Abstract
STUDY QUESTION
What doses of secretory phase progesterone (P) in women are associated with altered endometrial structure and/or function?
SUMMARY ANSWER
Consistently delayed histological maturation was seen at the lowest tested daily P dose (2.5 mg), whereas consistently altered functional response, as reflected by microarray analysis of gene expression was seen at both the 5 and 2.5 mg doses.
WHAT IS KNOWN ALREADY
Progesterone is absolutely required for normal embryo implantation and pregnancy survival. Progesterone supplementation is beneficial in ART cycles.
STUDY DESIGN, SIZE, DURATION
In this case–control experimental trial, 46 healthy young female volunteers (age 19–34) underwent a single modeled endometrial cycle after GnRH down-regulation or monitored in natural cycles.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In a university hospital, modeled cycles were obtained by GnRH agonist down-regulation, transdermal estradiol (E2) (0.2 mg/d), and daily injections of P in oil for 10 days: 2.5 mg (n = 6), 5 mg (n = 6), 10 mg (n = 12) or 40 mg (n = 12), after the 10th day of E2. Ten healthy, ovulatory women were used as controls. Endometrial biopsies were obtained on the 10th day of P exposure, or urinary LH surge (in controls). Analysis included histological dating, serum progesterone levels, microarray analysis of the whole genome, RT-PCR, western blot and comparison with the GEO database.
MAIN RESULTS AND THE ROLE OF CHANCE
In endometrial biopsies, a morphological delay appears in the 2.5 mg/day of P group. Higher sub-physiological levels of P (≥5 mg/day) resulted in normal histology, but aberrant gene expression. P levels required for consistent histological delay were lower than those in all ovulatory women. Gene expression abnormalities occurred at higher sub-physiological P concentrations, without a change in histology, a functional-morphological disassociation. The expression of some endometrial receptivity-associated genes appeared multiphasic, with peak or nadir of mean or median expression levels between the lowest and highest doses, suggesting sustained supraphysiological doses seen in ART treatment cycles may not be optimal.
LARGE SCALE DATA
GEO DataSets ID: 200056980; GSE 56980.
LIMITATIONS, REASONS FOR CAUTION
These results were obtained in fertile women, who may respond differently from infertile subjects.
WIDER IMPLICATIONS OF THE FINDINGS
The dose of P required for normal endometrial structure (5 mg/day) corresponds to a P concentration well below that seen in ovulatory women, suggesting that persistently delayed mid-secretory histology cannot be solely due to inadequate P concentrations in an ovulatory cycle. Endometrial gene expression is differentially regulated by different doses of progesterone. The apparent multiphasic response of some genes to P dose suggests the possibility that P concentration kinetics may play a role in normal endometrial preparation for receptivity. These findings strongly confirm that histologic development is not a reliable measure of endometrial P action.
STUDY FUNDING/COMPETING INTEREST(S)
Supported by The Eunice Kennedy Shriver National Institute for Child Health and Disease, National Institute of Health, USA (NICHD/NIH) (R01HD067721 and U54HD30476; SLY and BAL) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) 240239/2012-1 (RFS). All authors have no competing interests.
Keywords: endometrium, gene expression, progesterone, implantation, luteal phase, secretory phase
Introduction
Progesterone (P) is essential for endometrial receptivity to embryo implantation and pregnancy maintenance. Furthermore, luteal phase and early pregnancy P supplementation improves outcomes in cycles of IVF (van der Linden et al., 2015), compensating for compromised corpus luteum progesterone secretion. The timing of P exposure is also critical to normal implantation and reduced IVF success is associated with a premature rise in P, though the required threshold, concentration and duration remains uncertain (Huang et al., 2012; Venetis et al., 2013).
In 1949, Georgeanna Seegar Jones proposed the concept of luteal phase deficiency (LPD), hypothesizing that some human infertility was caused by inadequate P production or action (Jones, 1949). The existence of LPD remains controversial, primarily because there is no validated method for establishing luteal phase adequacy. Measurement of luteal P concentrations have limited clinical value because they can fluctuate 6-fold over a few hours (Filicori et al., 1984). Delayed histologic endometrial development (Noyes et al., 1950), previously the gold standard for LPD diagnosis has been shown to be highly imprecise and clinically irrelevant (Coutifaris et al., 2004; Murray et al., 2004).
Attention has since turned to molecular biomarkers, with demonstration of a number of secretory biomarkers in normal women differing significantly from that observed in women with disorders linked to impaired endometrial receptivity (Burney et al., 2007; Donaghay and Lessey, 2007; Savaris et al., 2011). However, no biomarker or group of biomarkers has yet been validated to reliably define distinguish a receptive from a non-receptive endometrium (Practice Committee of the ASRM, 2015). Whether or not LPD is clinically relevant, the underlying principle remains indisputable: since P is essential for successful embryo implantation and pregnancy, there must be a minimum P concentration required to induce normal secretory endometrial development and receptivity. Understanding this threshold will help to optimize ART, both for endometrial preparation and assessment of the significance of an early P rise during ovarian stimulation. To better define the concentration-dependent effects of P on endometrial structure and function, we used an experimental in vivo model to examine the effects of varying P concentrations on endometrial histologic development and gene expression in normal women. The results of our study provide the first direct experimental evidence that P has concentration-dependent effects on both endometrial structure and function, but also demonstrate that secretory endometrial histologic development and gene expression differ in their sensitivity to P concentrations.
Materials and Methods
Human subjects
Healthy women, ages 19–34 years, with a regular inter-menstrual interval between 25 and 35 days and no history of infertility or pelvic disease were invited to participate. Exclusion criteria were the following: (i) an inter-menstrual interval that varied by more than 3 days, (ii) the use of medication known to affect reproductive hormones or fertility within 60 days prior to enrollment, (iii) chronic disease, (iv) a body mass index >29.9 or <18.5, and (v) history of infertility, defined as a failure to conceive for 1 year or longer despite regular intercourse without contraception.
Ethical approval
The study was reviewed and approved by the Committee for the Protection of Human Subjects at the University of North Carolina at Chapel Hill.
Modeled endometrial cycles
In this study, we constructed modeled cycles as described previously (Usadi et al., 2008) with a few modifications. Subjects received treatment with leuprolide acetate (1 mg, s.c. daily; TAP Pharmaceuticals, Lake Forest, IL, USA), beginning in the midluteal phase of a natural cycle (calculated by cycle length and confirmed luteal by serum P > 3.0 ng/ml) and continuing throughout the study. After confirmation of effective down-regulation of the pituitary-ovarian axis (serum estradiol < 40 pg/ml, no ovarian follicle >10 mm), subjects received 0.2 mg/day transdermal estradiol (Vivelle Dot, Novartis, East Hanover, NJ), for a total of 20 days. After 10 days of estradiol treatment, subjects underwent transvaginal ultrasound to ensure endometrial thickness was at least 7 mm (all were > 7.4 mm) and then randomly allocated to one of four daily i.m. doses of P in oil (Pfizer, New York, NY): 2.5, 5.0, 10.0 mg, or 40.0. One subject was treated with 0 mg P (oil only) to control for effects of the carrier. Randomization was performed using sequential opaque envelopes with a marked group assignment slip. The dose given to each group was blinded to both participants and investigators until data analysis. With the exception of the varying doses of P, the induced artificial cycles were virtually identical to treatment regimens in common clinical use for women preparing to receive embryos derived from egg donation, which yield pregnancy rates per embryo transfer in excess of 50%, implying a highly receptive endometrium (Cardozo et al., 2016). Each controlled cycle was performed in a different individual.
Serum progesterone concentrations
Serum P concentrations in modeled cycles were measured at peak (2–3 h after injection) and trough (1–2 h before injection) on two separate occasions between 3 and 10 days of P treatment, allowing measurements under steady state conditions (Usadi et al., 2008). Serum P was measured using commercial immunoassay kits (Immulite DPC; Diagnostic Products Corp, Los Angeles, CA; inter- and intra-assay variability <4 and 6%, respectively). Serum P levels <5 ng/ml were re-measured by radioimmunoassay after ether extraction and Sephadex LH-20 column chromatography in the laboratory of Frank Stanczyk (University of Southern California), with those results replacing values from direct assays.
Endometrial biopsies
Endometrial biopsies were performed using an endometrial pipelle (Milex Product Inc., Chicago, IL) 10 days after detection of the midcycle urinary LH surge, as determined by once daily, self-testing of urine (Clearblue Easy Digital, Unipath Ltd., Bedford, UK), or on P Day 10 in modeled cycles (cycle Day 23). Endometrial tissue specimens were divided into two portions; one was fixed in formalin for histopathologic examination and the second was snap frozen in liquid nitrogen and stored at −80°C for later RNA and/or protein analysis.
Histological dating of endometrial biopsies
Histologic dating of endometrial biopsies was performed, blinded to cycle day, by two independent examiners (R.J.Z. and R.A.S.) according to the criteria of Noyes et al. (1950), with the observed histological date defined by the mean of the two results. The expected histologic date in samples from modeled cycles was defined as Day 23. For each tissue specimen, differences between the expected and observed histologic dates were calculated by subtracting the observed date from the expected day (Day 23). The examiners concurrently examined other samples from throughout the luteal phase, avoiding inadvertent unblinding. Day 23, conventionally regarded as the closing of the window of implantation, was chosen to allow comparison to our previous studies (Usadi et al., 2008).
RNA extraction, preparation, hybridization, scanning and microarray processing
Total RNA extraction and purification was performed as described (Usadi et al., 2008). The Illumina HumanWB-6 v3 Expression BeadChip kit (Illumina, San Diego, CA, USA) was used to generate transcriptomic profiles using the Illumina® TotalPrep Amplification Kit (Ambion, Austin, TX, USA), and hybridization according to the manufacturer's instructions.
Microarray data analysis
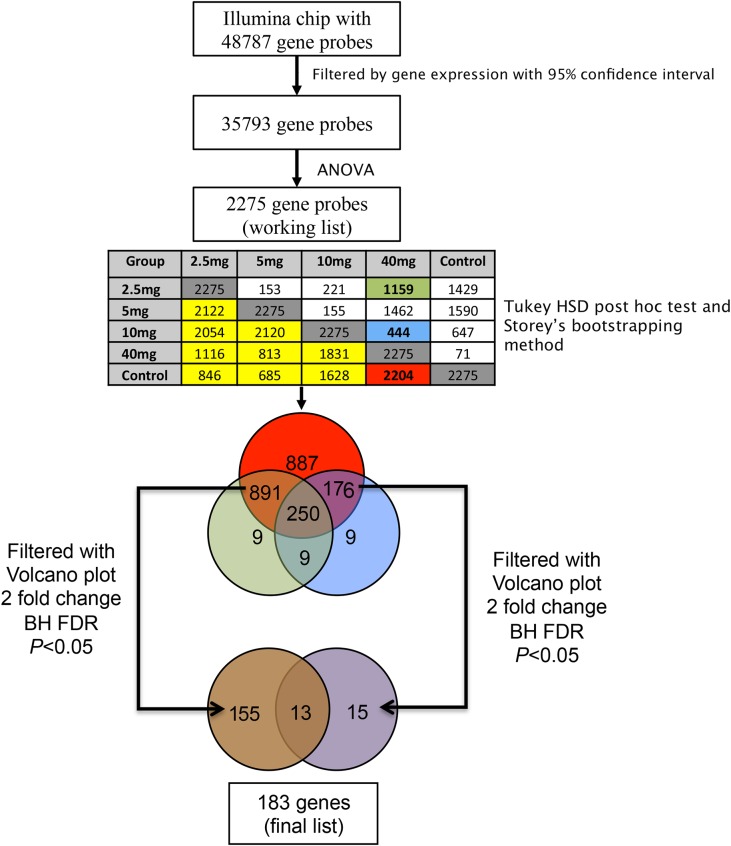
The microarray data analysis is summarized in Fig. 1 and detailed in the Supplementary Material.
Figure 1.
Flow chart of microarray analysis. From the Illumina HT-12 v4 BeadChip with 48 787 gene-probes, quality control of gene expression was performed by excluding genes outside the 95% CI. This filtering yielded an initial gene list with 35 793 genes. Next, this gene list was submitted to ANOVA statistical analysis, producing 2275 genes that were significantly different across the five groups. Post hoc analysis result is presented in the table. Dark gray cells represent the total number of significantly different genes among groups (2275). Cells on the upper right side of the gray cells are genes that are significantly different between two groups. Cells on the down left side of the gray cells are genes that are not significantly different (yellow). The intersection of cells Control versus 40 mg (red cell-2204 genes) represent the genes that were not significantly different in this comparison. These 2204 genes were presumed to be representative to the normal transcriptome of the mid-secretory phase. The intersection between group 40 mg versus 10 mg (green cell with 1159 genes) and intersection between 40 mg versus 10 mg (blue cell with 444 genes) represent the genes that were significantly different in the comparison above comparison. The gene list derived from the red (2204 genes), blue (444 genes) and green (1159 genes) cells were analysed in a Venn diagram. The intersections among groups (891, 250, 176, 9, 9, 9 genes) represent genes that are differently modulated with low levels of progesterone (≤10 mg), compared to normal cycling women (controls) and those who received 40 mg of progesterone. These lists of genes, derived from blue and green circles, were further filtered by Volcano plot for genes having at least 2-fold change and a P-value <0.05, with Benjamin Hochenbach (BH) as FDR. The final list of genes demonstrating differential expression by progesterone dose comprised a total of 183 genes (115 + 13; 13 + 15; 9 + 9 + 9). FDR, false discovery rate.
Validation of microarray data by RT-PCR
Genes with significantly different expression and fold change were selected for validation RT-PCR as described previously (Plante et al., 2012) and detailed in the Supplementary Material.
In silico comparison to previous studies
In order to create a table of genes likely involved in P action, we utilized data from previous studies, two of which listed genes considered critical for human endometrial receptivity (Altmäe et al., 2010; Díaz-Gimeno et al., 2011). We combined the two lists (497 unique genes) and compared the result to our final gene list (Supplementary Data, Table S1) and compiled these genes in Tables IA, IB, IC. After identification of common genes, the phase of the menstrual cycle, where these genes were significantly different from the proliferative phase, was examined using raw data from a study of natural cycles––GEO DataSet Record: GDS2052 (Talbi et al., 2006). Next, the expression of these genes, according to different levels of P, was analyzed by fluorescence values obtained from our microarray analysis and the selected gene expression patterns that were confirmed by RT-PCR. In addition, selected genes that have been validated as important in endometrial receptivity and P resistance (Burney et al., 2007; Savaris et al., 2011), including decay-accelerating factor for complement (DAF) and ERBB receptor feedback inhibitor 1 (MIG-6), were analysed by RT-PCR.
Table IA.
Genes that are down-regulated with 40 mg of progesterone, compared to 2.5 mg of progesterone.
| Gene name | Fold change | P-value |
|---|---|---|
| BX093803 Soares_pregnant_uterus_NbHPU Homo sapiens cDNA clone IMAGp998L054309. sequence | 4.0 | 0.004 |
| V-set domain containing T cell activation inhibitor 1 (VTCN1) | 3.8 | 0.011 |
| Claudin 22 (CLDN22) | 3.6 | 0.023 |
| Sodium channel, nonvoltage-gated 1, gamma (SCNN1G) | 3.1 | 0.003 |
| Immunoglobulin superfamily containing leucine-rich repeat 2 (ISLR2) | 3.1 | 0.004 |
| Aldehyde dehydrogenase one family, member L1 (ALDH1L1) | 3.1 | 0.008 |
| PREDICTED: Homo sapiens hypothetical protein LOC644571 (LOC644571) | 3.1 | 0.002 |
| Collagen, type IX, alpha 2 (COL9A2) | 2.6 | 0.004 |
| WAP four-disulfide core domain 2 (WFDC2), transcript variant 2 | 2.5 | 0.005 |
| Dipeptidyl-peptidase 6 (DPP6), transcript variant 1 | 2.4 | 0.006 |
| Collapsin response mediator protein 1 (CRMP1), transcript variant 1 | 2.4 | 0.003 |
| AGENCOURT_13462296 NIH_MGC_187 Homo sapiens cDNA clone IMAGE:30318122 5. sequence | 2.3 | 0.016 |
| Glycerophosphodiester phosphodiesterase domain containing 5 (GDPD5) | 2.3 | 0.005 |
| Chloride intracellular channel 6 (CLIC6) | 2.2 | 0.007 |
| Leucine rich repeat transmembrane neuronal 1 (LRRTM1) | 2.2 | 0.001 |
| Solute carrier family 29 (nucleoside transporters), member 4 (SLC29A4) | 2.1 | 0.004 |
| cDNA FLJ37143 fis, clone BRACE2024222 | 2.1 | 0.005 |
| Sodium channel, nonvoltage-gated 1, beta (SCNN1B) | 2.1 | 0.007 |
| Formin homology two domain containing 3 (FHOD3) | 2.1 | 0.003 |
| Dapper, antagonist of beta-catenin, homolog 2 (Xenopus laevis) (DACT2) | 2.0 | 0.006 |
Table IB.
Genes that are up-regulated with 40 mg of progesterone, compared to 2.5 mg of progesterone (full list in Supplementary Data, Table S1).
| Gene name | Fold change | P-value |
|---|---|---|
| Chemokine (C-X-C motif) ligand 13 (B-cell chemoattractant) (CXCL13) | 7.7 | 0.003 |
| Aldehyde oxidase 1 (AOX1) | 6.4 | 0.004 |
| Fibrinogen-like 2 (FGL2) | 5.3 | 0.006 |
| Scavenger receptor class A, member 5 (putative) (SCARA5) | 5.1 | 0.006 |
| G protein-coupled bile acid receptor 1 (GPBAR1), transcript variant 1 | 4.5 | 0.005 |
| MAM domain containing 2 (MAMDC2) | 4.3 | 0.004 |
| Sushi-repeat-containing protein, X-linked (SRPX) | 4.2 | 0.004 |
| Chondrolectin (CHODL) | 4.2 | 0.001 |
| Transmembrane protein 45A (TMEM45A) | 4.1 | 0.008 |
| G protein-coupled bile acid receptor 1 (GPBAR1), transcript variant 3 | 3.9 | 0.005 |
| Calcitonin receptor-like (CALCRL) | 3.5 | 0.004 |
| UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 13 (GalNAc-T13) (GALNT13) | 3.5 | 0.003 |
| Phospholipase A2, group IIA (platelets, synovial fluid) (PLA2G2A) | 3.4 | 0.009 |
| Prion protein (PRNP), transcript variant 2 | 3.3 | 0.006 |
| Cartilage intermediate layer protein, nucleotide pyrophosphohydrolase (CILP) | 3.2 | 0.010 |
| Phosphatidic acid phosphatase type 2B (PPAP2B), transcript variant 2 | 3.2 | 0.006 |
| Serglycin (SRGN) | 3.1 | 0.004 |
| PREDICTED: hypothetical protein LOC145837, transcript variant 2 (LOC145837) | 3.0 | 0.008 |
Table IC.
Genes up- and down-regulated with 40 mg of progesterone, compared to 10 mg of progesterone.
| Gene name | Fold change | P-value | Regulation with 40 mg of progesterone |
|---|---|---|---|
| Acyl-Coenzyme A oxidase 2, branched chain (ACOX2) | 2.4 | 0.045 | Up |
| ADAM metallopeptidase with thrombospondin type one motif, 1 (ADAMTS1) | 2.0 | 0.049 | Up |
| Arrestin domain containing 4 (ARRDC4) | 2.8 | 0.048 | Up |
| Chromosome four open reading frame 18 (C4orf18), transcript variant 2 | 2.7 | 0.045 | Up |
| CD36 molecule (thrombospondin receptor) (CD36), transcript variant 3 | 3.1 | 0.045 | Up |
| Cadherin 13, H-cadherin (heart) (CDH13) | 2.1 | 0.047 | Up |
| Collagen, Type IV, alpha 5 (COL4A5), transcript variant 2 | 2.3 | 0.041 | Up |
| Collagen, Type IV, alpha 6 (COL4A6), transcript variant A | 2.7 | 0.041 | Up |
| Chemokine (C-X-C motif) ligand 13 (B-cell chemoattractant) (CXCL13) | 6.0 | 0.047 | Up |
| Cytochrome P450, family 4, subfamily B, polypeptide 1 (CYP4B1), transcript variant 2 | 2.5 | 0.041 | Up |
| Hypothetical protein FLJ21986 (FLJ21986) | 2.0 | 0.041 | Up |
| UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 13 (GalNAc-T13) (GALNT13). | 2.5 | 0.049 | Up |
| Homeobox A10 (HOXA10), transcript variant | 2.1 | 0.049 | Up |
| KH domain containing, RNA binding, signal transduction associated 3 (KHDRBS3) | 2.1 | 0.041 | Up |
| Laminin, alpha 2 (LAMA2), transcript variant 1 | 2.6 | 0.047 | Up |
| Laminin, beta 1 (LAMB1) | 2.2 | 0.049 | Up |
| LIM and cysteine-rich domains 1 (LMCD1) | 2.8 | 0.049 | Up |
| PREDICTED: Homo sapiens hypothetical protein LOC144481, transcript variant 1 (LOC144481) | 2.2 | 0.041 | Up |
| MAM domain containing 2 (MAMDC2) | 3.3 | 0.049 | Up |
| Monooxygenase, DBH-like 1 (MOXD1), transcript variant 2 | 3.7 | 0.049 | Up |
| Monooxygenase, DBH-like 1 (MOXD1), transcript variant 2 | 3.1 | 0.047 | Up |
| NAD(P)H dehydrogenase, quinone 1 (NQO1), transcript variant 1 | 2.1 | 0.049 | Up |
| Phospholipase C-like 1 (PLCL1) | 2.6 | 0.048 | Up |
| RPE-spondin (RPESP) | 2.1 | 0.041 | Up |
| Solute carrier family 39 (zinc transporter), member 8 (SLC39A8) | 2.4 | 0.041 | Up |
| Sulfatase 1 (SULF1) | 2.1 | 0.041 | Up |
| Transmembrane 4L 6 family member 18 (TM4SF18) | 2.2 | 0.049 | Up |
| Vasoactive intestinal peptide receptor 2 (VIPR2) | 2.4 | 0.041 | Down |
Results
The median age of the subjects was 27 years (range: 19–34 years). Initially, 47 samples were obtained for analysis: 10 control samples obtained during the mid-secretory phase in natural cycles, 6 samples each from subjects receiving 2.5 and 5 mg of P daily, and 12 samples each from subjects receiving 10 and 40 mg of P daily. An endometrial biopsy was also obtained on a subject injected with oil alone, to serve as a carrier control (0 mg).
Serum P concentrations
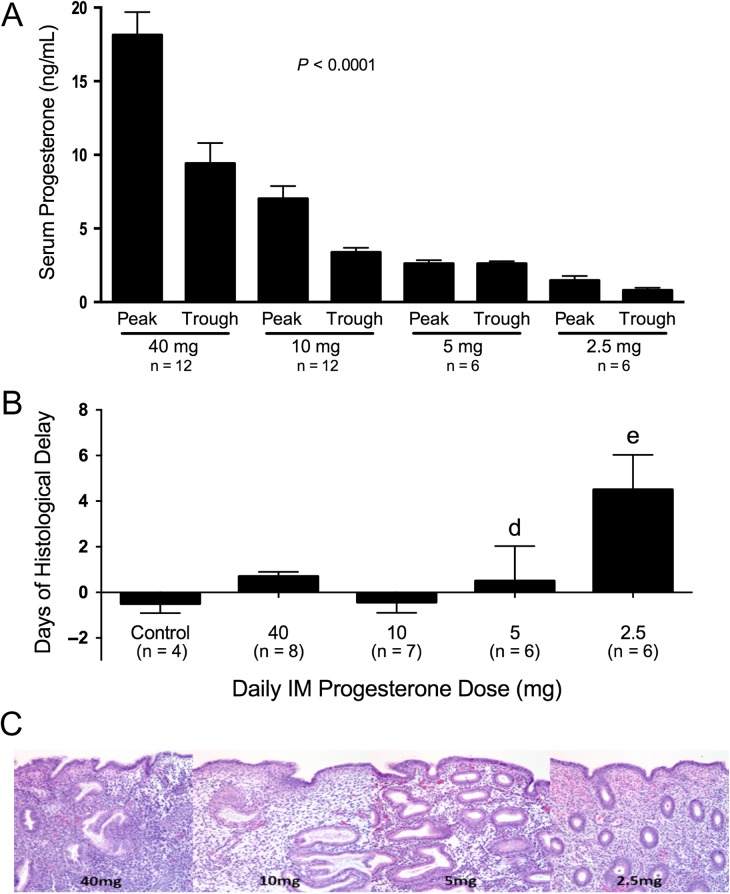
Peak and trough serum P concentrations (mean ± SEM) were different among the groups (Fig. 2A). In subjects receiving 40 mg, the peak (18.1 ± 5.1 ng/ml) and the trough (9.4 ± 4.8 ng/ml) concentrations were entirely within the range typically observed during the normal mid-secretory phase. In those receiving 10 mg P daily, the peak and trough serum P levels were 7 ± 2.9 ng/ml and 3.3 ± 1 ng/mL, respectively. In subjects receiving 5 and 2.5 mg P daily, respectively, peak and trough serum P concentrations were uniformly very low and not significantly different (Fig. 2A).
Figure 2.
(A) Progesterone levels (mean ± SEM) at each daily dose of intramuscular progesterone: 2.5, 5, 10, and 40 mg. Blood samples were obtained at Peak, 2–3 h after injection, and Trough, 1–2 h before the next injection. Significant difference was found among groups P < 0.0001. ANOVA with Tukey's post hoc test demonstrated differences between peaks and troughs for the 40 and 10 mg doses. The peak progesterone levels were different between 40 mg and all other peaks, as well as 10 mg versus 2.5 mg. The trough levels of 40 mg dose were different from all other trough levels. (B) Days of histological delay between the day of the menstrual cycle when endometrial biopsy was performed and histological dating according to Noyes criteria. Bars represent mean (SEM) of difference in days. A significant difference was seen between the 2.5 mg dose versus the 40 mg, 10 mg and control groups (ANOVA P < 0.0001, Tukey's post hoc test P < 0.05). (C) Histological photomicrography of endometrial biopsies performed at Day 10 of modeled. Note variability of histological maturation at the 5 mg group, and histological maturation delay in the 2.5 mg group (Magnification 400×).
Histology
Classical features of secretory histology were observed in all samples from natural and modeled cycles, at all P doses. No secretory changes were observed in the carrier control sample. Overall, we observed an increasing frequency of delayed endometrial histologic development (≥3 day difference between the expected and observed histologic dates) with decreasing doses of P (chi-square for trend; P = 0.01). However, a consistent delay in histological maturation was observed only in specimens from cycles receiving the lowest dose of P (2.5 mg), corresponding to mean peak and trough P concentrations that were uniformly <2 ng/ml; the extent of the observed delay in endometrial development varied among subjects (Fig. 2B and C).
Microarray data analysis
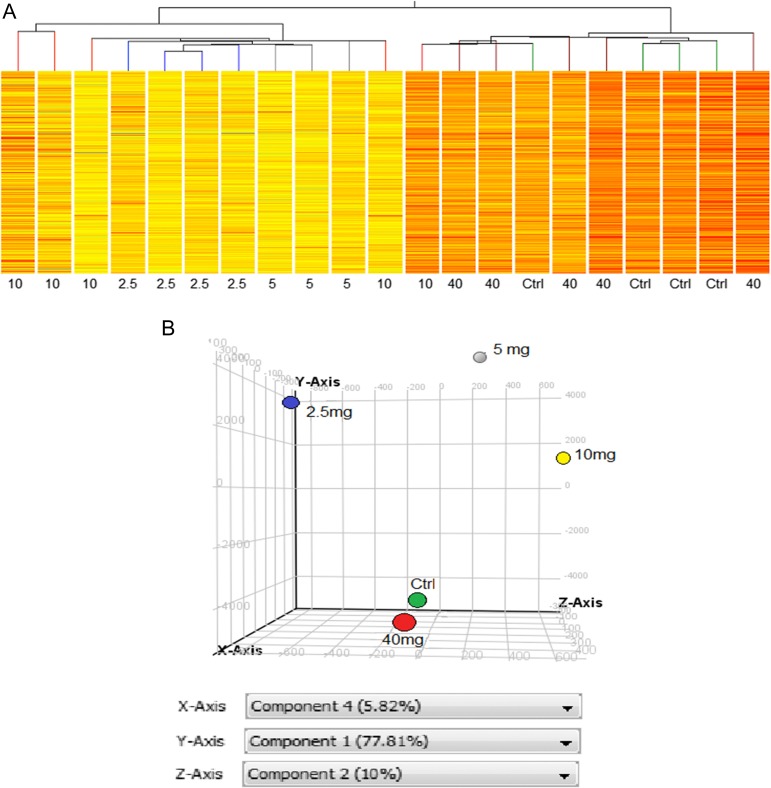
Six samples from each treatment group were randomly selected for microarray gene expression analysis. Raw data were normalized, nine samples rejected for quality, yielding 21 high-quality samples (2.5 mg group, n = 4; 5 mg group, n = 3; 10 mg group, n = 5, 40 mg group, n = 5; mid-secretory controls, n = 4), which were subjected to further analysis. All samples, including those rejected, were uploaded to GEO. After filtering to remove genes whose signal was outside the 95% CI, ANOVA was used to find genes whose expression was different among groups, providing a working list with 2275 genes (Fig. 1). Hierarchical clustering of the working gene list demonstrated a self-segregating pattern by dose of P administered (Fig. 3A). Controls and the group receiving 40 mg P were clustered together under one branch of the hierarchical tree and groups receiving 2.5 and 5 mg P were clustered under a separate branch. The group receiving 10 mg P was split into multiple branches (Fig. 3A), suggesting an inflection point for gene expression changes. The principal component analysis (PCA) analysis demonstrated results similar to the clustering analysis, with the controls and the group receiving 40 mg P daily exhibiting a similar expression pattern and the other groups distinct from each other (Fig. 3B).
Figure 3.
Microarray analysis. Unsupervised hierarchical clustering (A) sorted the 21 samples into two main branches, with the 40 mg dose and natural cycle controls clustered together and samples from the 5 and 2.5 mg groups clustered together. The 10 mg did not show consistent clustering. (B) PCA demonstrated results similar to the hierarchical clustering. Natural cycle controls (green) and the 40 mg dose group (red) cluster together in the PCA, while the 2.5 mg (blue), 5 mg (gray) and 10 mg (yellow) dose groups are at different points in the three dimensional space. PCA, principal component analysis.
Specific comparisons were used to generate groups of normally expressed mid-secretory genes (common genes between the control and 40 mg treatment groups—red group, Fig. 1), and those with different expression in 2.5 and 10 mg treatment groups compared to 40 mg group (green and blue groups, Fig. 1). Genes common to any two of red, green and blue groups represented candidate genes differentially modulated by lower doses of P (2.5 and 10 mg). Candidate genes with at least a 2-fold change and P-value < 0.05, comprised the final list of 183 genes (Tables IA, IB, IC, full list of genes provided in Supplementary Data, Table S1).
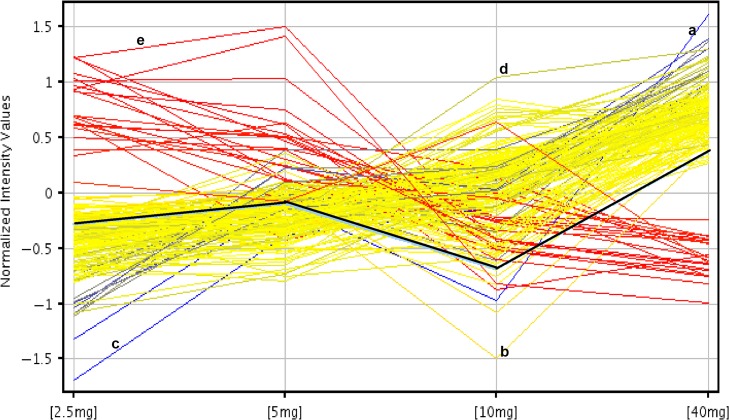
Treatment with 2.5 mg P daily yielded 20 down-regulated genes (Table IA) and 147 up-regulated (Table IB), compared to treatment with 40 mg P daily (Full list in Supplementary Data, Table S1). Treatment with 40 mg P daily yielded 26 up-regulated genes and only 1 down-regulated gene, vasoactive intestinal peptide receptor 2—VIPR2, compared to 10 mg P daily (Table IC). Treatment with 5 mg P daily was not specifically analyzed, as genes from that group would be expected to be included in the treatments with that of 2.5 and 10 mg P daily, compared to 40 mg (Tables IA, IB, IC). The expression pattern of genes across different concentrations of P is depicted in Fig. 4. Of note, some genes demonstrated a multiphasic effect of P dose on expression, as HOXA10 (black line; Fig. 4).
Figure 4.
Fold change analysis of the genes across dose groups. Each line represents an individual gene that demonstrates different patterns of expression by P dose. Blue lines are the genes that have a −6-fold change between 2.5/40 mg groups, red lines are those genes that have a 2-fold change between 2.5/40 mg groups. Yellow lines are those genes that have between −6 and −2-fold change in 2.5/40 mg group. Specific examples are labeled as follows: the black line represents HOXA10. The line marked with ‘a’ represents chemokine ligand 13 (CXCL13). This gene has a low expression with 2.5 mg of P, but high with 40 mg, a change that is −6 FC (2.5/40 mg group). Note that some genes do not have a linear correlation with progesterone dose: For example, monooxygenase, DBH-1 (MOXD1), labeled ‘b’ is down-regulated with 10 mg of progesterone, but up-regulated with 2.5 or 40 mg, Genes also show differential dose thresholds for up-regulation: Aldehyde Oxidase 1 (AOX1), labeled ‘c’ is up-regulated at 5 mg, as serglycin (SRGN), ‘d’, at 10 mg. Claudin 22, ‘e’, demonstrates linear down-regulation as progesterone dose increases.
Natural killer cell signaling
Ingenuity pathway analysis (IPA) (see Supplementary Data) identified two critical mediators of NK signaling differentially regulated by P dose. CD94 and NKG2A are both up-regulated at doses of 10 and 40 mg P daily, respectively (Supplemental Data Fig. S1B). IL-15 was also up-regulated in microarray analysis in the 40 mg group, as compared to 2.5 mg group, and the difference was confirmed by RT-PCR (Table II). CD94/NKG2A was over-expressed in normal cycle subjects, and in the 10 and 40 mg treatment groups, compared to reduced expression in the 5 and 2.5 mg treatment groups (Supplementary Data, Fig. S1B).
Table II.
Gene expression among different groups. ‘Dose’ represents the minimal threshold necessary to achieve not significantly different expression with control cycles. ‘Phase’ represents the phase of the menstrual cycle when the gene is significantly different from the proliferative phase, based from Talbi et al. Numbers are (mean ± SD). Genes with ‘†’ were validated by RT-PCR.
| Gene | Dose | Phase | 2.5 mg (A) | 5 mg (B) | 10 mg (C) | 40 mg (D) | P-value | Post hoc test |
|---|---|---|---|---|---|---|---|---|
| Genes in common between Altmae/Díaz-Gimeno and the 183-gene list | ||||||||
| CXCL13† | 40 | MSE | 0.5 ± 0.5 | 1.3 ± 0.5 | 0.4 ± 0.5 | 3.7 ± 1.8 | 0.0003 | D vs A |
| D vs B | ||||||||
| D vs C | ||||||||
| NP‡ | 2.5 | MSE | −0.7 ± 0.1 | −0.4 ± 0.3 | 0.05 ± 0.2 | 0.3 ± 0.3 | 0.0003 | D vs A |
| D vs B | ||||||||
| AOX1‡ | 2.5 | MSE | −1.6 ± 0.6 | −0.3 ± 1 | −0.1 ± 0.9 | 0.9 ± 0.4 | 0.001 | D vs A |
| KHDRBS3‡ | 2.5 | ESE | −0.1 ± 0.4 | −0.5 ± 0.4 | −0.4 ± 0.3 | 0.6 ± 0.3 | 0.002 | D vs A |
| D vs B | ||||||||
| D vs C | ||||||||
| ENPEP‡ | 2.5 | MSE | −0.3 ± 0.4 | 0.09 ± 0.2 | 0.1 ± 0.6 | 1.1 ± 0.4 | 0.002 | D vs A |
| D vs B | ||||||||
| D vs C | ||||||||
| SLC1A1‡ | 2.5 | MSE | −1 ± 0.5 | −0.7 ± 0.5 | 0.1 ± 0.5 | 0.4 ± 0.3 | 0.002 | D vs A |
| D vs B | ||||||||
| DEPDC6‡ | 2.5 | MSE | 0.6 ± 0.3 | 0.1 ± 0.4 | −0.007 ± 0.2 | −0.4 ± 0.2 | 0.003 | D vs A |
| KIF20A‡ | 2.5 | ESE | −0.4 ± 0.3 | −0.3 ± 0.3 | −0.1 ± 0.4 | 0.6 ± 0.2 | 0.005 | D vs A |
| D vs B | ||||||||
| D vs C | ||||||||
| HAL‡ | 2.5 | MSE | −0.5 ± 0.5 | −0.5 ± 0.6 | −0.06 ± 0.3 | 0.6 ± 0.2 | 0.005 | D vs B |
| D vs C | ||||||||
| LMCD1‡ | 2.5 | MSE | −0.3 ± 0.3 | 0.3 ± 0.3 | −0.4 ± 0.9 | 1 ± 0.2 | 0.006 | D vs A |
| D vs C | ||||||||
| PTGER2‡ | 2.5 | MSE | −0.5 ± 0.3 | −0.2 ± 0.7 | −0.04 ± 0.3 | 0.7 ± 0.5 | 0.007 | D vs A |
| ADAMTS1‡ | 2.5 | ESE | −0.4 ± 0.5 | 0.1 ± 0.08 | −0.2 ± 0.4 | 0.7 ± 0.4 | 0.008 | D vs A |
| D vs C | ||||||||
| KLRC1‡ | 2.5 | LSE | −0.4 ± 0.2 | −0.3 ± 0.4 | 0.6 ± 0.8 | 0.8 ± 0.2 | 0.008 | D vs A |
| D vs B | ||||||||
| EDNRB‡ | 2.5 | MSE | −0.6 ± 0.4 | −0.6 ± 0.7 | 0.6 ± 0.5 | 0.6 ± 0.7 | 0.008 | D vs A |
| D vs B | ||||||||
| CP‡ | 2.5 | MSE | −0.5 ± 0.2 | 0.02 ± 0.1 | −0.1 ± 0.4 | 0.4 ± 0.4 | 0.008 | D vs A |
| D vs C | ||||||||
| CLIC6† | 5 | MSE | 0.1 ± 0.1 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.003 | 0.01 | D vs A |
| C vs A | ||||||||
| NNMT‡ | 2.5 | ESE | −0.8 ± 0.5 | −0.1 ± 0.3 | −0.1 ± 0.5 | 0.3 ± 0.2 | 0.02 | D vs A |
| DKK1† | 5 | MSE | 2.3 ± 1.2 | 2.9 ± 1.1 | 4.4 ± 1.8 | 6 ± 2.3 | 0.03 | D vs A |
| Genes unique to Altmae/Díaz-Gimeno list | ||||||||
| LCN2† | 2.5 | Undefined | 0.1 ± 0.06 | 0.3 ± 0.07 | 0.2 ± 0.08 | 0.1 ± 0.04 | 0.005 | D vs B |
| D vs C | ||||||||
| IL-15† | 5 | MSE | 0.09 ± 0.02 | 0.2 ± 0.08 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.001 | D vs A |
| CXCL14† | 5 | MSE | 42.2 ± 28.3 | 83.1 ± 37.9 | 122.6 ± 37.8 | 122.6 ± 37.8 | 0.01 | D vs A |
| SPP1† | 2.5 | ESE | 3 ± 2.3 | 5.4 ± 2.8 | 13.9 ± 5.7 | 8.9 ± 6.2 | 0.04 | A vs C |
| VNN1† | 2.5 | ESE | 0.9 ± 0.5 | 1.5 ± 0.7 | 2.4 ± 0.9 | 1.6 ± 0.9 | 0.04 | A vs C |
| OGN† | 2.5 | MSE | 1.7 ± 0.6 | 1.1 ± 0.3 | 0.9 ± 0.1 | 1.2 ± 0.4 | 0.05 | |
| BCL6† | 2.5 | MSE | 2.2 ± 1.2 | 3.1 ± 0.4 | 1.5 ± 1.0 | 1.4 ± 1.2 | 0.1 | |
| SLC15A1† | 2.5 | MSE | 1.8 ± 0.8 | 1.2 ± 0.2 | 2.1 ± 0.4 | 2 ± 0.6 | 0.1 | |
| LIF† | 2.5 | MSE | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.7 | |
| VN1R4† | 2.5 | undefined | 2.9 ± 2.5 | 3 ± 1.4 | 4.8 ± 7.8 | 2.4 ± 2.4 | 0.8 | |
| CLEC1A† | 2.5 | ESE | 0.03 ± 0.01 | 0.04 ± 0.003 | 0.03 ± 0.01 | 0.03 ± 0.009 | 0.9 | |
| Gene unique to the 183-gene list | ||||||||
| HOXA10† | 5 | MSE | 0.5 ± 0.1 | 0.9 ± 0.1 | 0.5 ± 0.1 | 0.9 ± 0.2 | 0.004 | D vs A |
| D vs C | ||||||||
| B vs C | ||||||||
| Genes related to endometrial receptivity and progesterone resistance, not present in either list | ||||||||
| MIG-6† | 2.5 | ESE | 3.6 ± 1.5 | 1.9 ± 0.7 | 2 ± 0.7 | 1.7 ± 0.7 | 0.05 | |
| DAF† | 2.5 | MSE | 20.4 ± 10.5 | 21.8 ± 10.5 | 26 ± 6.0 | 18.3 ± 7.5 | 0.5 | |
| Genes validated from IPA | ||||||||
| IL-15† | 5 | MSE | 0.09 ± 0.02 | 0.2 ± 0.08 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.001 | D vs A |
| GP130† | 10 | MSE | 6.1 ± 0.5 | 6.3 ± 0.4 | 6.8 ± 0.5 | 7.3 ± 0.2 | 0.001 | D vs A |
| D vs B | ||||||||
† Analyzed with RT-PCR – quantification was made using 2−ΔCt between gene of reference and peptidylprolyl isomerase A (PPIA).
‡ Microarray data (normalized log scale) from Young et al.
MSE, mid-secretory; ESE, early secretory; IPA, ingenuity pathway analysis.
Validation of microarray data by RT-PCR and comparison with the literature
Our results identified 18 genes in common with the combined gene lists published by Altmäe et al. (2010) and Díaz-Gimeno et al. (2011). From the list of genes common to previous studies and ours (Table II), expression of three genes were examined by RT-PCR to validate the microarray findings: chemokine (C-X-C motif) ligand 13 (CXCL13), Dickkopf homolog 1—Xenopus laevis—(DKK1), and chloride intracellular channel 6 (CLIC6). RT-PCR results confirmed the microarray findings; CXCL13 is expressed in mid-secretory (MSE) and needs 40 mg of daily P to be similar to control cycles; CLIC6 and DKK1 are expressed in MSE and need lower levels of daily P, i.e. 5 mg/day, to be similar to control cycles, of note, 2.5 mg of P increases CLIC6 expression (Table II).
Nine genes presented in the Altmäe/Díaz-Gimeno analysis, but not included in our 183-gene list, were also analyzed for P dose regulation by RT-PCR analysis (Table II). Four of these nine, Lipocalin 2 (LCN2), chemokine (C-X-C motif) ligand 14 (CXCL14), Osteopontin (SPP1) and Vanin 1 (VNN1), were differentially expressed at different P doses. Of note, SPP1 expression is low in 2.5 and 40 mg groups, but high in 10 mg group (Table II). The remaining five genes, osteoglycin (OGN), B-cell CLL/lymphoma 6 (BCL6), solute carrier family 15 (oligo peptide transporter), member 1 (SLC15A1), LIF, vomeronasal one receptor 4 (VN1R4) and C-type lectin domain family 1, member A (CLEC1A), did not differ significantly among groups (Table II).
Genes unique to the 183 - gene list—genes differentially modulated by different concentrations of P
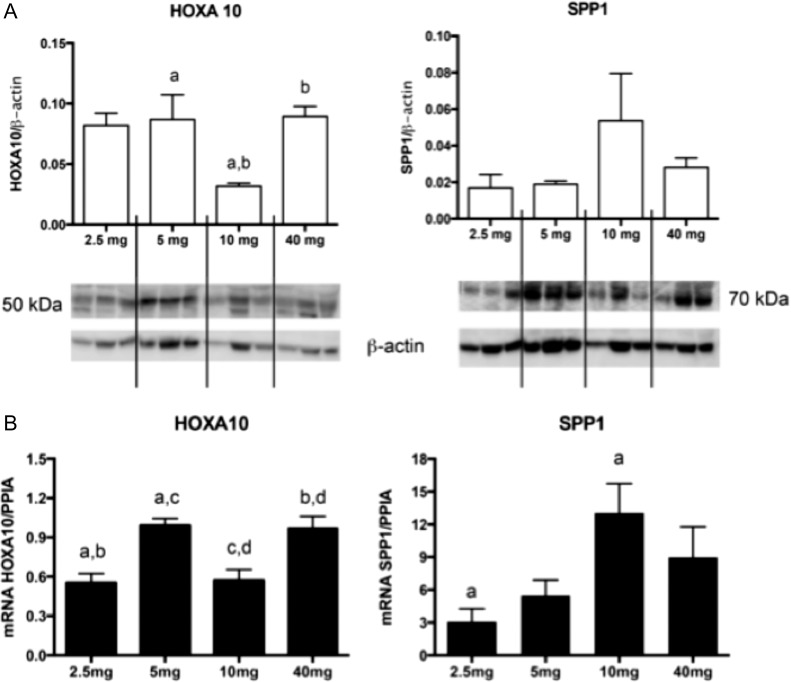
Three additional P-regulated genes were also analyzed with RT-PCR, Homeobox A10 (HOXA10), Mitogen-Inducible Gene 6 Protein (MIG-6) and decay-accelerating factor (DAF). HOXA10, a gene unique to the 183-gene list, was maximally expressed in subjects receiving 40 mg P daily, but expression was higher in the 5 mg group, compared to the 10 and 2.5 mg groups (Table II, Fig. 5). Neither MIG-6 or DAF mRNA expression were different among groups, confirming the microarray findings (Table II), but both were shown to be induced by all P doses as compared to proliferative controls (not shown). Protein expression was similar to mRNA expression for two genes chosen for western blot analysis, HOXA10 and SPP1 (Fig. 5).
Figure 5.
HOXA10 and ostepontin (SPP1) expression at different doses of progesterone using western blot (A) and mRNA (B). Western blot analysis of three samples in each group was normalized with beta-actin. HOXA10 demonstrates multiphasic protein expression by dose in the comparison among groups, P = 0.04 (a and b); SPP1 has higher mean expression at the 10 mg dose. (B) mRNA expression in different groups—(n = 4 in 2.5 and 5 mg group; n = 5 in 10 and 40 mg group). HOXA10 has multiphasic expression at different doses of progesterone; P = 0.03 (a); 0.02 (b); 0.02 (c); 0.01 (d). SPP1 has a higher expression at 10 mg, compared to 2.5 mg (P = 0.04) Bars represent mean (SEM) and significance among groups was identified by ANOVA with Tukey's post hoc test.
Discussion
The results support our hypothesis that P has concentration-dependent effects on secretory endometrial structure and function, as defined by histologic maturation and endometrial gene expression. The data demonstrate, for the first time, direct evidence that abnormally low P concentrations result in delayed endometrial development and abnormal patterns of gene expression. Strikingly, however, we also observed that histologic endometrial development can proceed normally even when P concentrations are well below those normally observed during the luteal phase of natural cycles. In contrast, we found that P has distinct concentration-dependent effects on endometrial gene expression. We also observed differential patterns of expression to increasing P concentrations. These findings are directly relevant to ovarian stimulation cycles during which a premature elevation of P concentration occurs, suggesting that even very low levels of P (between 0.3 and 2.5 ng/ml) can alter endometrial function.
We examined the effects of four different doses of experimentally induced P concentrations, ranging from levels normally observed during the luteal phase to those well below the accepted minimum concentration of luteal phase P (3 ng/ml). In subjects receiving 40 mg of P daily (mean peak and trough P concentrations 18.1 and 9.4 ng/ml, respectively), histologic endometrial dating was uniformly normal, similar to that observed in normally cycling women 10 days after the midcycle LH surge. In subjects, receiving 10 mg of P daily (mean peak and trough P concentrations 7.0 and 3.3 ng/ml, respectively), histologic endometrial dating was again uniformly normal. In subjects receiving 5 mg of P daily (mean peak and trough P concentrations 4.2 and 2.4 ng/ml, respectively), histologic dating varied, being normal in some and grossly delayed in others, suggesting that the threshold P concentration required for normal histologic development is at or very near the lower limit of the concentrations typically observed across the luteal phase of natural cycles. In subjects receiving 2.5 mg of P daily (mean peak and trough P concentrations 2.5 and 0.3 ng/ml, respectively), histologic dating was uniformly delayed by more than 3 days, in relation to the number of days of P exposure. The P levels required for normal histologic maturation in our experimentally modeled cycles are consistent with those suggested by observations in natural cycles: histologic dating was normal in 76% of women having a P concentration greater than 2 ng/ml and in 90% of women with a P concentration greater than 4.7 ng/ml (15 nmol/l) (Nadji et al., 1975). Taken together, our data provide compelling evidence to support the conclusions that endometrial histologic dating in untreated cycles does not accurately reflect differences seen in circulating P concentrations in ovulatory women and is, therefore, not a useful measure of luteal function.
In contrast to these histologic observations, we observed P concentration-dependent differences in patterns of endometrial gene expression within the range of P concentrations observed in ovulatory women in natural cycles. It is important to note that all tissue samples examined in our study who received progesterone demonstrated secretory histologic features; we did not compare the patterns of gene expression in secretory and proliferative endometrium. Hierarchical clustering analysis demonstrated that subjects receiving 40 mg P daily exhibited patterns of gene expression that were indistinguishable from those in naturally cycling women, confirming that the P levels generated in the 40 mg group were within the normal physiologic range. By comparison, samples obtained from women receiving 5.0 and 2.5 mg P daily clustered separately from each other and from those obtained from women receiving 40 mg P daily. Samples obtained from women receiving 10 mg P daily did not cluster together, instead resembling the patterns observed in women receiving either 5 mg or 40 mg P daily. Thus, the 10 mg treatment may represent serum P concentrations that straddle the effective threshold dose required for normal patterns of functional gene expression. Samples obtained from women receiving 10 and 5 mg P daily exhibited normal histologic development but aberrant patterns of gene expression. These observations demonstrate that abnormally low P concentrations can induce a morphologic-functional dissociation in the secretory endometrium. Women receiving 2.5 mg P daily exhibited both grossly, delayed histologic development and an aberrant pattern of gene expression.
Whereas many genes had a monophasic response to changes in P concentrations, a few exhibited likely multiphasic or variable response in the microarray data. Examples include SPP1 (OPN) and VNN1, whose mean and median expression with 2.5 mg P daily was similar to that with 40 mg, but mean and median expression by PCR appeared maximal with 10 mg. In the case of OPN PCR, the apparent decrease in expression from 10 to 40 mg did not achieve statistical significance (ANOVA), but protein expression appeared to mirror the PCR (Fig. 5). With VNN1, the lower mean and median at 40 mg versus 10 mg did not achieve statistical significance, but no protein studies were performed. Expression of HOXA10 was clearly multiphasic with PCR analysis; compared to the level observed in women receiving 40 mg P daily, expression was decreased with 10, 5 and 2.5 mg P daily, but lowest with a 10 mg daily dose. HOXA10 is an endometrial transcription factor required for embryo implantation (Taylor et al., 1998) and whose expression is reduced in women with disorders suspected to adversely affect implantation (Du and Taylor, 2015). The complex pattern of HOXA10 expression suggests the possibility that P actions may need to be finely tuned to optimize endometrial receptivity. To date, the cause(s) of the altered expression of P-regulated genes in some women has remained unclear, though they could theoretically result from abnormally low P concentrations, a defect of P signaling, or both. However, given that serum P levels during the luteal phase exhibit distinct pulsatile fluctuations (Filicori et al., 1984), P kinetics also may be important. Discussion of individual genes and pathways are in the Supplementary Data.
The patient population was normal without signs of endometrial dysfunction, broadening the generalizability of these findings. Other strengths of this study included a prospective design, randomization for P dose assignment, analysis of peak and trough P concentrations, use of liquid chromatography for low P concentrations, double-blinded histological dating, global assessment of gene expression, and validation using RT-PCR with western blot for two gene products. Provision of physiological P (40 mg/day) resulted in endometrial gene expression indistinguishable from that of normal volunteers 10 days after the urinary LH surge, supporting our approach.
Our study also had several limitations. While our study provides clear evidence of dose dependent regulation of endometrial function, it does not preclude the possibility that pulsatile progesterone secretion (Filicori et al., 1984) is physiologically important. Our study included only normal young women recruited from the general population, whom we would expect to have normally functioning endometrium. Further investigation will be necessary to determine if low P concentrations differentially affect the endometrium from infertile women compared to fertile controls. We did not consistently collect subject racial data and cannot exclude racial differences as a cause for variation between subjects. Additionally, we used transcriptomic analysis to investigate tissue function. Although changes in RNA abundance reflect changes in cellular activity, proteins are the primary functional molecules and there is not always a close correlation between changes in RNA and protein abundance. Thus, caution should be exercised in interpreting the functional significance of a variation in abundance of any specific transcript. Although the most important functional measure is embryo implantation rate, we would consider provision of suboptimal progesterone to women for that purpose to be unethical.
Our data demonstrate that observed delays in histologic endometrial maturation cannot be attributed solely to low P concentrations in ovulatory women. Moreover, our results advance on going efforts to identify clinically useful biomarkers of endometrial receptivity and provide essential normative data relating to P actions in normal endometrium, allowing a better understanding of the role of P concentrations in the pathogenesis and treatment of infertile women.
Supplementary data
Supplementary data are available at Human Reproduction online.
Authors’ roles
Study design: S.L.Y., B.A.L., M.A.F. Study execution: S.L.Y., U.B. Analysis: S.L.Y., R.F.S., B.A.L., A.M.S., R.J.Z., R.A.S. Manuscript preparation: S.L.Y., R.F.S., B.A.L. and M.A.F.
Funding
The Eunice Kennedy Shriver National Institute for Child Health and Disease, National Institute of Health, USA (NICHD/NIH) (R01HD067721 and U54HD30476; S.L.Y. and B.A.L.) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (240239/2012-1 to R.F.S.).
Conflict of interest
None declared.
Supplementary Material
Acknowledgement
We acknowledge the assistance of Janetta Phillips for organizing the samples and data, Dr Lingwen Yuan for preparing RNA and performing RT-PCR, and Drs Ringland Murray, Jessica Scotchie and Beth Plante for assisting with sample collection.
References
- Altmäe S, Martínez-Conejero JA, Salumets A, Simón C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod 2010;16:178–187. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007;148:3814–3826. [DOI] [PubMed] [Google Scholar]
- Cardozo ER, Karmon AE, Gold J, Petrozza JC, Styer AK. Reproductive outcomes in oocyte donation cycles are associated with donor BMI. Hum Reprod 2016;31:385–392. [DOI] [PubMed] [Google Scholar]
- Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MP et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril 2004;82:1264–1272. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the ASRM Current clinical irrelevance of luteal phase deficiency: a committee opinion. Fertil Steril 2015;103:e27–e32. [DOI] [PubMed] [Google Scholar]
- Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alamá P, Pellicer A, Simón C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril 2011;95:50–60. 60.e1–e15. [DOI] [PubMed] [Google Scholar]
- Donaghay M, Lessey BA. Uterine receptivity: alterations associated with benign gynecological disease. Semin Reprod Med 2007;25:461–475. [DOI] [PubMed] [Google Scholar]
- Du H, Taylor HS. The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb Perspect Med 2015;6:a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filicori M, Butler JP, Crowley WF Jr. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest 1984;73:1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-C, Lien Y-R, Chen H-F, Chen M-J, Shieh C-J, Yao Y-L, Chang C-H, Chen S-U, Yang Y-S. The duration of pre-ovulatory serum progesterone elevation before hCG administration affects the outcome of IVF/ICSI cycles. Hum Reprod 2012;27:2036–2045. [DOI] [PubMed] [Google Scholar]
- Jones GES. Some newer aspects of the management of infertility. J Am Med Assoc 1949;141:1123–1129. [DOI] [PubMed] [Google Scholar]
- Mingari MC, Ponte M, Bertone S, Schiavetti F, Vitale C, Bellomo R, Moretta A, Moretta L. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc Natl Acad Sci USA 1998;95:1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, Zeng D, Fritz MA. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril 2004;81:1333–1343. [DOI] [PubMed] [Google Scholar]
- Nadji P, Reyniak JV, Sedlis A, Szarowski DH, Bartosik D. Endometrial dating correlated with progesterone levels. Obstet Gynecol 1975;45:193–194. [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the Endometrial Biopsy. Fertil Steril 1950;1:3–25. [DOI] [PubMed] [Google Scholar]
- Plante BJ, Lessey BA, Taylor RN, Wang W, Bagchi MK, Yuan L, Scotchie J, Fritz MA, Young SL. G Protein-Coupled Estrogen Receptor (GPER) Expression in Normal and Abnormal Endometrium. Reprod Sci 2012;19:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaris RF, Groll JM, Young SL, DeMayo FJ, Jeong J-W, Hamilton AE, Giudice LC, Lessey BA. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab 2011;96:1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006;147:1097–1121. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 1998;101:1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadi RS, Groll JM, Lessey BA, Lininger RA, Zaino RJ, Fritz MA, Young SL. Endometrial development and function in experimentally induced luteal phase deficiency. J Clin Endocrinol Metab 2008;93:4058–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015;7:CD009154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update 2013;19:433–457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.