This study provides valuable information on differences in hepatitis C virus incidence by sex in people who inject drugs, controlling for differences in related exposures and behavioral factors, using well-characterized longitudinal data from geographically diverse prospective cohort studies.
Keywords: sex, hepatitis C virus, people who inject drugs, incidence, survival analysis
Abstract
Background
The objective of this study was to assess differences in hepatitis C virus (HCV) incidence by sex in people who inject drugs (PWID), using a large international multicohort set of pooled biological and behavioral data from prospective observational studies of incident human immunodeficiency virus (HIV) and HCV infections in high-risk cohorts (the InC3 Collaborative).
Methods
HCV infection date was estimated based on a hierarchy of successive serological (anti-HCV), virological (HCV RNA), and clinical (symptoms and/or liver function tests) data. We used a Cox proportional hazards model to calculate the crude and adjusted female to male (F:M) hazard ratio (HR) for HCV incidence using biological sex as the main exposure.
Results
A total of 1868 PWID were observed over 3994 person-years of observation (PYO). Unadjusted F:M HR was 1.38 (95% confidence interval [CI], 1.15–1.65) and remained significant after adjusting for behavioral and demographic risk factors (1.39 [95% CI, 1.12–1.72]). Although syringe and equipment sharing were associated with the highest HCV incidence rate in women (41.62 and 36.83 PYO, respectively), we found no sex differences attributed to these risk factors.
Conclusions
Our findings indicate that women who inject drugs may be at greater risk of HCV acquisition than men, independent of demographic characteristics and risk behaviors. Multiple factors, including biological (hormonal), social network, and differential access to prevention services, may contribute to increased HCV susceptibility in women who inject drugs.
Parenteral exposures to infected blood especially via injection drug use account for the majority of hepatitis C virus (HCV) infections in high- and middle-income countries [1]. There is a significant body of research assessing sex and gender differences in injection risk behavior among people who inject drugs (PWID); however, little or no research has investigated sex differences in HCV disease susceptibility.
Human immunodeficiency virus (HIV) incidence and prevalence have been shown to be modestly higher in female compared to male PWID, findings that have been associated with both behavioral and biological factors [2, 3]. While rates of illicit drug use, including injection use, are higher in men than for women worldwide [4, 5], many studies have shown that women report higher prevalence of injection-related risk behaviors than men, including receptive needle/syringe and equipment sharing [6–10]. Women are more likely to be initiated into injection drug use by their sex partners [11] and to have overlapping sexual and drug networks [12]. HCV prevalence is often higher in men compared to women, although in meta-analyses this has not been found [2, 13], Findings regarding prevalence differences are problematic to compare to incidence studies for several reasons: Studies use various measures of exposure (anti-HCV) and infection (RNA), and women are more likely to spontaneously clear HCV infection [14]. Sex differences in exposures to prevention programs, including medication-assisted treatment (MAT) programs for opioid dependence and needle and syringe programs (NSPs) [15], have also been associated with differences in HCV incidence. HCV transmission through sexual contact is very rare in heterosexual male-female couples (1 per 190000 sexual contacts) [16] and also low in HIV-infected men who have sex with men with presumable sexual acquisition (0.53%) [17] compared with parenterally acquired HCV infection.
HCV incidence differences by sex have not been well studied. Although some individual studies have shown nonsignificant sex differences in HCV incidence rates [18, 19], others have reported differential incidence among female PWID [7, 20, 21]. A recent systematic review and meta-analysis of longitudinal studies of HCV incidence found that women have a 36% higher risk of HCV infection [22]. However, that study did not control for individual-level demographic and injecting risk behavior, potentially confounding results. To further explore the relative risk of incident HCV by sex, we conducted analyses to assess incidence of primary HCV infection in susceptible HCV-negative female and male PWID, and calculate excess risk (female to male [F:M] ratio) among women independent of demographic characteristics, injection risk, and other risk and preventive exposures using individual-level data from the International Collaboration of Incident HIV and HCV in Injecting Cohorts (InC3) [23].
METHODS
The InC3 Collaborative and cohorts contributing behavioral and serological data have been described previously [23]. In brief, InC3 houses data from 10 individual prospective studies of HIV and HCV among PWID, which is harmonized across all studies: Amsterdam Cohort Study (ACS), Australian Trial in Acute Hepatitis C (ATAHC, Sydney), Boston Acute HCV Study: Transmission, Immunity, Outcomes Network (BAHSTION), Baltimore Before and After Acute Study in Hepatitis (BBAASH), Hepatitis C Virus Cohort (HCVC) Study (Sydney), Hepatitis C Incidence and Transmission–Prison Study (HITS-p) and Hepatitis C Incidence and Transmission–Community Study (HITS-c) (both Sydney), HepCo Study (Montreal), SuperMIX Study (Networks II and MIX studies, Melbourne), and U-Find-Out (UFO) Study (San Francisco). A principal goal of InC3 is to provide a platform for analyses from pooled data regarding HCV risk and outcomes that a single study may fail to answer due to small numbers of outcomes and insufficient statistical power. This study includes data from 7 of the 10 cohorts. Excluded cohorts included BAHSTION and BBAASH (which lacked systematically injection exposure data) and ATAHC (whose design limited HCV incidence estimation) [24]. Analyses were restricted to participants who reported any history of drug injection and did not report being transgender.
InC3 includes data previously collected under approved protocols from each participating institution. The University of New Mexico Institutional Review Board determined that InC3 was exempt from review as all data are de-identified.
Measurement of Incident Hepatitis C Virus Infection
We defined incident HCV cases based on anti-HCV and/or HCV RNA tests among previously HCV-naive persons using the following criteria: (1) a negative anti-HCV test followed by either a positive anti-HCV or HCV RNA test; or (2) evidence of seroconversion illness, including clinically documented jaundice or alanine aminotransferase (ALT) >400 IU/L and a history of high-risk exposure within 3 months of clinical manifestation of acute HCV [25]. Anti-HCV and HCV RNA testing assays varied between but not within cohorts and have been described previously [14].
HCV infection date was estimated using a hierarchy of successive serological (anti-HCV), virological (HCV RNA), and clinical (symptoms and/or liver function tests) data in 3 groups as follows [25]: (1) in acute seronegative viremic patients (anti-HCV negative/HCV RNA positive), HCV infection date was estimated from the date of that test minus 28 days (incubation period) [14]; (2) among those with a seroconversion illness (jaundice or ALT >400 IU/mL), HCV infection date was estimated as 6 weeks prior to recorded symptom date [26]; and (3) based on documented seroconversion, wherein infection date was estimated as the midpoint between date of the last negative anti-HCV test and the first positive anti-HCV or HCV RNA test. Person-years of observation (PYO) was calculated for each subject from their baseline study visit date to the estimated date of HCV infection for incident infections. Data from subjects who remained both anti-HCV and HCV RNA negative were censored at the last cohort visit date with available laboratory results.
Statistical Analyses
We conducted descriptive analyses of demographic characteristics and risk behaviors from baseline data in male and female participants and assessed differences using Wilcoxon rank-sum test or χ2 test. Then, we estimated the overall and sex-specific (female and male) HCV incidence rates using Kaplan-Meier methods. We used Cox proportional hazards models to calculate the crude and adjusted female-to-male (F:M) hazard ratio (HR) for HCV incidence using biological sex as the main exposure, controlling for demographic and recent injection related exposures (“recent” was either the 3- or 6-month interval prior to interview, depending on cohort). The following covariates were included in analyses: age (at baseline), race/ethnicity, education (less than high school or 12 years vs more), stable housing, employment status, incarceration (any history), MAT program (ever and recent), recent injection-related risks (receptive syringe sharing, receptive ancillary equipment sharing), NSP participation, recent injection of opioids (including heroin, speedball), psychostimulant (including amphetamines and methamphetamines) and cocaine use, obtained needles from safe source (NSP and/or pharmacy), recent number of sex partners (≥2 people vs less), ever engaged in transactional sex, recent alcohol use, duration of injection, and an indicator variable for site. Recent time frame was based on responses to the data collection interval: previous 3 or 6 months, depending on cohort.
Association Between Hepatitis C Virus Incidence and Injection-Related Risk Factors
We constructed multivariable Cox regression model to estimate the adjusted F:M HR using exposures as fixed and as time-varying covariates. This model was built as a saturated model that includes all behavioral risk exposure data in common for all sites. In addition to age at baseline and race/ethnicity, other variables associated with incident HCV and potentially sex were included: incarceration (ever) and recent MAT were considered as fixed covariates; and recent injecting, receptive syringe sharing, and stable housing were considered as time-varying covariates. Missing values were excluded from the adjusted model. The results of this multivariable model were compared with 2 other Cox regression models (model 2: saturated model [all covariates with <50% missing rate] that include all covariates in the model entering all variables to evaluate sensitivity of our reports; model 3: parsimonious model adjusting for covariates significant in bivariate analyses at P < .05). Survey and HCV testing data were collected at differing intervals, every 3 or 6 months (6 months: ACS, HCVC, HEPCO, HITS-c, and HITS-p; 3 months: SuperMIX and UFO) [23]. To address these differences in time frame and missing data, we conducted site-specific analyses and examined consistency between and over all sites.
Finally, we also assessed incidence rate (IR) and F:M incidence rate ratios (IRRs) stratified by subgroups (described below) and tested the heterogeneity by Mantel-Haenszel test. F:M IRRs were assessed in the following subgroups by those who reported the exposure vs those who did not: (1) recent receptive syringe exposure; (2) recent receptive ancillary equipment exposure; (3) any lifetime participation in MAT program; (3) any use of NSP services; and various factors associated with health disparities, including (4) education, (5) race/ethnicity, and (6) lifetime history of incarceration. We assessed interaction of HCV F:M HR and different behaviors and demographic characteristics using bivariate Cox proportional hazards regression. Then we did stratified Kaplan-Meier analysis to visualize possible interaction. Statistical hypotheses were tested using a 2-tailed P < .05 level of significance. All analyses were conducted using Stata version 13.1 (StataCorp LP, College Station, Texas).
RESULTS
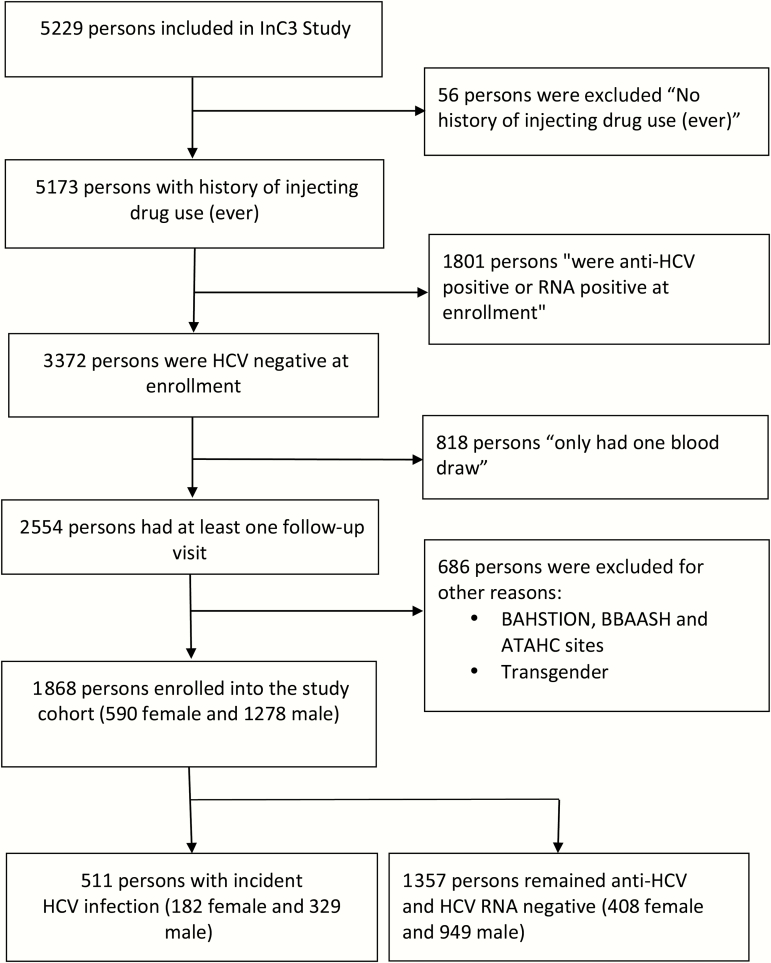
Figure 1 details eligibility criteria and data selection from the overall InC3 participant pool. Overall, 1868 seronegative PWID were enrolled in the 7 cohorts, 590 (31.58%) of whom were female. The baseline characteristics of the participants are presented in Table 1. Women were younger than men, and reported a shorter duration of injection drug use.
Figure 1.
Participant inclusion flowchart. Abbreviations: ATAHC, Australian Trial in Acute Hepatitis C; BAHSTION, Boston Acute HCV Study: Transmission, Immunity, Outcomes Network; BBAASH, Baltimore Before and After Acute Study in Hepatitis; HCV, hepatitis C virus; InC3, International Collaboration of Incident HIV and HCV in Injecting Cohorts.
Table 1.
Baseline Characteristics of 1868 Participants by Sex
| Variable | Category | Female, No. (%) | Male, No. (%) |
|---|---|---|---|
| Overall | 590 (31.58) | 1278 (68.42) | |
| Age, y, median (IQR) (n = 1868) | 24.37 (8.26) | 26.00 (9.00) | |
| Duration of injection, y, median (IQR) (n = 1866) | 6.00 (7.00) | 7.00 (8.00) | |
| Race/ethnicity (n = 1748) | Nonwhite | 138 (23.39) | 268 (20.97) |
| White | 407 (68.98) | 935 (73.16) | |
| Unknowna | 45 (7.63) | 75 (5.87) | |
| Educatedb (n = 1645) | No | 267 (45.25) | 550 (43.04) |
| Yes | 248 (42.03) | 580 (45.38) | |
| Unknowna | 75 (12.71) | 148 (11.58) | |
| Stable housing (n = 1136) | No | 189 (32.03) | 441 (34.51) |
| Yes | 168 (28.47) | 338 (26.45) | |
| Unknowna | 233 (39.49) | 449 (39.05) | |
| Unemployed (n = 1010) | No | 124 (21.02) | 320 (25.04) |
| Yes | 195 (33.05) | 371 (29.03) | |
| Unknowna | 271 (45.93) | 587 (45.93) | |
| Incarceration ever (n = 1779) | No | 224 (37.97) | 306 (23.94) |
| Yes | 333 (56.44) | 916 (71.67) | |
| Unknowna | 33 (5.59) | 56 (4.38) | |
| Medication-assisted treatment (ever) (n = 1272) | No | 255 (43.22) | 611 (47.81) |
| Yes | 132 (22.37) | 274 (21.44) | |
| Unknowna | 203 (34.41) | 393 (30.75) | |
| Recent injectionc (n = 1362) | No | 53 (8.98) | 132 (10.33) |
| Yes | 364 (61.69) | 813 (63.62) | |
| Unknowna | 173 (29.32) | 333 (26.06) | |
| Receptive syringe sharing (n = 1233) | No | 247 (41.86) | 617 (48.28) |
| Yes | 124 (21.02 | 245 (19.17) | |
| Unknowna | 219 (37.12) | 416 (32.55) | |
| Receptive ancillary equipment sharing (n = 1070) | No | 158 (26.78) | 460 (35.99) |
| Yes | 165 (27.97) | 287 (22.46) | |
| Unknowna | 267 (45.25) | 531 (41.55) | |
| Needle and syringe programs (n = 938) | No | 93 (15.76) | 199 (15.57) |
| Yes | 204 (34.58) | 442 (34.59) | |
| Unknowna | 293 (49.66) | 637 (49.84) | |
| Illicit opioid used (n = 781) | No | 57 (9.66) | 128 (10.02) |
| Yes | 198 (33.56) | 398 (31.14) | |
| Unknowna | 335 (56.78) | 752 (58.84) | |
| Medication-assisted treatment (recent) (n = 1207) | No | 238 (40.34) | 577 (45.15) |
| Yes | 127 (21.53) | 265 (20.74) | |
| Unknowna | 225 (38.14) | 436 (34.12) | |
| Obtained needles from safe source (n = 985) | No | 31 (5.25) | 64 (5.01) |
| Yes | 278 (47.12) | 612 (47.89) | |
| Unknowna | 281 (47.63) | 602 (47.10) | |
| Multiple sex partnerse (n = 822) | 0 or 1 | 110 (18.64) | 314 (24.57) |
| ≥2 | 123 (20.85) | 275 (21.52) | |
| Unknowna | 357 (60.51) | 689 (53.91) | |
| Ever traded sex for goods, drug, money, housing, or favors (n = 426) | No | 53 (8.98) | 300 (23.47) |
| Yes | 34 (5.76) | 39 (3.05) | |
| Unknowna | 503 (85.25) | 939 (73.47) | |
| Recent alcohol usec (n = 1048) | No | 79 (13.39) | 179 (14.01) |
| Yes | 239 (40.51) | 551 (43.11) | |
| Unknowna | 272 (46.10) | 548 (42.88) | |
| Collaborating sites | Amsterdam, Netherlands (ACS) | 52 (8.81) | 115 (9.00) |
| Sydney, Australia (HCVC) | 100 (16.95) | 156 (12.21) | |
| Montreal, Canada (HepCo) | 50 (8.47) | 218 (17.06) | |
| Sydney, Australia (HITS-c) | 37 (6.27) | 124 (9.70) | |
| Sydney, Australia (HITS-p) | 172 (29.15) | 322 (25.20) | |
| Melbourne, Australia (SuperMIX) | 43 (7.29) | 79 (6.18) | |
| San Francisco, California (UFO) | 136 (23.05) | 264 (20.66) |
Abbreviations: ACS, Amsterdam Cohort Studies; HCVC, Hepatitis C Virus Cohort Study; HepCo, St Luc Cohort; HITS-c, Hepatitis C Incidence and Transmission Study–Community; HITS-p, Hepatitis C Incidence and Transmission Study–Prison; IQR, interquartile range; SuperMIX, Networks 2 and MIX studies; UFO, U-Find-Out Study.
aData not collected by cohort or not reported by participant.
bEducated: completed high school and higher.
cReported at the interview prior to primary hepatitis C virus infection diagnosis, recent indicates last 3–6 months prior to interview. Interview interval varies for each site: 6 months: ACS, HCVC, HEPCO, HITS-c, and HITS-p; 3 months: SuperMIX and UFO.
dHeroin/other opioids includes heroin. other opioids. and speedball. Pychostimulants includes amphetamines (including methamphetamines) and cocaine.
eRecent number of sex partners (≥2 people vs less).
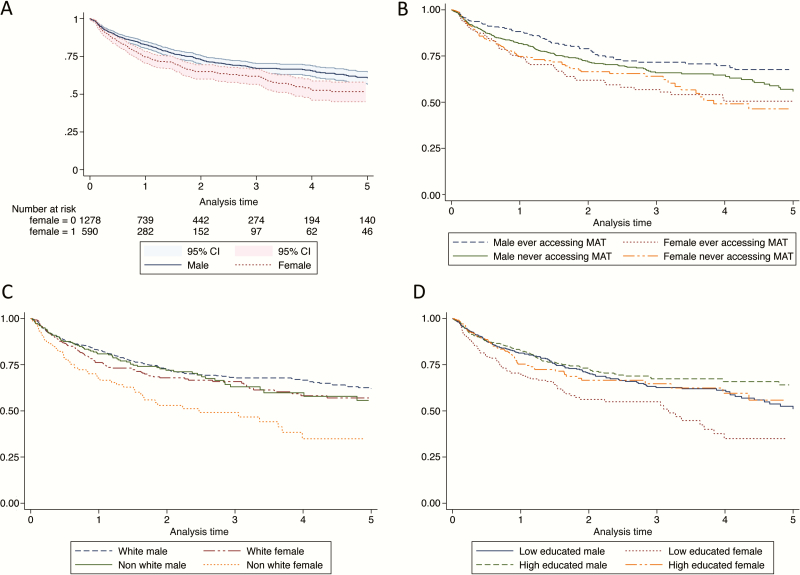
From the 1868 PWID, a total of 3994 PYO were collected over 8339 visits (Table 2). A total of 511 PWID were identified with incident HCV during follow-up, of whom 182 (31.5%) were female. Date of HCV infection was estimated in 106 of 511 (20.74%) based on acute seronegative viremia, 5 (0.98%) based on seroconversion illness, and 400 (78.28%) based on serial anti-HCV tests and documented anti-HCV seroconversion. HCV incidence (per 100 PYO) among women was 16.66 (95% confidence interval [CI], 14.41–19.27) and was significantly higher compared to men (11.34 [95% CI, 10.18–12.63]; P = .001). The crude F:M HR was 1.38 (95% CI, 1.15–1.65), corresponding to an attributable risk of 32.0% (95% CI, 18.0%–43.4%). In the adjusted model (Table 2), the F:M HR was 1.39 (95% CI, 1.12–1.72). The results are consistent with 2 other adjusted models (Supplementary Table 1). In analyses stratified by study site (Table 2), the highest HCV incidence rates among women were observed in the HCVC Study in Sydney (35.58/100 PYO) and the UFO Study in San Francisco (28.88/100 PYO). At all sites, after controlling for covariates, the F:M HR ratio was >1 except for in the ACS study (HR, 0.63), and it was not statistically significant. The highest adjusted HR ratio was observed in the HCVC Study in Sydney (HR, 2.11; P = .014). Figure 2A shows Kaplan-Meier time-to-event analyses of HCV incidence, stratified by sex.
Table 2.
Hepatitis C Virus Incidence by Sex Overall and by Site
| Participantsa | Rate per 100 PYO (95% CI) | Crude | Adjusted Modelb | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Valuec | ||
| All sites (n = 1868, 1616) | |||||
| Women | 16.66 (14.41–19.27) | 1.38 (1.15–1.65) | .001 | 1.39 (1.12–1.72) | .003 |
| Men | 11.34 (10.18–12.63) | ||||
| By sites | |||||
| ACS (n = 167, 72) | |||||
| Women | 3.72 (2.16–6.41) | 1.25 (.64–2.44) | .516 | 0.63 (.045–8.76) | .729 |
| Men | 2.80 (1.89–4.14) | ||||
| HCVC (n = 256, 159) | |||||
| Women | 35.58 (24.57–51.53) | 1.78 (1.04–3.05) | .036 | 2.11 (1.16–3.84) | .014 |
| Men | 20 (13.52–29.6) | ||||
| HepCo (n = 268, 262) | |||||
| Women | 14.44 (8.85–23.58) | 0.93 (.54–1.60) | .794 | 1.15 (.57–2.31) | .697 |
| Men | 15.48 (12.31–19.47) | ||||
| HITS-c (n=161, 141) | |||||
| Women | 7.59 (2.85–20.22) | 1.82 (.53–6.22) | .339 | 1.22 (.29–5.14) | .784 |
| Men | 4.1 (1.95–8.59) | ||||
| HITS-p (n = 494, 481) | |||||
| Women | 28.74 (22.45–36.79) | 1.69 (1.22–2.33) | .001 | 1.12 (.76–1.64) | .571 |
| Men | 14.78 (12.1–18.05) | ||||
| SuperMIX (n = 122, 119) | |||||
| Women | 7.39 (3.69–14.77) | 0.63 (.28–1.43) | .271 | 1.13 (.44–2.89) | .798 |
| Men | 11.26 (7.34–17.27) | ||||
| UFO Study (n = 400, 382) | |||||
| Women | 28.88 (21.89–38.1) | 1.38 (.97–1.96) | .075 | 1.23 (.81–1.87) | .331 |
| Men | 20.27 (16.32–25.17) | ||||
Crude and adjusted HR is shown for biological sex effect.
Abbreviations: ACS, Amsterdam Cohort Studies; CI, confidence interval; HCVC, Hepatitis C Virus Cohort Study (Sydney, Australia); HepCo, St Luc Cohort (Montreal, Canada); HITS-c, Hepatitis C Incidence and Transmission Study–Community; HITS-p, Hepatitis C Incidence and Transmission Study–Prison; HR, female to male hepatitis C virus hazard ratio; PYO, person-years of observation; SuperMIX, Networks 2 and MIX studies; UFO, U-Find-Out Study.
aSample sizes for crude and adjusted model, respectively, are shown.
bSaturated model that includes all common covariates (age at the time of screening, race/ethnicity, prison [ever], recent medication-assisted treatment and considering that following variables vary over time: recent injection, stable housing, receptive syringe sharing).
cThe values in bold were statistically significant based on a-priori P value determination of P < .05.
Figure 2.
Kaplan-Meier time-to-event analyses of hepatitis C virus (HCV) incidence by sex (A), and by assessing the effect modifier of medication-assisted treatment (MAT), race/ethnicity, and education (B–D). Women who did and did not access MAT (B), nonwhite women (C), and low-educated women (D) constantly had the highest rate of HCV infection during the 5-year follow-up. Abbreviations: CI, confidence interval; MAT, medication-assisted treatment.
In subgroup analyses (Table 3), we observed higher HCV incidence rates (per 100 PYO) in women (compared with men) who reported receptive syringe sharing (41.62 vs 24.51, respectively), and ancillary equipment sharing (36.83 vs 28.26, respectively). However, F:M IRR was not statistically different in exposed and unexposed group for receptive syringe and ancillary equipment sharing, education, incarceration, and NSP. The highest F:M IRR was observed among nonwhite women (2.15), which was significantly higher than nonwhite race/ethnicity (1.28; P = .013). Interaction effects between sex and alcohol, MAT, sex and education, and sex and race/ethnicity were also considered (Supplementary Table 2). Figure 2 shows Kaplan-Meier time-to-event analyses of HCV incidence, stratified by sex by MAT uptake (Figure 2B), sex by white vs nonwhite race/ethnicity (Figure 2C), and sex by low vs higher educational levels (Figure 2D). The F:M IRR was significantly higher in those who reported accessing MAT (2.18 [95% CI, 1.56–3.06]; P = .035) compared with those who did not access MAT (1.41 [95% CI, 1.10–1.81]) (Table 3). Women with lower education had higher risk of HCV than their male counterparts. No differences were found in F:M IRR for HCV associated with NSP participation or incarceration history.
Table 3.
Hepatitis C Virus Incidence Rate by Sex, and Incidence Rate Ratios by Different Subgroup Analyses (Selected Demographic Characteristics and Risk Exposures)
| Characteristic | Exposed/ Nonexposed | Female HCV Incidence /100 PYO (95% CI) | Male HCV Incidence/100 PYO (95% CI) | F:M Incidence Rate Ratio (95% CI) | Test of Homogeneity (M-H), P Value |
|---|---|---|---|---|---|
| Race/ethnicity | Other | 28.50 (21.99–36.95) | 13.23 (10.52–16.64) | 2.15 (1.50–3.09) | .013 |
| White | 13.97 (11.68–16.72) | 10.89 (9.61–12.34) | 1.28 (1.02–1.60) | ||
| Receptive syringe sharing | No | 19.23 (15.85–23.32) | 13.13 (11.40–15.13) | 1.46 (1.14–1.87) | .479 |
| Yes | 41.62 (32.25–53.72) | 24.51 (19.76–30.39) | 1.70 (1.20–2.40) | ||
| Receptive ancillary equipment sharing | No | 19.97 (15.74–25.32) | 11.83 (9.92–14.14) | 1.69 (1.23–2.29) | .234 |
| Yes | 36.83 (29.09–46.63) | 28.26 (23.36–34.19) | 1.30 (.95–1.78) | ||
| Completed high school and higher | No | 28.97 (23.72–35.38) | 16.02 (13.71–18.71) | 1.81 (1.39–2.34) | .078 |
| Yes | 17.71 (13.86–22.63) | 13.93 (11.73–16.54) | 1.27 (.93–1.73) | ||
| Incarceration ever | No | 16.40 (12.56–21.42) | 13.84 (10.74–17.82) | 1.19 (.81–1.74) | .099 |
| Yes | 19.30 (16.10–23.14) | 11.36 (10.04–12.86) | 1.70 (1.35–2.12) | ||
| Medication-assisted treatment (ever) | No | 20.64 (16.84–25.29) | 14.59 (12.74–16.72) | 1.41 (1.10–1.81) | .035 |
| Yes | 16.51 (13.14–20.73) | 7.56 (6.02–9.49) | 2.18 (1.56–3.06) | ||
| Needle and syringe programs | No | 17.55 (13.15–23.43) | 9.31 (7.42–11.68) | 1.89 (1.28–2.76) | .167 |
| Yes | 31.39 (26.02–37.88) | 22.66 (19.58–26.23) | 1.39 (1.08–1.77) |
Abbreviations: CI, confidence interval; F:M, female to male; HCV, hepatitis C virus; M-H, Mantel-Haenszel; PYO, person-years of observation.
DISCUSSION
Our findings, showing that women who inject, compared to men, have 38% higher risk of incident HCV, independent of injection-related exposures and covariates, provide important new perspective on HCV susceptibility, not previously studied in depth. This main finding is consistent with results of our recent systematic review and meta-analysis, which showed a 36% higher risk of HCV infection among female compared with male PWID [22]. The results point to the need to study additional factors not measured in these analyses including social, structural, and biological ones, that may contribute to higher HCV susceptibility in women, compared with men. Sexual dimorphism in HCV research has principally focused on differences following infection, wherein women show more favorable responses, including higher spontaneous clearance rates [14] and slower disease progression, including cirrhosis and hepatocellular carcinoma [27]. In the biological area, some researchers hypothesize that estrogen receptors and genetic variations in the estrogen receptor gene affect the pathology, infectivity, and progression of HCV infection [28, 29], and research showing slower fibrosis among women with higher estrogen levels and those on estrogen replacement therapy has supported this [30],. The effect of interferon-λ 4 genotype on spontaneous clearance of HCV has been shown to vary strongly by sex, with greater effects of CC homozygosity among women compared with men [14]. Differences in immune cell composition and activation between men and women may also contribute to observed sexual dimorphism following infection [31]; however, no studies have elucidated mechanisms for this to date. Our results showing higher HCV incidence, independent of individual injection risk behaviors, and the literature showing differences in response to infection, collectively, show a need to conduct further studies of potential biological, and genetic factors that may be associated with higher susceptibility to HCV in women.
Social and structural factors may also influence susceptibility. When examining differences in F:M incidence in stratified analyses, we found a strong differential in HCV incidence by exposure to MAT for opioids. MAT programs, including both methadone- and buprenorphine-based treatment for opioid dependence, have been shown to be associated with 40%–60% lower risk of incident HCV [13, 15, 32]. Our findings are consistent with these studies; however, we found that this protective effect was much less pronounced for women. MAT has significant potential to contribute reductions in HCV in opioid users, and increasing access to this important service is essential, especially in the United States, where opioid dependence and HCV infections are increasing [32]. Factors that may contribute to poor protective effects among women could include differences in how women compared to men reduce injection frequency while on MAT, or MAT dose and types of drugs used for women. In general, the prevalence of current illicit drug use is higher for men than women [4], and many studies report not only that higher numbers of men participate in MAT [15, 32], but also that women are less engaged and adherent to these programs than men [33, 34]. Factors contributing to these disparities include stigma, having children, and other social barriers [35, 36]. All of these factors should be studied further to better understand sex-related differences in risk and to maximize prevention effects of drug treatment programs and their potential to reduce acquisition of blood-borne viruses including HCV and HIV.
Differential risk for HCV among women was also found in association with race/ethnicity, education, and incarceration history. HCV disease has been shown to vary by race/ethnicity in studies of HCV natural history [27, 37] as well as incarceration history. Our recent systematic review and meta-analysis found 48% higher HCV IRR in women compared with men in correctional populations [22]. This difference has been hypothesized to be associated with age as well as with differential incarceration rates of women compared with men relative to their injecting career (women may be incarcerated later than men and thus more likely to be infected); differences in offenses for which women are incarcerated for, and differential access to drug treatment [38]. NSP programs are also important for HCV prevention [13]; however, we found no F:M differences in association with this exposure. Higher incidence of HCV in PWID involved with NSP has been associated with “volunteer bias” due to higher proportion of those injectors whose patterns of drug use place them at elevated risk of blood-borne viral infections [39], and also with low syringe coverage, a factor that varies by geographic site in this study [40]. Research shows that combination prevention approaches, especially those that synergize NSP and MAT, will have the highest population attributable protection impacts on HCV incidence [15]. Our results show the need to further study sex differences with respect to these important social and prevention dynamics, and further elucidate prevention opportunities.
Our findings have some limitations. InC3 includes data that were retrospectively merged after study initiation and data collection, leading to differences in study measures, including frequency of collection and exposure intervals. We addressed this issue by stratifying and adjusting for covariates by each center and combining variable categories to create harmonized variables. Not all studies collected the same data, and missingness emerges as a limitation. Subgroup analyses by relevant exposures and categories were conducted to present as much data as possible. Our analyses controlled for several demographic and risk exposures known to be associated with HCV risk, but there remains potential for unmeasured confounding, which could contribute to a higher F:M incidence ratio. For instance, factors difficult to measure and which could contribute to sex differences potentially include differential access to prevention services, gender-power dynamics, mental health factors, or social and injecting network mixing that introduce differential risk exposure.
Our findings provide important evidence that sex disparities in HCV acquisition exist independent of selected behavioral risk and demographic factors. Although several studies of PWID have shown that women report higher injection-associated risk behaviors, including receptive syringe and ancillary injecting equipment sharing, these exposures did not explain the higher incidence of HCV observed in women in these aggregated data. When considering HCV risk differential among women, multiple factors including biological, social, and network factors, as well as differential access to prevention services, need to be considered. We believe that these results and others strongly point to the need for more research and interventions designed specifically to assess and reduce HCV risk among women.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Drs John Doucette, Stephanie Factor, Hung-Mo Lin, Joseph Masci, and Juan Wisnivesky; the graduate school faculty and members of the multidisciplinary committee in Icahn School of Medicine at Mount Sinai; and journal editors and reviewers for comments that greatly improved the manuscript.
Disclaimer. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse (NIDA) or the National Institutes of Health (NIH).
Financial support. InC3 was funded by the NIDA (grant number R01DA031056). Research support for the InC3 cohorts includes The Netherlands National Institute for Public Health and the Environment to the ACS; BBAASH, NIH (grant number U19 AI088791); BAHSTION is funded under NIH National Institute of Allergy and Infectious Diseases grant U19 U19 AI066345; ATAHC, NIDA (grant number R01 DA 15999-01); HITS-p, National Health and Medical Research Council of Australia (NHMRC), Project No. 222887, Partnership No. 1016351, Program Nos. 510488 and 1053206; HITS-c, University of New South Wales Hepatitis C Vaccine Initiative and NHMRC Project Grant No. 630483; Networks/MIX, NHMRC Project Grants Nos. 331312 and 545891 and the Victorian Operational Infrastructure Support Programme (Department of Health, Victoria, Australia); HepCo, Canadian Institutes of Health Research (MOP-103138 and MOP-106468; Réseau SIDA Maladies Infectieuses, Fonds de la Recherche du Québec-Santé); HCVC, Australian National Health and Medical Research Council (project grant number 991357); UFO, NIH R01 DA016017. G. J. D., J. G., M. H., A. R. L., and L. M. receive fellowship support from the Australian National Health and Medical Research Council. N. H. S. receives support from the Fonds de la Recherche du Québec-Santé, Canada. In addition to the grant support for the InC3 research, K. P. receives support from 3R01 DA016017, 1ULTR001449, and U54GM104944.
Potential conflicts of interest. J. B., P. D., and M. H. have received funding from Gilead Sciences for work on HCV treatment unrelated to this study. P. D. has received funding from Reckitt Benckiser for work unrelated to this study. J. G. is a consultant/advisor and has received research grants from AbbVie, Bristol-Myers Squibb, Cepheid, Gilead Sciences, and Merck/MSD, and has received funding from AbbVie, Gilead, and Merck for HCV treatment research unrelated to this study. J. G. is supported by an Australian National Health and Medical Research Council Career Development Fellowship. M. H. also receives funding from AbbVie and BMS for investigator-initiated HCV research unrelated to this work. G. J. D. is a consultant/advisor and has received research grants from AbbVie, Bristol-Myers Squibb, Cepheid, Gilead, Merck, Janssen, and Roche. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Murphy EL, Bryzman SM, Glynn SA et al. . Risk factors for hepatitis C virus infection in United States blood donors. NHLBI Retrovirus Epidemiology Donor Study (REDS). Hepatology 2000; 31:756–62. [DOI] [PubMed] [Google Scholar]
- 2. Des Jarlais DC, Feelemyer JP, Modi SN, Arasteh K, Hagan H. Are females who inject drugs at higher risk for HIV infection than males who inject drugs: an international systematic review of high seroprevalence areas. Drug Alcohol Depend 2012; 124:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hurtado Navarro I, Alastrue I, Del Amo J et al. . Differences between women and men in serial HIV prevalence and incidence trends. Eur J Epidemiol 2008; 23:435–40. [DOI] [PubMed] [Google Scholar]
- 4. Lansky A, Finlayson T, Johnson C et al. . Estimating the number of persons who inject drugs in the United States by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One 2014; 9:e97596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. United Nations Office on Drugs and Crime. World drug report 2015. ISBN: 978-92-1-148282-9; eISBN: 978-92-1-057300-9. Available at: https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. Accessed May 2015. [Google Scholar]
- 6. Iversen J, Wand H, Topp L, Kaldor J, Maher L. Reduction in HCV incidence among injection drug users attending needle and syringe programs in Australia: a linkage study. Am J Public Health 2013; 103:1436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tracy D, Hahn JA, Fuller Lewis C et al. . Higher risk of incident hepatitis C virus among young women who inject drugs compared with young men in association with sexual relationships: a prospective analysis from the UFO Study cohort. BMJ Open 2014; 4:e004988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Viitanen P, Vartiainen H, Aarnio J et al. . Hepatitis A, B, C and HIV infections among Finnish female prisoners—young females a risk group. J Infect 2011; 62:59–66. [DOI] [PubMed] [Google Scholar]
- 9. Montgomery SB, Hyde J, De Rosa CJ et al. . Gender differences in HIV risk behaviors among young injectors and their social network members. Am J Drug Alcohol Abuse 2002; 28:453–75. [DOI] [PubMed] [Google Scholar]
- 10. Iversen J, Wand H, Gonnermann A, Maher L; Australian National Syringe Program Gender differences in hepatitis C antibody prevalence and risk behaviours amongst people who inject drugs in Australia 1998–2008. Int J Drug Policy 2010; 21:471–6. [DOI] [PubMed] [Google Scholar]
- 11. Gollub EL, Rey D, Obadia Y, Moatti JP; The Manif 2000 Study Group Gender differences in risk behaviors among HIV+ persons with an IDU history. The link between partner characteristics and women’s higher drug-sex risks. Sex Transm Dis 1998; 25:483–8. [DOI] [PubMed] [Google Scholar]
- 12. Latkin CA, Mandell W, Knowlton AR et al. . Gender differences in injection-related behaviors among injection drug users in Baltimore, Maryland. AIDS Educ Prev 1998; 10:257–63. [PubMed] [Google Scholar]
- 13. Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis 2011; 204:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grebely J, Page K, Sacks-Davis R et al. . InC3 Study Group The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014; 59:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turner KM, Hutchinson S, Vickerman P et al. . The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011; 106:1978–88. [DOI] [PubMed] [Google Scholar]
- 16. Terrault NA, Dodge JL, Murphy EL et al. . Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology 2013; 57:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS 2015; 29:2335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruneau J, Roy E, Arruda N, Zang G, Jutras-Aswad D. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction 2012; 107:1318–27. [DOI] [PubMed] [Google Scholar]
- 19. Vallejo F, Barrio G, Brugal MT et al. . Itinere Project Group High hepatitis C virus prevalence and incidence in a community cohort of young heroin injectors in a context of extensive harm reduction programmes. J Epidemiol Community Health 2015; 69:599–603. [DOI] [PubMed] [Google Scholar]
- 20. van de Laar TJ, Molenkamp R, van den Berg C et al. . Frequent HCV reinfection and superinfection in a cohort of injecting drug users in Amsterdam. J Hepatol 2009; 51:667–74. [DOI] [PubMed] [Google Scholar]
- 21. Maher L, Li J, Jalaludin B, Chant KG, Kaldor JM. High hepatitis C incidence in new injecting drug users: a policy failure?Aust N Z J Public Health 2007; 31:30–5. [DOI] [PubMed] [Google Scholar]
- 22. Esmaeili A, Mirzazadeh A, Carter GM et al. . Higher incidence of HCV in females compared to males who inject drugs: a systematic review and meta-analysis. J Viral Hepat 2017; 24:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grebely J, Morris MD, Rice TM et al. . InC Study Group Cohort profile: the international collaboration of incident HIV and hepatitis C in injecting cohorts (InC3) study. Int J Epidemiol 2013; 42:1649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cain KC, Harlow SD, Little RJ et al. . Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am J Epidemiol 2011; 173:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sacks-Davis R, Grebely J, Dore GJ et al. . InC3 study group Hepatitis C virus reinfection and spontaneous clearance of reinfection—the InC3 study. J Infect Dis 2015; 212:1407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hofer H, Watkins-Riedel T, Janata O et al. . Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology 2003; 37:60–4. [DOI] [PubMed] [Google Scholar]
- 27. Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014; 109:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai L, Gao C, Tang S et al. . Sex-specific association of estrogen receptor 2 polymorphisms with hepatitis C virus infection outcomes in a high-risk Chinese Han population. Infect Genet Evol 2014; 28:118–24. [DOI] [PubMed] [Google Scholar]
- 29. Ulitzky L, Lafer MM, KuKuruga MA, Silberstein E, Cehan N, Taylor DR. A new signaling pathway for HCV inhibition by estrogen: GPR30 activation leads to cleavage of occludin by MMP-9. PLoS One 2016; 11:e0145212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Martino V, Lebray P, Myers RP et al. . Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology 2004; 40:1426–33. [DOI] [PubMed] [Google Scholar]
- 31. Galligan CL, Fish EN. Sex differences in the immune response. In: Klein SL, Roberts CW. Sex and gender differences in infection and treatments for infectious diseases. 1st ed: Springer, 2015:1. [Google Scholar]
- 32. Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med 2014; 174:1974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simmonds L, Coomber R. Injecting drug users: a stigmatised and stigmatising population. Int J Drug Policy 2009; 20:121–30. [DOI] [PubMed] [Google Scholar]
- 34. Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J 2013; 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Page K, Tsui J, Maher L et al. . Biomedical HIV prevention including pre-exposure prophylaxis and opiate agonist therapy for women who inject drugs: state of research and future directions. J Acquir Immune Defic Syndr 2015; 69(suppl 2):S169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swan D, Long J, Carr O et al. . Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. AIDS Patient Care STDS 2010; 24:753–62. [DOI] [PubMed] [Google Scholar]
- 37. El-Serag HB, Kramer J, Duan Z, Kanwal F. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol 2014; 109:1427–35. [DOI] [PubMed] [Google Scholar]
- 38. Vescio MF, Longo B, Babudieri S et al. . Correlates of hepatitis C virus seropositivity in prison inmates: a meta-analysis. J Epidemiol Community Health 2008; 62:305–13. [DOI] [PubMed] [Google Scholar]
- 39. Hagan H, McGough JP, Thiede H, Hopkins SG, Weiss NS, Alexander ER. Volunteer bias in nonrandomized evaluations of the efficacy of needle-exchange programs. J Urban Health 2000; 77:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kwon JA, Iversen J, Maher L, Law MG, Wilson DP. The impact of needle and syringe programs on HIV and HCV transmissions in injecting drug users in Australia: a model-based analysis. J Acquir Immune Defic Syndr 2009; 51:462–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.