In 2 large Taiwanese cohorts, obesity had a harmful effect on tuberculosis mediated through diabetes but had a strong protective effect not mediated through diabetes. Individuals who were simultaneously obese and diabetic had a 70% reduction in tuberculosis risk.
Keywords: tuberculosis, cohort study, body mass index, diabetes
Abstract
Background
Mounting data have revealed that body mass index (BMI) is inversely associated with risk of active tuberculosis. The inverse association presents a “paradox” with regard to diabetes, because obesity is a major determinant of diabetes, and diabetes is a well-known risk factor for tuberculosis.
Methods
We conducted 2 population-based cohort studies involving 167392 participants. The main exposure was BMI and diabetes ascertained at baseline. Occurrence of incident tuberculosis was ascertained from Taiwan’s National Tuberculosis Registry. We conducted a causal mediation analysis and a joint effects analysis to characterize the relationship between BMI, diabetes, and tuberculosis.
Results
During a median of >7 years of follow-up, 491 individuals developed incident tuberculosis. Compared with normal-weight individuals, obese individuals (>30 kg/m2) had a 67% (95% confidence interval [CI], −3% to −90%) and 64% (31%–81%) reduction in tuberculosis hazard in the 2 cohorts. In the causal mediation analysis, obesity had a harmful effect on tuberculosis mediated through diabetes (0.8% and 2.7% increased odds in the 2 cohorts, respectively) but had a strongly protective effect not mediated through diabetes (72% and 67% decreased odds, respectively). Individuals who were simultaneously obese and diabetic had a lower but statistically insignificant risk of tuberculosis (adjusted hazard ratio, 0.30; 95% CI, .08–1.22) compared with nondiabetic normal-weight individuals.
Conclusions
Our analyses revealed that the relationship between obesity, diabetes, and risk of tuberculosis was complex and nonlinear. Better understanding of the interplay between host metabolism and tuberculosis immunology may lead to novel therapeutic or preventive strategies.
Nutritional status has long been recognized as a major determinant of tuberculosis. Being underweight is known to be an important tuberculosis risk factor [1]. Previous studies showed that obesity increased the risk of various types of infections, including postoperative and nosocomial infections [2]. On the other hand, available evidence on obesity and tuberculosis risk indicated a relationship in the opposite direction. A previous systematic review of 6 cohort studies showed an inverse relationship between body mass index (BMI) and tuberculosis incidence, and the incidence of tuberculosis was lower in the overweight and obese population than in those with normal weight [3]. However, only half of the studies in the review included participants with a BMI level of >30 kg/m2. The exact dose-response relationship between BMI and tuberculosis risk remains unclear, especially for those in the obese category.
The inverse association between BMI and tuberculosis also presents a “paradox” with regard to diabetes. Obesity is a major determinant of diabetes, and diabetes is a well-known risk factor for tuberculosis. It follows that the obese population should have an increased risk of tuberculosis because they are also more likely to be diabetic, but this is not consistent with available epidemiological data. Better understanding of the complex interplay between obesity and diabetes on the risk of tuberculosis has critical implications for global health, because the prevalence of obesity and diabetes has been on the rise steadily over the past decades, especially in countries where the burden of tuberculosis is still high [4, 5]. At the national level, health policymakers from the communicable disease and noncommunicable disease sectors need to jointly consider the potential impact of nutritional transition and diabetes, and the relevant interventions, such as weight reduction and glycemic control, on future tuberculosis epidemiology.
We analyzed data from 2 retrospective cohorts in Taiwan with the aim of investigating the association between BMI and tuberculosis risk, the mediation effect of diabetes between BMI and tuberculosis, and the joint association between BMI, diabetes, and risk of tuberculosis.
METHODS
Study Populations
The first cohort included adult participants from 3 rounds of National Health Interview Surveys (NHIS) conducted in 2001, 2005, and 2009 (total n = 53492). The NHIS is a cross-sectional survey that incorporated a multistage stratified systematic sampling scheme to obtain a nationally representative sample of the resident population in Taiwan. The second cohort consisted of participants from a community-based voluntary health screening service from 2005 to 2008 (n = 125865). The service is a multiple-disease screening program for adult residents in New Taipei City (NTC). Details of the 2 cohorts have been described in detail elsewhere [6, 7]. After exclusion of individuals with missing covariate information and those with a previous history of tuberculosis (10.4% of the NHIS cohort and 5.2% of the NTC cohort), there were 47937 and 119340 participants in the NHIS and NTC cohorts respectively (see Supplementary Figure S1 for study flow diagrams).
Measurement of BMI, Diabetes, and Other Covariates
The main exposure, BMI, was calculated by dividing weight (in kilograms) by squared of height (in meters). Weight and height were self-reported by the participants in the 2 cohorts. We accounted for major risk factors for tuberculosis in the analysis, based on a literature review of previous epidemiological studies. The baseline information on demographic variables (eg, sex, age, and level of education) and behavioral risk factors (eg, smoking and alcohol use) was obtained from structured questionnaires and personal interviews in the cohorts. The status of diabetes at baseline was determined using the following approaches: for the NHIS cohort, diabetes was defined based on self-reports of physician-diagnosed diabetes during the interview and information in the national health insurance database (prescription of hypoglycemic drugs for ≧28 days within 1 year before enrollment); for the NTC cohort, diabetes was defined based on fasting plasma glucose level (≥126 mg/dL) and information in the national health insurance database (prescription of hypoglycemic drugs for ≧28 days within 1 year before enrollment).
Measurement of Tuberculosis
The primary outcome of interest is incident active tuberculosis disease using the information from Taiwan’s National Tuberculosis Registry. In Taiwan, the diagnosis of tuberculosis is based on physical examination, bacteriologic evidence, chest radiography, and response to antituberculosis treatment [8]. We defined cases of tuberculosis by bacteriologically confirmed cases in the National Tuberculosis Registry. The incidence of active tuberculosis was ascertained by cross-matching the databases of the 2 cohorts to the National Tuberculosis Registry using the unique national identifier. The cohorts were followed up until the end of 2013.
Statistical Analysis
We categorized BMI levels following the World Health Organization classification: underweight (<18.5 kg/m2), normal (≥18.5 kg/m2 and <25 kg/m2), overweight (≥25 kg/m2 and <30 kg/m2), and obese (≥30 kg/m2). We used Cox proportional hazards regression models to estimate the adjusted hazard ratios (aHRs) of different levels of BMI and corresponding 95% confidence intervals (CIs), using the normal-weight group as the reference. We used causal diagrams to determine which variables to adjust for in the multivariable analysis [9]. We used restricted cubic spline regression to investigate the potential nonlinear relationship between BMI and tuberculosis, and the test for nonlinearity was done using the likelihood ratio test [10]. We performed subgroup analysis to estimate effect modification by age, sex, and smoking status. Cross-product terms were created and added to the multivariable regression model, and models with or without cross-product interaction terms were compared using the likelihood ratio test.
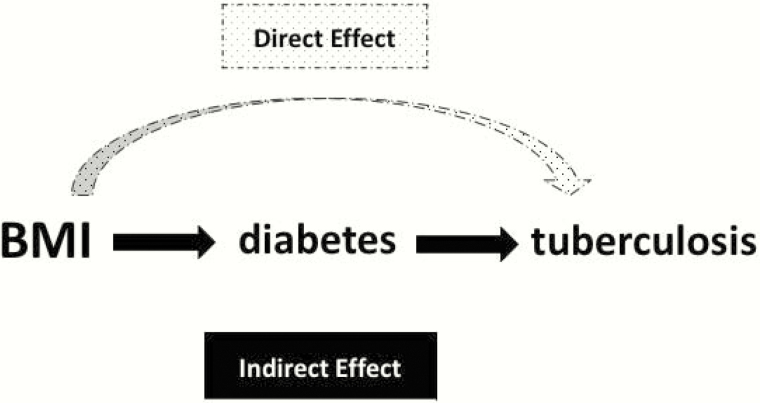
Because diabetes is probably on the causal pathway between BMI and tuberculosis, we did not adjust for diabetes in the main analysis in order to estimate the overall association between BMI and tuberculosis risk. We instead conducted a causal mediation analysis to characterize the relation between BMI, diabetes, and tuberculosis (see the causal diagram in Figure 1) [11, 12]. The mediation analysis was done by constructing 2 logistic regression models, with one regressing the risk of diabetes on BMI levels and the other regressing the risk of tuberculosis on BMI levels and diabetes status. The direct and indirect (mediated through the risk of diabetes) effects of BMI on the risk of tuberculosis were estimated using odds ratios (see Supplementary Materials for details) [13].
Figure 1.
Causal diagram on the hypothesized relationships between body mass index, diabetes, and tuberculosis. Abbreviation: BMI, body mass index.
In addition, we conducted a separate Cox proportional hazards regression analysis to estimate the joint effects of BMI and diabetes on tuberculosis risk. We conducted 2 sensitivity analyses, the first using culture-confirmed tuberculosis as the outcome and the second correcting for self-reporting bias of height and weight (see Supplementary Materials for details). All analyses were conducted using SAS (version 9.4; SAS Institute) and R (version 3.1.2; R project) software. The analyses of NHIS data accounted for sampling weights from the complex survey design.
RESULTS
The NHIS cohort (n = 48713 after accounting for sampling weights) had a median age of 42 years and a nearly equal sex ratio; 24.9% and 5.4% of the participants were overweight and obese, respectively, and 6.3% were underweight (Table 1). The NTC cohort (n = 119340) had a median age of 51 years and was predominantly female (64.4%); the proportions of overweight, obese, and underweight were 32.4%, 6.8%, and 2.8% respectively. In both cohorts, higher BMI was associated with male sex, older age, current tobacco and alcohol use, lower educational attainment, and higher prevalence of diabetes. In the NHIS cohort, higher BMI was also associated with lower household income. In other words, the prevalence of major tuberculosis risk factors increased with increasing BMI levels.
Table 1.
Baseline Characteristics of Participants From the National Health Interview Survey and New Taipei City Cohorts by Body Mass Index Level
| Characteristic | NHIS Cohort by BMI, kg/m2 | NTC Cohort by BMI, kg/m2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <18.5 | 18.5 to <25.0 | 25.0 to <30.0 | ≥30.0 | Overall | P Value | <18.5 | 18.5 to <25.0 | 25.0 to <30.0 | ≥30.0 | Overall | P Value | |
| Sample size, no. of participants | 3086 | 30 848 | 12 141 | 2638 | 48 713 | … | 3317 | 69 217 | 38 630 | 8176 | 11 9340 | … |
| Male sex, % | 25.1 | 48.0 | 63.0 | 58.4 | 50.8 | <.001 | 22.3 | 31.4 | 44.4 | 35.8 | 35.6 | <.001 |
| Age, median (IQR), y | 31 (25–44) | 40 (30–52) | 46 (36–56) | 42 (32–54) | 42 (31–53) | <.001 | 43 (36–56) | 50 (42–58) | 53 (45–62) | 53 (44–61) | 51 (43–59) | <.001 |
| Current smoker, % | 17.9 | 23.5 | 28.6 | 28.2 | 24.7 | <.001 | 15.2 | 14.0 | 16.5 | 15.7 | 14.9 | <.001 |
| Current alcohol use, % | 7.5 | 12.6 | 17.7 | 15.9 | 13.7 | <.001 | 4.2 | 6.4 | 8.6 | 7.3 | 7.1 | <.001 |
| Marital status, % | <.001 | |||||||||||
| Married or cohabiting | 43.2 | 62.0 | 70.5 | 62.5 | 63.0 | <.001 | 76.2 | 84.3 | 85.8 | 82.6 | 84.5 | |
| Single | 47.1 | 26.6 | 16.1 | 25.1 | 25.2 | 13.8 | 5.4 | 3.6 | 5.1 | 5.0 | ||
| Widowed, divorced, separated, or other | 9.76 | 11.3 | 13.4 | 12.4 | 11.8 | 10.0 | 10.3 | 10.6 | 12.3 | 10.5 | ||
| Educational level, % | <.001 | |||||||||||
| College or above | 43.3 | 34.5 | 25.7 | 23.4 | 32.3 | <.001 | 32.2 | 22.9 | 17.0 | 13.2 | 20.6 | |
| High school | 32.3 | 30.6 | 28.7 | 29.0 | 30.1 | 33.6 | 30.7 | 24.9 | 23.4 | 28.4 | ||
| Junior high school or below | 24.4 | 35.0 | 45.6 | 47.6 | 37.6 | 34.1 | 46.4 | 58.1 | 63.4 | 51.0 | ||
| Diabetes, % | 2.1 | 4.2 | 9.0 | 13.4 | 5.8 | <.001 | 3.0 | 6.1 | 12.4 | 20.4 | 9.0 | <.001 |
| Employed, % | 64.0 | 65.8 | 66.6 | 64.3 | 65.8 | .015 | NA | NA | NA | NA | NA | … |
| Low-income household, %a | 19.6 | 20.1 | 23.2 | 27.6 | 21.3 | <.001 | NA | NA | NA | NA | NA | … |
Abbreviations: BMI, body mass index; IQR, interquartile range; NA, not available (not measured in the NTC cohort); NHIS, National Health Interview Survey; NTC, New Taipei City.
aDefined as monthly household income <30000 Taiwan dollars.
The NHIS and the NTC cohorts were followed up for median periods of 8.3 and 7.3 years, respectively, and 169 and 322 cases of incident active tuberculosis developed during follow-up. The median times to incident tuberculosis were 4.82 and 3.72 years in the NHIS and NTC cohorts, respectively. In the NHIS cohort, the incidence rate of tuberculosis decreased with BMI; the rates were 82.8, 48.6, 30.1, and 15.7 per 100000 among the underweight, normal-weight, overweight, and obese populations, respectively. The incidence trend was similar in the NTC cohort, with the rates being 92.3, 41.8, 28.2, and 16.9 per 100000 in the 4 groups, respectively.
In the age-adjusted and multivariable-adjusted Cox regression analysis, the hazard of active tuberculosis decreased with BMI in both cohorts with a significant linear trend (multivariable-adjusted P for trend: P < .001 in both cohorts) (Table 2). With a 1-unit (1-kg/m2) increase in BMI, the hazard of tuberculosis decreased by 13.5% (95% CI, 9.1%–17.7%) in the NHIS and 14.6% (11.6%–17.5%) in the NTC cohort. The strong inverse association between BMI and tuberculosis persisted across all BMI levels in the spline regression, with evidence of departure from the linearity assumption in the NTC cohort likely due to its large sample size (P for nonlinearity: .40 in the NHIS and .04 in the NTC cohort) (Supplementary Figure S2). Compared with normal-weight individuals, the obese population had 67.2% and 63.5% reductions in tuberculosis hazard in the NHIS (aHR, 0.33; 95% CI, .10–1.03) and NTC (0.36; .19–.69) cohorts, respectively. Conversely, the underweight population had a 2-fold increase in tuberculosis risk compared with the normal-weight population in the NHIS (aHR, 2.28; 95% CI, 1.41–3.69) and NTC (2.44; 1.57–3.80) cohorts (Table 2). We did not find evidence of effect measure modification of the BMI-tuberculosis association by age, sex, or smoking status.
Table 2.
Body Mass Index at Baseline and the Risk of Tuberculosis in the National Health Interview Survey and the New Taipei City Cohortsa
| BMI Category, kg/m2 | ||||
|---|---|---|---|---|
| <18.5 | 18.5 to <25.0 | 25.0 to <30.0 | ≥30.0 | |
| NHIS cohort | ||||
| Participants, no. | 20 | 118 | 28 | 3 |
| Person-years | 24 168 | 242 725 | 93 130 | 19 091 |
| Incidence rate (per 100000) | 82.8 (52.0–125.5) | 48.6 (40.4–58.0) | 30.1 (20.4–42.9) | 15.7 (4.00–42.8) |
| Age-adjusted HR | 2.03 (1.26–3.27) | 1 | 0.51 (.34–.77) | 0.32 (.10–1.00) |
| Multivariable HRb | 2.28 (1.41–3.69) | 1 | 0.48 (.32–.73) | 0.33 (.10–1.03) |
| Multivariable HR, culture-confirmed tuberculosisb | 2.52 (1.53–4.14) | 1 | 0.53 (.34–.81) | 0.39 (.12–1.22) |
| Multivariable HR, corrected BMIb | 2.41 (1.49–3.91) | 1 | 0.43 (.28–.66) | 0.44 (.16–1.18) |
| NTC cohort | ||||
| Participants, no. | 22 | 211 | 79 | 10 |
| Person-years | 23 831 | 505 089 | 280 504 | 59 217 |
| Incidence rate (per 100000) | 92.3 (59.3–137.5) | 41.8 (36.4–47.7) | 28.2 (22.5–34.9) | 16.9 (8.6–30.1) |
| Age-adjusted HR | 2.33 (1.50–3.61) | 1 | 0.56 (.43–.73) | 0.35 (.18–.66) |
| Multivariable HRc | 2.44 (1.57–3.80) | 1 | 0.53 (.41–.68) | 0.36 (.19–.69) |
| Multivariable HR, culture-confirmed tuberculosisc | 2.33 (1.51–3.58) | 1 | 0.52 (.41–.67) | 0.33 (.17–.62) |
| Multivariable HR, corrected BMIc | 2.57 (1.65–4.00) | 1 | 0.53 (.41–.69) | 0.32 (.16–.63) |
Abbreviations: BMI, body mass index; HR, hazard ratio; NHIS, National Health Interview Survey; NTC, New Taipei City.
aParenthetical ranges represent 95% confidence intervals.
bAdjusted for age, sex, marital status, education, smoking, alcohol use, employment status, and household income.
cAdjusted for age, sex, marital status, education, smoking, and alcohol use.
In the sensitivity analysis using culture-confirmed tuberculosis as the outcome, the point estimates for a HRs were slightly different, but the overall associations between BMI and tuberculosis remained unchanged (Table 2). In a separate sensitivity analysis that corrected for self-reporting bias in BMI, the relations between BMI and risk of tuberculosis were also similar to the main analysis (Table 2).
In the mediation analysis, high BMI was associated with substantially increased odds of diabetes, and diabetes was associated with increased odds of active tuberculosis (Table 3). It followed that, in this diabetes-mediated pathway, obesity was associated with increased odds of tuberculosis compared with normal weight (0.8% increased odds in the NHIS and 2.7% increased odds in the NTC cohort), although some of the associations were not statistically significant. On the other hand, the direct pathway analysis revealed that obesity was associated with decreased odds of tuberculosis when compared with normal weight (71.9% decreased odds in the NHIS cohort and 67.3% decreased odds in the NTC cohort). The overall association between BMI and tuberculosis was dominated by the direct pathway (Table 3).
Table 3.
Results From Causal Mediation Analysis With Diabetes as Mediator Between Body Mass Index and Tuberculosis in the National Health Interview Survey and New Taipei City Cohorts
| Effect | Exposure level | Adjusted Odd Ratio (95% CI)a | |
|---|---|---|---|
| NHIS Cohort | NTC Cohort | ||
| Total effect of BMI on TBb | <18.5 | 2.28 (1.39–3.73) | 2.31 (1.48–3.63) |
| 18.5 to <25.0 | 1 | 1 | |
| 25.0 to <30.0 | 0.47 (.31–.72) | 0.52 (.40–.68) | |
| ≥30.0 | 0.29 (.09–.91) | 0.35 (.19–.67) | |
| Direct effect of BMI on TBb | <18.5 | 2.30 (.93–4.64) | 2.37 (1.46–3.58) |
| 18.5 to <25.0 | 1 | 1 | |
| 25.0 to <30.0 | 0.47 (.30–.69) | 0.51 (.39–.64) | |
| ≥30.0 | 0.28 (.00–.67) | 0.33 (.14–.56) | |
| Indirect effect of BMI on TBb | <18.5 | 0.999 (.995–1.002) | 0.995 (.990–.999) |
| 18.5 to <25.0 | 1 | 1 | |
| 25.0 to <30.0 | 1.003 (.996–1.010) | 1.010 (1.003–1.018) | |
| ≥30.0 | 1.008 (.988–1.032) | 1.027 (1.008–1.051) | |
| Effect of BMI on diabetes | <18.5 | 0.67 (.51–.86) | 0.54 (.44–.67) |
| 18.5 to <25.0 | 1 | 1 | |
| 25.0 to <30.0 | 1.83 (1.67–2.00) | 1.85 (1.77–1.94) | |
| ≥30.0 | 3.66 (3.19–4.18) | 3.53 (3.31–3.77) | |
| Effect of diabetes on TB | |||
| Diabetes | 1.23 (.74–1.97) | 1.62 (1.23–2.17) | |
| No diabetes | 1 | 1 | |
Abbreviations: BMI, body mass index; CI, confidence interval; NHIS, National Health Interview Survey; NTC, New Taipei City; TB, tuberculosis.
aThe mediation analysis was done by constructing 2 logistic regression models: (1) the first model regressed the risk of diabetes on BMI levels, adjusting for age(categorical), sex, marital status, education, smoking, alcohol use, employment status, and household income in the NHIS cohort and age(categorical), sex, marital status, education, smoking, and alcohol use in the NTC cohort and (2) the second model regressed the risk of tuberculosis on BMI levels and diabetes status, adjusting for age(categorical), sex, marital status, education, smoking, alcohol use, employment status, and household income in the NHIS cohort and age(categorical), sex, marital status, education, smoking, and alcohol use in the NTC cohort.
bSee Figure 1 and the main text for the definition of total, direct, and indirect effect.
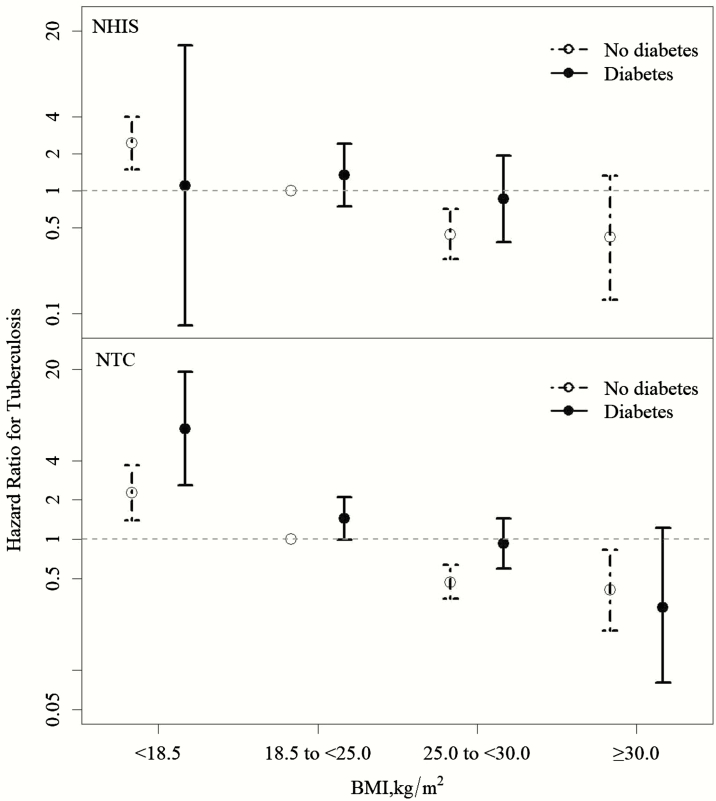
In the joint effects analysis of BMI and diabetes using multivariable Cox regression, overweight/obesity and diabetes had an opposite association with tuberculosis in both cohorts (Figure 2). Those who were simultaneously overweight and diabetic (2.23% of the total NHIS and 6.70% of the total NTC cohort) had similar risks of tuberculosis compared with normal-weight individuals without diabetes. There were limited data (<2 tuberculosis cases in both cohorts) for those who were simultaneously obese and diabetic (0.72% of the NHIS and 1.41% of the NTC cohort), but a statistically insignificant decrease in tuberculosis risk was observed in the NTC cohort among this subgroup (aHR, 0.30; 95% CI, .08–1.22).
Figure 2.
Joint effects of body mass index and diabetes mellitus on the risk of active tuberculosis in the National Health Interview Survey (NHIS) (upper panel) and New Taipei City (NTC) (lower panel) cohorts. Numbers represent multivariable hazard ratios with 95% confidence intervals. The hazard ratio in the obese and diabetic population in the NHIS cohort could not be determined because there were no tuberculosis cases in this subgroup. Adjusted for age, sex, marital status, education, smoking, alcohol use, employment status, and household income in the NHIS cohort and for age, sex, marital status, education, smoking, and alcohol use in the NTC cohort. Abbreviations: BMI, body mass index; NHIS, National Health Interview Survey; NTC, New Taipei City.
DISCUSSION
In the 2 retrospective Taiwanese cohorts of nearly 170000 individuals with >7 years of follow-up, we found that BMI at baseline was a strong predictor of subsequent development of active tuberculosis. The BMI was inversely associated with the risk of tuberculosis across all BMI levels, and the obese population had a two-thirds reduction in tuberculosis risk compared with normal-weight individuals. In the causal mediation analysis, high BMI had 2 different effects on tuberculosis: a harmful effect mediated through diabetes and a strongly protective effect not mediated through diabetes. The overall effect of high BMI and tuberculosis risk was dominated by the direct effect of BMI not mediated through diabetes. The protective effect of high BMI (>25 kg/m2) seemed stronger than the increase in risk due to diabetes; individuals who were simultaneously obese/overweight and diabetic had a similar or even lower risk of tuberculosis than nondiabetic individuals with normal weight.
The inverse association between BMI and tuberculosis risk in our study was consistent with the findings from a previous systematic review [3]. In a prospective cohort study of >42000 elderly persons in Hong Kong, overweight and obese individuals had a significantly lower risk of tuberculosis than normal-weight persons after adjustment for other tuberculosis risk factors [14]. In a US cohort based on the National Health and Nutrition Examination Survey, Cegielski et al [15] also reported an inverse association between BMI and tuberculosis risk after adjusting for confounders. Finally, in a UK cohort of 220000 diabetic patients and 1.2 million matched nondiabetic controls, a lower risk of tuberculosis among the overweight and obese individuals was also observed [16]. With the addition of 2 population-based Taiwanese cohorts and the consistent findings across these high-quality studies, we consider the evidence base for the inverse association between BMI and tuberculosis risk to be very strong (Supplementary Figure S3).
The inverse association between BMI and tuberculosis in the present analyses could not be explained by reverse causation (ie, being sick with tuberculosis leads to weight loss), because the BMI information was collected at baseline and both cohorts were followed up for >7 years. The association was also unlikely to be due to confounding, because we adjusted for major tuberculosis risk factors in the analyses. There is a possibility of residual confounding by socioeconomic status, which is a major determinant of tuberculosis and is difficult to measure precisely. In our study population, BMI was inversely associated with socioeconomic status; that is, the obese population is more likely to be associated with lower socioeconomic status than the nonobese population (Table 1). Therefore, the inverse association between BMI and tuberculosis would have been even stronger if the socioeconomic status had been fully adjusted for in our analyses.
A unique contribution of the present research is to delineate the role of diabetes in the BMI-tuberculosis association. We argue that diabetes should be considered as a causal intermediate between BMI and tuberculosis and should not be adjusted for if the goal is to determine the overall association between BMI and tuberculosis. In our causal mediation analysis, high BMI was associated with tuberculosis through 2 different pathways, a harmful pathway mediated through diabetes and a strongly protective pathway not mediated through diabetes. The phenomenon of direct and indirect effects from the same exposure operating in opposite directions has been reported in the literature [17]. Our mediation analysis confirmed and quantified the potential harmful effect of BMI and tuberculosis that was mediated through diabetes. We further quantified the direct protective effect of BMI that was not mediated through diabetes and found that the overall association between BMI and tuberculosis risk was dominated by this direct protective effect of BMI.
The biological mechanism of the decreased risk of tuberculosis among those with high BMI remains poorly understood. The adipose tissue has been suggested to be a reservoir where Mycobacterium tuberculosis could persist to avoid detection by the host immune system [18]. Leptin, an adipose tissue-derived energy-regulating hormone, is encoded by the obese (ob) gene. It has been shown that serum leptin concentration is positively associated with total body fat mass [19]. In addition to its effect on energy regulation, leptin might also affect the T-helper 1/T-helper 2 balance of the host immune system, subsequently altering the risk of tuberculosis infection [20, 21]. It was found that the genetically knocked out mice (the ob/ob mice), which were extremely obese but leptin deficient, had increased susceptibility to M. tuberculosis infection after intranasal challenge [22]. On the other hand, it has been reported that a cholesterol-rich diet accelerates bacteriologic sterilization in patients with pulmonary tuberculosis [23]. Further epidemiological and laboratory studies will help shed light on the role of leptin and hypercholesterolemia in the association between BMI and tuberculosis risk.
The present study has limitations. First, we used the simple but crude measurement of BMI to measure adiposity in the body. BMI cannot distinguish between weight from fat and weight from muscle and bone. In addition, the location of the fat (visceral vs peripheral) cannot be determined from the simple indicator of BMI. Cegielski et al [15] investigated the association between different anthropometric indicators of nutritional status and tuberculosis risk, including BMI, subcutaneous fat (measured by skinfold thickness), and lean skeletal muscle (measured by arm muscle area). They found that both subcutaneous fat and lean skeletal muscle were independently and inversely associated with tuberculosis risk, although the association for subcutaneous fat seemed to be stronger. The precise contribution of different components of BMI (fat vs muscle) to the BMI-tuberculosis association requires further investigation.
In addition, we did not have information on latent tuberculosis infection among the study participants. As a result, we could not determine whether the inverse association between BMI and active tuberculosis was due to the decreased risk in tuberculosis infection, the decreased risk of disease progression from latent infection, or both. In the aforementioned National Health and Nutrition Examination Survey study, the distribution of tuberculin skin test results did not differ significantly by BMI levels (data not shown), suggesting that the difference in tuberculosis risk across BMI levels was mainly driven by different risks of progression from latent tuberculosis infection [15]. Finally, we did not adjust for human immunodeficiency virus (HIV) status in our analysis. The very low prevalence of HIV (<1 per 1000) made it an unlikely confounder when investigating the association between BMI, diabetes, and tuberculosis [24].
Our results have important implications on the global triple epidemics of obesity, diabetes, and tuberculosis. In countries with a high tuberculosis burden, such as India, Indonesia, and China, the prevalence of overweight/obesity and diabetes has been rising steadily [4, 5]. The strong and protective overall effect of high BMI against tuberculosis in our study and others implies that high BMI may have a positive impact on tuberculosis epidemiology. This is not to diminish the importance of efforts to halt the rise of obesity, which is associated with a multitude of health problems. Nevertheless, both potential negative and positive effects of the nutritional transition need to be accounted for. Better understanding of the interplay between host metabolism and immunology associated with M. tuberculosis may lead to novel therapeutic or preventive strategies that target the host immune response and contribute to global tuberculosis elimination.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. H. H. L., C. Y. W., H. F., and Y. T. H. conceived the study and the analysis plan. H. H. L., C. Y. W., C. H. W., H. F., and Y. T. H. conducted the analysis. H. H. L. wrote the first draft of the manuscript. All authors critically revised the manuscript.
Acknowledgment. We thank Chia-Hsuin Chang for a helpful discussion on an earlier draft of the paper.
Disclaimer. The funder of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Financial support. This work was supported by the Taiwan Ministry of Science and Technology (grant MOST105-2628-B-002-025-MY3).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med 2009; 68:2240–6. [DOI] [PubMed] [Google Scholar]
- 2. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis 2006; 6:438–46. [DOI] [PubMed] [Google Scholar]
- 3. Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010; 39:149–55. [DOI] [PubMed] [Google Scholar]
- 4. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016; 387:1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016; 387:1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin HH, Ezzati M, Chang HY, Murray M. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med 2009; 180:475–80. [DOI] [PubMed] [Google Scholar]
- 7. Lee PH, Fu H, Lai TC, Chiang CY, Chan CC, Lin HH. Glycemic control and the risk of tuberculosis: a cohort study. PLoS Med 2016; 13:e1002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control, Ministry of Health and Welfare, R.O.C. (Taiwan). Taiwan guidelines for TB diagnosis & treatment. 5th ed Taipei, Taiwan: Centers for Disease Control, Ministry of Health and Welfare, R.O.C. (Taiwan); 2013. [Google Scholar]
- 9. Pearl J. Causal diagrams for empirical research. Biometrika 1995; 82:669–88. [Google Scholar]
- 10. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010; 29:1037–57. [DOI] [PubMed] [Google Scholar]
- 11. Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology 1992; 3:143–55. [DOI] [PubMed] [Google Scholar]
- 12. Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol 2010; 172:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013; 18:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leung CC, Lam TH, Chan WM et al. Lower risk of tuberculosis in obesity. Arch Intern Med 2007; 167:1297–304. [DOI] [PubMed] [Google Scholar]
- 15. Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional risk factors for tuberculosis among adults in the United States, 1971-1992. Am J Epidemiol 2012; 176:409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pealing L, Wing K, Mathur R, Prieto-Merino D, Smeeth L, Moore DA. Risk of tuberculosis in patients with diabetes: population based cohort study using the UK Clinical Practice Research Datalink. BMC Med 2015; 13:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang YT, Yang HI, Liu J, Lee MH, Freeman JR, Chen CJ. Mediation analysis of hepatitis B and C in relation to hepatocellular carcinoma risk. Epidemiology 2016; 27:14–20. [DOI] [PubMed] [Google Scholar]
- 18. Neyrolles O, Hernández-Pando R, Pietri-Rouxel F et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence?PLoS One 2006; 1:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimizu H, Shimomura Y, Hayashi R et al. Serum leptin concentration is associated with total body fat mass, but not abdominal fat distribution. Int J Obes Relat Metab Disord 1997; 21:536–41. [DOI] [PubMed] [Google Scholar]
- 20. Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol 2005; 174:3137–42. [DOI] [PubMed] [Google Scholar]
- 21. Brito Díaz B, Marcelino Rodríguez I, Almeida González D, Rodríguez Pérez M, Cabrera de León A. An overview of leptin and the Th1/Th2 balance. Open J Immunol 2014; 4:42–50. [Google Scholar]
- 22. Wieland CW, Florquin S, Chan ED et al. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int Immunol 2005; 17:1399–408. [DOI] [PubMed] [Google Scholar]
- 23. Pérez-Guzmán C, Vargas MH, Quiñonez F, Bazavilvazo N, Aguilar A. A cholesterol-rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis. Chest 2005; 127:643–51. [DOI] [PubMed] [Google Scholar]
- 24. Yang CH, Yang SY, Shen MH, Kuo HS. The changing epidemiology of prevalent diagnosed HIV infections in Taiwan, 1984-2005. Int J Drug Policy 2008; 19:317–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.