Summary
HCV-positive men have a higher risk of AMI than HCV-negative men at higher TC/LDL levels; this risk is more pronounced at a younger age. Lipid lowering therapy significantly reduces this risk, with more profound reduction among those with HCV.
Keywords: hepatitis C virus, acute myocardial infarction, lipid, cholesterol, ERCHIVES
Abstract
Background
Risk of acute myocardial infarction (AMI) among hepatitis C virus (HCV)-positive versus HCV-negative persons with similar lipid levels is unknown. We determined incident AMI rates among HCV-positive and HCV-negative men among various lipid strata.
Methods
We created a propensity score matched (PSM) cohort and a low cardiovascular disease (CVD) risk cohort. Primary outcome was incident AMI rates by HCV status in each lipid strata using National Cholesterol Program guidelines for lipid strata.
Results
We identified 85863 HCV-positive and HCV-negative men in the PSM population. The incidence rates/1000 patient-years (95% confidence interval [CI]) for AMI among total cholesterol (TC) 200–239 stratum were 5.3 (4.89, 5.71) for HCV-positive versus 4.71 (4.42, 5) for HCV-negative men (P = .02) and for TC >240 mg/dL were 7.38 (6.49, 8.26) versus 6.17 (5.64, 6.71) (P = .02). For low-density lipoprotein cholesterol (LDL) of 130–159 mg/dL, AMI rates were 5.44 (4.97, 5.91) for HCV-positive and 4.81 (4.48, 5.14) for HCV-negative men (P = .03). The rise in risk with increasing lipid levels was greater in younger HCV-positive than in HCV-negative men (e.g., TC > 240 mg/dL: age >50 HR 1.38 [HCV-positive] and 1.12 [HCV-negative]; age ≤50 HR 1.6 [HCV-positive] and 1.29 [HCV-negative]), and more profoundly altered in HCV-positive men by lipid lowering therapy (change in HR with lipid-lowering therapy for TC >240 mg/dL from 1.82 to 1.19 [HCV-positive] from 1.48 to 1.03 [HCV-negative]).
Conclusions
HCV-positive men have a higher risk of AMI than HCV-negative men at higher TC/LDL levels; this risk is more pronounced at a younger age. Lipid lowering therapy significantly reduces this risk, with more profound reduction among HCV-positive versus HCV-negative men at similar lipid levels.
(See the Editorial Commentary by Tien on pages 566–7.)
There is significant controversy about the association of hepatitis C virus (HCV) infection with coronary artery disease (CAD) [1–5]. Some studies have demonstrated a higher risk of CAD among HCV-infected persons and a higher degree and severity of angiographically proven CAD [1, 5], whereas others have not found such an association. The understanding of cardiovascular disease (CVD) risk in HCV-infected populations is complicated by the differing prevalence of various CVD risk factors in HCV-infected compared with uninfected persons and the poorly understood interaction of those factors. Of particular note, untreated HCV-infected persons have substantially lower total and low-density lipoprotein cholesterol (LDL) levels compared with demographically similar HCV-uninfected persons [6, 7]. Increasing severity of liver disease has also been associated with progressively lower lipid levels, further complicating the association [8]. It is unknown whether the lower serum lipid levels in HCV-infected persons confer lower risk of CVD events in this population independent of other CVD risk factors. Knowledge of any association, and how that might differ from HCV-uninfected persons, may be useful in developing targeted guidelines for lipid management in HCV-infected persons. The primary objective of our study was to determine the association of lipid levels with the risk of acute myocardial infarction (AMI) in HCV-infected and uninfected persons. We performed our analysis in propensity score matched HCV-infected and uninfected persons, as well as in persons free of most traditional CVD risk factors, so as to quantify the independent contribution of lipids to the risk of AMI in this population.
METHODS
Study Population
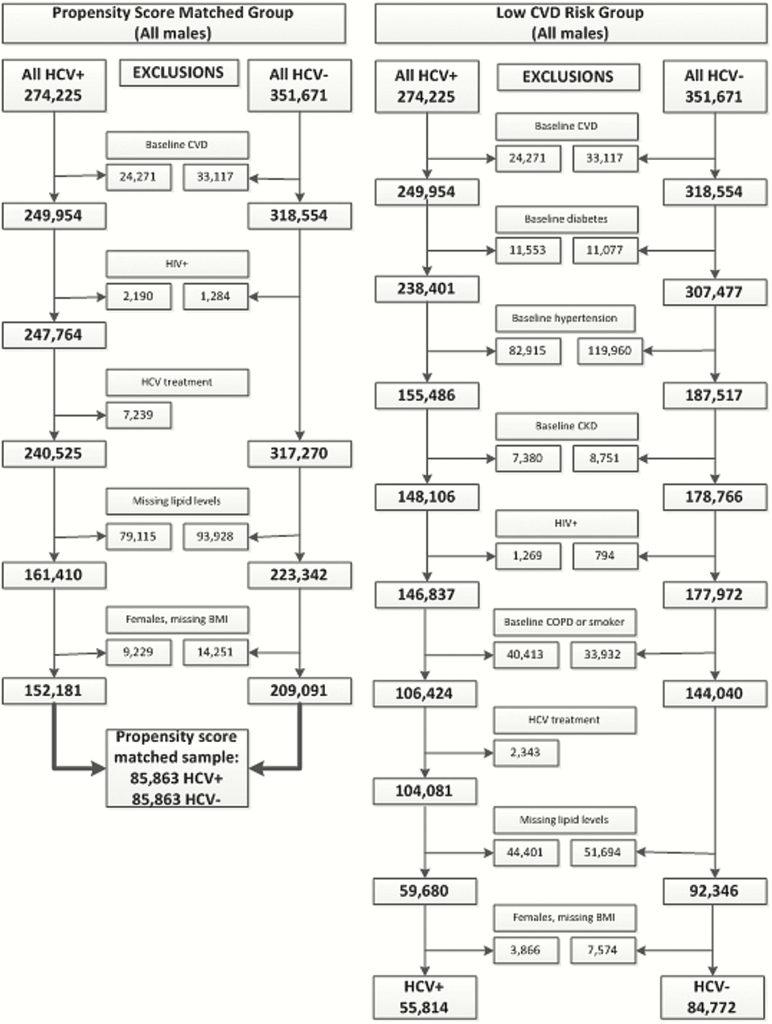
We used ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans), a well-established, previously described national cohort of HCV-infected Veterans and demographically matched HCV-uninfected controls for our study [9–13]. Demographic, clinical, pharmacy, laboratory, and mortality data are retrieved from multiple well-characterized sources in the Department of Veterans Affairs (VA) Healthcare System and merged using previously described methods [1, 14–16]. From the main ERCHIVES database spanning 2001–2015, we created 2 data sets. First, we created a “propensity score matched” data set as follows: among HCV-infected and uninfected persons we excluded those with baseline CVD (based on ICD-9 codes for AMI, stroke, heart failure, peripheral vascular disease, unstable angina, angioplasty, and coronary artery bypass graft surgery), human immunodeficiency virus (HIV) coinfection, those missing baseline lipid measurement or body mass index (BMI), and females (due to small numbers). Within the HCV-infected group, we also excluded those who received treatment for HCV for >2 weeks. We then matched HCV infected and uninfected persons on a difference of propensity score of <0.001 on age, race, BMI, receipt of lipid lowering therapy, hypertension, diabetes, smoking, chronic obstructive pulmonary disease, and stage of chronic kidney disease. Second, we created a “low CVD risk” data set as follows: among HCV-infected and uninfected persons, we excluded those with a diagnosis of CVD, diabetes mellitus, hypertension, stage 3–5 chronic kidney disease, HIV coinfection, chronic obstructive pulmonary disease and smoking at baseline. Those who received HCV treatment for >2 weeks, had missing baseline lipid levels or BMI measurement and women (due to small numbers) were also excluded.
Definitions
AMI was defined by the presence of at least one inpatient or two outpatient codes for AMI. These codes have been shown to have a high positive predictive value (PPV 96.9%) [17] and high correlation with adjudicated events based on chart reviews [18]. Other comorbidities (diabetes, hypertension, chronic kidney disease, smoking, chronic obstructive pulmonary disease, HIV infection) were identified at baseline (diagnosed any time prior and up to and 6 months after HCV diagnosis) using definitions provided in our prior publications [14, 19, 20].
Lipid levels were retrieved from the routine labs performed during the course of care. Total cholesterol, LDL, high-density lipoprotein cholesterol (HDL), and triglyceride (TG) levels were retrieved and divided into groups based on the desirable levels set forth by the National Cholesterol Education Program, whereby the optimal or the desirable level was used as the comparator group, except for high-density lipoprotein, for which the comparator group was low HDL (HDL < 40 mg/dL) [21].
Analyses
We separately analyzed the propensity score matched group and the low CVD risk group. Baseline characteristics and lipid levels were compared between HCV-infected and uninfected persons using χ2 test or t-test for normally distributed data and Wilcoxon-Mann-Whitney test for nonnormally distributed data, as appropriate. We calculated the incident AMI rates per 1000 patient-years of follow-up for each lipid stratum for HCV-infected and uninfected persons. We used a Cox proportional hazards model to determine the hazards ratios (HRs) for AMI diagnosis within each lipid stratum in the HCV-infected and uninfected groups separately, using the optimal level as the comparator group. Assumptions of proportionality were tested using the Schoenfeld residuals. When the assumptions were not met, we stratified the analysis by the variable.
Additionally, we compared AMI rates within the HCV infected propensity score matched and low CVD risk groups by HCV RNA positivity. We also determined the risk of AMI conferred by HCV infection by analyzing the combined (HCV-positive and HCV-negative) propensity score matched group and determining the HR for AMI for each lipid stratum among the HCV-positive using HCV-negative as the comparator.
We used SAS® (version 9.4, SAS Institute Inc., Cary, NC, USA) and Stata® version 11 (Stata Corp., College Station, TX, USA) for analyses. The study was approved by the Institutional Review Board at VA Pittsburgh Healthcare System, Pittsburgh, Pennsylvania.
RESULTS
Baseline Characteristics
From 274225 HCV infected and 351671 HCV uninfected persons in ERCHIVES, we identified 85863 HCV-infected and uninfected persons in propensity score matched population and 55814 HCV-infected and 84772 HCV-uninfected persons in the low CVD risk population. (Figure 1) In the propensity score matched population, the median age was 54 years (interquartile range [IQR] 49, 58), 63.3% were white, median BMI was 27.7 kg/m2 (IQR 24.8, 31.1), and 24.8% had received lipid lowering therapy. In the low CVD risk population, HCV-infected persons had a lower median BMI compared with HCV-uninfected (26.7 kg/m2 [IQR 23.8, 30.1] vs 28.3 kg/m2 [IQR 25.3, 31.8], P < .001), lower total cholesterol (TC), LDL, and TG levels. HDL levels were similar in both groups. HCV-infected persons were less likely to be on a lipid lowering agent compared with HCV-uninfected persons (11.1% vs 24.5%, P < .001). (Table 1)
Figure 1.
Creation of the data set for the current study from ERCHIVES. Abbreviations: BMI, body mass index; COPD, chronic obstructuve pulmonary disease; CVD, cardiovascular disease; CKD, chronic kidney disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Table 1.
Baseline Characteristics of the Final Evaluable Study Population
| Propensity Score Matched Population | Low Cardiovascular Disease Risk Population | |||||
|---|---|---|---|---|---|---|
| HCV Positive N = 85863 | HCV Negative N = 85863 | P-Value | HCV Positive N = 55814 | HCV Negative N = 84772 | P-Value | |
| Age, median (IQR) | 54 (49, 58) | 54 (49, 58) | Matched | 53 (48, 57) | 52 (46, 58) | <.001 |
| Race | ||||||
| White | 63.6% | 63.6% | Matched | 59.0% | 59.2% | .007 |
| Black | 26.2% | 26.2% | 22.0% | 22.0% | ||
| Hispanic | 1.7% | 1.7% | 4.6% | 4.9% | ||
| Other/Unknown | 8.54% | 8.5% | 14.3% | 13.9% | ||
| Body mass index, median (IQR) kg/m2 | 27.7 (24.8, 31.1) | 27.7 (24.8, 31.0) | Matched | 26.73 (23.85, 30.13) | 28.31 (25.27, 31.78) | <.001 |
| Lipid lowering therapy | 24.8% | 24.8% | Matched | 11.1% | 24.5% | <0.001 |
| Hypertension | 40.2% | 37.4% | <.001 | Excluded | Excluded | N/A |
| Diabetes | 3.7% | 3.2% | <.001 | Excluded | Excluded | N/A |
| Smoking | 26.3% | 25.4% | <.001 | Excluded | Excluded | N/A |
| Chronic obstructive pulmonary disease | 1.5% | 1.5% | .8 | Excluded | Excluded | N/A |
| Chronic kidney disease stage | ||||||
| 1 | 14.0% | 14.0% | Matched | 20.3 | 13.8 | N/A |
| 2 | 38.6% | 38.6% | 25.3 | 28.6 | ||
| 3 | 5.8% | 5.8% | Excluded | Excluded | ||
| 4–5 | 0.5% | 0.5% | Excluded | Excluded | ||
| Missing | 41.2% | 41.2% | 54.4 | 57.6 | ||
| Mean FIB-4 score (SD) | 2.2 (4.3) | 1.3 (1.3) | <.001 | 2.2 (5.1) | 1.2 (1.0) | <.001 |
| FIB-4 >3.5, % | 12.0 | 2.3 | <.001 | 11.9 | 1.5 | <.001 |
| Total cholesterol, median (IQR) mg/dL | 174.5 (150.5, 200.0) | 193.5 (169.0, 218.5) | <.001 | 174 (150, 200) | 195 (171, 221.5) | <.001 |
| <200 | 74.4% | 56.8% | 74.4% | 54.4% | ||
| 200–239 | 19.8% | 31.5% | 19.5% | 32.1% | ||
| >240 | 5.8% | 11.7% | 5.8% | 13.5% | ||
| LDL, median (IQR) mg/dL | 102.8 (81.5, 125.8) | 117.5 (95.7, 140.0) | <.001 | 102 (81, 126) | 120.5 (98, 144) | <.001 |
| <100 | 46.4% | 29.2% | 46.9% | 26.6% | ||
| 100–129 | 32.0% | 35.1% | 31.2% | 34.0% | ||
| 130–159 | 15.7% | 24.6% | 15.7% | 26.2% | ||
| ≥160 | 5.9% | 11.0% | 6.2% | 13.2% | ||
| HDL, median (IQR) mg/dL | 43 (35.0,53.0) | 44 (36.5,54.0) | <.001 | 44 (36, 54) | 44 (37, 53) | <.001 |
| <40 | 39.8% | 35.4% | 36.7% | 35.3% | ||
| 40–59 | 44.5% | 48.1% | 46.0% | 50.1% | ||
| ≥60 | 15.7% | 16.5% | 17.4% | 14.5% | ||
| Triglycerides, median (IQR) mg/dL | 115 (81.0, 168.0) | 128 (87.0, 192.0) | <.001 | 107 (76, 156) | 127 (86, 191) | <.001 |
| <150 | 68.3% | 60.5% | 72.6% | 60.7% | ||
| 150–199 | 14.9% | 16.5% | 13.5% | 16.5% | ||
| 200–499 | 15.9% | 21.6% | 13.3% | 21.5% | ||
| ≥500 | 0.9% | 1.3% | 0.6% | 1.3% | ||
Abbreviations: HCV, hepatitis C virus; HDL, high density lipoprotein cholesterol; IQR, interquartile range; LDL, low density lipoprotein cholesterol; N/A, not applicable; SD, standard deviation.
Propensity Score Matched Population
In the propensity score matched population, a total of 2710 AMI events were identified in the HCV-infected and 3239 in the HCV-uninfected persons. The incidence rate for AMI/1000 patient years was higher among HCV-infected persons with higher TC levels compared with the HCV-uninfected persons in the same TC level strata (e.g., for TC >240 mg/dL rates [95% CI] were 7.38 [6.49, 8.26] for HCV-infected vs 6.17 [5.64, 6.71] for HCV-uninfected; P = .02). For LDL stratum of 130–159 mg/dL, AMI rates (95% CI) were 5.44 (4.97, 5.91) for HCV-infected and 4.81 (4.48, 5.14) for HCV uninfected persons (P = .03). For TC <200 mg/dL and LDL <100 mg/dL, AMI rates were lower in the HCV-positive compared with HCV-negative population (4.23 vs 4.57, P = .02 for TC; 4.16 vs 4.71, P = .007 for LDL). For the HDL stratum of <40 mg/dL, AMI rates (95% CI) were conversely higher among HCV-uninfected persons (6.23 [5.91, 6.55]) than HCV-infected persons (5.49 [5.18, 5.79]), P = .001. There were no significant differences between HCV-infected and uninfected persons for any of the TG strata (Table 2).
Table 2.
Incidence Rate of Acute Myocardial Infarction (per 1000 Patient Years) at Various Lipid Levels Among Hepatitis C Virus Infected and Uninfected Persons in the Propensity Score Matched Population
| Variable | Category | HCV-Positive N = 85863 | HCV-Negative N = 85863 | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Follow-up Years | N* | AMI Rate (95% CI) | Follow-up Years | N* | AMI Rate (95% CI) | |||
| Total cholesterol, mg/dL | <200 | 424 412 | 1796 | 4.23 (4.04, 4.43) | 370 421 | 1693 | 4.57 (4.35, 4.79) | .02 |
| 200–239 | 121 891 | 646 | 5.30 (4.89, 5.71) | 220 430 | 1038 | 4.71 (4.42, 5.00) | .02 | |
| >240 | 36 330 | 268 | 7.38 (6.49, 8.26) | 82 290 | 508 | 6.17 (5.64, 6.71) | .02 | |
| LDL cholesterol, mg/dL | <100 | 259 519 | 1079 | 4.16 (3.91, 4.41) | 187 414 | 882 | 4.71 (4.40, 5.02) | .007 |
| 100–129 | 190 096 | 856 | 4.50 (4.20, 4.80) | 237 368 | 1061 | 4.47 (4.20, 4.74) | .89 | |
| 130–159 | 95 362 | 519 | 5.44 (4.97, 5.91) | 170 974 | 822 | 4.81 (4.48, 5.14) | .03 | |
| ≥160 | 37 656 | 256 | 6.80 (5.97, 7.63) | 77 384 | 474 | 6.13 (5.57, 6.68) | .19 | |
| HDL cholesterol, mg/dL | <40 | 228 234 | 1252 | 5.49 (5.18, 5.79) | 237 457 | 1479 | 6.23 (5.91, 6.55) | .001 |
| 40–59 | 262 394 | 1160 | 4.42 (4.17, 4.68) | 326 685 | 1400 | 4.29 (4.06, 4.51) | .45 | |
| ≥60 | 92 006 | 298 | 3.24 (2.87, 3.61) | 108 999 | 360 | 3.30 (2.96, 3.64) | .83 | |
| Triglycerides, mg/dL | <150 | 390 367 | 1575 | 4.03 (3.84, 4.23) | 398 761 | 1603 | 4.02 (3.82, 4.22) | .93 |
| 150–199 | 89 362 | 494 | 5.53 (5.04, 6.02) | 112 019 | 618 | 5.52 (5.08, 5.95) | 1.00 | |
| 200–499 | 97 124 | 590 | 6.07 (5.58, 6.56) | 153 112 | 936 | 6.11 (5.72, 6.50) | .93 | |
| ≥500 | 5781 | 51 | 8.82 (6.40, 11.2) | 9248 | 82 | 8.87 (6.95, 10.8) | .95 | |
Abbreviations: AMI, acute myocardial infarction; CI, confidence interval; HCV, hepatitis C virus; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol.
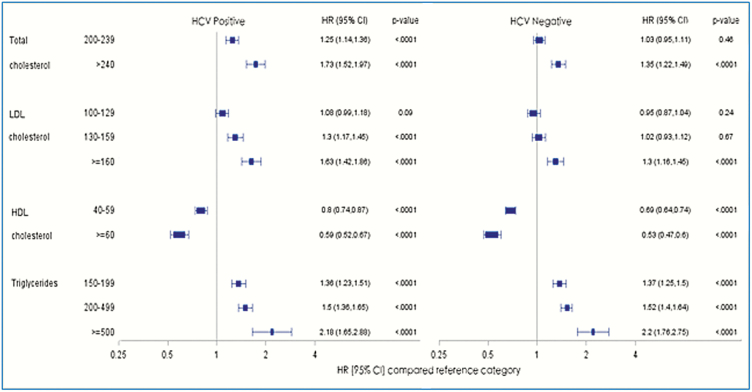
The risk of AMI was higher with increasing TC, LDL, and TG levels, and lower with increasing HDL levels compared with optimal levels within HCV-infected and uninfected persons (Figure 2). The HRs were numerically higher in the HCV-infected persons with higher TC and LDL levels but not for TG levels.
Figure 2.
Risk of acute myocardial infarction in the propensity score matched population in various lipid groups (Cox proportional hazards model). Reference categories were total cholesterol < 200, LDL cholesterol < 100, HDL cholesterol <40, and triglycerides < 150 mg/dL. Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HDL, high density lipoprotein cholesterol; HR, hazards ratio; LDL, low density lipoprotein cholesterol.
Low Cardiovascular Disease Risk Population
In the low CVD risk population, a total of 1231 AMI events were identified in the HCV infected and 2222 in the HCV-uninfected persons. The incidence rates of AMI/1000 patient years of follow-up were higher and statistically significant among HCV-infected versus HCV uninfected for the TC stratum of 200–239 mg/dL (4.01 [3.57, 4.44] vs 3.47 [3.22, 3.71], P = .03) and numerically higher but not statistically significant for the TC stratum of >240 mg/dL (5.13 [4.24, 6.03] vs 4.35 [3.94, 4.77], P = .12) (Table 3). For LDL stratum of ≥160 mg/dL, the rates were higher for HCV-infected versus HCV-uninfected persons (5.43 [4.54, 6.32] vs 4.30 [3.88, 4.72], P = .02). For TC <200 mg/dL and LDL <100 mg/dL, AMI rates were lower in the HCV-positive compared with HCV-negative population (2.69 vs 2.97, P = .04 for TC; 2.5 vs 3.08, P < .01 for LDL). For HDL and TG, there were no significant differences among HCV infected versus HCV-infected persons at the higher strata.
Table 3.
Incidence Rate of Acute Myocardial Infarction (per 1000 patient-years) at Various Lipid Levels Among Hepatitis C Virus Infected and Uninfected Persons in the Low CVD Risk Population
| Variable | Category | HCV Positive | HCV Negative | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| N = 55814 | N = 84772 | |||||||
| Follow-up Years | Number of Events | AMI Rate (95% CI) | Follow-up Years | Number of Events | AMI Rate (95% CI) | |||
| Total cholesterol, mg/dL | <200 | 289073.3 | 777 | 2.69 (2.50, 2.88) | 348627.5 | 1034 | 2.97 (2.79, 3.15) | .04 |
| 200–239 | 81853.31 | 328 | 4.01 (3.57, 4.44) | 222195.6 | 771 | 3.47 (3.22, 3.71) | .03 | |
| >240 | 24556.29 | 126 | 5.13 (4.24, 6.03) | 95795.58 | 417 | 4.35 (3.94, 4.77) | .12 | |
| LDL cholesterol, mg/dL | <100 | 178697.4 | 447 | 2.50 (2.27, 2.73) | 168587.5 | 519 | 3.08 (2.81, 3.34) | <.01 |
| 100–129 | 125517.4 | 397 | 3.16 (2.85, 3.47) | 226242.5 | 683 | 3.02 (2.79, 3.25) | .48 | |
| 130–159 | 64937.96 | 244 | 3.76 (3.29, 4.23) | 179057.9 | 621 | 3.47 (3.20, 3.74) | .31 | |
| ≥160 | 26330.07 | 143 | 5.43 (4.54, 6.32) | 92730.85 | 399 | 4.30 (3.88, 4.72) | .02 | |
| HDL cholesterol, mg/dL | Ref <40 | 143122 | 519 | 3.63 (3.31, 3.94) | 234886.4 | 992 | 4.22 (3.96, 4.49) | .01 |
| 40–59 | 183583 | 557 | 3.03 (2.78, 3.29) | 336803.4 | 998 | 2.96 (2.78, 3.15) | .67 | |
| ≥60 | 68777.86 | 155 | 2.25 (1.90, 2.61) | 94928.99 | 232 | 2.44 (2.13, 2.76) | .47 | |
| Triglycerides, mg/dL | <150 | 280482.4 | 775 | 2.76 (2.57, 2.96) | 393124.9 | 1095 | 2.79 (2.62, 2.95) | .88 |
| 150–199 | 55523.38 | 211 | 3.80 (3.29, 4.31) | 112145.6 | 410 | 3.66 (3.30, 4.01) | .68 | |
| 200–499 | 57018.39 | 233 | 4.09 (3.56, 4.61) | 152075.5 | 660 | 4.34 (4.01, 4.67) | .45 | |
| ≥500 | 2458.74 | 12 | 4.88 (2.12, 7.64) | 9272.63 | 57 | 6.15 (4.55, 7.74) | .56 | |
Abbreviations: AMI, acute myocardial infarction; CI, confidence interval; HCV, hepatitis C virus; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol.
To further understand the contribution of HCV infection to the risk of AMI, we determined the hazards of AMI among the overall group at each lipid stratum using the HCV-uninfected group in the same stratum as the comparator group in the propensity score matched cohort. The hazards were higher for TC strata of 200–239 mg/dL (1.13 [1.02, 1.25]) and >240 mg/dL (1.19 [1.03, 1.38]) and LDL stratum of 130–159 mg/Dl (1.13 [1.01, 1.26] (Table 4). The association was not significant for higher TG or HDL levels.
Table 4.
Risk of Acute Myocardial Infarction Associated With Hepatitis C Virus (HCV) Infection at Various Lipid Levels in the Overall Propensity Score Population, With HCV Uninfected in the Same Lipid Stratum Serving as the Comparator (Cox Proportional Hazards Model)
| Variable | N = 171 726 | HCV+ (Ref HCV-) | P-Value |
|---|---|---|---|
| Category | HR (95% CI) | ||
| Total cholesterol, mg/dL | <200 | 0.93 (0.87, 0.99) | .03 |
| 200–239 | 1.13 (1.02, 1.25) | .02 | |
| >240 | 1.19 (1.03, 1.38) | .02 | |
| LDL cholesterol, mg/dL | <100 | 0.88 (0.81, 0.97) | .01 |
| 100–129 | 1.01 (0.93, 1.11) | .76 | |
| 130–159 | 1.13 (1.01, 1.26) | .03 | |
| ≥160 | 1.11 (0.96, 1.29) | .17 | |
| HDL cholesterol, mg/dL | <40 | 0.88 (0.82, 0.95) | .001 |
| 40–59 | 1.04 (0.96, 1.12) | .36 | |
| ≥60 | 0.98 (0.84, 1.15) | .83 | |
| Triglycerides, mg/dL | <150 | 1.01 (0.94, 1.08) | .81 |
| 150–199 | 1.00 (0.89, 1.13) | .97 | |
| 200–499 | 0.99 (0.9, 1.10) | .90 | |
| ≥500 | 1.00 (0.71, 1.42) | .99 |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HDL, high density lipoprotein cholesterol; HR, hazard ratio; LDL, low density lipoprotein cholesterol.
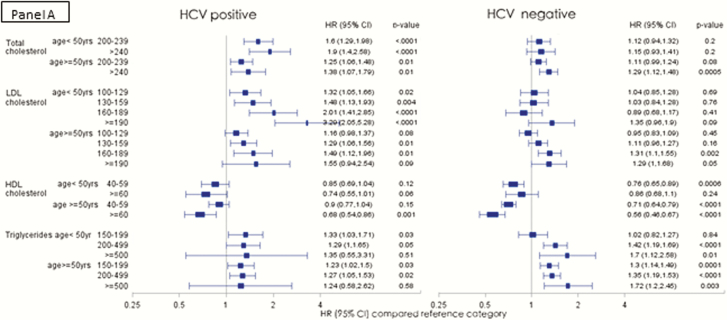
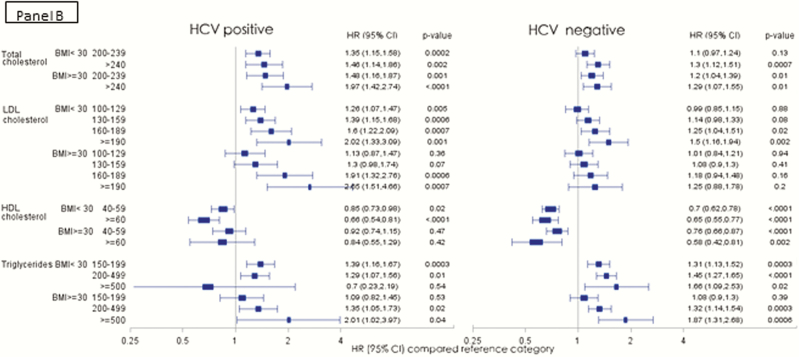
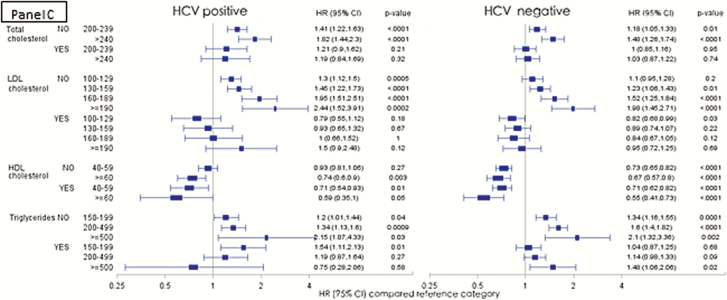
We further analyzed the risk of AMI among HCV-infected and uninfected persons in the low CVD risk population stratified by age (<50 vs ≥50 years; Figure 3A), BMI (<30 vs ≥30; Figure 3B) and receipt of lipid lowering therapy (Figure 3C). Among the HCV-infected, the risk of AMI with increasing TC and LDL levels was higher among the younger age group, whereas in the HCV-uninfected persons, a more traditional risk profile with increasing risk in the older age group was observed (Figure 3A). When stratified by BMI categories, the risk profile was also more traditional in the HCV-infected group with higher risk of AMI observed in those with higher BMI (Figure 3B). Those who received lipid lowering agents experienced a significant protective effect compared with those who did not receive lipid lowering therapy in the same lipid level strata in both the HCV-infected and uninfected groups (Figure 3C).
Figure 3.
Risk of acute myocardial infarction in the low cardiovascular disease risk population in various lipid groups, stratified by age groups (A), BMI (B), and receipt of lipid lowering therapy (C). (Adjusted Cox proportional hazards model, adjusted for age, race, BMI, and receipt of lipid lowering agents, excluding adjustment for outcome of interest in each model). Abbreviations: BMI, body mass index; CI, confidence interval; HCV, hepatitis C virus; HDL, high density lipoprotein cholesterol; HR, hazards ratio; LDL, low density lipoprotein cholesterol.
Supplementary Analyses
We also determined the incidence rate of AMI at various lipid levels among the full cohort of HCV-infected and uninfected persons after excluding those with baseline CVD, HIV coinfection, >2 weeks of HCV treatment, missing lipid levels, and females (Supplementary Table 1). The trends were similar to those in the propensity score matched and low CVD risk populations.
To determine the possible impact of excluding patients for missing information, we compared the demographics and lipid levels of subjects who were included in the dataset with those who were excluded for the comorbidities listed in Figure 1 in the low CVD risk population. The results were similar (Supplementary Table 2).
Among the full cohort, baseline HCV RNA data were available for 113503 persons in the HCV-infected group, of whom 26946 (23.7%) were HCV RNA negative (Supplementary Table 3). Among the propensity score matched population, HCV RNA levels were available for 39101 persons in the HCV-infected group. Of those, 9275 (23.7%) were HCV RNA negative (Supplementary Table 4). In both analyses, there were no significant differences in the rates of incident AMI among HCV RNA positive versus HCV RNA negative persons in the highest lipid level strata (Supplementary Tables 3 and 4). We also analyzed AMI rates among various groups after excluding those with baseline FIB-4 score >3.5. Results were similar to those provided in the main analyses (Supplementary Tables 5a and 5b).
DISCUSSION
In our large cohort of HCV-infected and uninfected persons, HCV-infected persons had a higher risk of AMI compared with HCV-uninfected persons with TC and LDL levels in the higher strata. This higher risk was seen in the propensity score matched population as well as those at a low risk of CVD due to exclusion of most traditional CVD risk factors. In the within-group (HCV-infected and HCV-uninfected) analyses, rate of AMI was lower in HCV-positive persons with optimal TC and LDL levels compared with HCV-negative persons in the same stratum; however, the risk of AMI increased more rapidly with each subsequent higher lipid stratum in the HCV-infected group than observed in the HCV-uninfected group, suggesting that incremental increases in lipid levels may have greater implications for risk for AMI in HCV-infected persons than in HCV-uninfected persons. Rates of AMI were lower in HDL <40 mg/dL stratum for HCV-positive compared with HCV-negative persons. In the low CVD risk population, a higher risk was observed in the younger age group (<50 years old) compared with older persons within the TC and LDL strata, and this was more pronounced in the HCV-infected compared with HCV-uninfected persons. Additionally, treatment with lipid lowering agents was associated with a greater reduction in risk of AMI among HCV-infected persons compared with HCV-uninfected persons with similar baseline TC, LDL, and TG levels.
There is increasing evidence that HCV is associated with a higher risk of CAD [1, 2, 22]. The increased risk associated with HCV extends to stroke and heart failure [23, 24]. Proposed mechanisms for this increased risk include a pro-inflammatory state with higher levels of pro-inflammatory cytokines associated with a higher risk of CVD events [22], severity of liver disease directly contributing to the risk of myocardial injury [25], and possible direct effects of the virus on the myocardium and endothelium [25, 26]. Infection with HCV is associated with alteration of the lipid profile, but the independent contribution of this to the risk of AMI has been unclear thus far. Our results suggest that higher TC (>200) and LDL (>100) levels among HCV-infected persons should prompt clinicians into evaluating the risk of AMI more carefully in the HCV-infected persons and considering a more aggressive approach to CVD prevention strategies in these persons.
The vast majority of the HCV-infected subjects who had HCV RNA results were HCV RNA positive. Because subjects who received any treatment were excluded, this represents true chronic infection. Our analysis did not demonstrate a consistent higher risk of AMI among HCV RNA positive versus HCV RNA negative and similar lipid levels. This suggests a role of HCV infection itself and not necessarily ongoing viral replication in the genesis of risk of AMI in this population. Future studies should assess the impact of successful HCV eradication upon future risk of AMI events.
The demonstration of a higher risk of AMI among the younger HCV-infected persons is a cause for concern. It is somewhat counterintuitive that younger persons with a similar risk profile would have a higher risk. One possible explanation of this observation is that with increasing age more people may have altered their lifestyle toward healthier behaviors. However, we adjusted for at least 1 possible effect of such behavior, namely, BMI. It is also possible that HCV-infected persons may have reached a censoring event at a younger age (namely, AMI, death), thus biasing the remaining older persons toward a lower risk of events. Also, when we analyzed the risk by BMI categories, the higher BMI categories were associated with a higher risk of AMI. Finally, these data may suggest that lipid levels are a more significant risk factor at a younger age, and with aging, other factors begin to contribute more to the risk of AMI in HCV-infected persons.
Lipid lowering therapy had profound effects on the risk of AMI in all lipid strata (except TG levels 150–199 mg/dL). In each of those strata, the risk of AMI dropped to insignificant levels compared with the baseline stratum in those taking lipid lowering agents. For several strata, the absolute difference in HRs was larger among HCV-infected persons compared with HCV-uninfected persons. Although the reasons for this are unclear, these findings have potential implications in management of HCV-infected persons. Our data suggest that guidelines for treatment of lipid abnormalities in HCV-infected persons may need to be revised to suggest earlier and more aggressive treatment of hyperlipidemia.
Certain limitations need to be considered when interpreting the results from our study. Lipid levels were measured in the course of routine clinical care, so it is unclear how many of these values were collected in a fasting state, although lipid levels change minimally with fasting and even nonfasting lipid levels are associated with cardiovascular events [27]. We used ICD-9 codes to determine AMI. Although this has been used and validated in previous studies, there is a potential for a bias when relying of such codes. It has also been suggested that Veterans with acute, life-threatening conditions like AMI may not present to the VA for their acute care and may seek care at the nearest facility, which may not be a VA facility. We have studied this extensively in the setting of AMI and have found that these codes have high positive predictive values and demonstrate good agreement with formal chart review adjudication processes [18]. The ICD-9 codes used in our study included both inpatient and outpatient records, allowing us to capture events that occurred outside the VA as the Veterans sought follow-up care in the VA system. Finally, our results may not be generalizable to women because we excluded women due to small numbers.
In conclusion, HCV-infected persons may be at a higher risk of AMI than HCV-uninfected persons with similar TC and LDL levels, and this risk may be more pronounced at a younger age. Lipid lowering therapy significantly alters the risk of AMI, with a more profound lowering of risk among HCV-infected persons than HCV-uninfected persons at similar lipid levels. These data suggest that there is a need to revise the guidelines for management of lipid levels in HCV-infected persons.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. This material is the result of work supported with resources and the use of facilities at the VA Pittsburgh Healthcare System and the central data repositories maintained by the VA Information Resource Center, including the National Patient Care Database, Decisions Support System Database, and Pharmacy Benefits Management Database.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Funding and authorship statement. The creation of the database that evolved into ERCHIVES was initially funded by the National Institutes of Health/National Institute on Drug Abuse (K23 DA016175-03; 2003–2008, PI: Butt)
Over subsequent years, parts of ERCHIVES have been funded through investigator initiated or collaborative grants from Valeant, Merck, Gilead Sciences, and AbbVie. Current analyses were not funded by any specific grant or entity. A. A. B had complete access to data at all times and accepts the responsibility of the integrity of this article.
Potential conflict of interests. A. A. B. Grant support to the institution from Gilead Sciences, AbbVie, and Merck. K. W. C.: Grant support to the institution from Merck and Gilead. All other authors have nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis 2009; 49:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roed T, Lebech AM, Kjaer A, Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging 2012; 32:421–30. [DOI] [PubMed] [Google Scholar]
- 3. Pothineni NV, Rochlani Y, Vallurupalli S et al. Comparison of angiographic burden of coronary artery disease in patients with versus without hepatitis C infection. Am J Cardiol 2015; 116:1041–4. [DOI] [PubMed] [Google Scholar]
- 4. Wong RJ, Kanwal F, Younossi ZM, Ahmed A. Hepatitis C virus infection and coronary artery disease risk: a systematic review of the literature. Dig Dis Sci 2014; 59:1586–93. [DOI] [PubMed] [Google Scholar]
- 5. Satapathy SK, Kim YJ, Kataria A et al. Higher prevalence and more severe coronary artery disease in hepatitis C virus-infected patients: a case control study. J Clin Exp Hepatol 2013; 3:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corey KE, Kane E, Munroe C, Barlow LL, Zheng H, Chung RT. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology 2009; 50:1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Floris-Moore M, Howard AA, Lo Y, Schoenbaum EE, Arnsten JH, Klein RS. Hepatitis C infection is associated with lower lipids and high-sensitivity C-reactive protein in HIV-infected men. AIDS Patient Care STDS 2007; 21:479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cicognani C, Malavolti M, Morselli-Labate AM, Zamboni L, Sama C, Barbara L. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med 1997; 157:792–6. [PubMed] [Google Scholar]
- 9. Mohanty A, Erqou S, McGinnis KA et al. Therapy for hepatitis C virus infection increases survival of patients with pretreatment anemia. Clin Gastroenterol Hepatol 2013; 11:741–7.e3. [DOI] [PubMed] [Google Scholar]
- 10. Chew KW, Bhattacharya D, McGinnis KA et al. ; ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans) Study. Short communication: coronary heart disease risk by Framingham risk score in hepatitis C and HIV/hepatitis C-coinfected persons. AIDS Res Hum Retroviruses 2015; 31:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butt AA, Yan P, Shaikh OS, Chung RT, Sherman KE; ERCHIVES study Sofosbuvir-based regimens in clinical practice achieve SVR rates closer to clinical trials: results from ERCHIVES. Liver Int 2016; 36:651–8. [DOI] [PubMed] [Google Scholar]
- 12. Butt AA, Yan P, Bonilla H et al. ; ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans) Study Team. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: results from ERCHIVES. Hepatology 2015; 62:365–74. [DOI] [PubMed] [Google Scholar]
- 13. Butt AA, Yan P, Lo Re V 3rd et al. ; ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans) Study Team. Liver fibrosis progression in hepatitis C virus infection after seroconversion. JAMA Intern Med 2015; 175:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Butt AA, Wang X, Fried LF. HCV infection and the incidence of CKD. Am J Kidney Dis 2011; 57:396–402. [DOI] [PubMed] [Google Scholar]
- 15. Butt AA, Yan P, Simon TG, Chung RT, Abou-Samra AB; ERCHIVES study team Changes in circulating lipids level over time after acquiring HCV infection: results from ERCHIVES. BMC Infect Dis 2015; 15:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rogal SS, Yan P, Rimland D et al. ; Electronically Retrieved Cohort of HCV Infected Veterans Study Group. Incidence and progression of chronic kidney disease after hepatitis C seroconversion: results from ERCHIVES. Dig Dis Sci 2016; 61:930–6. [DOI] [PubMed] [Google Scholar]
- 17. Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med 1999; 14:555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freiberg MS, Chang CC, Skanderson M et al. ; Veterans Aging Cohort Study. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes 2011; 4:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butt AA, McGinnis K, Rodriguez-Barradas MC et al. ; Veterans Aging Cohort Study. HIV infection and the risk of diabetes mellitus. AIDS 2009; 23:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butt AA, UA K, McGinnis KA, Skanderson M, Kwoh CK. Co-morbid medical and psychiatric illness and substance abuse in HCV-infected and uninfected veterans. J Viral Hepat 2007; 14: 890–6. [DOI] [PubMed] [Google Scholar]
- 21. NCEP. Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–421. [PubMed] [Google Scholar]
- 22. Oliveira CP, Kappel CR, Siqueira ER et al. Effects of hepatitis C virus on cardiovascular risk in infected patients: a comparative study. Int J Cardiol 2013; 164:221–6. [DOI] [PubMed] [Google Scholar]
- 23. Liao CC, Su TC, Sung FC, Chou WH, Chen TL. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One 2012; 7:e31527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, Shlipak M. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Card Fail 2009; 15:451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maruyama S, Koda M, Oyake N et al. Myocardial injury in patients with chronic hepatitis C infection. J Hepatol 2012; 58: 11–5. [DOI] [PubMed] [Google Scholar]
- 26. Boddi M, Abbate R, Chellini B et al. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol 2010; 47:72–5. [DOI] [PubMed] [Google Scholar]
- 27. Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation 2008; 118:2047–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.