:40 ratio of HIV infection to noninfection.
Incidence rates of influenza-associated ILI were calculated by dividing the number of events by the number of person-years of follow-up. Poisson regression models were used to estimate IRRs and 95% confidence intervals (CIs) for the effect of HIV and other risk factors on influenza. Age, sex, and HIV infection were included as potential confounders in the multivariable models. Population average Poisson regression models using generalized estimating equations were constructed for recurrent events.
In both studies, stepwise backward elimination of covariates with P values < .20 was used to rationalize the multivariable models. We limited the number of covariates in a multivariable model to maintain a limit of 10 events per variable [21]. Two-way interactions were evaluated in all final models. All available case information was used in each univariable analysis. In the multivariable models, we excluded patients with missing data for included variables (data were >95% complete for all variables). The impact of ART and CD4+ cell count was assessed in subgroup analyses of HIV-infected individuals.
For the case-control study, a sample size of 57 case patients and 114 unmatched controls provided 80% power to detect an odds ratio (OR) of ≥2.5. Based on observed influenza prevalences of 11% and 16.4% in adults presenting to QECH with severe or mild acute respiratory illness, respectively, in our sentinel surveillance (unpublished data), we estimated recruitment of approximately 518 adults with hospitalized LRTI and 695 adults with ILI.
We estimated the OR of having HIV infection and other potential risk factors for severe influenza in case patients and controls, controlling for confounders, using unconditional logistic regression models. HIV status and recruitment season were included a priori in the multivariable model. Population-attributable fractions of modifiable risk factors for severe influenza were estimated from the prevalence of exposure in case patients and adjusted OR from the multivariable logistic regression model [22]. Ethical approval was provided by the University of Malawi College of Medicine Research Ethics Committee (Study No. P.11/12/1310) and the Research Ethics Committee of the University of Liverpool (Study No. 12.43).
RESULTS
Cohort Study: Participant Characteristics
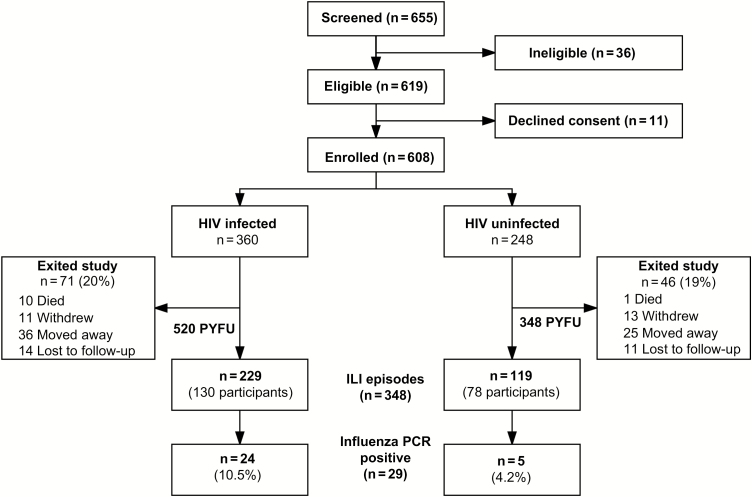
In total, 608 adults were enrolled; 360 (59%) had HIV infection (Figure 3 and Table 1). Compared with HIV-uninfected participants, HIV-infected participants were older (median age, 37 vs 31 years; P < .001), and a higher proportion were female (69% vs 55%; P = .001). Chronic lung disease and smoking were uncommon in both groups. A significantly higher proportion of HIV-infected participants reported previous tuberculosis (25% vs 2%; P < .001) and pneumonia in the past 5 years (16% vs 5%; P < .001).
Figure 3.
Recruitment and progress of cohort participants. Flow diagram for recruitment, loss to follow-up, and influenza-like illness events among cohort participants. Reasons for ineligibility included intention to relocate out of Blantyre (n = 10), inability to attend regular study visits (n = 6), inability to give written informed consent (n = 1), evidence of active acute respiratory disease at enrollment (n = 17), and enrollment of another household member in the study (n = 2). Reasons for declining consent included not having time (n = 3), not interested (n = 2), fear of participation (n = 4), and wished to seek spouse’s consent (n = 2). Abbreviations: HIV, human immunodeficiency virus; ILI, influenzalike illness; PCR, polymerase chain reaction; PYFU, person-years follow-up.
Table 1.
Demographic, Clinical, Household, and Socioeconomic Characteristics of the Cohort Participants
| Characteristic | Participants, No./Total (%)a | P Value for Differenceb | |
|---|---|---|---|
| HIV Infected (n = 360) | HIV Uninfected (n = 248) | ||
| Male sex | 113/360 (31) | 111/248 (45) | .001 |
| Age, median (IQR), y | 37 (31–45) | 31 (25–39) | <.001c |
| Medical history | |||
| Asthma | 18/360 (5) | 8/248 (3) | .29 |
| Chronic lung disease | 1/360 (0.3) | 0/248 (0) | 1.0 |
| Chronic cardiac disease | 2/360 (0.6) | 1/248 (0.4) | .80 |
| Chronic kidney disease | 0/360 (0) | 0/248 (0) | …d |
| Chronic liver disease | 2/359 (0.6) | 0/248 (0) | .24 |
| Pregnant (at enrollment) | 4/359 (1) | 4/248 (2) | .84 |
| Previous pulmonary tuberculosis | 90/359 (25) | 6/248 (2) | <.001 |
| Pneumonia in past 5 y | 59/358 (16) | 13//248 (5) | <.001 |
| Current smoker | 11/360 (3) | 4/248 (2) | .14e |
| Drinks alcohol | 44/359 (12) | 35/248 (14) | .47 |
| Body mass index <18.5 kg/m2 | 38/346 (11) | 17/242 (7) | .18 |
| Household and socioeconomic factors | |||
| No. of children aged <5 y in household | |||
| 0 | 219/358 (61) | 132/247 (54) | |
| 1 | 114/358 (32) | 83/247 (33) | |
| ≥2 | 25/358 (7) | 32/247 (13) | .03 |
| No. of individuals aged >5 y in household | |||
| 0–2 | 89/358 (25) | 56/247 (23) | |
| 3–4 | 161/358 (45) | 91/247 (37) | |
| ≥5 | 108/358 (30) | 100/247 (41) | .03 |
| Crowding indexf | |||
| <1.5 | 110/360 (30) | 66/248 (27) | |
| 1.5–2.4 | 144/360 (40) | 95/248 (38) | |
| >2.5 | 106/360 (30) | 87/248 (35) | .33 |
| Sanitation facility | |||
| None/non-VIP toilet | 327/359 (91) | 205/248 (83) | |
| VIP toilet/flush toilet | 32/359 (9) | 43/248 (17) | .002 |
| Water supply | |||
| River/stream/borehole | 75/354 (21) | 44/246 (18) | |
| Public tap/standpipe | 212/354 (60) | 131/246 (53) | |
| Piped to dwelling | 67/354 (19) | 71/246 (29) | .02 |
| Principal cooking fuel/energy source | |||
| Firewood | 77/357 (22) | 44/246 (18) | |
| Charcoal | 244/357 (68) | 131246 (53) | |
| Electricity | 36/357 (10) | 71/246 (29) | .66 |
| Highest level of education | |||
| Never attended school | 18/359 (5) | 10/248 (4) | |
| Primary | 152/359 (42) | 89/248 (36) | |
| Secondary/tertiary | 189/359 (53) | 149/248 (60) | .19 |
| Unemployed | 87/359 (24) | 47/248 (19) | .13 |
| Assets ownedg | |||
| 1–2 | 141/360 (39) | 83/248 (33) | |
| 3 | 151/360 (42) | 113/248 (46) | |
| 4–5 | 68/360 (19) | 52/248 (21) | .36 |
| Difficulties obtaining food | |||
| Never | 137/356 (38) | 133/247 (54) | |
| 1–2 times/mo | 149/356 (42) | 86/247 (35) | |
| >2 times/mo | 70/356 (20) | 28/247 (11) | <.001 |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; VIP, ventilated improved pit latrine.
aData represent No./total (%) of participants unless otherwise specified.
bDetermined with Mantel-Haenszel χ2 test unless stated otherwise.
cWilcoxon rank sum test.
dFor Chronic kidney disease: Chi-square or Fisher’s exact test not possible when neither group has the exposure of interest.
eFisher exact test.
fThe crowding index was calculated as the number of persons in the household divided by the number of sleeping rooms.
gNumber of the following assets owned in household: working refrigerator, radio, mobile phone, bed, and car or motorbike.
HIV-uninfected participants had larger households, but crowding was similar in the 2 groups. HIV-uninfected participants had better sanitation facilities, water supply, and food security. No significant differences were observed in education level, employment, or asset ownership.
Of the 360 HIV-infected participants, 234 (65.0%) were receiving ART at enrolment, the majority (82%) for >12 months. The median CD4+ cell count at enrollment was 390/µL (interquartile range, 244–547/µL).
Eleven participants died during the study (HIV infected, n = 10); none had reported respiratory symptoms before death. Sixty-one participants (10%) migrated out of Blantyre, 24 (3.9%) withdrew consent, and 25 (4.1%) were lost to follow-up (Figure 3). There was no differential loss to follow-up by HIV status. The total person-time follow-up was 520 and 348 PY in the HIV-infected and HIV-uninfected cohorts, respectively.
Impact of HIV Infection on Influenza-Associated ILI Incidence
We recorded 348 ILI episodes in 208 participants (HIV infected, n = 130) (clinical characteristics show in Supplementary Table S1). The incidences of ILI presentation were 442 and 341 per 1000 PY in the HIV-infected and HIV-uninfected participants, respectively (IRR, 1.21; 95% CI, .99–1.48). Twenty-nine ILI episodes (8.3%) had PCR results positive for influenza, including influenza A(H3N2) (n = 11) and influenza B (n = 18) and occurring in 24 (10.5%) of 229 HIV-infected and 5 (4.2%) of 119 HIV-uninfected participants. The incidence of laboratory-confirmed influenza-associated ILI per 1000 PY was 46.0 (95% CI, 30.8–68.6) in HIV-infected and 14.5 (6.0–34.7) in HIV-uninfected adults (IRR, 3.21; 95% CI, 1.22–8.41) (Table 2).
Table 2.
Risk Factors for Laboratory-confirmed Influenza Illness Among Cohort Participants
| Characteristic | Incidence Rate per 1000 PY of Follow-up (95% CI) | Univariable Analysisa | Multivariable Analysisa,b | ||
|---|---|---|---|---|---|
| IRR (95% CI) | P Value | IRR (95% CI) | P Value | ||
| Sex | |||||
| Male | 31.4 (16.9–58.3) | 1 | … | 1 | … |
| Female | 34.6 (22.1–54.2) | 1.21 (0.52–2.84) | .66 | 0.88 (0.40–1.93) | .74 |
| Age group, y | |||||
| 18–29 | 20.9 (8.7–50.2) | 1 | … | 1 | … |
| 30–39 | 39.9 (23.2–68.7) | 1.73 (0.60–4.97) | … | 1.55 (0.54–4.43) | … |
| ≥40 | 36.3 (20.1–65.6) | 1.33 (0.43–4.05) | .30 | 1.42 (0.47–4.28) | .70 |
| HIV status | |||||
| Uninfected | 14.5 (6.0–34.7) | 1 | … | 1 | … |
| Infected | 46.0 (30.8–68.6) | 3.21 (1.22–8.41) | .02 | 2.75 (1.02–7.44) | .03 |
| Medical history | |||||
| Previous pulmonary tuberculosis | 28.2 (23.3–51.1) | 0.82 (0.28–2.43) | .87 | … | … |
| Pneumonia in past 5 y | 54.3 (24.4–120.9) | 1.86 (0.75–4.57) | .14 | … | … |
| Body mass index <18.5 kg/m2 | 40.3 (13.0–125.0) | 0.81 (0.25–2.68) | .73 | … | … |
| Housing characteristics | |||||
| No. of children aged <5 y in household | … | … | … | ||
| 0 | 36.2 (22.8–57.5) | 1 | … | … | |
| 1 | 27.8 (13.9–55.6) | 0.77 (0.33–1.77) | … | ||
| ≥2 | 37.3 (12.0–115.5) | 1.03 (0.30–3.50) | .80 | … | |
| No. of individuals aged ≥5 y in household | … | … | … | ||
| 0–2 | 25.5 (10.6–61.3) | 1 | … | … | |
| 3–4 | 28.1 (15.1–52.3) | 1.10 (0.38–3.22) | … | … | |
| ≥5 | 44.7 (26.5–75.4) | 1.76 (0.63–4.87) | .41 | … | |
| Crowding indexc | |||||
| <1.5 | 16.3 (6.12–43.5) | 1 | … | 1 | … |
| 1.5–2.4 | 47.1 (28.8–76.8) | 2.88 (0.96–8.62) | … | 3.41 (1.12–10.36) | … |
| ≥2.5 | 32.0 (16.6–61.5) | 1.95 (0.60–6.35) | .11 | 2.06 (0.62–6.83) | .06 |
| Socioeconomic characteristics | |||||
| Highest level of education | … | … | … | ||
| Never attended/primary | 47.6 (30.0–75.6) | 1 | … | … | |
| Secondary/tertiary | 22.5 (12.5–40.7) | 0.47 (0.22–1.00) | .05 | … | |
| Employment | … | … | … | ||
| No | 49.6 (28.8–85.4) | 1 | … | … | |
| Yes | 26.4 (16.2–43.1) | 0.53 (0.26–1.11) | .09 | … | |
| Food security: difficulty obtaining food | |||||
| Never | 25.9 (13.9–48.2) | 1 | … | 1 | … |
| 1–2 times/mo | 20.7 (9.9–43.5) | 0.80 (0.30–2.10) | … | 0.71 (0.27–1.88) | … |
| >2 times/mo | 87.2 (49.5–153.6) | 3.35 (1.45–7.76) | .005 | 3.09 (1.30–7.36) | .006 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IRR, incidence rate ratio; PY, person-years.
aIRRs estimated for the incidence of laboratory-confirmed influenza, using Poisson regression.
bAdjusted for sex, age group, HIV status, crowding index, and food security.
cThe crowding index was calculated as the number of persons in the household divided by the number of sleeping rooms.
In the univariable analysis, history of previous pneumonia, household crowding, lower education level, unemployment, and food insecurity were associated with influenza-related ILI (Table 2). After adjustment for age, sex, household crowding and food security, HIV-infected adults had an approximately 3-fold increased rate of influenza-associated ILI compared with HIV-uninfected adults (adjusted IRR [aIRR], 2.75; 95% CI, 1.02–7.44; Pearson’s goodness-of-fit test, P = .12).
Among HIV-infected participants, individuals with enrollment CD4+ cell counts <200/µL had a higher incidence of influenza-associated ILI than those with counts >200/µL, but the association was nonsignificant (79.1 vs 40.5 per 1000 PY; IRR, 1.95; 95% CI, .78–4.92). The effect of HIV on influenza did not differ by ART status.
Other Risk Factors for Influenza Infection
A household crowding index (number of persons in the household divided by the number of sleeping rooms) of 1.5–2.4 was associated with a 3-fold increased risk of influenza-associated ILI, compared with households with <1.5 persons per sleeping room (aIRR, 3.41; 95% CI, 1.12–10.36). The increased risk was not observed in participants who lived in household with a crowding index >2.5 (2.06; .62–6.83) (Table 2). Participants who reported difficulties accessing food more than twice per month had a 3-fold increased risk of influenza-associated ILI, compared with those with no food access difficulties (aIRR, 3.09; 95% CI, 1.30–7.36). Food insecurity was also a possible effect modifier of the impact of HIV infection on influenza (P = .01). Among those with frequent difficulty obtaining food, HIV infection was associated with 10-fold increased incidence of influenza-associated ILI (IRR, 9.50; 95% CI, 1.27–70.99), but no association was evident in those with little or no food insecurity (IRR, 1.18; 95% CI, .32–4.39). No interaction was demonstrated with the other covariates.
Case-Control Study: Patient Characteristics
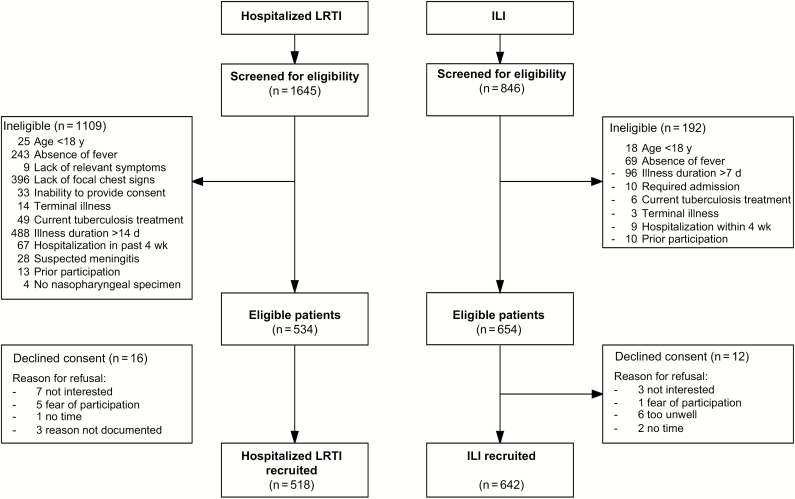
A total of 1645 patients were assessed for eligibility for hospitalized LRTI and 846 for ILI, with subsequent recruitment of 518 and 642 patients, respectively (Figure 4). Patients with severe respiratory presentation were older (median age, 35 vs 32 years; P < .001), predominantly male (62% vs 43%; P < .001), and had a substantially higher prevalence of HIV infection (77% vs 30%; P < .001) (Table 3). They also had a higher prevalence of previous pulmonary tuberculosis (18% vs 7%), pneumonia within the past 5 years (22% vs 7%), smoking (11% vs 6%), and regular alcohol intake (26% vs 12%) (all P < .001). Sanitation facility, water supply, cooking fuel, asset ownership, education level, and food security were also worse among patients with hospitalized LRTI.
Figure 4.
Case-control study recruitment. Abbreviations: ILI, influenza-like illness; LRTI, lower respiratory tract infection.
Table 3.
Demographic, Clinical, Household, and Socioeconomic Characteristics of Adults Enrolled With Hospitalized Lower Respiratory Tract Infection and Mild Influenza-like Illness
| Characteristic | Participants, No./Total (%)a | P Value for Differenceb | |
|---|---|---|---|
| Hospitalized LRTI (n = 518) | ILI (n = 642) | ||
| Male sex | 323/518 (62) | 273/642 (43) | <.001 |
| Age, median (IQR), y | 35 (30–42) | 32 (25–43) | <.001c |
| Prior treatment | |||
| Attended another health facilityd | 312/513 (61) | 62/635 (10) | <.001 |
| Antibiotics within 2 wke | 309/509 (61) | 70/630 (11) | <.001 |
| Antimalarials within 2 wk | 83/511 (16) | 13/630 (2) | <.001 |
| HIV status | |||
| HIV infected | 396/517 (77) | 192/640 (30) | <.001 |
| CD4+ cell count, median (IQR) cells/µL | 101 (46–196) | 313 (167–450) | <.001c |
| Receiving ART at enrollmentf | 188/233 (81) | 68/87 (78) | .62 |
| Medical history | |||
| Chronic lung disease | 18/503 (4) | 15/635 (2) | .23 |
| Chronic cardiac disease | 2/503 (0.4) | 1/635 (0.2) | .59g |
| Hypertension | 9/503 (2) | 19/635 (3) | .19 |
| Chronic kidney disease | 1/503 (0.2) | 0/503 (0) | .44g |
| Chronic liver disease | 1/503 (0.2) | 0/503 (0) | .44g |
| Pregnant | 2/195 (0) | 10/369 (3) | .19g |
| Previous pulmonary tuberculosis | 90/514 (18) | 45/638 (7) | <.001 |
| Pneumonia in past 5 y | 113/512 (22) | 46/638 (7) | <.001 |
| Body mass index <18.5 kg/m2 | 147/495 (30) | 67/638 (11) | <.001 |
| Current smoker | 58/513 (11) | 39/638 (6) | <.001 |
| Drinks alcohol | 135/512 (26) | 78/638 (12) | <.001 |
| Household and socioeconomic factors | |||
| No. of children aged <5 y in household | |||
| 0 | 296/513 (58) | 381/638 (60) | |
| 1 | 156/513 (30) | 195/638 (30) | |
| ≥2 | 61/513 (12) | 62/638 (10) | .48 |
| Crowding indexh | |||
| <1.5 | 174/497 (35) | 219/612 (36) | |
| 1.5–2.4 | 203/497 (41) | 258/612 (42) | |
| ≥2.5 | 120/497 (24) | 135/612 (22) | .71 |
| Sanitation facility | |||
| None/non-VIP toilet | 284/514 (55) | 260/637 (41) | |
| VIP toilet | 188/514 (37) | 252/637 (39) | |
| Flush toilet | 42/514 (8) | 125/637 (20) | <.001 |
| Water supply | |||
| River/stream/borehole | 150/514 (29) | 134/638 (21) | |
| Public tap/standpipe | 272/514 (53) | 308/638 (48) | |
| Piped to dwelling | 92/514 (18) | 196/638 (31) | <.001 |
| Principal cooking fuel/energy source | |||
| Firewood | 140/514 (27) | 129/637 (20) | |
| Charcoal | 325/514 (63) | 421/637 (66) | |
| Electricity | 49/514 (10) | 87/637 (14) | .006 |
| Highest level of education | |||
| Never attended school | 49/508 (10) | 52/638 (8) | |
| Primary | 274/508 (54) | 266/638 (42) | |
| Secondary/tertiary | 185/508 (36) | 320/638 (50) | <.001 |
| Unemployed | 68/514 (13) | 91/638 (14) | .61 |
| Assets ownedi | |||
| 0 | 84/515 (16) | 75/638 (12) | |
| 1 | 106/515 (20) | 112/638 (18) | |
| 2 | 122/515 (24) | 165/638 (26) | |
| 3 | 158/515 (31) | 206/638 (32) | |
| 4–5 | 35/515 (9) | 80/638 (12) | .04 |
| Difficulties obtaining food | |||
| Never | 247/514 (48) | 367/638 (58) | |
| 1–2 times/mo | 226/514 (44) | 250/638 (39) | |
| >2 times/mo | 41/514 (8) | 31/638 (3) | <.001 |
| Season of recruitment | |||
| January–March | 99/518 (19) | 204/642 (32) | |
| April–June | 100/518 (19) | 180/642 (28) | |
| July–September | 161/518 (31) | 145/642 (24) | |
| October–December | 15/518 (31) | 102/642 (16) | <.001 |
Abbreviations: ART, antiretroviral treatment; HIV, human immunodeficiency virus; ILI, influenzalike illness; IQR, interquartile range; LRTI, lower respiratory tract infection, VIP, ventilated improved pit latrine.
aData represent No./total (%) unless otherwise specified.
bDetermined with Mantel-Haenszel χ2 test unless stated otherwise.
cWilcoxon rank sum test.
dIncluding attendance at another hospital, health center, private clinic, traditional healer, or pharmacy.
eExcluding cotrimoxazole prophylaxis in HIV-infected individuals.
fDenominator is the number of patients with known HIV infection at enrollment.
gFisher exact test.
hThe crowding index was calculated by the number of persons in the household divided by the number of sleeping rooms.
iNumber of the following assets owned in household: working refrigerator, radio, mobile phone, bed, and car/motorbike.
Contribution of Influenza to Mild and Severe Respiratory Infection
Influenza was identified in 56 (10.8%) patients with hospitalized LRTI (case patients) and 88 (13.7%) patients with ILI (controls) (Table 4). Influenza A virus was detected in 30 (53.6%) of the case patients and 35 (39.8%) of the controls; all had influenza A(H3N2) except 1 control (unsubtyped). Two controls were positive for both influenza A(H3N2) and B. Influenza A(H1N1)pdm09 was not detected during the study period.
Table 4.
Risk Factors for Severe Influenza Presentation in Influenza-Positive Case Patients and Controls
| Characteristic | Participants, No./Total (%) | Univariable Analysisc | Multivariable Analysisc,d | |||
|---|---|---|---|---|---|---|
| Case Patients (n = 56)a | Controls (n = 88)b | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Male sex | 28/56 (50) | 43/88 (49) | 0.96 (0.49–1.88) | .89 | … | … |
| Age group, y | … | … | … | |||
| 18–29 | 17/56 (30) | 36/88 (41) | 0.75 (0.32–1.73) | .32 | … | |
| 30–39 | 22/56 (39) | 25/88 (28) | 1.40 (0.61–3.22) | … | … | |
| ≥40 | 17/56 (30) | 27/88 (31) | 1 | … | … | |
| HIV infected | 39/56 (70) | 26/88 (30) | 5.47 (2.63–11.36) | <.001 | 4.98 (2.09–11.88) | <.001 |
| Medical history | … | |||||
| Previous pulmonary tuberculosis | 10/56 (18) | 5/88 (6) | 3.61 (1.16–11.20) | .03 | … | … |
| Pneumonia in past 5 y | 17/55 (31) | 7/88 (8) | 5.18 (1.98–13.53) | .001 | 6.49 (1.95–21.25) | .001 |
| Body mass index <18.5 kg/m2 | 12/53 (23) | 9/88 (10) | 2.57 (1.00–6.60) | .05 | … | |
| Housing and socioeconomic characteristics | ||||||
| Sanitation facility | ||||||
| None/non-VIP toilet | 34/55 (61) | 37/88 (42) | 2.12 (1.08–4.22) | .03 | 3.14 (1.25–7.84) | .01 |
| VIP toilet/flush toilet | 22/55 (40) | 51/88 (58) | 1 | … | 1 | … |
| Water supply | … | … | … | |||
| River/stream/borehole | 15/56 (27) | 19/88 (22) | 2.54 (0.93–6.98) | .07 | … | |
| Public tap/standpipe | 32/56 (57) | 40/88 (45) | 2.58 (1.07–6.22) | … | … | |
| Piped to dwelling | 9/56 (16) | 29/88 (33) | 1 | … | … | |
| Highest level of education | … | … | … | |||
| Never attended | 5/55 (9) | 15/88 (17) | 1 | … | … | |
| Primary | 34/55 (62) | 28/88 (32) | 3.64 (1.18–11.27) | … | … | |
| Secondary/tertiary | 16/55 (29) | 45/88 (51) | 1.07 (0.33–3.41) | .002 | … | |
| Difficulties obtaining food | ||||||
| Never | 28/56 (50) | 58/88 (66) | 1 | … | 1 | … |
| 1–2 times/mo | 21/56 (38) | 29/88 (33) | 1.50 (0.73–3.08) | … | 1.15 (0.47–2.84) | … |
| >2 times/mo | 7/56 (12) | 1/88 (1) | 14.4 (1.70–123.65) | .007 | 20.85 (1.97–221.16) | .01 |
| Month of recruitment | ||||||
| January–March | 16/56 (29) | 49/88 (56) | 1 | … | 1 | … |
| April–June | 15/56 (27) | 18/88 (20) | 2.55 (1.05–5.20) | … | 3.36 (1.13–9.95) | … |
| July–September | 12/56 (21) | 9/88 (10) | 4.08 (1.45–11.46) | … | 6.34 (1.69–23.80) | … |
| October–December | 13/56 (23) | 12/88 (14) | 3.32 (1.25–8.72) | .01 | 3.60 (1.07–12.10) | .01 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; PCR, polymerase chain reaction; VIP, ventilated improved pit latrine.
aCase had severe (hospitalized) lower respiratory tract infection and a positive PCR result for influenza.
bControls had influenza-like illness and a positive PCR result for influenza.
cUnconditional logistic regression.
dAdjusted for HIV status, history of pneumonia within 5 years, month of recruitment, sanitation facility, and food security.
Risk Factors for Severe Influenza Presentation
Among the 56 case patients and 88 controls, no difference in age and sex was observed (Table 4). Thirty nine (69.6%) of the case patients and 26 (29.6%) of the controls were HIV-infected (P < .001). Compared with HIV-infected controls, HIV-infected case patients had more advanced immunosuppression (median CD4+ cell count, 140/µL vs 265/µL; P = .03) and were more likely to be receiving ART (35.7 vs 9.1%; P < .001). Case patients were more likely to have a low body mass index and report a history of tuberculosis and pneumonia. Controls had better sanitation, education, and food security. However, household exposure to children <5 years old or crowding did not differ according to case-control status.
In the univariable analysis, HIV infection, reported history of tuberculosis, pneumonia within past 5 years, low body mass index (<18.5 kg/m2), month of recruitment, type of water supply, sanitation facility, lower education level, and food insecurity were associated with being a case patient (Table 4). Month of recruitment was included in the multivariable model a priori owing to seasonal discrepancy in the recruitment of patients with hospitalized LRTI or ILI. In the multivariable model, HIV infection was strongly associated with severe influenza (adjusted OR, 4.98; 95% CI, 2.09–11.88; P < .001). In addition, reported pneumonia within 5 years (adjusted OR, 6.49; 95% CI, 2.00–21.07; P < .001), poor sanitation facility (3.14; 1.25–7.84; P = .01); and frequent difficulty accessing food (20.85; 1.97–221.16; P = .01) were independent risk factors for severe influenza.
In a subgroup analysis of HIV-infected case patients and controls, there was a trend toward lower CD4+ cell counts in case patients (OR, 2.72; 95% CI, .97–7.60; CD4+ cell count, <200/µL vs >200/µL). No association was found between ART status and influenza severity.
Population-Attributable Fraction
The highest proportions of hospitalized influenza cases were attributed to HIV infection (adjusted population-attributable fraction, 56.7%; 95% CI, 42.3–67.4) and poor sanitation (40.9%; 20.5–56.0) (Table 5). Frequent difficulty accessing food accounted for 12% (95% CI, 10.6–13.6) of hospitalized influenza cases.
Table 5.
Population-Attributable Fraction of Modifiable Risk Factors for Severe Influenza
| Risk Factor | Proportion of Case Patients Exposed, % | Adjusted OR (95% CI) | P Value | Adjusted Population-Attributable Fraction (95% CI), % |
|---|---|---|---|---|
| HIV infection | 70 | 4.98 (2.09–11.88) | <.001 | 56.7 (42.3–67.4) |
| Sanitation facility | ||||
| None/non-VIP toilet | 39 | 3.14 (1.25–7.84) | .01 | 40.9 (20.5–56.0) |
| VIP/flush toilet | 61 | 1 | ||
| Difficulties obtaining food | ||||
| Never | 50 | 1 | … | |
| 1–2 times/wk | 38 | 1.15 (0.47–2.84) | 6 (−40.9 to 38.0) | |
| >2 times/wk | 12 | 20.85 (1.97–221.16) | .01 | 12.0 (10.3–13.7) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; VIP, ventilated improved pit latrine.
DISCUSSION
In this urban African adult population, HIV infection is an important risk factor for both symptomatic influenza and severe illness. Compared with HIV-uninfected adults, HIV-infected adults had a 3 times higher incidence of influenza-associated ILI, and a 5-fold greater odds of severe influenza disease. Furthermore, nearly 60% of influenza-related hospitalized LRTI cases were attributable to HIV. Although neither study was powered to examine the effect of HIV at different levels of immunosuppression, our data suggest higher incidence and greater disease severity among those with CD4+ cell counts <200/µL.
Previous population-level surveillance and retrospective studies in South Africa and Kenya that have examined the association between HIV and influenza found a higher disease burden [8–10] and an increased risk of influenza-associated hospitalization in HIV-infected persons [11–13], particular among those with severe immunosuppression [13]. The current studies provide robust evidence to support these findings having prospectively ascertained HIV status, CD4+ cell count, and information on ART, as well as other exposures, including household and socioeconomic characteristics that may confound the association between HIV and influenza.
We found a higher incidence of influenza-associated ILI among HIV-infected than among HIV-uninfected individuals. Although this could indicate an increased susceptibility to infection, it may instead, or also, reflect a greater propensity of HIV-infected individuals to develop symptomatic illness after influenza infection, which has been described elsewhere [23]. This is a pertinent finding in high–HIV prevalence settings, because HIV-infected individuals may play an important role in the community transmission of influenza.
Taken together, data from the 2 studies present a persuasive argument for a strong association between HIV and influenza. The finding that more than half of hospitalized influenza presentations in Malawian adults were attributable to HIV further emphasizes its critical role in severe influenza in this population. Because pneumonia is the most common cause of adult medical admissions at QECH [24], effective influenza preventive strategies could substantially reduce the burden of acute respiratory infections in Malawi and other similar resource-limited settings.
Inactivated influenza vaccines have demonstrated safety and efficacy in HIV-infected persons [25]. Clinical efficacy of 75.5% has been reported in South African HIV-infected adults, but the trial excluded patients with comorbid conditions and ART-naive patients with CD4+ cell counts <100/μL [26]. Influenza vaccines are not widely deployed in most African countries [2, 27]. Before consideration of HIV-infected persons as a target group for immunization, policy makers will require evidence of vaccine efficacy in the context of advanced immunosuppression and/or comorbid conditions, as well as the potential public health impact and cost-effectiveness of vaccinating HIV-infected individuals, compared with other target groups (eg, pregnant women, young children). The acceptability of annual vaccination, feasibility of vaccine administration at ART clinics, and optimal timing of vaccination in the absence of clear seasonality will require elucidation. Numerous regulatory, logistical, and financial obstacles will need to be overcome if targeted influenza vaccination policies are to be successfully and sustainably implemented in the region.
The introduction of ART was associated with a dramatic decline in influenza-attributable hospitalizations in the United States [28], and improved survival after pandemic influenza A(H1N1) infection in Mexico [29]. However, this beneficial effect has not been observed in Africa. Cohen et al [30] found no difference in case fatality ratio by ART status among HIV-infected individuals with influenza-positive severe acute respiratory illness [30]. A Malawian study demonstrated poor reconstitution of influenza-specific CD4+ T-cell response in HIV-infected adults after 12 months of highly active ART, despite a rise in CD4+ cell count [31]. Hence the impact of ART on the relationship between HIV and influenza severity requires further evaluation.
Identification of household crowding, poor sanitation, and food insecurity as risk factors for influenza highlight the importance of current public health interventions to alleviate hunger and poverty and improve access to clean water and sanitation [32]. Food insecurity emerged as a previously unrecognized risk factor for both influenza illness and severity. More in-depth evaluation of food insecurity and its association with influenza and other respiratory infections is warranted [33].
Our results, along with other data from the region, contrast with those from developed settings [6]. This highlights the limitations of extrapolating findings from developed settings to inform influenza control policies in Africa. Differences in the observed impact of HIV on influenza burden and severity may be due to more advanced immunosuppression, poorer access to ART, higher prevalence of comorbid conditions, poverty-related factors identified in this study, compared with other regions.
Our study has several limitations. First, it was conducted in a single urban center, which may limit the generalizability of our findings to rural populations. Second, this study was not powered to assess the impact of CD4+ cell count or ART on influenza incidence and severity. CD4+ cell counts were measured during acute illness in the case-control study; the degree of immunosuppression may not be accurately represented because CD4+ cell count depression can occur during acute illness. Third, passive surveillance was used for case ascertainment in both studies; underascertainment of ILI episodes, and therefore influenza cases, is conceivable. Biased estimates away from the null could have resulted if HIV-infected cohort participants were more likely than uninfected individuals to present with ILI . Similar bias could have arisen if HIV-infected individuals had a higher propensity to attend hospitals with severe respiratory symptoms in the case-control study. However, there was no significant difference in the incidence of ILI between HIV-infected and uninfected cohorts, and a greater proportion of HIV-related ILI cases had severe clinical signs (Supplementary Table S1). Furthermore, the majority of case-control study participants were unaware of their HIV status at enrollment. Finally, we were unable to control for bacterial coinfection in the case-control study, because diagnostic tests for bacteria were undertaken in case patients but not controls.
We have comprehensively evaluated the association between HIV and influenza, identifying HIV-infected persons at particular risk of symptomatic influenza and severe disease. Influenza-preventive strategies should be an important aspect of the management of HIV-infected adults in sub-Saharan Africa. Further studies are needed in Malawi and other high–HIV prevalence settings to determine influenza vaccine efficacy in persons with advanced immunosuppression and evaluate the potential public health impact ahead of operational research addressing the logistical barriers to implementing large-scale vaccination programs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Marc-Alain Widdowson and Meredith McMorrow (US Centers for Disease Control and Prevention) provided input into study implementation. Nico Nagelkerke (Malawi–Liverpool–Wellcome Trust Clinical Research Programme) provided statistical analysis support.
Financial support. This work was supported by the Wellcome Trust (grant 097464/Z/11/A).
Potential conflicts of interest. N. F. has received grant support from GlaxoSmithKine outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Influenza (seasonal). Fact sheet No. 211 Available at: http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed 20 September 2012.
- 2. Duque J, McMorrow ML, Cohen AL. Influenza vaccines and influenza antiviral drugs in Africa: are they available and do guidelines for their use exist?BMC Public Health 2014; 14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Public health research needs for influenza in Africa. public health research agenda for influenza. Geneva, Switzerland: WHO, 2012:1–10. [Google Scholar]
- 4. Geretti AM, Brook G, Cameron C et al. British HIV Association guidelines for immunization of HIV-infected adults 2015. HIV Medicine 2016; 9:795–848. [DOI] [PubMed] [Google Scholar]
- 5. Grohskopf LA, Sokolow LZ, Broder KR et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2017-18 influenza season. MMWR Recomm Rep 2017; 66:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheth AN, Althoff KN, Brooks JT. Influenza susceptibility, severity, and shedding in HIV-infected adults: a review of the literature. Clin Infect Dis 2011; 52:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheth AN, Patel P, Peters PJ. Influenza and HIV: lessons from the 2009 H1N1 influenza pandemic. Curr HIV/AIDS Rep 2011; 8:181–91. [DOI] [PubMed] [Google Scholar]
- 8. Feikin DR, Njenga MK, Bigogo G et al. Etiology and Incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PloS One 2012; 7:e43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen C, Moyes J, Tempia S et al. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009–2011. Emerg Infect Dis 2013; 19:1766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray J, Cohen A, Walaza S et al. Determining the provincial and national burden of influenza-associated severe acute respiratory illness in South Africa using a rapid assessment methodology. PLoS One 2015; 10:e0132078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ope MO, Katz MA, Aura B et al. Risk factors for hospitalized seasonal influenza in rural western Kenya. PLoS One 2011; 6:e20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abadom TR, Smith AD, Tempia S, Madhi SA, Cohen C, Cohen AL. Risk factors associated with hospitalisation for influenza-associated severe acute respiratory illness in South Africa: a case-population study. Vaccine 2016; 34:5649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tempia S, Walaza S, Moyes J et al. Risk Factors for influenza-associated severe acute respiratory illness hospitalization in South Africa, 2012–2015. Open Forum Infect Dis 2017; 4:ofw262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tempia S, Walaza S, Viboud C et al. Deaths associated with respiratory syncytial and influenza viruses among persons ≥5 years of age in HIV-prevalent area, South Africa, 1998–2009. Emerg Infect Dis 2015; 21:600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen C, Moyes J, Tempia S et al. Mortality amongst patients with influenza-associated severe acute respiratory illness, South Africa, 2009–2013. PloS One 2015; 10:e0118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Statistics Office (NSO), ICF Macro. Malawi Demographic and Health Survey 2010. Zomba, Malawi, and Calverton, Maryland: NSO & ICF Macro, 2011:437. [Google Scholar]
- 17. Peterson I, Bar-Zeev N, Kennedy N et al. Respiratory virus-associated severe acute respiratory illness and viral clustering in Malawian children in a setting with a high prevalence of HIV infection, malaria, and malnutrition. J Infect Dis 2016; 214:1700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. Interim guidance on specimen collection, processing, and testing for patients with suspected novel influenza A(H1N1) virus Infection Available at: https://www.cdc.gov/h1n1flu/specimencollection.htm. Accessed 25 February 2013.
- 19. World Health Organization. Rapid HIV tests: guidelines for use in HIV testing and counselling services in resource-constrained settings. Geneva, Switzerland: World Health Organization, 2004:48. [Google Scholar]
- 20. World Health Organization. CDC protocol of realtime RTPCR for influenza A(H1N1). Atlanta, GA: WHO Collaborating Centre for Influenza, Centers for Disease Control and Prevention, 2009. [Google Scholar]
- 21. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49:1373–9. [DOI] [PubMed] [Google Scholar]
- 22. Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J 2013; 13:672–98. [Google Scholar]
- 23. Tempia S, Walaza S, Moyes J et al. Attributable fraction of influenza virus detection to mild and severe respiratory illnesses in HIV-infected and HIV-uninfected patients, South Africa, 2012–2016. Emerg Infect Dis 2017; 23:1124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. SanJoaquin MA, Allain TJ, Molyneux ME et al. Surveillance Programme of IN-patients and Epidemiology (SPINE): implementation of an electronic data collection tool within a large hospital in Malawi. PLoS Med 2013; 10:e1001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Remschmidt C, Wichmann O, Harder T. Influenza vaccination in HIV-infected individuals: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness and safety. Vaccine 2014; 32:5585–92. [DOI] [PubMed] [Google Scholar]
- 26. Madhi SA, Maskew M, Koen A et al. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis 2011; 52:128–37. [DOI] [PubMed] [Google Scholar]
- 27. Palache A, Oriol-Mathieu V, Abelin A, Music T. Seasonal influenza vaccine dose distribution in 157 countries (2004–2011). Vaccine 2014; 32:6369–76. [DOI] [PubMed] [Google Scholar]
- 28. Neuzil KM, Coffey CS, Mitchel EF Jr, Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr 2003; 34:304–7. [DOI] [PubMed] [Google Scholar]
- 29. Ormsby CE, de la Rosa-Zamboni D, Vazquez-Perez J et al. Severe 2009 pandemic influenza A (H1N1) infection and increased mortality in patients with late and advanced HIV disease. AIDS 2011; 25:435–9. [DOI] [PubMed] [Google Scholar]
- 30. Cohen C, Walaza S, Moyes J et al. Epidemiology of severe acute respiratory illness (SARI) among adults and children aged ≥5 years in a high HIV-prevalence setting, 2009–2012. PloS One 2015; 10:e0117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jambo KC, Sepako E, Glennie SJ et al. Naturally-acquired influenza-specific CD4+ T-cell proliferative responses are impaired in HIV-infected African adults. PLoS One 2012; 7:e38628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. United Nations. Sustainable development goals Available at: http://www.un.org/sustainabledevelopment/sustainable-development-goals/. Accessed 21 September 2015.
- 33. Jones AD, Ngure FM, Pelto G, Young SL. What are we assessing when we measure food security? a compendium and review of current metrics. Adv Nutr 2013; 4:481–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.