Summary
Despite having access to clinical care, disparities in the occurrence of chronic kidney disease, type 2 diabetes, and hypertension persist between sex and racial/ethnic groups, among antiretroviral therapy–experienced individuals aging with HIV.
Keywords: noncommunicable disease, disparities, aging, HIV
Abstract
Background.
There remains concern regarding the occurrence of noncommunicable diseases (NCDs) among individuals aging with human immunodeficiency virus (HIV), but few studies have described whether disparities between demographic subgroups are present among individuals on antiretroviral therapy (ART) with access to care.
Methods.
We assessed the first documented occurrence of type 2 diabetes mellitus (DM), chronic kidney disease (CKD), and treated hypertension (HTN) by age, sex, and race within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). HIV-infected adults (≥18 years) who initiated ART were observed for first NCD occurrence between 1 January 2000 and 31 December 2013. Cumulative incidences as of age 70 were estimated accounting for the competing risk of death; Poisson regression was used to compare rates of NCD occurrence by demographic subgroup.
Results.
We included >50000 persons with >250000 person-years of follow-up. Median follow-up was 4.7 (interquartile range, 2.4–8.1) years. Rates of first occurrence (per 100 person-years) were 1.2 for DM, 0.6 for CKD, and 2.6 for HTN. Relative to non-black women, the cumulative incidences were increased in black women (68% vs 51% for HTN, 52% vs 41% for DM, and 38% vs 35% for CKD; all P < .001); this disparity was also found among men (73% vs 60% for HTN, 44% vs 34% for DM, and 30% vs 25% for CKD; all P < .001).
Conclusions.
Racial disparities in the occurrence of DM, CKD, and HTN emphasize the need for prevention and treatment options for these HIV populations receiving care in North America.
Human immunodeficiency virus (HIV)–infected individuals are living longer due to the use of effective antiretroviral therapy (ART) [1]. Principally because of this increased life expectancy, it is projected that by the end of 2017, 50% of the US population living with HIV/AIDS will be >50 years old [2]. This public health success is tempered by concerns for long-term health and quality of life. Noncommunicable diseases (NCDs) typically associated with aging in the general population have emerged as significant sources of clinical concern [3].
Type 2 diabetes mellitus (DM), chronic kidney disease (CKD), and hypertension (HTN) are important NCDs that result in significant morbidity. They have great implications on cardiovascular disease, a leading cause of death in those with HIV [4], having been identified as risk factors for atherosclerotic heart and other vascular diseases [5, 6]. However, whether and to what extent disparities of these NCDs exist in HIV patients receiving care is unclear. Prospective data are lacking among specific demographic subgroups with HIV previously identified as being differentially at risk for age-related diseases [7]. The objective of this study was to estimate the rates of first documented occurrence of HTN, DM, and CKD, by age, sex, and race, among ART-experienced adults living with HIV.
METHODS
Study Population
We analyzed data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite U.S. and Canadian cohorts of HIV-infected adults that has been described previously [8]. In brief, cohort participants eligible for inclusion in NA-ACCORD were required to have at least 2 HIV care visits within 12 months. Each cohort has standardized methods of data collection and submits data on enrolled participant characteristics, diagnoses, laboratory measures, prescribed medications, and vital status to the Data Management Core (University of Washington, Seattle). The completeness and accuracy of data are evaluated before data elements are harmonized across cohorts. Data are then sent to the Epidemiology/Biostatistics Core (Johns Hopkins University, Baltimore, Maryland), where additional quality control procedures are executed and analytic files are created.
For this analysis, clinical cohorts were included if data elements to ascertain HTN, DM, and CKD were readily available. There were 16 cohorts that contributed data for HTN, 20 cohorts for DM, and 22 cohorts for CKD. Because our interest was in an ART-treated population, as these individuals are most likely to age with HIV and be susceptible to these NCDs, the study population was restricted to participants who were ART-experienced and contributed data at least twice between 1 January 2000 and 31 December 2013 (see Supplementary Figure 1 for more details on inclusion criteria).
Outcomes: Type 2 Diabetes Mellitus, Chronic Kidney Disease, and Treated Hypertension
The outcomes of interest were the first documented occurrence of DM, CKD, and HTN during the study period. Any DM was defined as having either a glycosylated hemoglobin (HgbA1c) level of ≥6.5%; documented use of a diabetes-specific medication; or documented use of a diabetes-related medication in addition to a diagnosis of diabetes. CKD was laboratory-based, defined as 2 values of estimated glomerular filtration rate (eGFR) <60 mL/minute/1.73 m2 (>90 days apart without an intervening normal value) and calculated by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation [9]. HTN was defined as ever having a diagnosis of hypertension in conjunction with documented use of antihypertensive medication, thus capturing treated hypertension.
We attempted to account for detection bias by excluding prevalent cases defined as evidence of these outcomes prior to, at, or within 9 months after study entry. For our CKD analysis, to account for a bias in the rate of CKD progression between individuals entering the study with low vs high renal function, participants were required to have an eGFR >90 mL/minute/1.73 m2 within 6 months prior to or after they began contributing person-time to the analysis.
Covariates
Sex, race (“black” for those reporting black or black Hispanic, and “non-black” otherwise, due to the small number of events that occur within more granular racial strata), and injection drug use as a risk factor for HIV transmission (categorized as self-reported injection drug use, or injection drug use and men who have sex with men) were reported at enrollment into the NA-ACCORD. For our study, HIV transmission risk group was collapsed by injection drug use status because of previous evidence to suggest that injection drug users (IDUs) are more disadvantaged in terms of health, receiving less than optimal healthcare compared to other HIV subpopulations [10].
Smoking status was assigned based on all self-reported and medical record data contributed to NA-ACCORD. Any individuals who reported using tobacco or had a clinician-documented diagnosis of smoking were designated as smokers, individuals with no evidence of smoking were categorized as nonsmokers, and individuals with a missing report or diagnosis were deemed missing. Body mass index (BMI), calculated as (kg) / [height (m)]2, was obtained no more than 6 months prior to when a participant began contributing data to the analysis. Calendar year at ART initiation was categorized as 1996–1999, 2000–2005, or 2006–2013. ART prescription was defined consistent with US guidelines as a regimen of ≥3 antiretroviral agents from at least 2 classes or a triple nucleoside/nucleotide reverse transcriptase inhibitor regimen containing abacavir or tenofovir [11]. A history of an AIDS diagnosis at entry into our study was defined according to 1993 criteria from the Centers for Disease Control and Prevention, excluding the CD4 T-lymphocyte count (CD4) <200 cells/µL criterion to avoid collinearity when adjusting for time-varying CD4 in our analysis [12].
CD4 was categorized as <200, 200–349, 350–499, and ≥500 cells/µL. A combined time-varying measure of viral suppression (HIV RNA value ≤400 copies/mL) and ART use was created due to the collinearity of these variables, and was categorized as (1) ART-experienced but not currently prescribed ART, (2) currently prescribed ART but not virally suppressed, and (3) currently prescribed ART and virally suppressed.
Statistical Analyses
Differences in demographic and clinical characteristics were explored with Pearson χ2 tests for categorical variables.
We calculated incidence rates (IRs) per 100 person-years (PY) and 95% confidence intervals (CIs) for each disease outcome. To examine the association between sex–race subgroup (non-black women, black women, non-black men, and black men) and NCDs, crude and adjusted incidence rate ratios (IRRs and aIRRs, respectively) and 95% CIs were estimated using Poisson regression models. The person-time contributed by an individual under observation for the outcome of interest was accrued from study entry, defined as the later date of either ART initiation or 1 January 2000. Participants were followed until the outcome of interest, death, 1 year after the last CD4 or HIV RNA measure, 70 years of age, or 31 December 2013, whichever came first. We censored person-time at and beyond age 70 to avoid skewed disease rates and cumulative incidence estimates as these individuals had small risk set sample sizes.
Cumulative incidence estimates as of age 70 were obtained for each disease outcome accounting for the competing risk of death [13]. We report our findings using age as the time metric to compare people of similar age, thus having the greatest possible control for age as a confounder [14]. In a supplement, we present our data using a scale that is comparable with other studies (time metric = time on study) [15–19]. The proportional subdistribution hazard assumption was assessed by including an interaction term between age and sex–race in our competing risk regression model. Finally, to account for confounding by BMI, we conducted a subgroup analysis restricting to those with BMI measurements.
Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, North Carolina) and Stata software version 12.1 (StataCorp, College Station, Texas). All statistical tests were 2-sided and a P value < .05 guided statistical interpretation.
RESULTS
Study Population Characteristics
For each analysis, the majority of the study population was <50 years of age at study entry, male, non-black, did not report injection drug use, had not experienced AIDS, had a recent CD4 of <200 cells/µL, and was on ART but not virally suppressed (Table 1). Among participants with BMI measured at study entry, less than a fifth were overweight or obese for the HTN and DM analysis, but almost half were overweight or obese in the CKD analysis. Participants who developed any one of the NCDs during the study period differed by demographic and clinical characteristics at study entry compared with persons who remained event-free. Older age, black race, injection drug use, a history of smoking, and having initiated ART in earlier calendar periods were each associated with increased rates of first occurrence of HTN, DM, and CKD (Table 2).
Table 1.
Characteristics of Antiretroviral Therapy–Experienced, HIV-Infected Adults at Study Entry During 2000–2013
| Variable | HTN (n = 9547) |
No HTN (n = 58858) |
DM (n = 5881) |
No DM (n = 80908) |
CKD (n = 1785) |
No CKD (n = 50626) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PY = 41291 | PY = 323788 | PY = 23918 | PY = 452258 | PY = 10053 | PY = 288382 | |||||||
| Age, y | ||||||||||||
| <40 | 2734 | 29 | 27002 | 46 | 1488 | 25 | 33831 | 42 | 430 | 24 | 24012 | 47 |
| 40–49 | 4201 | 44 | 21555 | 37 | 2530 | 43 | 30270 | 37 | 782 | 44 | 18553 | 37 |
| 50–59 | 2234 | 23 | 8673 | 15 | 1601 | 27 | 13789 | 17 | 480 | 27 | 7044 | 14 |
| 60–69 | 378 | 4 | 1628 | 3 | 262 | 4 | 3018 | 4 | 93 | 5 | 1017 | 2 |
| Sex | ||||||||||||
| Male | 8464 | 89 | 48908 | 83 | 4842 | 82 | 67621 | 84 | 1481 | 83 | 42200 | 83 |
| Race | ||||||||||||
| Non-black | 4836 | 51 | 35330 | 60 | 2893 | 49 | 47838 | 59 | 884 | 50 | 28592 | 56 |
| Black | 4711 | 49 | 23528 | 40 | 2988 | 51 | 33070 | 41 | 901 | 50 | 22034 | 44 |
| Injection drug use as HIV transmission risk | ||||||||||||
| No | 6829 | 72 | 47617 | 81 | 4230 | 72 | 63921 | 79 | 1191 | 67 | 39948 | 79 |
| Smokinga | ||||||||||||
| Never | 717 | 8 | 5927 | 10 | 397 | 7 | 9153 | 11 | 146 | 8 | 5653 | 11 |
| Ever | 2615 | 27 | 20798 | 35 | 1565 | 27 | 30142 | 37 | 646 | 36 | 19692 | 39 |
| Missing | 6215 | 65 | 32133 | 55 | 3919 | 67 | 41613 | 51 | 993 | 56 | 25281 | 50 |
| BMI, kg/m2 | ||||||||||||
| <18.5 | 913 | 10 | 10268 | 17 | 429 | 7 | 14001 | 17 | 28 | 2 | 727 | 1 |
| 18.5–24.9 | 685 | 7 | 5961 | 10 | 408 | 7 | 8688 | 11 | 284 | 16 | 9293 | 18 |
| 25–29.9 | 334 | 3 | 2184 | 4 | 353 | 6 | 3401 | 4 | 150 | 8 | 5655 | 11 |
| 30–40 | 58 | 1 | 304 | 1 | 73 | 1 | 473 | 1 | 55 | 3 | 2391 | 5 |
| >40 | 57 | 1 | 851 | 1 | 35 | 1 | 1102 | 1 | 12 | 1 | 401 | 1 |
| Missing | 7500 | 79 | 39290 | 67 | 4583 | 78 | 53243 | 66 | 1256 | 70 | 32159 | 64 |
| AIDS | ||||||||||||
| No | 7811 | 82 | 47252 | 80 | 4668 | 79 | 65016 | 80 | 1341 | 75 | 40770 | 81 |
| Calendar period of ART initiation | ||||||||||||
| 1996–1999 | 4651 | 49 | 17707 | 30 | 2804 | 48 | 25534 | 32 | 851 | 48 | 14895 | 29 |
| 2000–2005 | 3669 | 38 | 21231 | 36 | 2361 | 40 | 29892 | 37 | 766 | 43 | 19204 | 38 |
| 2006–2013 | 1101 | 12 | 17595 | 30 | 638 | 11 | 22624 | 28 | 138 | 8 | 14962 | 30 |
| CD4 count, cells/µL | ||||||||||||
| >500 | 1684 | 18 | 10208 | 17 | 1257 | 21 | 14474 | 18 | 284 | 16 | 9345 | 18 |
| 350–499 | 1219 | 13 | 8223 | 14 | 852 | 14 | 11790 | 15 | 197 | 11 | 7701 | 15 |
| 200–349 | 1772 | 19 | 11964 | 20 | 1021 | 17 | 17167 | 21 | 351 | 20 | 11118 | 22 |
| <200 | 4870 | 51 | 28458 | 48 | 2751 | 47 | 37471 | 46 | 953 | 53 | 22459 | 44 |
| ART and HIV RNA, copies/mL | ||||||||||||
| ≤400, on ART | 2848 | 30 | 16091 | 27 | 1933 | 33 | 23031 | 28 | 439 | 25 | 14279 | 28 |
| >400, on ART | 3620 | 38 | 26026 | 44 | 2202 | 37 | 35650 | 44 | 853 | 48 | 24334 | 48 |
| Off ART | 207 | 2 | 1235 | 2 | 187 | 3 | 1936 | 2 | 60 | 3 | 1120 | 2 |
| Missing | 2872 | 30 | 15506 | 26 | 1559 | 27 | 20291 | 25 | 433 | 24 | 10893 | 22 |
Data are presented as No. (%) unless otherwise indicated. Statistical differences were assessed comparing cases to noncases for each NCD. All covariates were statistically significant (P < .05) with the exception of sex and BMI for the CKD analysis.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; HIV, human immunodeficiency virus; HTN, hypertension; NCD, noncommunicable diseases; PY, person-years.
Smoking data in NA-ACCORD included observed and imputed observations. Across all 3 study populations, 3% of participants were imputed to be never smokers, 10% were imputed to be ever smokers, 7%–8% were observed as never smokers, 24%–29% were observed as ever smokers, and 50%–56% had missing smoking data. Results of our analyses pooled smoking coefficients across 5 datasets with imputed smoking status, and we obtained valid inferences by accounting for associated covariance matrices between imputed datasets.
Table 2.
Noncommunicable Disease Rates (per 100 person-years) Among Antiretroviral Therapy–Experienced, HIV-Infected Adults, 2000–2013
| Variable | Treated Hypertension (n = 68405) | Type 2 Diabetes (n = 86789) | Chronic Kidney Disease (n = 52411) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | PY | IR | (95% CI) | Events | PY | IR | (95% CI) | Events | PY | IR | (95% CI) | |
| Age, y | ||||||||||||
| <40 | 1388 | 109425 | 1.3 | (1.2–1.3) | 806 | 130208 | 0.6 | (.6–.7) | 167 | 92767 | 0.2 | (.2–.2) |
| 40–49 | 3804 | 148131 | 2.6 | (2.5–2.7) | 2232 | 190276 | 1.2 | (1.1–1.2) | 605 | 122913 | 0.5 | (.5–.5) |
| 50–59 | 3407 | 87322 | 3.9 | (3.8–4.0) | 2190 | 124127 | 1.8 | (1.7–1.8) | 694 | 68786 | 1.0 | (.9–1.1) |
| 60–69 | 948 | 20202 | 4.7 | (4.4–5.0) | 653 | 31565 | 2.1 | (1.9–2.2) | 319 | 13968 | 2.3 | (2.0–2.5) |
| Sex | ||||||||||||
| Male | 8464 | 310127 | 2.7 | (2.7–2.8) | 4842 | 399860 | 1.2 | (1.2–1.2) | 1481 | 248835 | 0.6 | (.6–.6) |
| Female | 1083 | 54953 | 2.0 | (1.9–2.1) | 1039 | 76315 | 1.4 | (1.3–1.4) | 304 | 49599 | 0.6 | (.6–.7) |
| Race | ||||||||||||
| Non-black | 4836 | 220407 | 2.2 | (2.1–2.3) | 2893 | 283628 | 1.0 | (1.0–1.1) | 884 | 169906 | 0.5 | (.5–.6) |
| Black | 4711 | 144672 | 3.3 | (3.2–3.4) | 2988 | 192547 | 1.6 | (1.5–1.6) | 901 | 128529 | 0.7 | (.7–.8) |
| Injection drug use as HIV transmission risk | ||||||||||||
| No | 6829 | 287518 | 2.4 | (2.3–2.4) | 4230 | 370127 | 1.1 | (1.1–1.2) | 1191 | 230247 | 0.5 | (.5–.6) |
| Yes | 2718 | 77562 | 3.5 | (3.4–3.6) | 1651 | 106048 | 1.6 | (1.5–1.6) | 594 | 68187 | 0.9 | (.9–.9) |
| Smoking | ||||||||||||
| Never | 729 | 41021 | 1.8 | (1.7–1.9) | 297 | 57707 | 0.7 | (.6–.8) | 146 | 35927 | 0.4 | (.4–.5) |
| Ever | 2636 | 125111 | 2.1 | (2.0–2.2) | 1565 | 179512 | 0.9 | (.8–.9) | 646 | 119984 | 0.5 | (.5–.6) |
| Missing | 6365 | 212274 | 3.0 | (2.9–3.1) | 3919 | 238956 | 1.6 | (1.6–1.7) | 993 | 142524 | 0.7 | (.7–.7) |
| BMI at entry, kg/m2 | ||||||||||||
| <18.5 | 913 | 60520 | 1.5 | (1.4–1.6) | 429 | 82159 | 0.5 | (.5–.6) | 28 | 3834 | 0.7 | (.5–1.1) |
| 18.5–24.9 | 685 | 34790 | 2.0 | (1.8–2.1) | 408 | 51176 | 0.8 | (.7–.9) | 284 | 55107 | 0.5 | (.5–.6) |
| 25–29.9 | 334 | 11874 | 2.8 | (2.5–3.1) | 353 | 19503 | 1.8 | (1.6–2.0) | 150 | 33341 | 0.5 | (.4–.5) |
| 30–40 | 58 | 1533 | 3.8 | (2.9–4.9) | 73 | 2673 | 2.7 | (2.2–3.4) | 55 | 13568 | 0.4 | (.3–.5) |
| >40 | 57 | 4314 | 1.3 | (1.0–1.7) | 35 | 5606 | 0.6 | (.4–.9) | 12 | 2338 | 0.5 | (.3–.9) |
| Missing | 7500 | 252049 | 3.0 | (2.9–3.0) | 4583 | 315057 | 1.5 | (1.4–1.5) | 1256 | 190246 | 0.7 | (.6–.7) |
| AIDS at entry | ||||||||||||
| No | 7811 | 298095 | 2.6 | (2.6–2.7) | 4668 | 383941 | 1.2 | (1.2–1.3) | 1341 | 240786 | 0.6 | (.5–.6) |
| Yes | 1736 | 66984 | 2.6 | (2.5–2.7) | 1213 | 92234 | 1.3 | (1.2–1.4) | 444 | 57648 | 0.8 | (.7–.9) |
| Calendar year at ART initiation | ||||||||||||
| 2006–2013 | 1101 | 67973 | 1.6 | (1.5–1.7) | 638 | 80535 | 0.8 | (.7–.9) | 138 | 53031 | 0.3 | (.2–.3) |
| 2000–2005 | 3669 | 141696 | 2.6 | (2.5–2.7) | 2361 | 189319 | 1.3 | (1.2–1.3) | 766 | 123619 | 0.6 | (.6–.7) |
| 1996–1999 | 4651 | 146738 | 3.2 | (3.1–3.3) | 2804 | 195250 | 1.4 | (1.4–1.5) | 851 | 116228 | 0.7 | (.7–.8) |
| ART and HIV RNA, copies/mL | ||||||||||||
| ≤400, on ART | 5493 | 183209 | 3.0 | (2.9–3.1) | 3387 | 251583 | 1.4 | (1.3–1.4) | 1034 | 162144 | 0.6 | (.6–.7) |
| >400, on ART | 1487 | 46523 | 3.2 | (3.0–3.4) | 925 | 61277 | 1.5 | (1.4–1.6) | 320 | 39789 | 0.8 | (.7–.9) |
| Off ART | 2447 | 131500 | 1.9 | (1.8–1.9) | 467 | 34259 | 1.4 | (1.2–1.5) | 152 | 21030 | 0.7 | (.6–.8) |
| Missing | 120 | 3847 | 3.1 | (2.6–3.7) | 1102 | 129056 | 0.9 | (.8–.9) | 279 | 75472 | 0.3 | (.3–.4) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; IR, incidence rates per 100 person-years; PY, person-years.
Overall, rates of first occurrence for each disease were as follows: 2.6/100 PY for HTN (n = 9547 events; n = 4198 for non-Hispanic whites, n = 4711 for non-Hispanic blacks, n = 487 for Hispanics, and n = 151 for other), 1.2/100 PY for DM (n = 5881 events; n = 2275 for non-Hispanic whites, n = 2988 for non-Hispanic blacks, n = 498 for Hispanics, n = 120 for other), and 0.6/100 PY for CKD (n = 1785 events; n = 742 for non-Hispanic whites, n = 901 for non-Hispanic blacks, n = 100 for Hispanics, n = 42 for others). The median follow-up time for each analysis was 4.4 (interquartile range [IQR], 2.1–7.8) years, 4.8 (IQR, 2.8–8.0) years, and 5.0 (IQR, 2.5–8.5) years, respectively. Median age at diagnosis for HTN, DM, and CKD for the observed cases was 48 (IQR, 43–54), 49 (IQR, 43–55), and 51 (IQR, 45–57) years, respectively. Median follow-up for those with and without HTN was 3.6 (IQR, 2.0–6.2) and 4.6 (IQR, 2.2–8.1) years, respectively; for those with and without DM was 3.3 (IQR, 1.5–6.0) and 5.0 (IQR, 2.4–8.1) years, respectively; and for those with and without CKD was 5.3 (IQR, 3.2–8.0) and 5.1 (IQR, 2.5–8.5) years, respectively.
Estimation of Outcomes by Subgroup of Sex and Race
We observed disparities by sex and race after adjusting for age, risk behavior, and HIV-related factors in our Poisson analyses. Regardless of sex, black race was significantly associated with higher rates of HTN (relative to non-black men: aIRR, 1.8 [95% CI, 1.6–2.1] for black women and 1.5 [95% CI, 1.4–1.7] for black men). For DM, black men, black women, and non-black women experienced significantly higher rates of DM relative to non-black men (aIRRs, 1.4 [95% CI, 1.2–1.6], 2.0 [95% CI, 1.6–2.3], and 1.4 [95% CI, 1.2–1.7], respectively). Last, black and non-black women had significantly higher CKD rates compared with non-black men (aIRRs, 1.5 [95% CI, 1.2–1.9] and 1.5 [95% CI, 1.1–2.0], respectively). Study entry characteristics by sex–race subgroup are presented in Supplementary Table 1.
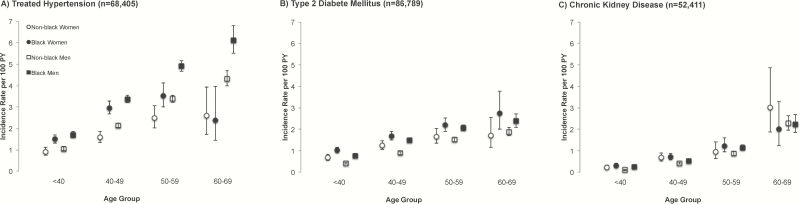
We also assessed whether the relationship between age and NCD was significantly different by sex–race subgroup (Figure 1). Adjusted rates of NCD occurrence increased with older age for each sex–race subgroup (Table 3), and was significantly different between sex–race subgroups for HTN and DM (all P ≤ .02).
Figure 1.
Unadjusted rates of first occurring treated hypertension (A), type 2 diabetes mellitus (B), and chronic kidney disease (C), stratified by sex and race group. Vertical bars represent 95% confidence intervals for rates. Abbreviation: PY, person-years.
Table 3.
Crude and Adjusted Incidence Rate Ratios for Noncommunicable Diseases Among Antiretroviral Therapy–Experienced, HIV-Infected Adults by Age, Sex, and Race, 2000–2013
| Age Group, y | Univariate Analysis | Multivariable Analysisa | ||
|---|---|---|---|---|
| IRR | (95% CI) | aIRR | (95% CI) | |
| Hypertension (n = 68405) | ||||
| Non-black women | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 1.7 | (1.3–2.3) | 1.6 | (1.2–2.2) |
| 50–59 | 2.8 | (2.1–3.7) | 2.5 | (1.7–3.5) |
| 60–69 | 2.9 | (1.8–4.6) | 2.2 | (1.2–4.1) |
| Black women | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 2.0 | (1.7–2.3) | 1.6 | (1.3–1.9) |
| 50–59 | 2.4 | (1.9–2.9) | 1.6 | (1.2–2.2) |
| 60–69 | 1.6 | (1.0–2.7) | 1.1 | (.5–2.5) |
| Non-black men | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 2.0 | (1.9–2.2) | 1.9 | (1.7–2.2) |
| 50–59 | 3.2 | (2.9–3.5) | 2.5 | (2.2–2.9) |
| 60–69 | 4.1 | (3.7–4.6) | 3.0 | (2.4–3.8) |
| Black men | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 2.0 | (1.8–2.2) | 1.8 | (1.5–2.1) |
| 50–59 | 2.9 | (2.6–3.2) | 2.4 | (1.9–2.9) |
| 60–69 | 3.6 | (3.1–4.1) | 2.4 | (1.0–1.4) |
| Type 2 diabetes (n = 86789) | ||||
| Non-black women | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 1.8 | (1.4–2.4) | 1.4 | (.9–2.1) |
| 50–59 | 2.4 | (1.8–3.3) | 2.4 | (1.5–3.7) |
| 60–69 | 2.5 | (1.6–4.0) | 1.9 | (1.0–3.9) |
| Black women | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 1.7 | (1.4–2.0) | 1.5 | (1.1–2.1) |
| 50–59 | 2.1 | (1.8–2.6) | 1.6 | (1.1–2.3) |
| 60–69 | 2.7 | (1.9–3.8) | 2.8 | (1.7–4.8) |
| Non-black men | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 2.2 | (1.9–2.5) | 2.0 | (1.6–2.5) |
| 50–59 | 3.7 | (3.2–4.2) | 3.4 | (2.7–4.3) |
| 60–69 | 4.6 | (3.9–5.3) | 4.7 | (3.5–6.2) |
| Black men | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 1.9 | (1.7–2.2) | 1.3 | (1.0–1.8) |
| 50–59 | 2.7 | (2.3–3.1) | 1.8 | (1.3–2.5) |
| 60–69 | 3.1 | (2.6–3.7) | 2.8 | (1.9–4.2) |
| Chronic kidney disease (n = 52411) | ||||
| Non-black women | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 3.2 | (1.9–5.6) | 2.7 | (1.3–5.6) |
| 50–59 | 4.5 | (2.4–8.3) | 3.9 | (1.7–8.9) |
| 60–69 | 14.3 | (7.4–27.8) | 16.7 | (6.9–40) |
| Black women | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 2.5 | (1.7–3.6) | 1.7 | (1.0–2.8) |
| 50–59 | 4.3 | (2.9–6.4) | 3.6 | (2.1–6.2) |
| 60–69 | 7.0 | (3.9–12.5) | 8.1 | (3.9–16.6) |
| Non-black men | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 4.1 | (3.0–5.5) | 3.7 | (2.4–5.5) |
| 50–59 | 8.4 | (6.2–11.4) | 7.3 | (4.8–11.1) |
| 60–69 | 22.1 | (16.1–30.4) | 16.8 | (10.4–26.9) |
| Black men | ||||
| <40 | 1 | Ref | 1 | Ref |
| 40–49 | 2.0 | (1.5–2.7) | 1.6 | (1.0–2.6) |
| 50–59 | 4.5 | (3.4–5.9) | 4.0 | (2.5–6.6) |
| 60–69 | 8.8 | (6.4–12.0) | 9.6 | (5.2–17.5) |
Confidence intervals that do not overlap 1 are shown in bold.
Abbreviations: aIRR, adjusted incidence rate ratio; CI, confidence interval; IRR, incidence rate ratio; Ref = reference.
Adjusted Poisson analyses controlled for injection drug use, history of smoking, calendar year at antiretroviral therapy (ART) initiation, cohort, AIDS at entry, time-updated CD4 count, and the combined variable for ART and viremia status (where viral suppression ≤400 copies/mL).
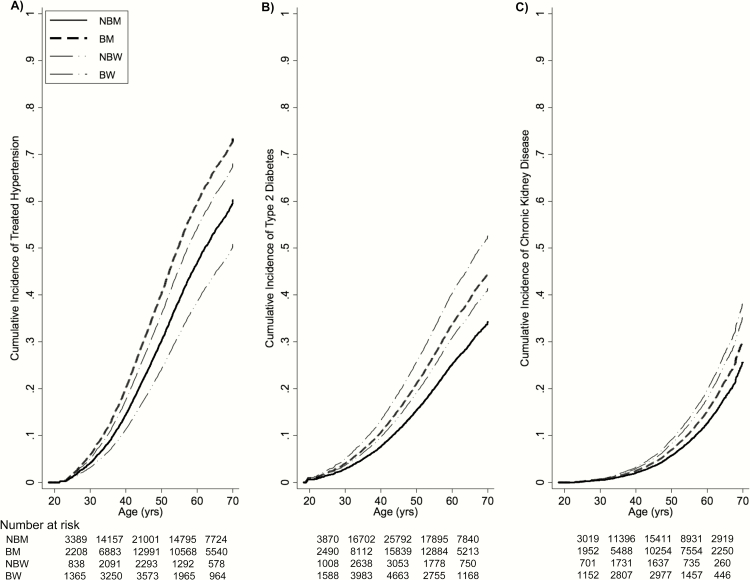
Evidence for disparities in the cumulative incidences between sex–race group was observed. Using age as the time metric, cumulative incidence estimates between sex–race groups for HTN by age 70 ranged from 51% to 73% (Figure 2A), from 34% to 52% for DM (Figure 2B), and from 25% to 38% for CKD (Figure 2C). As an example of interpreting an estimate from Figure 2A, among black men who have reached the age of 55, the probability of experiencing HTN is 51%, conditional on their surviving free of HTN up until that age. Disparities between groups of sex–race were also observed when using time on study as the time metric (results not shown; Supplementary Figure 2). The proportional hazards assumption was met (all P ≥ .9).
Figure 2.
Cumulative incidence curves for treated hypertension (A), type 2 diabetes mellitus (B), and chronic kidney disease (C), stratified by sex and race. Abbreviations: BM, black men; BW, black women; NBM, non-black men; NBW, non-black women.
Given the potential influence of BMI on NCD development, we conducted a subanalysis for the minority of participants for whom BMI data were available at study entry (n = 21615 for HTN, n = 28963 for DM, n = 18996 for CKD). Sex–race disparities persisted after adjusting for BMI (results not shown).
DISCUSSION
Sex–race disparities in the occurrence of HTN, DM, and CKD persisted among men and women aging with HIV with access to care. As ART-treated adults age with HIV, it becomes increasingly important to describe NCD epidemiology [20]. Estimating the occurrence of HTN, DM, and CKD by specific sex–race subgroups of adults aging with HIV further develops the evidence of the persistent racial disparity as shown in other studies of incidence by age, sex, or race [15, 17, 18, 20, 21]. In our study, black adults experienced at least a 1.4-fold higher rate of HTN, DM, and CKD compared with non-black adults. Our findings are a call to action to (1) better understand the drivers of these disparities that persist in an environment with equal access to care in attempts to meet the Healthy People 2020 goals and (2) fill the gaps in NCD prevention services among adults aging with HIV [22].
Our study population experienced lower rates of HTN and CKD, and rates of DM within range of those reported in other HIV-infected populations. In previous literature, HTN, DM, and CKD rates have been reported to range from 3.44 to 22/100 PY, from 0.31 to 2.6/100 PY, and from 0.88 to 1.12/100 PY, respectively; among HIV-uninfected individuals, ranges were 0.59–9.11/100 PY, 0.62–0.71/100 PY, and 1.04–4.46/100 PY, respectively [15–21, 23–28]. Heterogeneity in measurement of the outcomes is partially responsible for the large ranges of incidence estimates. We focused on treated HTN and did not include fasting glucose measures in our DM definition. Additionally, high eGFRs at study entry restricted the rate of decline.
Given our broad study period, temporal changes in the effectiveness, tolerability profiles, and convenience of ART regimens may play an important role in our observed outcome rates. In our population, a high proportion of individuals was prescribed ART but not virally suppressed at study entry. However, this was a function of our study entry definition, which allowed the inclusion of a heterogeneous population of individuals: (1) those initiating ART and who thus have unsuppressed viral load at initiation, and (2) those who may have been exposed to earlier ART formulations. Earlier ART regimens may predispose individuals to NCD development compared with contemporary regimens [11], underscoring the continued importance of monitoring HTN, DM, and CKD [28]. For this reason, in our study it is possible that rates of NCD occurrence were higher in earlier years, but declined following the introduction and uptake of newer ARTs. Potential differences in ART availability by calendar period, and therefore differences in risk of NCD occurrence due to type of ART exposure, were adjusted for in our analyses by controlling for era of ART initiation.
As observed in other studies, the rates of NCDs were frequently higher among black compared with non-black persons [16, 18, 29]. Despite blacks comprising only 12% of the US population, blacks have a disproportionate burden of HIV with infection rates 8 times higher than those of whites [30]. The confluence of this disproportionate burden of HIV infection and the associations of race with NCDs indicates an important clinical and public health issue. The already complex clinical management of HIV infection [31], and evidence supporting that disease preventive services are less common among disadvantaged communities [32], suggest a need for primary and secondary disease prevention strategies directed at minorities.
Consistent with accumulating evidence identifying women at greater risk for several adverse health outcomes compared with men [7], women in our study experienced higher rates of DM and CKD. In the setting of HIV infection, reasons for sex disparities are likely multifactorial, and may include biological differences [33] and differences in care retention [34]. In light of this, women aging with HIV may require approaches to care that are distinct from those routinely used for men in terms of devising effective prevention and treatment plans for NCDs.
The foreseeable growth of NCDs among HIV-infected individuals will have implications for clinical care and healthcare resources [20]. As HIV-infected individuals age, an increasing number of treatable NCDs may give rise to issues related to polypharmacy, with management of both ARTs and treatment of NCDs [35]. To date, there is a dearth of guidelines on how best to clinically manage older individuals with multiple diseases [36], and it is unclear whether undertaking earlier or intensified screening for specific age-related diseases is justifiable [37]. Appropriate healthcare delivery models that are diverse in medical expertise will be fundamental to the care of HIV-infected persons.
Our results should be interpreted in acknowledgement of their limitations. As our study population excludes persons who did not successfully access HIV care, our findings that HTN, DM, and CKD are more likely to be diagnosed among blacks may reflect an underestimate of true disparity, reinforcing the urgency of linking minorities to care [38]. We may have underestimated HTN and CKD events by excluding individuals with untreated hypertension, and excluding individuals who may have progressed to CKD more rapidly by requiring an eGFR >90 mL/minute/1.73 m2 at study entry, respectively. It is also feasible that NCD rates are higher among individuals being followed for care for longer periods of time. But we contend that it is important to describe what is being seen in clinical settings as individuals seek care. The extent of missing data on BMI is another limitation. However, our subanalyses confirmed the persistence of the sex–race disparity after adjusting for BMI. Given previous evidence to suggest that BMI after ART initiation is relatively stable [39], adjusting for BMI in a time-varying manner may not add more than what we have shown. Given that our goal was to quantify rates of first occurrence for HTN, DM, and CKD, the analysis for each outcome was independent and we reported results that did not adjust for concomitant disease. Finally, although an HIV-uninfected comparison group would have provided estimates as to the role of HIV in the disparities shown, the objective was not to examine the HIV effect, but rather to describe the epidemiology of NCDs among individuals with access to HIV clinical care.
In summary, we found increased rates of HTN, DM, and CKD, especially among HIV-infected black men and women. Minimizing sex–race health disparities through more proactive preventive care, such as smoking cessation, physical activity, and nutritional assistance programs, will shape the quality of extended life made possible by effective ART in this population engaged in medical care. Understanding North American rates of NCDs in HIV-infected persons, and sex–race disparities in NCD occurrence, is a necessary step for responding to the U.S. National HIV/AIDS Strategy’s call to improve long-term health outcomes among HIV-infected individuals and to prepare for the clinical and public health challenges that may lie ahead [38].
Supplementary Material
APPENDIX
NA-ACCORD Collaborating Cohorts and Representatives: AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch; AIDS Link to the IntraVenous Experience: Gregory D. Kirk; Fenway Health HIV Cohort: Stephen Boswell, Kenneth H. Mayer, and Chris Grasso; HAART Observational Medical Evaluation and Research: Robert S. Hogg, P. Richard Harrigan, Julio S. G. Montaner, Angela Cescon, and Karyn Gabler; HIV Outpatient Study: Kate Buchacz and John T. Brooks; HIV Research Network: Kelly A. Gebo and Richard D. Moore; Johns Hopkins HIV Clinical Cohort: Richard D. Moore; John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez; Kaiser Permanente Mid-Atlantic States: Michael A. Horberg; Kaiser Permanente Northern California: Michael J. Silverberg; Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne; Multicenter Hemophilia Cohort Study–II: Charles Rabkin; Multicenter AIDS Cohort Study: Lisa P. Jacobson and Gypsyamber D’Souza; Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein; Ontario HIV Treatment Network Cohort Study: Sean B. Rourke, Anita R. Rachlis, Jason Globerman, and Madison Kopansky-Giles; Retrovirus Research Center, Bayamon Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor; Southern Alberta Clinic Cohort: M. John Gill; Study of the Consequences of the Protease Inhibitor Era: Steven G. Deeks and Jeffrey N. Martin; Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Pragna Patel and John T. Brooks; University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig; University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik; University of Washington HIV Cohort: Mari M. Kitahata, Heidi M. Crane, and Daniel R. Drozd; Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, Megan Turner, Sally Bebawy, and Ben Rogers; Veterans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin; Women’s Interagency HIV Study: Stephen J. Gange and Kathryn Anastos.
NA-ACCORD Study Administration: Executive Committee: Richard D. Moore, Michael S. Saag, Stephen J. Gange, Mari M. Kitahata, Keri N. Althoff, Rosemary G. McKaig, and Aimee M. Freeman; Administrative Core: Richard D. Moore, Aimee M. Freeman, and Carol Lent; Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Daniel R. Drozd, Liz Morton, Justin McReynolds, and William B. Lober; Epidemiology and Biostatistics Core: Stephen J. Gange, Keri N. Althoff, Alison G. Abraham, Bryan Lau, Jinbing Zhang, Jerry Jing, Sharada Modur, Cherise Wong, Brenna Hogan, Fidel Desir, Bin Liu, and Bin You.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention or the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (grant numbers U01AI069918, F31DA037788, G12MD007583, K01AI093197, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, M01RR000052, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA011602, R01DA012568, R24AI067039, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA03629, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, T32-AG000247, Z01CP010214, Z01CP010176, and pilot grant R24AG044325); the Centers for Disease Control and Prevention (contract numbers CDC-200-2006-18797 and CDC-200-2015-63931); the Agency for Healthcare Research and Quality (contract number 90047713); the Health Resources and Services Administration (contract number 90051652); the Canadian Institutes of Health Research (grant numbers CBR-86906, CBR-94036, HCP-97105, and TGF-96118); the Ontario Ministry of Health and Long Term Care; the Government of Alberta, Canada; the National Cancer Institute; the National Institute of Mental Health; and the National Institute on Drug Abuse.
Potential conflicts of interest. J. T. has served on the scientific board with AbbVie and as a consultant for Gilead Sciences and NightstaRx, and has grants pending from National Eye Institute and Allergan, Inc. F. J. P. has received honoraria for advice or public speaking with Gilead Sciences Inc, Janssen Pharmaceuticals, Merck and Co, and Bristol-Myers Squibb. A. G. A. has received/pending grants from the National Institute on Aging, National Institutes of Health (NIH), has served as a consultant for Mount Sinai, lectured with the Johns Hopkins University Summer Institute, and has served on the advisory board for the National Institute of Diabetes and Digestive and Kidney Diseases Observational Study Monitoring Board. K. N. A. has served on the medical advisory board for Gilead Sciences Inc. J. M. G. has served on national HIV advisory boards to Merck, Gilead, and ViiV Healthcare. M. J. S. has received research grants from Merck and Pfizer. H. C. has grants received/pending from the Patient-Centered Outcomes Research Institute, NIH. P. F. R. has grants received/pending from the National Institute of Allergy and Infectious Diseases, NIH. R. D. M., R. B., C. W., S. N., S. J. G, A. J., J. K., M. A. H. have grants received/pending from the NIH. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Samji H, Cescon A, Hogg RS, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. HIV surveillance report. Diagnoses of HIV infection in the United States and dependent areas, 2013. Atlanta, GA: CDC, 2015; 25. [Google Scholar]
- 3. High KP, Brennan-Ing M, Clifford DB, et al. ; OAR Working Group on HIV and Aging HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH office of AIDS research by the HIV and aging working group. J Acquir Immune Defic Syndr 2012; 60:S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith CJ, Ryom L, Weber R, et al. ; D:A:D Study Group Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 5. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG. Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int 2010; 78:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchacz K, Baker RK, Palella FJ, Jr, et al. ; HIV Outpatient Study Investigators Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther 2013; 18:65–75. [DOI] [PubMed] [Google Scholar]
- 8. Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanna DB, Buchacz K, Gebo KA, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiologic Databases to Evaluate AIDS Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis 2013; 56:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed 22 June 2015.
- 12. Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41:1–19. [PubMed] [Google Scholar]
- 13. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 14. Lamarca R, Alonso J, Gómez G, Muñoz A. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci 1998; 53:M337–43. [DOI] [PubMed] [Google Scholar]
- 15. Thiébaut R, El-Sadr WM, Friis-Møller N, et al. ; Data Collection of Adverse Events of Anti-HIV Drugs Study Group Predictors of hypertension and changes of blood pressure in HIV-infected patients. Antivir Ther 2005; 10:811–23. [DOI] [PubMed] [Google Scholar]
- 16. Factor SH, Lo Y, Schoenbaum E, Klein RS. Incident hypertension in older women and men with or at risk for HIV infection. HIV Med 2013; 14:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the Multicenter AIDS Cohort Study. Arch Intern Med 2005; 165:1179–84. [DOI] [PubMed] [Google Scholar]
- 18. Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis 2008; 197:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mocroft A, Kirk O, Reiss P, et al. ; EuroSIDA Study Group Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS 2010; 24:1667–78. [DOI] [PubMed] [Google Scholar]
- 20. Smit M, Brinkman K, Geerlings S, et al. ; ATHENA observational cohort Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hasse B, Ledergerber B, Furrer H, et al. ; Swiss HIV Cohort Study Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53:1130–9. [DOI] [PubMed] [Google Scholar]
- 22. US Department of Health and Human Services and Office of Disease Prevention and Promotion. Healthy People 2020. Available at: https://www.healthypeople.gov/2020/topics-objectives Accessed 24 April 2016.
- 23. Vargas CM, Ingram DD, Gillum RF. Incidence of hypertension and educational attainment: the NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am J Epidemiol 2000; 152:272–8. [DOI] [PubMed] [Google Scholar]
- 24. Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 2011; 57:1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bash LD, Coresh J, Köttgen A, et al. Defining incident chronic kidney disease in the research setting: the ARIC study. Am J Epidemiol 2009; 170:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention. Diagnosed diabetes, age adjusted rate (per 100), adults—2013. Available at: http://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html Accessed 5 October 2015.
- 27. Chronic Kidney Disease (CKD) Surveillance Project. Incidence of individual CKD stages/eGFR categories by stage, 2012. Available at: https://nccd.cdc.gov/ckd/detail.aspx?Qnum=Q89 Accessed 2 December 2015.
- 28. Okeke NL, Davy T, Eron JJ, Napravnik S. Hypertension among HIV-infected patients in clinical care, 1996–2013. Clin Infect Dis 2016; 63:242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Wit S, Sabin CA, Weber R, et al. ; Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study Incidence and risk factors for new-onset diabetes in HIV-infected patients: the data collection on adverse events of anti-HIV drugs (D:A:D) study. Diabetes Care 2008; 31:1224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention. Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. HIV surveillance supplemental report. Atlanta, GA: CDC, 2012; 17. [Google Scholar]
- 31. Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA 2013; 309:1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agency for Healthcare Research and Quality. 2014 national healthcare quality and disparities report: key findings. Available at: http://www.ahrq.gov/research/findings/nhqrdr/nhqdr14/key1.html Accessed 5 February 2016.
- 33. Womack JA, Brandt CA, Justice AC. Primary care of women aging with HIV. J Midwifery Womens Health 2015; 60:146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr 2013; 62:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krentz HB, Gill MJ. The impact of non-antiretroviral polypharmacy on the continuity of antiretroviral therapy (ART) among HIV patients. AIDS Patient Care STDS 2016; 30:11–7. [DOI] [PubMed] [Google Scholar]
- 36. Boyd C, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public Health Rev 2011; 32:451–74. [Google Scholar]
- 37. Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA; Infectious Diseases Society of America Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–34. [DOI] [PubMed] [Google Scholar]
- 38. White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States updated to 2020. Washington, DC: White House Office of National AIDS Policy, 2015. [Google Scholar]
- 39. Koethe JR, Jenkins CA, Lau B, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.