Summary
There is an age effect on human postvaccination responses to seasonal influenza vaccines. Prior exposure history, especially H1 influenza encounter, affected cross-reactivity of vaccination-induced H3-specific hemagglutination inhibition antibody responses, and consequently might affect vaccine effectiveness.
Keywords: Seasonal influenza vaccine, H3N2 antibody cross-reactivity, antigenic map, prior exposure, vaccine strain selection.
Abstract
Background.
Effectiveness of seasonal influenza vaccines mainly depends upon how well vaccine strains represent circulating viruses; mismatched strains can lead to reduced protection. Humans have complex influenza exposure histories that increase with age, which may lead to different postvaccination responses to emerging influenza variants. Recent observational studies also suggest that prior vaccination may influence the performance of current seasonal vaccines.
Methods.
To elucidate the effects of age and influenza preexposures on cross-reactivity of vaccination-induced human antibodies, we generated antigenic maps based on postvaccination hemagglutination inhibition titers against representative H3 variants circulating during the 2015–2016, 2014–2015, and 2012–2013 influenza seasons.
Results.
Antigenic maps determined using sera from subjects 18–64 and ≥65 years of age correlated well with each other but poorly with those determined using sera from children. Antigenic maps derived from human postvaccination sera with H1 influenza preexposure also correlated poorly with those derived from sera with neither H1 nor type B influenza preexposure, and the correlation lessened considerably over time. In contrast, antigenic maps derived from human postvaccination sera with only type B influenza preexposure consistently showed good correlation with those derived from sera with neither H1 nor type B influenza preexposure.
Conclusions.
Our results suggest an age-specific difference in human postvaccination responses. Our findings also suggest that prior exposure to H1 or type B influenza may differentially affect cross-reactivity of vaccination-induced H3-specific hemagglutination inhibition antibody responses, and consequently might affect vaccine effectiveness. Our study highlights the need to study the impact of prior exposure on influenza vaccine performance.
Influenza is a major public health concern. Vaccination is the most effective and economic approach for stopping influenza virus transmission, preventing infections, and reducing the severity of associated complications. Annual influenza vaccination has been recommended for persons aged ≥6 months in the United States since 2010 [1]. However, considerable variation in vaccine effectiveness (VE) has been reported for different flu seasons, especially reduced VE against recent A/H3N2 viruses [2–7].
The performance of influenza vaccines depends predominantly on how well vaccine strains match with circulating viruses. Influenza hemagglutinin (HA) is the primary target of vaccination and its antigenicity varies considerably between different strains. Thus, vaccine strains used for manufacturing seasonal influenza vaccines must be evaluated and updated annually. In the vaccine strain evaluation process, standard reference ferret antisera play the determinant role in the final selection of vaccine strains; these antisera are raised by infecting influenza-seronegative ferrets with reference viruses representing recent and emergent isolates [8, 9]. Despite tremendous efforts by World Health Organization (WHO) collaborative centers, a mismatched vaccine strain sometimes is unavoidable and results in poor vaccine performance, as seen with the 2014–2015 Northern Hemisphere influenza vaccines [2, 9, 10].
Such mismatches are at least partially attributed to the fact that standard reference ferret antisera and human postvaccination sera “see” emerging H3 variants differently [9, 11]. One key difference is that the immunity of ferrets used in the influenza vaccine selection process reflects exposure to either current vaccine virus or a representative emergent variant, but human immunity reflects and is greatly influenced by previous influenza infections and/or vaccinations [9, 12, 13].
During this and the past century, humans have been exposed to a variety of influenza viruses. After the 1918 “Spanish flu” pandemic, A/H1N1 virus prevailed in the environment for almost 40 years until its disappearance in 1957, when the A/H2N2 “Asian flu” pandemic began [14]. In 1968, a novel H3N2 virus derived from reassortment between human H2N2 virus and an avian H3 virus emerged, resulting in “Hong Kong flu” pandemic, which continues to circulate in an endemic pattern [14]. In 1977, 20 years since its last appearance, H1N1 virus from the 1918 lineage reemerged and began to co-circulate with H3N2 and type B virus in the environment [14]. And in 2009, a novel H1N1 virus, a descendant of the 1918 linage, caused the “swine flu” pandemic [14]. Thus, it is conceivable that the influenza antibody repertoire in children born after 2009 would be very distinct from that in older persons who have different exposure histories.
In addition, prior influenza vaccination also can shape preexisting immunity by inducing cross-reactive antibodies against antigenic variant viruses [15–19]. Lower VE in individuals with repeated annual vaccination compared with those not vaccinated in previous season has been reported, suggesting that residual protection from prior vaccination may interfere with the performance of current vaccines [3, 6, 7, 19–22]. From 2009 to 2016, H1N1 A/California/07/2009-like virus and B/Brisbane/60/2008-like virus remained as WHO-recommended vaccine prototype viruses for trivalent or quadrivalent influenza vaccines, whereas the H3 vaccine component has been updated several times. It is unclear how annual vaccination with unchanged H1N1 and B vaccine components might affect vaccine performance.
In this study, we assessed H3 cross-reactivity of seasonal influenza vaccines and elucidated the effects of age and prior influenza exposures on vaccination-induced human cross-reactive hemagglutination inhibition (HAI) antibodies by antigenic mapping.
METHODS
Human Postvaccination Sera
Archived human pre- and postvaccination sera were provided by government, industry, and international partners. All human samples were analyzed anonymously at the Center for Biologics Evaluation and Research, US Food and Drug Administration, in support of WHO annual influenza vaccine strain selection, which is considered to be a public health, nonresearch, regulatory activity that is exempt from human subjects review. Under institutional review board approval by individual participating institutes and with written informed consent, serum samples were collected from healthy persons in 3 age groups: children (6–36 months of age), younger adults (18–64 years of age), and older adults (≥65 years of age). Persons in each age group had been immunized with egg-based inactivated, nonadjuvanted, quadrivalent influenza vaccines (QIVs) for the 2015–2016 or 2014–2015 influenza seasons, or trivalent influenza vaccines (TIVs) for the 2012–2013 season. All vaccines were formulated for use in the Northern Hemisphere according to WHO recommendations.
Viruses
The egg-grown viruses included H1N1 strain A/California/07/2009 (CA/07e), H3N2 strains A/Switzerland/9715293/2013 (SWZ/13e), A/Hong Kong/4801/2014 (HK/4801e), A/Texas/50/2012 (TX/50e), A/Victoria/361/2011 (VIC/361e), and A/North Carolina/13/2014 (NC/13e), and type B viruses B/Brisbane/60/2008 (B/Bris/60, Victoria lineage) and B/Phuket/3073/2013 (B/Pht/3073, Yamagata lineage). The cell-grown viruses included H3N2 strains SWZ/13c, TX/50c, A/Michigan/15/2014 (MI/15c), A/Wisconsin/20/2015 (WI/20c), A/Ohio/02/2012 (OH/02c), A/Delaware/15/2012 (DE/15c), and A/South Carolina/16/2012 (SC/16c).
HAI Assay and Data Analysis
Sera were pretreated with receptor-destroying enzyme (Denka-Seiken) and serially 2-fold diluted from the initial 1:10 dilution. Viruses were adjusted to 8 HA units/50 µL in phosphate-buffered saline. The HAI assays were performed, using 0.5% turkey red blood cells (RBCs) for H1N1 and type B viruses and 0.75%–1.00% guinea pig RBCs for H3N2 viruses, according to previously described procedures [9, 16, 17]. RBC agglutination was difficult with the most recent H3N2 isolates (eg, WI/20c) [23]; thus, the H3N2-specific HAI assays for the 2015–2016 season were performed in the presence of 20 nM oseltamivir, as recommended by the WHO vaccine strain selection workgroup, unless otherwise specified. HAI titers were expressed as the reciprocal of the highest serum dilution that resulted in complete HAI. A titer of 5 was assigned if no inhibition was observed at the starting 1:10 serum dilution.
Geometric mean titers (GMTs) for prevaccination and postvaccination HAI titers were determined. Calculations were also made for seroprotection rates (% with postvaccination HAI titer of ≥40) and seroconversion rates (% with 4-fold increase in postvaccination HAI titer with either a prevaccination HAI titer of ≤1:10 and a postvaccination HAI titer of ≥1:40 or a prevaccination HAI titer of >1:10 and a minimum 4-fold rise in postvaccination HAI antibody titer).
Antigenic Map Construction
To minimize the biases caused by low reactor values (HAI titer ≤1:10), low-rank matrix completion was conducted before generating the antigenic maps (http://sysbio.cvm.msstate.edu/AntigenMap) [24, 25]. Each HAI titer is normalized by the overall maximum value max(Hij) and the maximum value for each column max(Hj), and the normalized value will be transformed into
A 2-dimensional map with multidimensional scaling was used to display antigenic distances between H3 viruses characterized [9]. Each gridline (horizontal and vertical) in the map is one antigenic unit distance corresponding to a 2-fold difference in HAI titers.
Correlation Coefficient and Bootstrap Analyses
Pearson product-moment correlation coefficient was adopted as the correlation coefficient for any 2 sets of antigenic distances derived from antigenic cartography generated by HAI data:
in which CC is correlation coefficient, X and Y are 2 observed antigenic distances, and are mean antigenic distances, and n is the number of antigens used in the HAI assay. Correlation coefficient values range from −1 to 1, in which 1 indicates a perfect positive correlation and −1 denotes a perfect negative correlation; 0 suggests no correlation.
Bootstrap analyses were performed to minimize potential biases from small sample sizes by randomly selecting 100 sub-HAI tables from a corresponding parent table to calculate antigenic distances, and determine the correlation coefficients.
Statistical Analysis
P < .05, determined by 2-way analysis of variance using Prism 6.02 software (GraphPad), was considered statistically significant.
RESULTS AND DISCUSSION
Age Effect on H3-Specific HAI Cross-reactivity of 2015–2016 QIV
Immunization with 2015–2016 QIV achieved reasonable HAI antibody titers for each vaccine component in all 3 age groups (Figure 1 and Supplementary Table 1). Of note, nearly all persons, regardless of age, included in this 2015–2016 QIV evaluation had a prevaccination HAI antibody titer of ≥40 against SWZ/13e, the prototype virus for the H3 vaccine component of 2015–2016 QIV (Figure 1A–C), suggesting these persons had already been exposed to SWZ/13-like virus before receiving the vaccination. This finding is not surprising because the SWZ/13-like virus was predominant during 2014–2015, and the H3N2 mismatch of the 2014–2015 Northern Hemisphere influenza vaccines had resulted in low VE [2, 9]. Despite apparent preexposure to SWZ/13-like virus, each age group showed an H3-specific seroconversion rate of nearly 50% or greater after vaccination with 2015–2016 QIV (Figure 1A–C). However, all 3 populations had a >50% reduction in postvaccination GMTs for antibodies against recent representative 3C.2a variants, including MI/15c and WI/20c, compared with those against SWZ/13e (3C.3a) (Figure 1D). These reduced GMTs suggest that the antibodies induced by the SWZ/13e vaccine component of 2015–2016 QIV might be inefficient in neutralizing newly emergent 3C.2a variants [9, 26, 27].
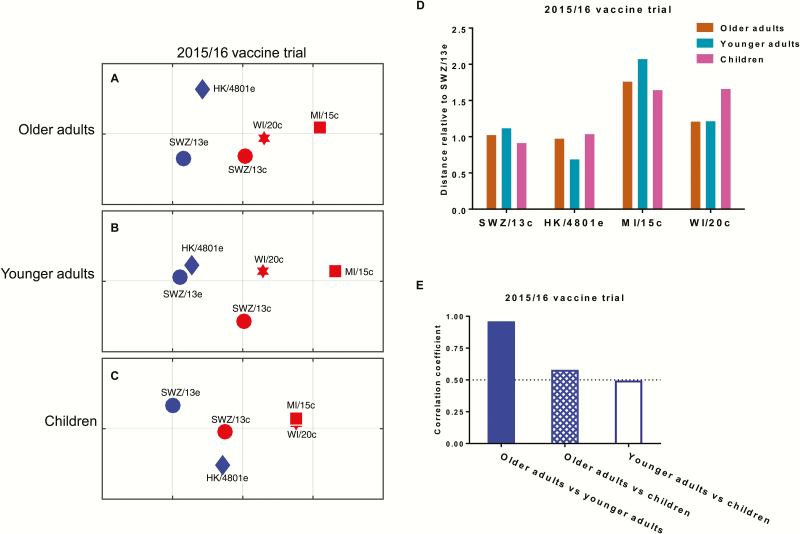
Figure 1.
Immunogenicity and cross-reactivity of the 2015–2016 Northern Hemisphere seasonal influenza vaccine against H3N2 variants. A–C. Proportions of study participants with prevaccination (pre-vac) or postvaccination (post-vac) hemagglutination inhibition (HAI) titers of ≥40, ≥80, ≥160, or ≥320 against egg-grown H3 vaccine prototype virus, A/Switzerland/9715293/2013 (SWZ/13e); % seroconversion is based on individual age groups. A, Older adults (≥65 years of age; n = 54). B, Younger adults (18–64 years of age; n = 54). C, Children (6–36 months of age; n = 24). All participants were vaccinated with 2015–2016 Northern Hemisphere quadrivalent vaccine. H3 variants in the testing panel included egg-grown A/Hong Kong/4801/2014 (HK/4801e) and cell-grown SWZ/13c, A/Wisconsin/20/2015 (WI/20c), and A/Michigan/15/2014 (MI/15c). D, Cross-reactive post-vac geometric mean titers (GMTs) against H3 variants (expressed as % of SWZ/13e post-vac HAI GMT) for each participant age group. The dotted lines indicate 50% of SWZ/13e post-vac HAI GMT.
The postvaccination H3 HAI titers for study participants who had a prevaccination HAI titer of <40 against the H1N1 and type B vaccine prototype viruses of 2015–2016 QIV were used to construct age-specific antigenic maps (Figure 2A–C) and calculate antigenic distances relative to SWZ/13e, respectively (Figure 2D). Regardless of the participants’ ages, the new 3C.2a variants WI/20c and MI/15c were distant from SWZ/13e; however, the 2016–2017 H3 vaccine prototype virus HK/4801e (3C.2a) was close to SWZ/13e (Figure 2A–D). For the 5 H3 strains characterized, antigenic distances determined using postvaccination sera from children correlated poorly with those determined using sera from younger adults (Figure 2E). In contrast, the antigenic distances of the same H3 strains determined using postvaccination sera from younger and older adults were highly correlated (Figure 2E). This age-specific difference in antigenic characterization can probably be explained by the fact that young children do not have the complex influenza exposure histories that adults have. This difference in exposure histories is especially true for persons born before 1957; this population of adults experienced all 3 pandemics in recent human history, the 1957–1958 H2N2 “Asian flu,” the 1968 H3N2 “Hong Kong flu,” and the 2009 H1N1 “swine flu” pandemics [14].
Figure 2.
Age effect on antigenic distances of H3N2 variants determined by postvaccination sera from persons vaccinated with the 2015–2016 Northern Hemisphere seasonal vaccine. A–C, Antigenic maps constructed using postvaccination hemagglutination inhibition (HAI) titers from healthy persons who had been vaccinated with 2015–2016 Northern Hemisphere quadrivalent vaccine. A, Older adults (≥65 years of age; n = 54). B, Younger adults (18–64 years of age; n = 54). C, Children (6–36 months of age; n = 24). H3 strains in the testing panel including egg-grown A/Switzerland/9715293/2013 (SWZ/13e) and A/Hong Kong/4801/2014 (HK/4801e) and cell-grown SWZ/13c, A/Wisconsin/20/2015 (WI/20c), and A/Michigan/15/2014 (MI/15c). Each gridline (horizontal and vertical) in the maps represents 1 antigenic unit distance corresponding to a 2-fold difference in HAI titers. D, Antigenic distances of 4 tested H3 variants relative to the vaccine prototype virus SWZ/13e. E, Correlation between antigenic maps for the 3 participant age groups; correlation coefficients of 1, −1, and 0 represent a perfect positive correlation, a perfect negative correlation, and no correlation, respectively.
Effect of Prior H1 or Type B Virus Exposure on Antigenic Characterization Derived From Human Postvaccination Sera
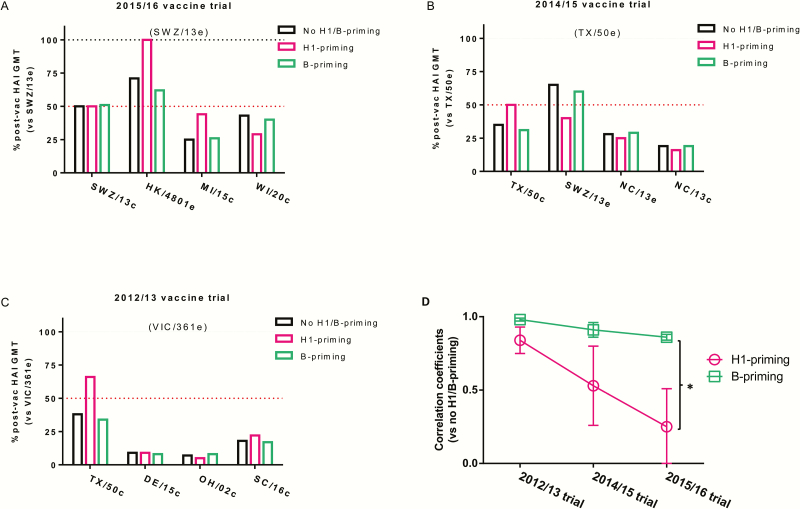
Because exposure history may contribute to age-specific differences in H3 antigenic characterization, we were curious whether previous H1 or type B virus exposure had an equal effect on antigenic distances of the H3 viruses characterized. All younger and older adults of the 2015–2016 QIV trial were combined into one group because of the high correlation between the antigenic maps derived from their postvaccination sera, and were then divided according to their paired prevaccination HAI titers against H1N1 and type B vaccine prototype viruses, as follows: (1) no H1/B-priming (prevaccination HAI titer of <40 against H1N1 and type B virus), (2) H1-priming (prevaccination HAI titer of ≥40 against H1N1 but of <40 against type B virus), and (3) B-priming (prevaccination HAI titer of ≥40 against type B but of <40 against H1N1 virus). Because all human sera analyzed were from archived samples with no infections/vaccinations documented, we used seroprotective HAI titer of ≥40 before vaccination to define previous exposures [9, 16, 17]. H3-specific postvaccination responses in participants with H1-priming differed from those with no H1/B-priming or B-priming only (Figure 3A). Specifically, H1-priming participants showed no reduction in postvaccination GMTs against HK/4801e compared with participants with no H1/B-priming or B-priming who had a 29%–38% GMT reduction (Figure 3A). In addition, H1-priming participants showed more GMT reduction against newly emergent WI/20c, but less GMT reduction against early isolated MI/15c (54%) than participants with no H1/B-priming or B-priming (Figure 3A).
Figure 3.
H3N2 cross-reactivity of the 2015–2016, 2014–2015, and 2012–2013 Northern Hemisphere influenza vaccines. A–C, Post-vaccination (post-vac) hemagglutination inhibition (HAI) titers against epidemic H3N2 variants circulating during each influenza season are expressed as the percentage of post-vac geometric mean titers (GMTs) against corresponding vaccine prototype virus. Adult participants in the 2015–2016, 2014–2015, and 2012–2013 Northern Hemisphere influenza vaccine trials were grouped based on their paired prevaccination HAI titers against H1N1 virus and 2 B vaccine prototype viruses as follows: (1) no H1/B-priming (prevaccination HAI titer <40 against H1N1 and type B virus); (2) H1-priming (prevaccination HAI titer ≥40 against H1N1 but <40 against type B virus); (3) B-priming (prevaccination HAI titer ≥40 against B virus but <40 against H1N1). A, The 2015–2016 vaccine trial (43 participants with no H1/B-priming, 5 with H1-priming, and 38 with B-priming). B, The 2014–2015 vaccine trial (10 participants with no H1/B-priming, 3 with H1-priming, and 26 with B-priming). C, The 2012–2013 vaccine trial (66 participants with no H1/B-priming, 9 with H1-priming, and 40 with B-priming). The H3 viruses tested were egg-grown A/Switzerland/9715293/2013 (SWZ/13e), A/Hong Kong/4801/2014 (HK/4801e), A/Texas/50/2012 (TX/50e), A/North Carolina/13/2014 (NC/13e), and A/Victoria/361/2011 (VIC/361e) and cell-grown SWZ/13c, A/Wisconsin/20/2015 (WI/20c), A/Michigan/15/2014 (MI/15c), TX/50c, A/Ohio/02/2012 (OH/02c), A/Delaware/15/2012 (DE/15c), and A/South Carolina/16/2012 (SC/16c). D, Correlation coefficients of antigenic distances relative to corresponding vaccine prototype virus for the H1-priming and B-priming groups, as compared with the no H1/B-priming group, for the 2012–2013, 2014–2015, and 2015–2016 vaccine trials. *P < .05 as determined by 2-way analysis of variance.
Accordingly, postvaccination HAI titer-derived H3 antigenic distances differed for participants in the 3 subgroups: WI/20c showed a longer distance to SWZ/13e in the map derived from H1-priming sera than those derived from no H1/B-priming or B-priming sera (Table 1 and Supplementary Figure 1A–C). On the other hand, MI/15c showed a much shorter distance to SWZ/13e in the map derived from H1-priming sera than those derived from no H1/B-priming or B-priming sera (Table 1 and Supplementary Figure 1A–C). The correlation coefficient analysis revealed that the H3 antigenic distances derived from no H1/B-priming sera correlated well with those derived from B-priming sera, but had a poor correlation with those derived from H1-priming sera (Table 1). To minimize the bias potentially associated with the small sample size, the bootstrap analyses were conducted and indicated that the H3 antigenic distances derived from B-priming sera correlated significantly better than those derived from H1-priming sera (Table 1; P < .05), suggesting that previous H1 virus exposure might affect 2015–2016 human postvaccination responses to H3 variants more than previous type B virus exposure.
Table 1.
Antigenic Distances and Results of Correlation Coefficient and Bootstrap Analyses for Influenza H3 Variants Characterized by Human Postvaccination Sera
| H3N2 Virusa | Antigenic Distanceb | H1-Priming vs no H1/B-Priming | B-Priming vs no H1/B-Priming | H1/Bris-Priming vs no H1/B-Priming | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No H1/B-Primingc | H1-Priming | B-Priming | H1/Bris-Priming | Correlation Coefficientd | Bootstrap Analysise | Correlation Coefficient | Bootstrap Analysis | Correlation Coefficient | Bootstrap Analysis | |||||
| Individual | Average | Individual | Average | Individual | Average | Individual | Average | |||||||
| SWZ/13e (2015–2016 vaccine prototype) | ||||||||||||||
| HK/4801e | 0.86 | 1.14 ± 0.33 | 0.54 | 1.12 ± 0.35 | 0.82 | 1.08 ± 0.27 | 0.40 | 1.00 ± 0.33 | 0.29 | 0.25 ± 0.26f,g | 0.86 | 0.86 ± 0.02f | 0.56 | 0.49 ± 0.19 |
| SWZ/13c | 1.06 | 1.14 | 1.20 | 0.97 | ||||||||||
| MI/15c | 1.80 | 1.08 | 1.62 | 1.19 | ||||||||||
| WI/20c | 1.19 | 1.66 | 1.15 | 1.43 | ||||||||||
| TX/50e (2014–2015 vaccine prototype) | ||||||||||||||
| SWZ/13e | 0.92 | 1.38 ± 0.44 | 1.25 | 1.33 ± 0.63 | 1.04 | 1.44 ± 0.36 | 1.18 | 1.43 ± 0.51 | 0.70 | 0.53 ± 0.27 | 0.95 | 0.91 ± 0.05 | 0.88 | 0.85 ± 0.07 |
| NC/13e | 1.76 | 1.77 | 1.81 | 1.80 | ||||||||||
| TX/50c | 1.37 | 0.88 | 1.53 | 1.46 | ||||||||||
| NC/13c | 2.22 | 2.60 | 2.19 | 2.55 | ||||||||||
| VIC/361e (2012–2013 vaccine prototype) | ||||||||||||||
| TX/50c | 1.42 | 2.02 ± 0.89 | 0.72 | 2.24 ± 1.15 | 1.64 | 1.98 ± 0.84 | 0.81 | 2.22 ± 1.07 | 0.90 | 0.84 ± 0.09g | 0.98 | 0.98 ± 0.01 | 0.90 | 0.87 ± 0.07 |
| DE/15c | 3.26 | 3.25 | 3.29 | 3.33 | ||||||||||
| OH/02c | 3.52 | 4.14 | 3.37 | 3.85 | ||||||||||
| SC/16c | 2.35 | 2.24 | 2.43 | 1.91 | ||||||||||
aThe H3N2 vaccine prototype virus and variants used for the 2015–2016 Northern Hemisphere influenza vaccine trial include: egg-grown A/Switzerland/9715293/2013 (SWZ/13e) and A/Hong Kong/4801/2014 (HK/4801e), and cell-grown SWZ/13c, A/Michigan/15/2014 (MI/15c), and A/Wisconsin/20/2015 (WI/20c). The H3N2 vaccine prototype virus and variants used for the 2014–2015 Northern Hemisphere influenza vaccine trial include: egg-grown A/Texas/50/2012 (TX/50e), SWZ/13e, and A/North Carolina/13/2014 (NC/13e), and cell-grown TX/50c and NC/13c. The H3N2 vaccine prototype virus and variants used for the 2012/13 Northern Hemisphere influenza vaccine trial include egg-grown A/Victoria/361/2011 (VIC/361e) and cell-grown TX/50c, A/Delaware/15/2012 (DE/15c), A/Ohio/02/2012 (OH/02c), and A/South Carolina/16/2012 (SC/16c).
bAntigenic distances of individual H3N2 variants to the vaccine prototype virus were calculated based on the postvaccination hemagglutination inhibition (HAI) titers. Average distances are shown as mean ± standard error of the means (SEM).
cHuman postvaccination sera were selected based on their paired prevaccination HAI titers against the corresponding vaccine strains: (1) No H1/B-priming (prevaccination HAI titer of <40 against H1N1 and B vaccine strains); (2) H1-priming (prevaccination HAI titer of ≥40 against H1N1 but <40 against type B virus); (3) B-priming (prevaccination HAI titer of ≥40 against type B but <40 against H1N1; (4) H1/Bris-priming (prevaccination HAI titer of ≥40 against H1N1 including those with prevaccination HAI titer of ≥40 against B/Brisbane/60/2008 [Bris] vaccine strain). There are 43 no H1/B-priming subjects, 5 H1-priming subjects, 38 B-priming subjects, and 9 H1/Bris-priming subjects identified for the 2015–2016 vaccine trial. There are 18 no H1/B-priming subjects, 3 H1-priming subjects, 30 B-priming subjects, and 13 H1/Bris-priming subjects identified for the 2014–2015 vaccine trial. There are 20 no H1/B-priming subjects, 5 H1-priming subjects, 23 B-priming subjects, and 9 H1/Bris-priming subjects identified for the 2012–2013 vaccine trial.
dCorrelation coefficient values were determined by Pearson product-moment correlation coefficient analysis on the antigenic distances of H3 variants characterized by human postvaccination sera with H1-priming, B-priming, or H1/Bris-priming as compared to those with no H1/B-priming. A correlation coefficient of 1 suggests a perfect positive correlation, –1 indicates a perfect negative correlation, and 0 denotes no correlation.
eBootstrap analyses, which were performed to minimize the potential biases from small sample sizes, randomly selected 100 sub-HAI tables from a corresponding parent table to calculate the correlation coefficient values ± SEMs on the H3 antigenic distances derived.
f P < .05 vs 2015–2016 B-priming group as determined by 2-way analysis of variance (ANOVA).
g P < .05 vs 2012–2013 H1-priming group, as determined by 2-way ANOVA.
To determine if this observation was a general trend that could be applied to other influenza seasons, we conducted the same analysis on H3 cross-reactivity of the 2014–2015 and 2012–2013 Northern Hemisphere vaccines. TX/50e (clade 3C.1), the H3 prototype virus of the 2014–2015 Northern Hemisphere influenza vaccines, has been a mismatch to the prevalent 3C.2a and 3C.3a viruses, and it was replaced by SWZ/13e (3C.3a) in the 2015–2016 Northern Hemisphere vaccine formulation [2, 9, 10, 28]. The participants with no H1/B-priming or B-priming had less GMT reduction against egg-grown SWZ/13e, but greater GMT reduction against cell-grown TX/50c than H1-priming participants (Figure 3B). Cell-grown TX/50c is antigenically different from its egg-grown counterpart, TX/50e, due to egg-adapted mutations at G186V and S219F in H3 antibody binding sites B and D [4, 9, 29]. However, the distance between TX/50c and TX/50e was much shorter in the map derived from H1-priming sera than those derived from no H1/B-priming and B-priming sera (Table 1 and Supplementary Figure 1E–G). Additionally, TX/50c appeared much closer to SWZ/13e in the map derived from H1-priming sera than those derived from no H1/B-priming and B-priming sera (Supplementary Figure 1E–G). These results suggested that H1-priming sera from the 2014–2015 QIV trial “see” TX/50c (3C.1) and SWZ/13e (3C.3a) as antigenically related, whereas no H1/B-priming and B-priming sera “see” these 2 strains as antigenically distinct. The antigenic distances determined with B-priming sera correlated obviously better with those determined with no H1/B-priming sera than those determined with H1-priming sera (Table 1). This result was similar to that observed in the 2015–2016 QIV trial (Table 1).
For the 2012–2013 TIV trial, all 3 participant groups had >50% reduction in postvaccination GMTs against the 4 representative circulating viruses, including TX/50c, which is in the same clade as VIC/361e (3C.1), the H3 vaccine prototype virus of 2012–2013 Northern Hemisphere TIV (Figure 3C). This reduction occurred because IVR-165, the actual vaccine reassortant used for 2012–2013 vaccine production, has gained 1 additional egg-adapted mutation (H156Q), which, in addition to mutations G186V and S219Y in VIC/361e, changed its original antigenicity and consequently caused it to become distinct from VIC/361e [4, 9, 29]. Thus, it is not surprising that 2012–2013 postvaccination sera responded poorly to the representative H3N2 variants, even though the original vaccine prototype virus, VIC/361e, matched most circulating H3N2 strains during the 2012–2013 season [4]. The antigenic distance between TX/50c and VIC/361e as determined using H1-priming sera was much shorter than that determined using no H1/B-priming sera or B-priming sera (Table 1). The antigenic maps derived from 2012–2013 no H1/B-priming sera and B-priming sera were highly correlated with each other; whereas the antigenic distances determined with 2012–2013 H1-priming sera showed less though good correlation with those determined with no H1/B-priming sera (Table 1). This trend was consistent with those observed during 2015–2016 and 2014–2015 vaccine trials.
Preexisting immunity can promote host resistance through cross-reactive antibodies against emerging viruses, the benefits of which have been well documented for the 2009 H1N1 pandemic [13, 16, 17, 30–34]. Prior H1N1 or H3N2 infection was also found to significantly augment HAI responses in laboratory animals to subsequent immunization of inactivated heterosubtypic vaccines, and the minimum dose required to induce detectable HAI titers was significantly reduced in hamsters with prior heterosubtypic infections compared with naive animals [35]. On the other hand, preexisting immunity can also potentially interfere with influenza vaccination due to “original antigenic sin,” a phenomenon by which immune memory is recalled to cross-react with historically related viruses rather than current vaccine strains [3, 36–38]. Postvaccination response has been reported to be inversely associated with the number of prior influenza vaccination, and reduced VE has been observed in patients with repeated annual vaccination [3, 7, 20–22].
The average antigenic distances of H3 variants determined using no H1/B-priming sera, H1-priming sera, or B-priming sera were essentially identical during each influenza season, although the averages decreased over time (Table 1). However, individual antigenic distances determined with H1-priming sera vs no H1/B-priming sera became considerably less correlated over the period studied, but B-priming groups remained highly correlated with no H1/B-priming groups across the seasons (Figure 3D). This finding suggests that the antigenic differences among H3 variants “seen” by H1-priming sera went in different directions from those “seen” by no H1/B-priming sera or B-priming sera.
H1N1 and H3N2 viruses have co-circulated along with type B viruses since 1977. H3N2 vaccine viruses have evolved from A/Uruguay/716/2007-like (clade 1) to HK/4801-like (3C.2a) since 2009, but H1N1 CA/07-like and B/Bris/60-like (Victoria lineage) viruses have remained the vaccine components to date. Indeed, we observed that approximately 22%–30% of persons enrolled in 2012–2013, 2014–2015, or 2015–2016 vaccine trials had preexisting HAI titers ≥40 against CA/07 and B/Bris/60 (defined as H1/Bris-priming). The H3 antigenic maps derived from postvaccination sera of these H1/Bris-priming participants became less correlated with those derived from no H1/B-priming sera over time, following a similar trend to those determined using H1-priming sera (Table 1). These results suggest that H1 virus preexposure could influence the H3-specific postvaccination response more than type B virus preexposure, which could be due to the fact that H1 virus generally causes more severe illness, thus having a greater effect on the immune system [39]. Apparently the longer CA/07-like virus is in circulation, the bigger the effect.
CONCLUSIONS
Our results indicate that human immunity to influenza virus is shaped significantly by exposure history, the complexity of which cannot be duplicated by standard reference ferret antisera with single virus infection [9, 12, 13]. Attention should be given to prior influenza infection/vaccination history of volunteers whose postvaccination sera are used for annual vaccine strain selection. Our study also highlights the need to investigate the impact of prior exposure, especially repeated annual vaccination on influenza vaccine performance.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. H. X. and X.-F. W. conceived the ideas. H. X. and Z. Y. designed the HAI serology experiments. H. X., Z. Y., X. L., E. P. P., O. Z., Y. Zhao, X. J., Z. L., T. K., M.-J. C., C. L. F., M. K., A. Z., and Y. Zhu performed the HAI assays. H. X. analyzed the HAI data. L. L. and X.-F. W. generated the cartographies and conducted correlation coefficient and bootstrap analyses. H. X. performed the statistical analyses and wrote the manuscript.
Acknowledgments. The authors sincerely appreciate Dr Xiyan Xu of Centers for Diseases Control and Prevention for providing influenza viruses. We also thank Drs Marian Major and Vladimir Lugovtsev of the Center for Biologics Evaluation and Research (CBER) for critical review of this manuscript.
Disclaimer. The findings and conclusions in this article have not been formally disseminated by US Food and Drug Administration and should not be construed to represent any Agency determination or policy.
Financial support. This project was supported by the intramural research fund of CBER, US Food and Drug Administration. X.-F. W. and L. L. were supported by the National Institutes of Health (grant number R01AI116744 to X.-F. W.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fiore AE, Uyeki TM, Broder K, et al. ; Centers for Disease Control and Prevention (CDC) Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62. [PubMed] [Google Scholar]
- 2. Flannery B, Clippard J, Zimmerman RK, et al. ; Centers for Disease Control and Prevention Early estimates of seasonal influenza vaccine effectiveness—United States, January 2015. MMWR Morb Mortal Wkly Rep 2015; 64:10–5. [PMC free article] [PubMed] [Google Scholar]
- 3. McLean HQ, Thompson MG, Sundaram ME, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37. [DOI] [PubMed] [Google Scholar]
- 6. Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Russell CA, Jones TC, Barr IG, et al. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine 2008; 26suppl 4:D31–4. [DOI] [PubMed] [Google Scholar]
- 9. Xie H, Wan XF, Ye Z, et al. H3N2 mismatch of 2014-15 Northern Hemisphere influenza vaccines and head-to-head comparison between human and ferret antisera derived antigenic maps. Sci Rep 2015; 5:15279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Mello T, Brammer L, Blanton L, et al. ; Centers for Disease Control and Prevention (CDC) Update: influenza activity—United States, September 28, 2014-February 21, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:206–12. [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta V, Earl DJ, Deem MW. Quantifying influenza vaccine efficacy and antigenic distance. Vaccine 2006; 24:3881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hensley SE. Challenges of selecting seasonal influenza vaccine strains for humans with diverse pre-exposure histories. Curr Opin Virol 2014; 8:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Myers JL, Bostick DL, et al. Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J Exp Med 2013; 210:1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010; 7:440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fonville JM, Wilks SH, James SL, et al. Antibody landscapes after influenza virus infection or vaccination. Science 2014; 346:996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie H, Jing X, Li X, et al. Immunogenicity and cross-reactivity of 2009–2010 inactivated seasonal influenza vaccine in US adults and elderly. PLoS One 2011; 6:e16650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xie H, Li X, Gao J, et al. Revisiting the 1976 “swine flu” vaccine clinical trials: cross-reactive hemagglutinin and neuraminidase antibodies and their role in protection against the 2009 H1N1 pandemic virus in mice. Clin Infect Dis 2011; 53:1179–87. [DOI] [PubMed] [Google Scholar]
- 18. Reber AJ, Kim JH, Coleman LA, et al. Seasonal influenza vaccination of children induces humoral and cell-mediated immunity beyond the current season: cross-reactivity with past and future strains. J Infect Dis 2016; 214:1477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohmit SE, Petrie JG, Malosh RE, Fry AM, Thompson MG, Monto AS. Influenza vaccine effectiveness in households with children during the 2012–2013 season: assessments of prior vaccination and serologic susceptibility. J Infect Dis 2015; 211:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson MG, Naleway A, Fry AM, et al. Effects of repeated annual inactivated influenza vaccination among healthcare personnel on serum hemagglutinin inhibition antibody response to A/Perth/16/2009 (H3N2)-like virus during 2010–11. Vaccine 2016; 34:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis 2013; 57:474–6. [DOI] [PubMed] [Google Scholar]
- 23. Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015-16 season and composition of the 2016-17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–75. [DOI] [PubMed] [Google Scholar]
- 24. Cai Z, Zhang T, Wan XF. A computational framework for influenza antigenic cartography. PLoS Comput Biol 2010; 6:e1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barnett JL, Yang J, Cai Z, Zhang T, Wan XF. AntigenMap 3D: an online antigenic cartography resource. Bioinformatics 2012; 28:1292–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kishida N, Fujisaki S, Yokoyama M, et al. Evaluation of influenza virus A/H3N2 and B vaccines on the basis of cross-reactivity of postvaccination human serum antibodies against influenza viruses A/H3N2 and B isolated in MDCK cells and embryonated hen eggs. Clin Vaccine Immunol 2012; 19:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Deem MW. Influenza evolution and H3N2 vaccine effectiveness, with application to the 2014/2015 season. Protein Eng Des Sel 2016; 29:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chambers BS, Parkhouse K, Ross TM, Alby K, Hensley SE. Identification of hemagglutinin residues responsible for H3N2 antigenic drift during the 2014-2015 influenza season. Cell Rep 2015; 12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Z, Zhou H, Jin H. The impact of key amino acid substitutions in the hemagglutinin of influenza A (H3N2) viruses on vaccine production and antibody response. Vaccine 2010; 28:4079–85. [DOI] [PubMed] [Google Scholar]
- 30. Skountzou I, Koutsonanos DG, Kim JH, et al. Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus. J Immunol 2010; 185:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012; 109:9047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM. Recipients of vaccine against the 1976 “swine flu” have enhanced neutralization responses to the 2009 novel H1N1 influenza virus. Clin Infect Dis 2010; 50:1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Houser KV, Pearce MB, Katz JM, Tumpey TM. Impact of prior seasonal H3N2 influenza vaccination or infection on protection and transmission of emerging variants of influenza A(H3N2)v virus in ferrets. J Virol 2013; 87:13480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller MS, Gardner TJ, Krammer F, et al. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med 2013; 5:198ra07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jennings R, Potter CW, McLaren C. Effect of preinfection and preimmunization on the serum antibody response to subsequent immunization with heterotypic influenza vaccines. J Immunol 1974; 113:1834–43. [PubMed] [Google Scholar]
- 36. Fazekas de St G, Webster RG. Disquisitions on original antigenic sin. II. Proof in lower creatures. J Exp Med 1966; 124:347–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol 2009; 183:3294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Webster RG. Original antigenic sin in ferrets: the response to sequential infections with influenza viruses. J Immunol 1966; 97:177–83. [PubMed] [Google Scholar]
- 39. Pan K, Subieta KC, Deem MW. A novel sequence-based antigenic distance measure for H1N1, with application to vaccine effectiveness and the selection of vaccine strains. Protein Eng Des Sel 2011; 24:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.