Prognostic models developed and validated using data from 1699 adults with tuberculous meningitis (TBM), with or without human immunodeficiency virus infection, performed well and could be used in clinical practice to identify patients with TBM at high risk of death.
Keywords: tuberculous meningitis, prognostic models, mortality, HIV
Abstract
Background
Tuberculous meningitis (TBM) is the most severe form of extrapulmonary tuberculosis. We developed and validated prognostic models for 9-month mortality in adults with TBM, with or without human immunodeficiency virus (HIV) infection.
Methods
We included 1699 subjects from 4 randomized clinical trials and 1 prospective observational study conducted at 2 major referral hospitals in Southern Vietnam from 2001–2015. Modeling was based on multivariable Cox proportional hazards regression. The final prognostic models were validated internally and temporally and were displayed using nomograms and a Web-based app (https://thaole.shinyapps.io/tbmapp/).
Results
951 HIV-uninfected and 748 HIV-infected subjects with TBM were included; 219 of 951 (23.0%) and 384 of 748 (51.3%) died during 9-month follow-up. Common predictors for increased mortality in both populations were higher Medical Research Council (MRC) disease severity grade and lower cerebrospinal fluid lymphocyte cell count. In HIV-uninfected subjects, older age, previous tuberculosis, not receiving adjunctive dexamethasone, and focal neurological signs were additional risk factors; in HIV-infected subjects, lower weight, lower peripheral blood CD4 cell count, and abnormal plasma sodium were additional risk factors. The areas under the receiver operating characteristic curves (AUCs) for the final prognostic models were 0.77 (HIV-uninfected population) and 0.78 (HIV-infected population), demonstrating better discrimination than the MRC grade (AUC, 0.66 and 0.70) or Glasgow Coma Scale score (AUC, 0.68 and 0.71) alone.
Conclusions
The developed models showed good performance and could be used in clinical practice to assist physicians in identifying patients with TBM at high risk of death and with increased need of supportive care.
Tuberculous meningitis (TBM) accounted for about 1%–5% of the 10.4 million new tuberculosis cases in 2015 and is the most severe manifestation of the disease, killing or disabling about half of those afflicted [1]. TBM is especially common in children and those infected with human immunodeficiency virus (HIV), in whom outcomes are poor [2]. In a trial conducted in Vietnam, approximately 30% of participants died during the 9-month study period, and 40% of the survivors had disabilities [3].
The British Medical Research Council (MRC) constructed the first TBM severity grades for use in the 1948 trial of streptomycin [4]. Patients were subdivided, based on clinical experience rather than statistical derivation, into “early” (no clinical signs of meningitis or focal neurology and fully conscious), “medium” (patient’s condition falling between early and advanced) and “advanced” (extremely ill, in deep coma). With the introduction of the Glasgow Coma Scale (GCS) in 1974, these categories were modified to the following grading system: grade I (GCS score 15; no focal neurological signs), grade II (GCS score 11–14 or 15 with focal neurological signs), or grade III (GCS score ≤10) [5]. This grading system has become the most widely used classification for TBM severity. Despite the age of the MRC scale, and its lack of statistical derivation, improved and robust prediction models for poor outcomes (death and/or neurological deficit) in TBM based on large cohort studies and rigorous statistical methods are still lacking. Among prognostic studies in TBM published in the last 20 years, the majority were based on a small number of subjects (ranging from 23 to 507), and modern prognostic modeling tools for handling missing data or model validation were rarely used [6–9].
The primary objective of this study was to develop and validate novel robust prognostic models for 9-month mortality in adult patients with TBM, with or without HIV coinfection. The models were based on a large data set of 1699 subjects (951 HIV uninfected, 748 HIV infected) enrolled in 4 randomized controlled trials and 1 prospective cohort study conducted in Vietnam. In addition, we compared the predictive performance of the developed models with the MRC grading system and the GCS, both widely used in the assessment of TBM severity.
METHODS
Study Population
Study Participants
The study population comprised subjects enrolled in 5 TBM studies conducted between 2001 and 2015 at 2 tertiary referral centers in Ho Chi Minh City, Vietnam: Pham Ngoc Thach Hospital and the Hospital for Tropical Diseases [3, 10–13]. Detailed descriptions of the studies have been published elsewhere [3, 10–13] and are summarized in Supplementary Table S1. All studies were approved by the Oxford Tropical Research, Hospital for Tropical Diseases, and Pham Ngoc Thach Hospital ethics committees.
Inclusion Criteria
The inclusion criteria for the 5 studies are provided in Supplementary Table S1. In brief, all 5 studies included adult subjects with a clinical diagnosis of TBM, defined as having >5 days of meningitis symptoms, nuchal rigidity, and cerebrospinal fluid (CSF) abnormalities suggestive of TBM; additional criteria were radiological evidence of tuberculosis on chest radiograph or brain scan or microbiological evidence of tuberculosis from specimens other than CSF. Diagnostic categories of definite, probable, or possible TBM were defined according to study-specific diagnostic criteria because the uniform TBM case definition became available only in 2010 [14]. All study participants were included in the pooled analysis except if they had a confirmed alternative diagnosis, a study drug administration error, or an unknown HIV status.
Laboratory Investigation
CSF specimens were stained and cultured by standard methods for pyogenic bacteria, mycobacteria, and fungi. In the last study [3], CSF was also tested with the Xpert MTB/RIF assay (Cepheid). Isolates of M. tuberculosis were tested for susceptibility to isoniazid, rifampin, ethambutol, and streptomycin with the mycobacterial growth indicator tube method [15]. Baseline peripheral blood CD4 cell counts were measured for all HIV-infected adults, using flow cytometry.
Antituberculosis and Adjunctive Treatment
Unless study participants were randomized to an experimental antituberculosis treatment, participants received a standard antituberculosis regimen consisting of isoniazid (5 mg/kg/d; maximum, 300 mg/d), rifampin (10 mg/kg/d; maximum, 600 mg/d), pyrazinamide (25 mg/kg/d; maximum, 2 g/d) and ethambutol (20 mg/kg/d; maximum, 1.2 g/d) or streptomycin (20 mg/kg/d; maximum, 1 g/d) for 3 months, followed by rifampin and isoniazid at the same doses for another 6 months. Since 2005, all patients received adjunctive dexamethasone for the first 6–8 weeks of treatment [3].
Primary Outcome
The primary end point was overall survival during a 9-month follow-up period. Patients without documented death during the follow-up period were censored at 9 months or at the last date they were known to be alive, whichever was earlier.
Candidate Predictors
Candidate predictors were initially selected based on clinical judgment, their status as established risk factors in previous publications [6–9, 16–18], and completeness of the data. The number of predictors was further restricted based on a rule of thumb of requiring at least 10 events for each included predictor variable or degree of freedom [19]. The final list of candidate predictors is presented in Supplementary Table S2. For all laboratory parameters and radiology assessments, we used the value recorded closest to enrollment (up to ±7 days from enrollment). Of note, CSF total white blood cell count and CSF total lymphocyte count were strongly correlated; therefore, only CSF lymphocyte count was included as candidate predictor.
Resistance is known to be an important independent predictor of death in subjects with TBM [20, 21]. However, unless earlier isolates are available or rapid molecular tests are performed, information about a patients’ tuberculosis drug susceptibility is not available at enrollment. The main models therefore did not include resistance, but models with resistance were depicted in the Supplementary Materials. The cohort variable was included in the full model to represent the change in patient management, treatment, and standard of health care over the 15-years time span of the included studies. We decided to construct separate prognostic models for the HIV-uninfected and HIV-infected TBM populations, because they are clinically distinct populations and we expected that predictors may differ between them.
Statistical Analysis
Full details of the statistical analysis are described in the Supplementary Appendix S1. In brief, incomplete data were multiply imputed using multivariable imputation by chained equations (MICE) [22]. The statistical model of choice was multivariable Cox proportional hazards regression including all prespecified candidate predictors. We tested for potential nonlinear effects of continuous candidate predictors and for interactions, that is, effect modifications, between age and other candidate predictors. Two variable selection methods were used to simplify the model: (1) backward stepwise selection with a stopping rule based on Akaike’s information criterion and (2) the least absolute shrinkage and selection operator (LASSO) [23].
We combined model selection with bootstrapping and based the final models on the most frequently (≥60%) included variables across all imputed and bootstrap data sets [24]. We used the area under the cumulative/dynamic receiver operating characteristic (ROC) curve (AUC) [25] for 9-month mortality risk prediction to assess the discrimination of the models and calibration plots [26] and to visually assess how closely the predicted mortality probabilities agree with the observed mortality probabilities. Internal bootstrap validation was performed to correct measures of model performance for overfitting. In addition, we performed temporal validation, wherein data from the most recent trial served as the test data set to validate models developed based on earlier studies. The final prognostic models, that is, the variable-selected models with the highest AUC, were implemented in a Web-based mortality calculator and graphically depicted using nomograms [26]. All analyses were conducted using R software, version 3.3.1 [27].
RESULTS
Baseline Characteristics of Study Participants
The 5 studies included a total of 1734 subjects, of which 1699 were included for prognostic modeling (Supplementary Figure S1). The reasons for excluding 35 subjects were a confirmed other diagnosis (n = 16), unknown HIV status (n = 16), or a study drug administration error (n = 3).
Table 1 presents the baseline characteristics of all included subjects; 951 were HIV uninfected (56.0%), and 748 (44.0%) were HIV infected. The median age was 34 years (interquartile range, 27–45 years), and 823 subjects (48.4%) had definite, microbiologically confirmed TBM. The MRC grade was I in 588 subjects (34.6%), II in 743 (43.8%), and III in 367 (21.6%). Among HIV-infected subjects, the median CD4 cell count was 41 cells/μL, and 124 (16.6%) were receiving antiretroviral therapy (ART) at baseline. Compared with HIV-uninfected subjects, those infected with HIV tended to be younger (median age, 31 vs 40 years), were more frequently male (85.6% vs 62.3% male) and were more likely to be MRC grade III (25.6% vs 18.5%).
Table 1.
Baseline Characteristics of Patients With Tuberculous Meningitis Included in the Pooled Database, Overall and By Human Immunodeficiency Virus Status
| Characteristic | All Patients (N = 1699) | HIV Uninfected (n = 951) | HIV Infected (n = 748) | |||
|---|---|---|---|---|---|---|
| Total No.a | No. (%)b | Total No.a | No. (%)b | Total No.a | No. (%)b | |
| Cohort | 1699 | 951 | 748 | |||
| Dexamethasone trial | 534 (31.4) | 436 (45.9) | 98 (13.1) | |||
| Fluoroquinolone trial | 56 (3.3) | 53 (5.6) | 3 (0.4) | |||
| TBM HIV cohort | 58 (3.4) | 0 (0) | 58 (7.7) | |||
| ART timing trial | 248 (14.6) | 0 (0) | 248 (33.2) | |||
| Intensified treatment trial | 803 (47.3) | 462 (48.6) | 341 (45.6) | |||
| Age, median (IQR), y | 1698 | 34 (27–45) | 951 | 40 (27–56) | 747 | 31 (26–35) |
| Female sex | 1699 | 514 (30.25) | 951 | 406 (42.7) | 748 | 108 (14.4) |
| Weight, median (IQR), kg | 1695 | 46 (41–51) | 951 | 46 (42–52) | 744 | 45 (40–50) |
| Received dexamethasone treatment | 1699 | 1436 (84.5) | 951 | 742 (78.0) | 748 | 694 (92.8 ) |
| Receiving ART at enrollment | 745 | 124 (16.6) | … | … | 745 | 124 (16.6) |
| MRC gradec | 1698 | 951 | 747 | |||
| Grade I | 588 (34.6) | 327 (34.4) | 261 (34.9) | |||
| Grade II | 743 (43.8) | 448 (47.1) | 295 (39.5) | |||
| Grade III | 367 (21.6) | 176 (18.5) | 191 (25.6) | |||
| Illness duration at study entry, median (IQR), d | 1685 | 15 (10–30) | 948 | 15 (10–26) | 737 | 15 (9–30) |
| History of previous tuberculosis treatment | 1658 | 236 (14.2) | 922 | 88 (9.5) | 736 | 148 (20.1) |
| Focal neurological signs present | 1683 | 818 (48.6) | 951 | 517 (54.4) | 732 | 301 (41.1) |
| Temperature, median (IQR), °C | 1697 | 37.6 (37.2–38.5) | 950 | 37.7 (37.2–38.5) | 747 | 37.5 (37.2–38.5) |
| Occurrence of seizures | 1685 | 50 (3.0) | 950 | 25 (2.6) | 735 | 25 (3.4) |
| Laboratory values, median (IQR) | ||||||
| Plasma sodium, mmol/L | 1537 | 129 (124–133) | 843 | 130 (125–134) | 694 | 127 (123–132) |
| CSF lymphocyte count, cells/μL | 1614 | 85 (27.6–197.5) | 919 | 94 (32.5–200) | 695 | 73 (23.3–188.8) |
| CSF protein, g/L | 1624 | 1.3 (0.70–1.94) | 909 | 1.2 (0.7–2.0) | 715 | 1.3 (0.7–1.9) |
| CSF glucose, mmol/L | 1636 | 1.60 (1.05–2.30) | 920 | 1.52 (1.00–2.30) | 716 | 1.70 (1.17–2.33) |
| Ratio of CSF to blood glucose | 1470 | 0.30 (0.21–0.41) | 830 | 0.30 (0.20–0.40) | 640 | 0.30 (0.21–0.42) |
| Microbiologically confirmed/definite TBM | 1699 | 823 (48.4) | 951 | 361 (38.0) | 748 | 462 (61.8) |
| Resistanced | 1699 | 951 | 748 | |||
| No isoniazid or rifampin resistance | 479 (28.2) | 203 (21.4) | 276 (36.9) | |||
| Isoniazid monoresistance | 172 (10.1) | 63 (6.6) | 109 (14.6) | |||
| Rifampin monoresistance/MDR | 35 (2.1) | 10 (1.1) | 25 (3.3) | |||
| Unknown resistance | 1013 (59.6) | 675 (71.0) | 338 (45.2) | |||
| Miliary tuberculosis present on chest radiograph | 1525 | 279 (18.3) | 890 | 165 (18.5) | 635 | 114 (17.9) |
| Peripheral blood CD4 count, median (IQR), cells/μL | 646 | 41 (16–108) | … | … | 646 | 41 (16–108) |
Abbreviations: ART, antiretroviral therapy; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IQR, interquartile range; MDR, multidrug resistance; MRC, Medical Research Council; TBM, tuberculous meningitis.
aNo. of subjects with nonmissing data for the respective characteristic.
bData represent no. (%) of subjects unless otherwise specified.
cMRC grade I is defined as Glasgow Coma Scale (GCS) score 15 with no focal neurological signs; grade II, GCS score 11–14, or 15 with focal neurological signs; grade III, GCS score ≤10.
dIsoniazid monoresistance is defined as resistance to isoniazid but not to rifampin; MDR, as resistance to at least isoniazid and rifampin; unknown resistance, as drug-susceptibility test results not available. In all categories, resistance to other drugs may be present.
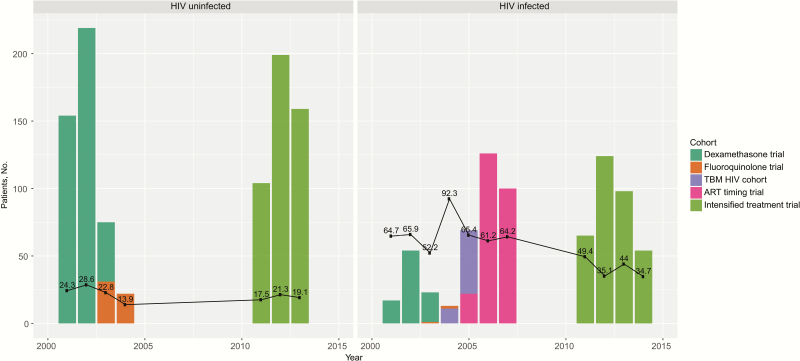
During 9-month follow-up, 219 of 951 HIV-uninfected subjects with TBM (23.0%) died, compared with 384 of 748 HIV-infected subjects (51.3%). Among the 1096 survivors, 998 (91.1%) were followed up for ≥260 days. Yearly recruitment into the 5 studies and observed mortality rate by HIV status are shown in Figure 1. The figure displays a decline in mortality rate over time, which is particularly pronounced in HIV-infected subjects. In this subpopulation, the estimated 9-month mortality rate dropped from 52%–92% during 2001–2007 to 35%–50% during 2011–2015. Coincidently, the number of study participant receiving ART at enrollment was only 7 of 407 (1.7%) in the first period and increased to 117 of 341 (34.3%) in the second period.
Figure 1.
Annual recruitment into the 5 contributing studies by human immunodeficiency virus status. Black lines show estimated 9-month mortality rates (as percentages) for each calendar year, based on the Kaplan-Meier method. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; TBM, tuberculous meningitis.
Prognostic Models
Univariable analyses of the effect of all candidate predictors for survival are shown in Supplementary Tables S3 and S4. Results of the multivariable analyses are shown in Table 2 and Supplementary Table S5. The final models identified higher MRC disease severity grade and lower CSF lymphocyte cell counts as risk factors for death in both HIV-uninfected and HIV-infected subjects (Table 2). Importantly, the mortality rate in MRC grade III (GCS score ≤10) was 3-fold higher in HIV-uninfected patients and 4-fold higher in HIV-infected patients compared with MRC grade I (GCS score 15; no focal neurological signs). Mortality rates for MRC grade II (GCS score 11–14, or 15 with focal neurological signs) was intermediate in both populations.
Table 2.
Final Cox Regression Models for 9-Month Survival in Each Human Immunodeficiency Virus Populationa
| Variable | HIV-Uninfected TBM Population | HIV-Infected TBM Population | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Model | Final Modelb | Full Model | Final Modelb | |||||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age (per +10 y) | 1.24 | 1.15–1.34 | <.001 | 1.24 | 1.15–1.34 | <.001 | 0.99 | .85–1.16 | .89 | … | … | … |
| Male sex | 1.07 | .79–1.44 | .67 | … | … | … | 0.95 | .70–1.30 | .77 | … | … | … |
| Weight (per +10 kg) | 0.88 | .73–1.06 | .17 | … | … | … | 0.72 | .61–.85 | <.001 | 0.73 | .63–.85 | <.001 |
| MRC gradec | ||||||||||||
| Grade I | 1 | … | … | 1 | … | … | 1 | … | … | 1 | … | … |
| Grade II | 1.36 | .87–2.13 | .17 | 1.41 | .9–2.19 | .13 | 1.71 | 1.24–2.35 | .001 | 1.88 | 1.43–2.48 | <.001 |
| Grade III | 2.97 | 1.83–4.83 | <.001 | 3.05 | 1.92–4.86 | <.001 | 3.76 | 2.69–5.26 | <.001 | 4.08 | 3.07–5.41 | <.001 |
| Illness duration at study entry (d)d | 1.03 | .91–1.17 | .61 | … | … | … | 1.02 | .93–1.11 | .73 | … | … | … |
| History of previous tuberculosis treatment | 1.46 | 1.00–2.13 | .05 | 1.57 | 1.09–2.26 | .02 | 1.26 | .97–1.65 | .09 | … | … | … |
| Focal neurological signs present | 1.80 | 1.22–2.64 | .003 | 1.65 | 1.15–2.39 | .007 | 1.15 | .87–1.53 | .33 | … | … | … |
| Temperature (°C) | 0.96 | .79–1.17 | .71 | … | … | … | 1.09 | .96–1.24 | .18 | … | … | … |
| Occurrence of seizures | 0.96 | .41–2.22 | .92 | 0.95 | .58–1.56 | .83 | ||||||
| No dexamethasone: treatment | 1.67 | 1.15–2.40 | .006 | 1.97 | 1.46–2.67 | <.001 | 1.12 | .68–1.85 | .65 | … | … | … |
| Plasma sodium (per +10 mmol/L)e | 1.01 | .85–1.21 | .89 | … | … | … | … | … | <.001 | … | … | <.001 |
| Plasma sodium, 135 vs 125 mmol/L | 1.01 | .85–1.21 | … | … | … | … | 1.09 | .91–1.31 | … | 1.07 | .90–1.28 | … |
| Plasma sodium, 115 vs 125 mmol/L | 0.99 | .83–1.17 | … | … | … | … | 1.63 | 1.31–2.04 | … | 1.06 | 1.29–2.00 | … |
| CSF lymphocyte count (cells/μL)d | 0.88 | .82–.94 | <.001 | 0.86 | .81–.92 | <.001 | 0.93 | .87–.99 | .02 | 0.93 | .88–.98 | .004 |
| CSF protein (g/L)d | 0.95 | .84–1.07 | .38 | … | … | … | 0.95 | .85–1.06 | .37 | … | … | … |
| CSF glucose (mmol/L) | 1.03 | .87–1.23 | .70 | … | … | … | 1.08 | .98–1.19 | .10 | … | … | … |
| Ratio of CSF to blood glucosed | 1.02 | .81–1.28 | .88 | … | … | … | 0.91 | .77–1.08 | .30 | … | … | … |
| Miliary tuberculosis present on chest radiograph | 0.82 | .56–1.18 | .29 | … | … | … | 0.95 | .70–1.29 | .73 | … | … | … |
| Peripheral blood CD4 (cells/μL)d | … | … | … | … | … | … | 0.91 | .85–.98 | .01 | 0.9 | .84–.96 | .002 |
| Receiving ART at enrollment | … | … | … | … | … | … | 0.87 | .60–1.26 | .45 | … | … | … |
| Cohort | ||||||||||||
| Intensified trial | 1 | … | … | … | … | … | 1 | … | … | 1 | … | … |
| Dexamethasone trial | 1.46 | .94–2.27 | .09 | … | … | … | 1.96 | 1.23–3.14 | .005 | 1.72 | 1.25–2.37 | <.001 |
| Fluoroquinolone trial | 1.12 | .51–2.46 | .77 | … | … | … | … | … | … | … | … | … |
| TBM HIV cohort | … | … | … | … | … | … | 2.58 | 1.63–4.06 | <.001 | 2.64 | 1.81–3.84 | <.001 |
| ART timing trial | … | … | … | … | … | … | 1.78 | 1.28–2.47 | <.001 | 1.71 | 1.34–2.19 | <.001 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; HR, hazard ratio. MRC, Medical Research Council; TBM, tuberculous meningitis.
aEstimates were pooled across multiply imputed data sets.
bThe final model for the HIV-uninfected population was selected using the least absolute shrinkage and selection operator (LASSO) method. The final model for the HIV-infected population was selected using stepwise backward model selection. The 95% CIs and P values for final models do not take into account the uncertainty of model selection.
cMRC grade I is defined as Glasgow Coma Scale (GCS) score 15 with no focal neurological signs); grade II, GCS score 11–14, or 15 with focal neurological signs); grade III, GCS score ≤10.
dHR per 2-fold increase.
eIn HIV-infected subjects, the effect of sodium levels on mortality rates was significantly nonlinear and modeled with a restricted cubic spline function with 2 df. To simplify interpretation of the corresponding regression coefficients, only HRs for 2 derived sodium contrasts from that model are given. P values for sodium values In the HIV-infected population are based on overall Wald tests of the restricted cubic spline function.
In HIV-uninfected subjects, older age, previous tuberculosis, not receiving adjunctive dexamethasone, and focal neurological signs were additional predictors of higher mortality risk. Of note, the mortality rate was increased by >50% in patients with previous tuberculosis and those with focal neurological signs, and almost doubled for patients who did not receive dexamethasone.
In the HIV-infected population, lower weight, lower peripheral blood CD4 cell count, and abnormal plasma sodium were additional predictors of higher mortality. Plasma sodium had a significant nonlinear association with survival, and both decreased and elevated sodium values were associated with a higher predicted mortality rate compared with intermediate value values. Ongoing ART at enrollment was associated with a more favorable outcome in univariable analysis (Supplementary Table S4) but was no longer significantly associated with survival after adjustment for other factors. There was no clear evidence that age modified the effect of any of the other predictors (overall interaction test, P = .14 in HIV-uninfected and P =.10 in HIV-infected subjects).
Model Validation
The final models showed good discrimination between TBM survivors and deaths at 9 months; the corresponding AUCs in internal validation (corrected for overfitting) were 0.77 in the HIV-uninfected and 0.78 in the HIV-infected population (Table 3). The addition of drug resistance as a covariate resulted in only a slightly improved model discrimination, with AUC values ranging from 0.77 to 0.79 (Supplementary Table S7).
Table 3.
Discrimination of Candidate Models for Tuberculous Meningitis Mortality By Human Immunodeficiency Virus Population Measured By Area Under the Curve at 9 Months
| Model | Internal Validation | Temporal Validation: AUC (95% CI) | |
|---|---|---|---|
| Apparent AUC (95% CI)a | Optimism-Corrected AUCb | ||
| HIV-uninfected population | |||
| Full model | 0.79 (.76–.83) | 0.76 | 0.77 (.72–.83) |
| Model selected by stepwise backward model selection | 0.78 (.74–.81) | 0.76 | 0.77 (.71–.82) |
| Model selected by LASSO methodc | 0.78 (.75–.82) | 0.77 | 0.82 (.77–.87) |
| MRC graded | 0.66 (.62–.70) | 0.66 | 0.70 (.64–.75) |
| GCS | 0.68 (.64–.72) | 0.68 | 0.68 (.62–.75) |
| HIV-infected population | |||
| Full model | 0.79 (.75–.83) | 0.77 | 0.76 (.70–.82) |
| Model selected by stepwise backward model selectionc | 0.79 (.75–.82) | 0.78 | 0.73 (.67–.79) |
| Model selected by LASSO method | 0.78 (.74–.82) | 0.77 | 0.75 (.69–.81) |
| MRC graded | 0.70 (.66–.74) | 0.70 | 0.69 (.63–.75) |
| GCS | 0.71 (.67–.75) | 0.71 | 0.68 (.62–.74) |
Abbreviations: AUC, area under the cumulative/dynamic receiver operating characteristic curve; CI, confidence interval; GCS, Glasgow Coma Scale; HIV, human immunodeficiency virus; LASSO, least absolute shrinkage and selection operator; MRC, Medical Research Council.
aPerformance estimated directly from the original 45 imputed data sets used to develop the prediction models.
bAdjusted performance corrected for overoptimism through internal bootstrap validation.
cFinal simplified model.
dMRC grade I is defined as GCS score 15 with no focal neurological signs; grade II, GCS score 11–14, or 15 with focal neurological signs; and grade III, GCS score ≤10.
In internal validation, the models showed good agreement between predicted and observed mortality (Supplementary Figure S2A and S2C). In temporal validation, calibration in the HIV-uninfected groups remained satisfactory, whereas in the HIV infected group models developed based on earlier studies systematically overestimated the actual mortality in the most recent trial (Supplementary Figure S2B and S2D).
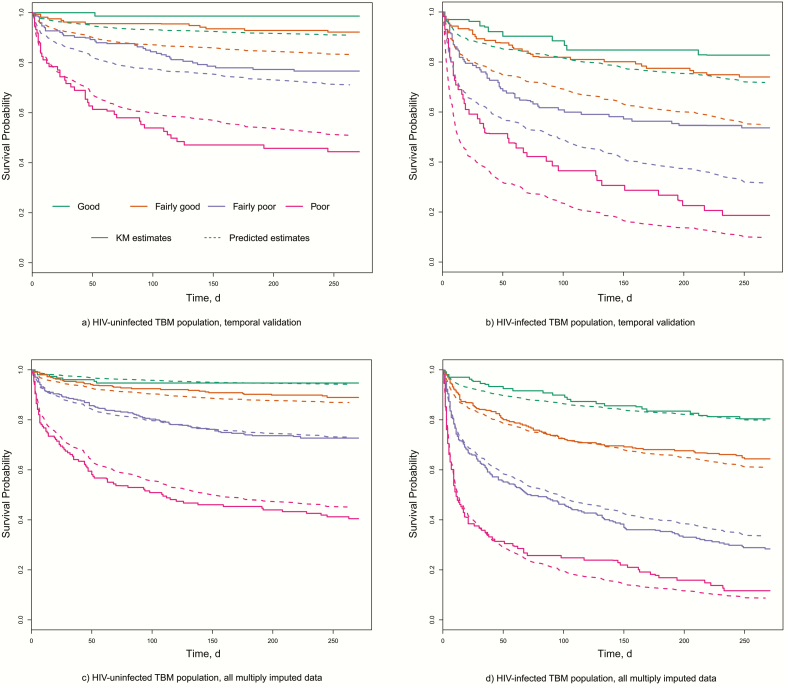
Figure 2 shows predicted survival curves of the final simplified models in 4 risk groups defined using cutoff points at the 16th, 50th, 84th percentiles of the prognostic index generated by these models [28]. They show strong prognostic separation across risk strata, good agreement between predicted survival curves and Kaplan-Meier estimates in the full data set, and mild to substantial risk overestimation in temporal validation in HIV-uninfected and HIV-infected subjects
Figure 2.
Comparison between predicted survival with the final models and observed Kaplan-Meier estimates. Risk groups are defined using cutoff points at the 16th, 50th, and 84th percentiles of the prognostic index as generated by the final models (defining “good,” “fairly good,” “fairly poor,” and “poor” prognostic subgroups). (A) HIV-uninfected TBM population, temporal validation; (B) HIV-infected TBM population, temporal validation; (C) HIV-uninfected TBM population, all multiply imputed data; (D) HIV-infected TBM population, all multiply imputed data. Abbreviations: HIV, human immunodeficiency virus; KM, Kaplan-Meier; TBM, tuberculous meningitis.
Comparison of Prognostic Models With MRC Grade and GCS Alone
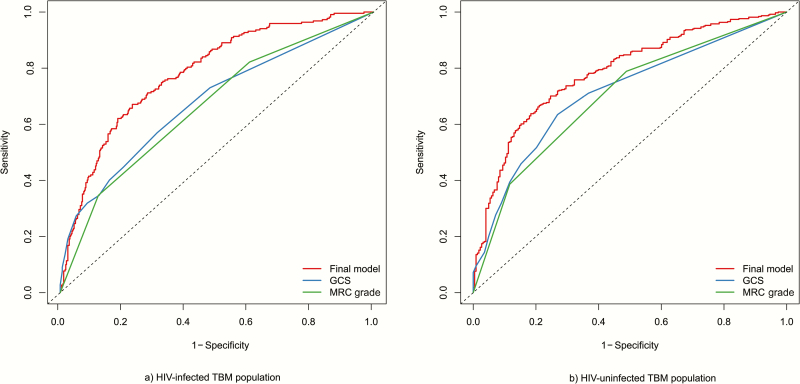
MRC grade and GCS predicted mortality rate similarly well (AUC, 0.66–0.71) (Table 3). The corresponding ROC curves for MRC grade, GCS score, and the final models are shown in Figure 3. MRC grade and GCS score were both clearly inferior to the developed multivariable models in terms of discrimination (for all comparisons, P < .001 for tests of equality between AUC values), whereas discrimination did not differ significantly between MRC grade and GCS score (P = .23 in HIV-uninfected and P = .19 in HIV-infected subjects).
Figure 3.
Cumulative/dynamic receiver operating characteristic curves (apparent estimate) for mortality rates evaluated at 9 months for the final prognostic model, Glasgow Coma Scale (GCS) score, and Medical Research Council (MRC) grade in the tuberculous meningitis populations with or without human immunodeficiency virus infection. MRC grade I is defined as GCS score 15 with no focal neurological signs; grade II, GCS score 11–14, or 15 with focal neurological signs; grade III, GCS score ≤0. (A) HIV-uninfected TBM population; (B) HIV-infected TBM population. Abbreviations: GCS, Glasgow Coma Scale; HIV, human immunodeficiency virus; MRC, Medical Research Council; TBM, tuberculous meningitis.
Nomograms and Web App for Mortality Rate Prediction
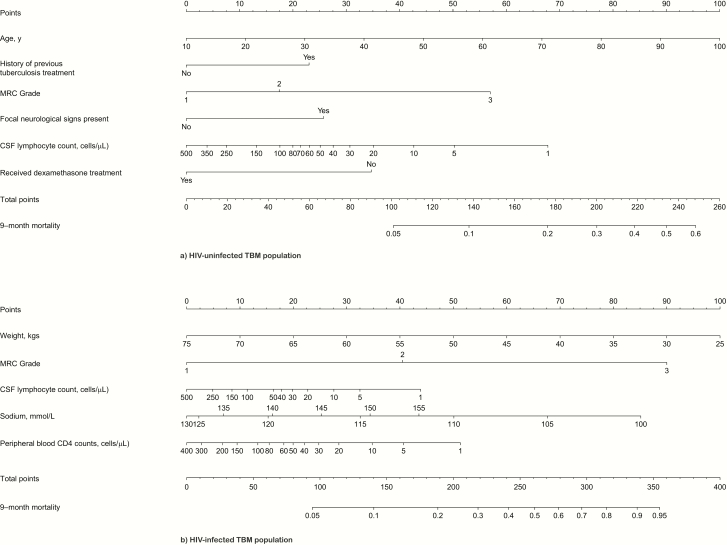
Figure 4 visualizes the final models as nomograms. For clinical use, this model has also been implemented as a user-friendly Web app (available at https://thaole.shinyapps.io/tbmapp/). We also generated nomograms for use when resistance information is available (Supplementary Figure S3). Of note, for creation of the nomogram and the Web app in the HIV-infected population, the cohort variable was chosen as our most recent trial [3], because this is most relevant for future prediction.
Figure 4.
Nomograms for the prediction of 9-month mortality based on the final prognostic models for the tuberculous meningitis populations without (A) or with (B) human immunodeficiency virus (HIV) infection. To derive a prediction, locate the value of each predictor on the corresponding variable line, read the corresponding points assigned on the 0–100 scale, and sum all of these points to a total point score. Then read the result on the “total points” scale and its corresponding prediction below. For HIV-infected population, the cohort variable was chosen as the most recent trial [3], because this is most relevant for future prediction. Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; MRC, Medical Research Council; TBM, tuberculous meningitis.
DISCUSSION
We developed and validated prognostic models for 9-month mortality after TBM diagnosis in a large data set of 951 HIV-uninfected and 748 HIV-infected patients with TBM. We found that a higher MRC grade and lower CSF lymphocyte cell counts. Predictors of death in both groups. MRC grade assesses the severity of neurological impairment and numerous studies have shown its strong association with poor outcome [29, 30]. The predictive value of lower CSF lymphocyte counts is intriguing. Others have recently found that higher CSF neutrophil count predicted death from TBM [31]; both findings reflect that the importance of neuroinflammation in determining outcome from TBM is poorly understood.
Additional risk factors for mortality in HIV-uninfected patients were older age, previous tuberculosis treatment, not receiving dexamethasone, and having focal neurological signs. In HIV-infected subjects, additional risk factors were lower weight, lower CD4 cell count, and abnormal plasma sodium level. These finding are consistent with results from previous published studied conducted in India [32], Hong Kong [17], Taiwan [8], Turkey [33], South Africa [34], and Brazil [35]. The inclusion of different predictors in the 2 HIV populations may suggest important differences in disease pathogenesis and outcomes between these subgroups.
The main analysis did not include drug resistance as a covariate because this information is often not available at enrollment. Alternative models including drug resistance showed only relatively little improvement in model performance, especially in HIV-uninfected subjects. However, we note that the low prevalence in our cohort of multidrug-resistant tuberculosis, an important predictor of poor outcome in TBM [20, 21], may have contributed to this finding.
The final prediction models were carefully developed and validated with proper statistical methods [36]. They showed good discrimination between TBM survivors and patients who died in both internal and temporal validation, with substantial improvement over the MRC grade or the GCS score alone. Although our models had excellent calibration in internal validation, they overestimated mortality rates in temporal validation where the model was developed from earlier studies and tested on subjects included in the most recent trial. This overestimation was mild in HIV-uninfected subjects but substantial in HIV-infected subjects. It may be explained by improvements in treatment, especially the availability of ART, and patients’ supportive care over time. Importantly, because ART did not become widely available in Vietnam until 2005 [37] and receiving ART was an exclusion criterion for the ART timing trial, the intensified treatment trial [3] was the only study that included subjects receiving ART at TBM diagnosis. Therefore, the effect of ART was neglected in the temporal validation, which may have contributed to the observed overestimation. Temporal heterogeneity was addressed by including the cohort as a covariate in the statistical regression models. Survival for future patients is anticipated to be close to predictions adjusted to our most recent study.
Our study has several limitations, mostly owing to the characteristics of our database. First, CSF lactate, which may be an important risk factor [38], was not taken into account in our final model because it was missing in >40% of subjects. Second, previous studies suggested that leukotriene A4 hydrolase (LTA4H) genotype is a determinant of the inflammatory response in HIV-uninfected adults with TBM and consequently might predict who benefits from adjunctive corticosteroid treatment [39]. This factor may therefore influence survival in HIV-uninfected subjects with TBM. Because the information of LTA4H genotype was not available for all included studies, we did not consider this covariate in our analyses. Third, the models were developed and validated based on data from 2 large hospitals in Southern Vietnam only. Thus, it would be desirable to validate and possibly improve our models in an independent external database. Finally, our models included only risk factors available at TBM diagnosis. However, changes in biomarkers repeatedly measured during the disease course may carry important information that could allow an initial prognosis to be updated and improved during tuberculosis treatment. The value of such longitudinally measured biomarkers will be examined in a future research project.
Our final models were displayed as nomograms and implemented in a user-friendly Web-based risk calculator (https://thaole.shinyapps.io/tbmapp/) for use in clinical practice to improve prognostic stratification in patients with TBM. Patients at high risk could be identified early and then monitored more strictly to receive appropriate counseling or supportive care, or enrolled in future clinical trials exploring novel treatments targeted to severe TBM.
Our models are based on one of the largest sources of prospectively collected clinical data for TBM disease worldwide. The final prognostic models included variables that are usually available for patients with TBM. Overall, our prediction models have good discrimination and calibration performance, and they clearly outperformed the MRC grading score.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank all the study physicians and nurses of Pham Ngoc Thach Hospital and the Hospital for Tropical Diseases for their attentive care to the patients, and the staff of the Clinical Trials Unit and Tuberculosis Group of the Oxford University Clinical Research Unit for their enduring support; we also gratefully acknowledge all the patients and their relatives for their participation in the included studies.
Financial support. This work was supported by the Academy of Medical Sciences and the Health Foundation (Clinician Scientist Fellowship to M. E. T.), the National Institute of Health Research Cambridge Biomedical Research Centre (M. E. T), and a Wellcome Trust Intermediate Fellowship (grant number WT097147MA) to J.D.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report. Geneva: 2015. [Google Scholar]
- 2. Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 2013; 12:999–1010. [DOI] [PubMed] [Google Scholar]
- 3. Heemskerk AD, Bang ND, Mai NT et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med 2016; 374:124–34. [DOI] [PubMed] [Google Scholar]
- 4. Streptomycin in Tuberculosis Trials Commitee, Medical Research Council. Streptomycin treatment of tuberculous meningitis. Lancet 1948; 251:582–96. [PubMed] [Google Scholar]
- 5. Thwaites GE, Tran TH. Tuberculous meningitis: many questions, too few answers. Lancet Neurol 2005; 4:160–70. [DOI] [PubMed] [Google Scholar]
- 6. Erdem H, Ozturk-Engin D, Tireli H et al. Hamsi scoring in the prediction of unfavorable outcomes from tuberculous meningitis: results of Haydarpasa-II study. J Neurol 2015; 262:890–8. [DOI] [PubMed] [Google Scholar]
- 7. Hosoğlu S, Ayaz C, Geyik MF, Kökoğlu OF, Ceviz A. Tuberculous meningitis in adults: an eleven-year review. Int J Tuberc Lung Dis 1998; 2:553–7. [PubMed] [Google Scholar]
- 8. Hsu PC, Yang CC, Ye JJ, Huang PY, Chiang PC, Lee MH. Prognostic factors of tuberculous meningitis in adults: a 6-year retrospective study at a tertiary hospital in northern Taiwan. J Microbiol Immunol Infect 2010; 43:111–8. [DOI] [PubMed] [Google Scholar]
- 9. Misra UK, Kalita J, Roy AK, Mandal SK, Srivastava M. Role of clinical, radiological, and neurophysiological changes in predicting the outcome of tuberculous meningitis: a multivariable analysis. J Neurol Neurosurg Psychiatry 2000; 68:300–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thwaites GE, Nguyen DB, Nguyen HD et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004; 351:1741–51. [DOI] [PubMed] [Google Scholar]
- 11. Thwaites GE, Bhavnani SM, Chau TT et al. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother 2011; 55:3244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torok ME, Chau TT, Mai PP et al. Clinical and microbiological features of HIV-associated tuberculous meningitis in Vietnamese adults. PLoS One 2008; 3:e1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Török ME, Yen NT, Chau TT et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)–associated tuberculous meningitis. Clin Infect Dis 2011; 52:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marais S, Thwaites G, Schoeman JF et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 2010; 10:803–12. [DOI] [PubMed] [Google Scholar]
- 15. Ardito F, Posteraro B, Sanguinetti M, Zanetti S, Fadda G. Evaluation of BACTEC Mycobacteria Growth Indicator Tube (MGIT 960) automated system for drug susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol 2001; 39:4440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thwaites GE, Tran TH. Tuberculous meningitis: many questions, too few answers. Lancet Neurol 2005; 4:160–70. [DOI] [PubMed] [Google Scholar]
- 17. Lau KK, Yu IT, Chan AC et al. ; Hong Kong Tuberculous Meningitis Study Group A registry of tuberculous meningitis in Hong Kong. Int J Tuberc Lung Dis 2005; 9:1391–7. [PubMed] [Google Scholar]
- 18. Yasar KK, Pehlivanoglu F, Sengoz G. Predictors of mortality in tuberculous meningitis: a multivariate analysis of 160 cases. Int J Tuberc Lung Dis 2010; 14:1330–5. [PubMed] [Google Scholar]
- 19. Harrell F, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. 1996; 15:361–87. [DOI] [PubMed] [Google Scholar]
- 20. Tho DQ, Török ME, Yen NT et al. Influence of antituberculosis drug resistance and Mycobacterium tuberculosis lineage on outcome in HIV-associated tuberculous meningitis. Antimicrob Agents Chemother 2012; 56:3074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thwaites GE, Lan NT, Dung NH et al. Effect of antituberculosis drug resistance on response to treatment and outcome in adults with tuberculous meningitis. J Infect Dis 2005; 192:79–88. [DOI] [PubMed] [Google Scholar]
- 22. van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate imputation by chained equations in R. J Stat Softw 2011; 45:1–67. [Google Scholar]
- 23. Hastie T, Tibshirani R, Friedman J.. The elements of statistical learning. New York: Springer New York, 2009. [Google Scholar]
- 24. Heymans MW, van Buuren S, Knol DL, van Mechelen W, de Vet HC. Variable selection under multiple imputation using the bootstrap in a prognostic study. BMC Med Res Methodol 2007; 7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blanche P, Latouche A, Viallon V. Time-dependent AUC with right-censored data: a survey. In: Lee MLT, Gail M, Pfeiffer R, Satten G, Cai T, Gandy A, eds. Risk assessment and evaluation of predictions. New York: Springer New York, 2013:239–51. [Google Scholar]
- 26. Harrell F. Regression modeling strategies with applications to linear models, logistic and ordinal regression, and survival analysis. New York: Springer New York, 2015. [Google Scholar]
- 27. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 28. Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol 2013; 13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalita J, Misra UK. Outcome of tuberculous meningitis at 6 and 12 months: a multiple regression analysis. Int J Tuberc Lung Dis 1999; 3:261–5. [PubMed] [Google Scholar]
- 30. Misra UK, Kalita J, Srivastava M, Mandal SK. Prognosis of tuberculous meningitis: a multivariate analysis. J Neurol Sci 1996; 137:57–61. [DOI] [PubMed] [Google Scholar]
- 31. van Laarhoven A, Dian S, Ruesen C et al. Clinical parameters, routine inflammatory markers, and LTA4H genotype as predictors of mortality among 608 patients with tuberculous meningitis in Indonesia. J Infect Dis 2017; 215:1029–39. [DOI] [PubMed] [Google Scholar]
- 32. Yasar KK, Pehlivanoglu F, Sengoz G. Predictors of mortality in tuberculous meningitis: a multivariate analysis of 160 cases. Int J Tuberc Lung Dis 2010; 14:1330–5. [PubMed] [Google Scholar]
- 33. Hosoglu S, Geyik MF, Balik I et al. Predictors of outcome in patients with tuberculous meningitis. Int J Tuberc Lung Dis 2002; 6:64–70. [PubMed] [Google Scholar]
- 34. Marais S, Pepper DJ, Schutz C, Wilkinson RJ. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS One 2011; 6:e20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Croda MG, Vidal JE, Hernández AV, Dal Molin T, Gualberto FA, de Oliveira ACP. Tuberculous meningitis in HIV-infected patients in Brazil: clinical and laboratory characteristics and factors associated with mortality. Int J Infect Dis 2010; 14:e586–91. [DOI] [PubMed] [Google Scholar]
- 36. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015; 162:55–63. [DOI] [PubMed] [Google Scholar]
- 37. Nguyen DB, Do NT, Shiraishi RW et al. Outcomes of antiretroviral therapy in Vietnam: results from a national evaluation. PLoS One 2013; 8:e55750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thwaites GE, Simmons CP, Than Ha Quyen N et al. Pathophysiology and prognosis in vietnamese adults with tuberculous meningitis. J Infect Dis 2003; 188:1105–15. [DOI] [PubMed] [Google Scholar]
- 39. Thuong NTT, Heemskerk D, Tram TTB et al. Leukotriene A4 hydrolase genotype and HIV infection influence intracerebral inflammation and survival from tuberculous meningitis. J Infect Dis 2017; 215:1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.