Summary
Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) CC398 was an increasing cause of bacteremia in Denmark during 2010–2015. Most patients lived in rural areas but had no contact to livestock. Whole-genome sequencing supported that Danish pigs are the most likely source of human LA-MRSA CC398 infections.
Keywords: MRSA, humans, livestock, zoonosis, bacteremia
Abstract
Background
Livestock-associated methicillin-resistant Staphylococcus aureus clonal complex 398 (LA-MRSA CC398) is causing an increasing number of skin and soft tissue infections (SSTIs) in Denmark and other European countries with industrial pig production. Yet, its impact on MRSA bloodstream infections (BSIs) has not been well studied.
Methods
We investigated the clinical epidemiology of all human cases of LA-MRSA CC398 BSI during 2010–2015. Cases of LA-MRSA CC398 BSI were compared to cases of BSI caused by other types of MRSA and cases of SSTI caused by LA-MRSA CC398. Whole-genome sequence analysis was used to assess the phylogenetic relationship among LA-MRSA CC398 isolates from Danish pigs and cases of BSI and SSTI.
Results
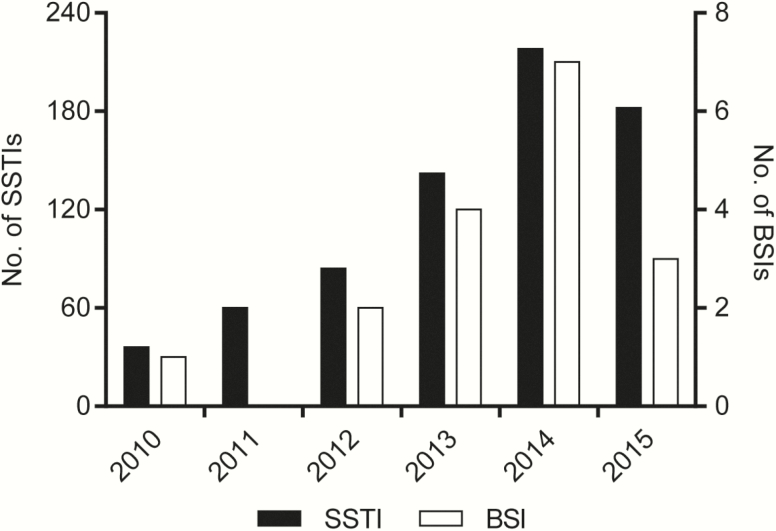
The number of LA-MRSA CC398 BSIs and SSTIs increased over the years, peaking in 2014, when LA-MRSA CC398 accounted for 16% (7/44) and 21% (211/985) of all MRSA BSIs and SSTIs, corresponding to 1.2 and 37.4 cases of BSI and SSTI per 1000000 person-years, respectively. Most patients with LA-MRSA CC398 BSI had no contact to livestock, although they tended to live in rural areas. LA-MRSA CC398 caused 24.3 BSIs per 1000 SSTIs among people with no livestock contact, which is similar to the ratio observed for other types of MRSA. Whole-genome sequence analysis showed that most of the BSI and SSTI isolates were closely related to Danish pig isolates.
Conclusions
This study demonstrates that the increasing number of LA-MRSA CC398 BSIs occurred in parallel with a much larger wave of LA-MRSA CC398 SSTIs and an expanding pig reservoir.
Since the early 2000s, zoonotic transmission of livestock-associated methicillin-resistant Staphylococcus aureus clonal complex 398 (LA-MRSA CC398) has led to an increasing number of human infections in Denmark and other European countries with industrial pig production [1, 2]. LA-MRSA CC398 is primarily associated with skin and soft tissue infections (SSTIs) among otherwise young and healthy livestock workers but also in the general population, which includes elderly and immunocompromised people with an elevated risk of developing invasive staphylococcal illnesses [1]. Yet, its impact on MRSA bloodstream infections (BSIs) is poorly understood. In Denmark, the first case of LA-MRSA CC398 BSI was identified in November 2010 in an 11-year-old boy with leukemia. The patient’s father owned a pig farm and was found to be colonized with LA-MRSA CC398. Subsequently, LA-MRSA CC398 has been described as a sporadic cause of BSIs and deaths in patients with no connection to pigs or pig farmers [3].
The objectives of this study were to: (1) investigate the clinical epidemiology of cases of LA-MRSA CC398 BSI; (2) compare cases of LA-MRSA CC398 BSI to cases of BSI caused by other types of MRSA and cases of SSTI caused by LA-MRSA CC398; and (3) infer the phylogenetic relationship among LA-MRSA CC398 isolates from Danish pigs and cases of BSI and SSTI.
METHODS
The study population included all Danish patients who were registered as having had an episode of MRSA BSI or SSTI between 1 January 2010 and 31 December 2015. Detailed data on the characteristics of MRSA isolates from each episode of BSI and information on the patients at the time of diagnosis were extracted from the national S. aureus bacteremia (SAB) database. For comparative purposes, the national MRSA database was reviewed to identify and abstract information on patients with MRSA SSTIs. The MRSA database contains strain typing results and information about all individuals colonized or infected with MRSA but only at the time when a given MRSA CC is first detected, except from subsequent episodes of BSI, which are recorded in the SAB database. The regional clinical microbiology laboratories performed S. aureus identification and established methicillin resistance by use of standard laboratory methods and forwarded the isolates to the National Reference Laboratory for Staphylococci at Statens Serum Institut. LA-MRSA CC398 was identified using a 3-step procedure. First, MRSA identification was confirmed by polymerase chain reaction (PCR) detection of mecA and spa [4]. Second, MRSA CC398 was differentiated from other types of MRSA using spa typing and PCR detection of the S. aureus CC398-specific sau1-hsdS1 variant. Third, LA-MRSA CC398 was distinguished from the human variant of MRSA CC398 using a dual-probe real-time PCR assay [1]. The following data were collected for each patient’s medical record: sex, age, livestock contact (only available for patients with LA-MRSA CC398 BSI or SSTI), residential address, hospitalization dates, and Charlson comorbidity score (only available for patients with MRSA BSI) [5]. An episode of MRSA BSI was defined as community-onset if the patient had a positive blood culture within the first 48 hours after admission or hospital-onset if the first positive blood culture was obtained ≥48 hours after admission. Data collection was approved by the Danish Data Protection Agency (protocol number 2001-14-0021). Incidence rates for LA-MRSA CC398 BSIs and SSTIs were calculated by using all cases identified in 2014 and Danish census population estimates for 2014 [6]. Pearson correlation coefficient (r) was used to evaluate temporal trends, and patient variables were compared between groups using Student’s t test for continuous data and Fisher’s exact test for categorical data (GraphPad Prism software, version 5, GraphPad, La Jolla, California). The significance level was set at α = .05. Whole-genome phylogenetic tools were used to study the relationship among the following S. aureus CC398 isolates: (1) all 17 BSI isolates collected between 2010 and 2015; (2) a subset of 66 SSTI isolates, including all 36 isolates from 2010 and every third isolate according to isolation date from 2015; (3) a subset of 50 LA-MRSA CC398 isolates collected from Danish pig production farms (1 isolate per farm) in 2010 and 2014 by the Danish Veterinary and Food Administration [7, 8]; and (4) 89 international S. aureus CC398 isolates from humans and various animal species [9]. Six LA-MRSA CC398 isolates from the international collection (2007-70-91-4-SPA, 2008-60-970, 9B, 3-S-1, 2008-60-3254, and 7_2007-70-77-4) originated from Danish pigs and were categorized as such in the analysis. Methodological details are provided in the Supplementary Materials.
RESULTS AND DISCUSSION
LA-MRSA CC398 accounted for 17 cases of BSI, 700 cases of SSTI, and 76 cases with other infections between 2010 and 2015, whereas other types of MRSA caused a total of 145 BSIs, 3897 SSTIs, and 570 other infections during the study period. Cases of LA-MRSA CC398 BSI (cases A–Q) and laboratory findings are described in Table 1. The number of LA-MRSA CC398 BSIs and SSTIs increased over the years, peaking in 2014, where LA-MRSA CC398 accounted for 16% (7/44) and 21% (211/985) of all MRSA BSIs and SSTIs, corresponding to 1.2 and 37.4 cases of BSI and SSTI per 1000000 person-years, respectively (Figure 1). The correlation between the annual numbers of LA-MRSA CC398 BSIs and SSTIs was statistically significant (r = 0.88, P = .02).
Table 1.
Danish Patients With Livestock-Associated Methicillin-Resistant Staphylococcus aureus CC398 Bloodstream Infection and Laboratory Findings
| Case/ Isolate | Sex | Age,y | Isolation Date | Charlson Comorbidity Score | Residence | Disease Onset | Outcomea | Livestock Exposure | Other Epidemiologic Findings | spa Type | Shortest Distance to a Danish Pig LA-MRSA CC398 Isolate, SNPs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 11 | 10 Sept 2010 | 6 | Rural | Community | Yes | t034 | 7 | ||
| B | F | 57 | 7 Apr 2012 | 5 | Urban | Community | No | Possibly foodborne transmission [12] | t899 | 90 | |
| C | F | 51 | 6 Oct 2012 | 4 | Rural | Community | Died | No | t034 | 22 | |
| D | M | 65 | 10 June 2013 | 1 | Rural | Community | Yes | t034 | 8 | ||
| E | M | 63 | 11 Nov 2013 | 3 | Rural | Community | Died | No | Hospital outbreak A [14] | t011 | 16 |
| F | F | 86 | 23 Nov 2013 | 3 | Rural | Community | Died | No | t034 | 7 | |
| G | F | 0 | 4 Dec 2013 | 0 | Rural | Community | Yes | t034 | 9 | ||
| H | F | 60 | 8 Apr 2014 | 2 | Rural | Community | Yes | t034 | 15 | ||
| I | M | 74 | 14 Apr 2014 | 4 | Rural | Community | Died | No | Nursing home outbreak [14] | t034 | 19 |
| J | M | 69 | 9 June 2014 | 1 | Rural | Community | No | t034 | 13 | ||
| K | M | 72 | 5 July 2014 | 0 | Rural | Hospital | Died | No | t571 | 16 | |
| L | M | 31 | 14 Sept 2014 | 6 | Rural | Community | Yes | t034 | 10 | ||
| M | M | 48 | 30 Dec 2014 | 0 | Rural | Community | Yes | t034 | 11 | ||
| N | M | 49 | 31 Dec 2014 | 0 | Rural | Community | Yes | t034 | 13 | ||
| O | F | 67 | 6 Mar 2015 | 3 | Rural | Community | No | t034 | 8 | ||
| P | F | 68 | 4 Sept 2015 | 0 | Rural | Hospital | No | Hospital outbreak B | t034 | 14 | |
| Q | M | 77 | 11 Oct 2015 | 2 | Rural | Hospital | Died | No | Hospital outbreak B | t034 | 14 |
Abbreviations: CC, clonal complex; LA-MRSA, livestock-associated methicillin-resistant Staphylococcus aureus; SNP, single-nucleotide polymorphism.
aDied within 30 days after diagnosis of LA-MRSA CC398 bloodstream infection.
Figure 1.
Temporal trends of livestock-associated methicillin-resistant Staphylococcus aureus CC398 bloodstream infections (BSIs) and skin and soft tissue infections (SSTIs) in Denmark.
Thirty-two percent (221/700) of the LA-MRSA CC398 SSTIs occurred in people with no livestock contact, confirming previous findings that LA-MRSA CC398 is spreading into the community [1]. This is worrisome because the general population includes a higher proportion of elderly and immunocompromised people at elevated risk of developing invasive staphylococcal illnesses, compared to people with livestock contact who consist primarily of healthy adults of working age. In fact, 59% (10/17) of patients with LA-MRSA CC398 BSI had no contact to livestock, although they tended to live in rural areas where livestock is raised (Table 1). In addition, LA-MRSA CC398 caused 45.2 BSIs per 1000 SSTIs (10/221) among people with no livestock contact, compared to only 14.6 BSIs per 1000 SSTIs (7/449) among livestock workers and their family members. Death attributable to LA-MRSA CC398 BSI was also more common in the general population in that none of the 6 patients, who died within 30 days after diagnosis of LA-MRSA CC398 BSI, had contact to livestock (Table 1).
LA-MRSA CC398 lacks many of the adhesion and virulence-associated determinants found in other types of MRSA, which has raised questions about its pathogenicity in humans [10, 11]. However, we found no differences between cases of LA-MRSA CC398 BSI and cases with other types of MRSA BSI with regard to age (mean, 56 years vs 61 years; P = .3), Charlson comorbidity score (mean, 2.4 vs 2.2; P = .8), the total number of BSIs per 1000 SSTIs (24.3 vs 37.2; P = .1), or the 30-day case-fatality rate (35% [6/17] vs 21% [31/145]; P = .2). Although the small numbers make it difficult to draw definitive conclusions, these findings indicate that LA-MRSA CC398 may be as capable as other types of MRSA of causing serious illness and even death in elderly and immunocompromised people.
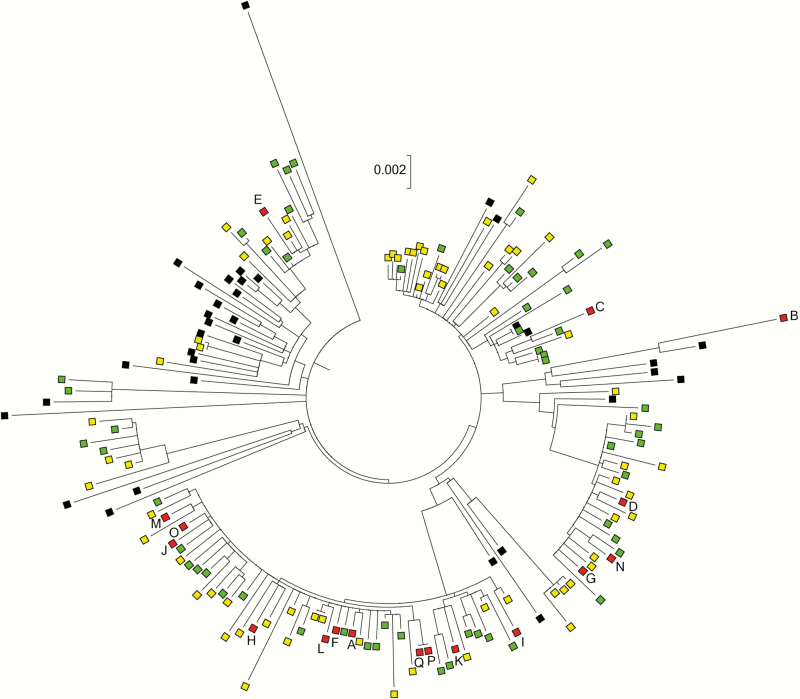
Pigs are the primary host of LA-MRSA CC398 in Denmark [12]. During the study period, the prevalence of Danish pig farms that tested positive for LA-MRSA CC398 increased from 16% in 2010 to >60% in 2014 [7, 8]. Thus, pigs constitute a likely source of both LA-MRSA CC398 BSIs and SSTIs in Denmark. However, LA-MRSA CC398 can also spread between countries, for example via contaminated meat products and colonized individuals [12, 13]. We used whole-genome phylogenetic tools to determine whether LA-MRSA CC398 isolates from cases of BSI and SSTI were most closely related to each other, to LA-MRSA CC398 isolates from Danish pigs, or to S. aureus CC398 isolates from the international collection. The maximum-likelihood phylogenetic tree showed that LA-MRSA CC398 isolates from Danish pigs and cases of SSTI and BSI often grouped together in several distinct clusters (Figure 2 and Supplementary Figure 1). The average single-nucleotide polymorphism (SNP) distance between the 17 BSI isolates and their closest SSTI and Danish pig LA-MRSA CC398 counterparts was 18 (range, 8–86 SNPs) and 17 (range, 7–90 SNPs), respectively, whereas the average shortest SNP distance to an S. aureus CC398 isolate from the international collection was 48 SNPs (range, 32–58 SNPs) (P < .0001). Likewise, the 66 SSTI isolates had significantly shorter average SNP distances to Danish pig isolates than to S. aureus CC398 isolates from the international collection (18 SNPs [range, 2–61] vs 42 SNPs [range, 15–75]; P < .0001). In total, 16 of the 17 BSI isolates and 59 of the 66 SSTI isolates were more closely related to LA-MRSA CC398 isolates from Danish pigs than to isolates from the international collection (Figure 2 and Supplementary Figure 1). Thus, Danish pigs and cases of BSI and SSTI seem to share a common pool of LA-MRSA CC398 isolates, which supports that the increasing number of SSTIs and BSIs is a result of zoonotic transmission from the expanding pig reservoir. The remaining BSI isolate originated from a 57-year-old urban-dwelling woman with no history of livestock exposure (case B). It had spa type t899 and was most closely related to a spa type t899 pig MRSA isolate from France (Figure 2 and Supplementary Figure 1). This isolate from case B was analyzed in a recent study, which showed that it belongs to a poultry-adapted genotype that consists of a CC398 chromosomal backbone and a smaller CC9 region [12]. The CC9/CC398 genotype has never been found in Danish livestock, whereas it occurs with some frequency in poultry and poultry meat produced in other European countries as well as in chicken meat products imported to Denmark [12]. These findings support that the isolate from case B originates from a poultry reservoir outside Denmark, rather than from Danish pigs.
Figure 2.
Part of the maximum-likelihood phylogeny containing the bloodstream infection (BSI) and skin and soft tissue infection (SSTI) isolates (see Supplementary Figure 1 for a full version of the phylogeny, including bootstrap values). Red boxes, BSI isolates; yellow boxes, SSTI isolates; green boxes, Danish pig livestock-associated methicillin-resistant Staphylococcus aureus CC398 isolates, including 6 isolates from the international collection; black boxes, other livestock-associated Staphylococcus aureus CC398 isolates from the international collection. Scale bar represents the number of nucleotide substitutions per site.
Three patients (cases K, P, and Q) had hospital-onset LA-MRSA CC398 BSI, 2 of whom, a 68-year-old woman (case P) and a 77-year old man (case Q), were epidemiologically linked to a nosocomial outbreak in 2015 (Table 1). Pairwise SNP comparison showed that the isolates were identical to each other (Figure 2 and Supplementary Figure 1), which supports transmission between these patients or acquisition from a common hospital source. In addition, 2 patients with community-onset LA-MRSA CC398 BSI, a 63-year-old man receiving outpatient dialysis (case E) and a 74-year-old male nursing home resident (case I), were previously reported to be part of 2 distinct healthcare outbreaks [14]. All 5 isolates from cases E, P, and Q were closely related to Danish pig isolates (range, 14–16 SNPs). These observations substantiate those of previous studies from the Netherlands showing that LA-MRSA CC398 is able to migrate into healthcare facilities where it can cause outbreaks among high-risk patients [15–17].
The findings of our study pertain to CC398, which is the dominant LA-MRSA strain type in pigs in Denmark and other European countries. Other LA-MRSA strain types are dominant in pigs elsewhere, such as CC9 in Asia and some areas of the United States [18, 19], and their impact on human health is currently less well described.
In summary, this study demonstrates that the increasing number of LA-MRSA CC398 BSIs observed in Denmark between 2010 and 2015 occurred in parallel with a much larger wave of LA-MRSA CC398 SSTIs and an expanding pig reservoir with increased spread into the community and healthcare settings. However, Denmark is still considered to have low-endemic levels of MRSA compared to other European countries [20]. This is illustrated by the fact that MRSA accounted for only 44 of 1964 episodes of S. aureus BSI in Denmark in 2014, when the number of LA-MRSA CC398 BSIs peaked at 7 [21]. Nonetheless, the number of serious infections and deaths is expected to increase in the near future if LA-MRSA CC398 is allowed to spread into the general population. Thus, it is important to identify and implement effective control measures to prevent transmission of LA-MRSA CC398 between pig farms and from pigs to humans.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Members of the Danish MRSA study group. Helle Krogh Johansen (Rigshospitalet, Copenhagen), Henrik Westh (Hvidovre Hospital), Michael Pedersen (Herlev Hospital), Ulrich Stab Jensen (Slagelse Hospital), Marie Louise Slott Jensen (Odense University Hospital), Ming Chen (Hospital of Southern Jutland, Sønderborg), Steffen Strøbæk (Hospital South West Jutland, Esbjerg), Claus Østergaard (Lillebælt Hospital, Vejle), Steen Lomborg and Svend Ellermann-Eriksen (Aarhus University Hospital), and Pernille Ripadal (Aalborg University Hospital).
Author contributions. J. L., A. P., A. R. L., M. S., A. K., and R. L. S. were members of the MRSA surveillance team. J. L., M. S., L. B. P., and R. L. S. designed the study and prepared the initial manuscript. A. P., A. R. L., R. N. S., A. K., and F. M. A. contributed to the subsequent editorial revisions. J. L., A. P., and A. K. performed epidemiological investigations. J. L., R. N. S., and M. S. conducted whole-genome sequencing and phylogenetic analysis of the isolates. F. M. A. performed initial genotypic characterization of the Danish pig isolates collected in 2010 and 2014. The Danish MRSA Study Group collected and shared isolates and patient data and commented on the manuscript. All authors proofread the article.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant number 1R01AI101371-01A1 to J. L., A. R. L., M. S., L. B. P., and R. L. S.) and the Ministry of Environment and Food of Denmark through the Danish Agrifish Agency (33010-NIFA-14–612 to J. L., A. R. L., R. N. S., A. K., and R. L. S.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
for the Danish MRSA Study Group:
Helle Krogh Johansen, Henrik Westh, Michael Pedersen, Ulrich Stab Jensen, Marie Louise Slott Jensen, Ming Chen, Steffen Strøbæk, Claus Østergaard, Steen Lomborg, Svend Ellermann-Eriksen, and Pernille Ripadal
References
- 1. Larsen J, Petersen A, Sørum M et al. . Methicillin-resistant Staphylococcus aureus CC398 is an increasing cause of disease in people with no livestock contact in Denmark. Euro Surveill 2015; 20. doi:10.2807/1560-7917.ES.2015.20.37.30021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Cleef BA, Monnet DL, Voss A et al. . Livestock-associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emerg Infect Dis 2011; 17:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. EPI-NYT. MRSA CC398-epidemiologien i Danmark. Danish Available at: http://www.ssi.dk/Aktuelt/Nyhedsbreve/EPI-NYT/2014/Uge%2024a%20-%202014.aspx. Accessed 4 April 2016.
- 4. Larsen AR, Stegger M, Sørum M. spa typing directly from a mecA, spa and pvl multiplex PCR assay-a cost-effective improvement for methicillin-resistant Staphylococcus aureus surveillance. Clin Microbiol Infect 2008; 14:611–4. [DOI] [PubMed] [Google Scholar]
- 5. Quan H, Li B, Couris CM et al. . Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–82. [DOI] [PubMed] [Google Scholar]
- 6. Statistics Denmark. Population and elections Available at: http://www.dst.dk/en/Statistik/emner?subject=02. Accessed 4 December 2016.
- 7. Danish Integrated Antimicrobial Resistance Monitoring and Research Programme. DANMAP 2010: use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark Available at: http://danmap.org/~/media/Projekt%20sites/Danmap/DANMAP%20reports/Danmap_2010.ashx. Accessed 4 December 2016.
- 8. Danish Integrated Antimicrobial Resistance Monitoring and Research Programme. DANMAP 2014: use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark Available at: http://danmap.org/~/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%202014/Danmap_2014.ashx. Accessed 4 December 2016.
- 9. Price LB, Stegger M, Hasman H et al. . Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio 2012; 3:e00305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hallin M, De Mendonça R, Denis O et al. . Diversity of accessory genome of human and livestock-associated ST398 methicillin resistant Staphylococcus aureus strains. Infect Genet Evol 2011; 11:290–9. [DOI] [PubMed] [Google Scholar]
- 11. Argudín MA, Tenhagen BA, Fetsch A et al. . Virulence and resistance determinants of German Staphylococcus aureus ST398 isolates from nonhuman sources. Appl Environ Microbiol 2011; 77:3052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsen J, Stegger M, Andersen PS et al. . Evidence for human adaptation and foodborne transmission of livestock-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2016; 63:1349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grøntvedt CA, Elstrøm P, Stegger M et al. . Methicillin-resistant Staphylococcus aureus CC398 in humans and pigs in Norway: a “One Health” perspective on introduction and transmission. Clin Infect Dis 2016; 63:1431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nielsen RT, Kemp M, Holm A et al. . Fatal septicemia linked to transmission of MRSA clonal complex 398 in hospital and nursing home, Denmark. Emerg Infect Dis 2016; 22:900–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wulf MW, Markestein A, van der Linden FT, Voss A, Klaassen C, Verduin CM. First outbreak of methicillin-resistant Staphylococcus aureus ST398 in a Dutch hospital, June 2007. Euro Surveill 2008; 13 pii:8051. [PubMed] [Google Scholar]
- 16. Fanoy E, Helmhout LC, van der Vaart WL et al. . An outbreak of non-typeable MRSA within a residential care facility. Euro Surveill 2009; 14 pii:19080. [PubMed] [Google Scholar]
- 17. Verkade E, Bosch T, Hendriks Y, Kluytmans J. Outbreak of methicillin-resistant Staphylococcus aureus ST398 in a Dutch nursing home. Infect Control Hosp Epidemiol 2012; 33:624–6. [DOI] [PubMed] [Google Scholar]
- 18. Chuang YY, Huang YC. Livestock-associated meticillin-resistant Staphylococcus aureus in Asia: an emerging issue? Int J Antimicrob Agents 2015; 45:334–40. [DOI] [PubMed] [Google Scholar]
- 19. Smith TC. Livestock-associated Staphylococcus aureus: the United States experience. PLoS Pathog 2015; 11:e1004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) Stockholm: ECDC; 2015. [Google Scholar]
- 21. Petersen A, Skov RL, Larsen AR; Danish Staphylococcus aureus bacteraemia group Staphylococcus aureus bacteraemia cases in Denmark 2014 Available at: http://www.ssi.dk/~/media/Indhold/DK%20-%20dansk/Smitteberedskab/Referencelaboratorier/Stafylokoklaboratoriet/SAB%202014.ashx. Accessed 4 December 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.