This study found that combination antiretroviral therapy (cART) regimens differentially affect estradiol levels in pregnancy. Exposure to lopinavir/ritonavir was associated with an increase in estradiol, while exposure to efavirenz was associated with a decrease in estradiol, relative to cART-naive HIV-infected women.
Keywords: estradiol, progesterone, HIV, pregnancy, combination antiretroviral therapy
Abstract
Background
Combination antiretroviral therapy (cART) use in pregnancy has been associated with hormonal dysregulation. We performed a secondary retrospective analysis of longitudinal progesterone and estradiol levels in pregnancy using specimens from the Protease Inhibitors to Reduce Malaria Morbidity in HIV-infected Pregnant Women study, which randomized Ugandan human immunodeficiency virus (HIV)–infected ART-naive women to initiate either lopinavir/ritonavir (LPV/r)–based or efavirenz (EFV)–based cART.
Methods
Three hundred twenty-six women (160 randomized to the EFV arm and 166 women to the LPV/r arm) with at least 1 plasma sample collected during pregnancy were included. Enrollment samples collected prior to cART initiation were used as a cART-naive comparator group. Hormone levels were quantified by enzyme-linked immunosorbent assay.
Results
Estradiol levels were differentially affected by the 2 cART regimens. Exposure to LPV/r was associated with an increase in estradiol (P < .0001), whereas exposure to EFV was associated with a decrease in estradiol (P < .0001), relative to the cART-naive gestationally matched comparator group. Lower estradiol levels correlated with small for gestational age (SGA) (P = .0019) and low birth weight (P = .019) in the EFV arm, while higher estradiol levels correlated with SGA in the LPV/r arm (P = .027). Although progesterone levels were similar between treatment arms, we observed an association between SGA and lower progesterone in the LPV/r arm (P = .04). No association was observed between hormone levels and preterm birth in either arm. Levels of progesterone and estradiol were lower in cases of stillbirth, and levels of both hormones declined immediately prior to stillbirth in 5 of 8 cases.
Conclusions
Combination ART regimens differentially affect estradiol levels in pregnancy, a hormone critical to the maintenance of a healthy pregnancy. Identifying cART regimens that minimize perinatal HIV transmission without contributing to hormonal dysregulation represents an urgent public health priority.
Clinical Trials Registration
(See the Major Article by Balogun et al on pages 420–7.)
World Health Organization (WHO) guidelines recommend combination antiretroviral therapy (cART) for all human immunodeficiency virus (HIV)–infected (HIV+) women during pregnancy and breastfeeding to promote maternal health and prevent vertical HIV transmission [1]. Both protease inhibitor (PI)–based and nonnucleoside reverse transcriptase inhibitor (NNRTI)–based therapy are commonly used in HIV+ pregnant women. Efavirenz (EFV)–based cART (NNRTI-based) is the WHO-recommended first-line therapy, while lopinavir/ritonavir (LPV/r)–based cART (PI-based) is recommended as second-line therapy in case of virologic failure on first-line therapeutics, and has been widely used in high-resource settings. While the benefits of cART for both infant and mother are clear, the use of highly potent drugs in pregnancy has potential risks. Several studies suggest that antiretroviral use in pregnancy is associated with increased incidence of adverse birth outcomes including preterm birth (PTB), small for gestational age birth (SGA), and stillbirth, where others do not [2, 3]. Increased regimen complexity (cART vs monotherapy), preconception exposure, and PI-based cART may increase the risk of adverse birth outcomes [2, 4–7].
The steroid hormones progesterone and estradiol are critical to the maintenance and progression of normal pregnancy, and regulation of parturition [8, 9]. Levels of both hormones increase across a healthy pregnancy and are closely tied to birth outcome [10–12]. Reduced progesterone and estradiol are associated with ectopic pregnancy, placental abnormalities, spontaneous abortion, fetal distress, intrauterine growth restriction, and preterm delivery [12–17]. Endocrine dysfunction has been reported in HIV+ individuals both in the pre- and post-cART eras [18]; however, data in pregnancy are limited. Lower levels of progesterone have been observed in a Canadian cohort of HIV+ pregnant women receiving PI-based cART compared with HIV-negative (HIV–) controls, and reduced progesterone was associated with lower birth weight centile in this cohort [19, 20]. A transient increase in the estradiol precursor dehydroepiandrosterone sulphate (DHEAS) was observed in neonates exposed in utero to LPV/r-based cART [21], though to date no published studies have examined the relationship between cART regimens and estradiol in pregnancy.
More than 1.5 million HIV+ pregnant women receive cART each year, and this number is expected to increase. The majority of these women reside in resource-constrained settings where adverse birth outcomes disproportionally contribute to neonatal mortality. To ensure optimal maternal and infant health outcomes, it is imperative that mechanism underlying adverse outcomes among women receiving cART in pregnancy be identified. The objectives of this study were to examine estradiol and progesterone levels across pregnancy and to investigate associations between these hormones and adverse birth outcomes among HIV+ women participating in a previously completed trial in Tororo, Uganda [22, 23], randomized to initiate either LPV/r-based or EFV-based cART.
MATERIALS AND METHODS
Study Population
Maternal plasma samples collected from HIV+ pregnant women participating in the Protease Inhibitors to Reduce Malaria Morbidity in HIV-infected Pregnant Women (PROMOTE) trial in Uganda were used for this secondary retrospective analysis. The PROMOTE trial was a randomized controlled trial whereby ART-naive HIV+ pregnant women (≥16 years of age, between 12 and 28 weeks’ gestation) were randomized (1:1) to receive either LPV/r-based (400 mg/100 mg twice daily; increased to 600 mg/150 mg twice daily from gestational week 30) or EFV-based (600 mg once daily) cART. Both groups received zidovudine/lamivudine (300 mg/150 mg twice daily). Women at all CD4 counts were eligible. The PROMOTE trial study design and results have been published previously [23]. Blood collection took place at enrollment (between 12 and 28 weeks’ gestation) and all subsequent antenatal visits. Samples were drawn at least 4 weeks apart. All available plasma samples from women with a singleton pregnancy and known birth outcome and a minimum of a single sample collected in 1 of 6 gestational age categories (16 to <20, 20 to <24, 24 to <28, 28 to <32, 32 to <36, and 36 to <37 weeks) were tested for progesterone and estradiol levels. Plasma samples collected at enrollment from participants who had not yet initiated cART were used as a cART-naive comparator group (Supplementary Table 1). The median number of samples tested per participant was 3.
Ethics Statement
Ethical approval from review boards at Makerere University School of Medicine, Uganda National Council for Science and Technology and the National Drug Authority (Kampala, Uganda), University of California, San Francisco (San Francisco, California), and University Health Network (Toronto, Canada). Signed informed consent was obtained from all participants enrolled in the parent trial to permit blood collection for marker analysis.
Hormone Assays
Maternal peripheral ethylenediaminetetraacetic acid (EDTA) plasma samples were collected and stored at –80°C prior to testing. Plasma samples were assessed for estradiol and progesterone levels using precoated enzyme-linked immunosorbent assay plates purchased from DRG International (Springfield, New Jersey). Assays were performed blinded to the patient trial arm, and according to the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was performed using Stata version 12 software (StataCorp, College Station, Texas), SPSS version 20 (IBM), GraphPad Prism version 6 (GraphPad, La Jolla, California) and R version 3.2.1 (R Foundation for Statistical Computing). Descriptive data were summarized using median (interquartile range [IQR]), or number and percentage. Baseline characteristics were compared between trial arms using χ2 or Fisher exact test where appropriate. A random-slope, random-intercept linear mixed-effects (LME) model (R package “lme4,” linear mixed-effects models using Eigen and S4_. R package version 1.1–9) was used to examine longitudinal changes in loge-transformed estradiol and progesterone levels across gestation by trial arm. The model included a random intercept for each participant and a by-participant random slope for the effect of gestational age (GA). Fixed effects were GA at sample collection and treatment group. We shifted the GA covariate to provide a meaningful intercept by subtracting the lowest GA from all GA values. The model was constrained so that treatment groups would have the same intercept, with subsequent testing for interaction of GA and treatment arm. Adding quadratic and cubic terms for GA significantly improved the model fit for the estradiol analysis (Supplementary Table 2). Q-Q and residual plots revealed departure from normality; therefore, 50 samples that were over the limit of detection for the assay (all from the LPV/r group) were excluded. We validated our approach with a sensitivity analysis; excluding these values did not change the effect size (Supplementary Figure 1). Stepwise likelihood ratio significance tests compared the model with the treatment group to a partial model without the effect of treatment. Associations between loge-transformed hormone levels across gestational bins of sample collection and birth outcomes (PTB, SGA, LBW, stillbirth) were assessed for each treatment arm using 2-way analysis of variance with Holm posttest. Only samples obtained from participants who already initiated cART were used for the birth outcome analyses. PTB was defined as delivery prior to 37 weeks’ gestation (based on last menstrual period with ultrasound biometry) [24]. SGA was defined as birth weight <10th percentile based on gestational age and infant sex [25]. LBW was defined as birth weight <2500 g. Stillbirth was defined as birth without signs of life on or after 28 weeks of gestation, according to the WHO definition.
RESULTS
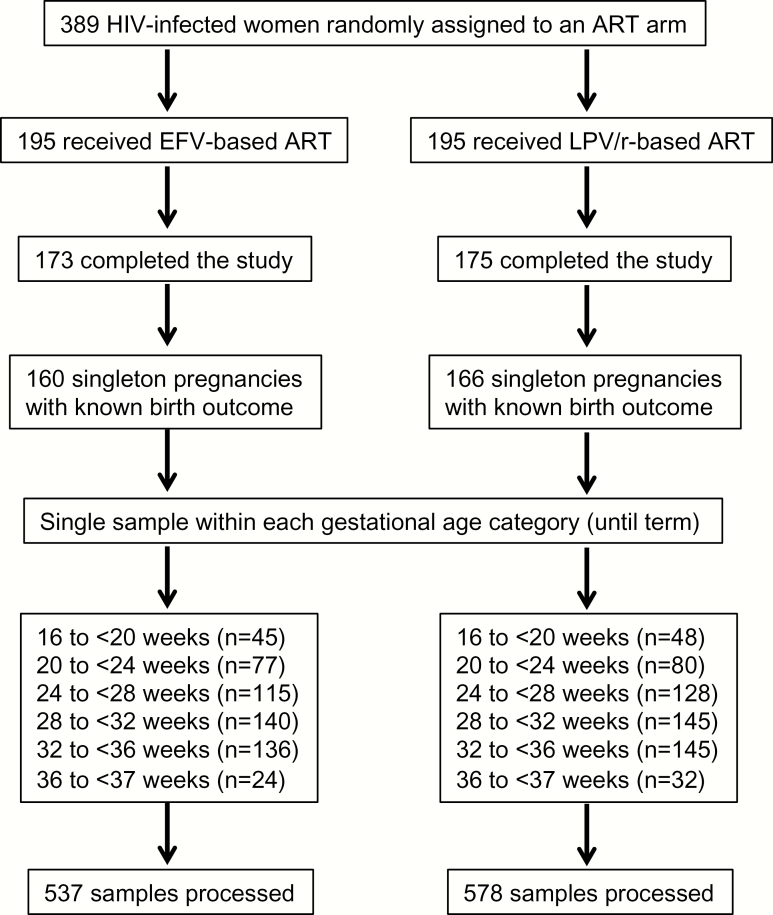
Of the 389 women who participated in the PROMOTE trial, 160 women from the EFV arm and 166 women from the LPV/r arm had at least 1 blood sample collected and known birth outcome, and were included in this study (Figure 1 and Supplementary Table 1). A total of 105 women contributed samples prior to randomization and cART initiation that were used as cART-naive controls. All women initiated cART between gestational week (GW) 12 and GW28. There were no significant differences in baseline characteristics or clinical outcomes of the study population by trial arm (Table 1). The demographics of the study cohort did not differ from those of the parent cohort [23].
Figure 1.
Flowchart of study participants and maternal plasma samples processed in this study. Abbreviations: ART, antiretroviral therapy; EFV, efavirenz; HIV, human immunodeficiency virus; LPV/r, lopinavir/ritonavir.
Table 1.
Characteristics of the Study Population by Trial Arm
| Characteristic | Treatment Arm | P Value | |
|---|---|---|---|
| EFV-Based ART (n = 160) | LPV/r-Based ART (n = 166) | ||
| Baseline characteristics | |||
| Age, y, mean ± SD | 29.5 ± 4.8 | 29.2 ± 5.3 | .61 |
| BMI, kg/m2, median (IQR) | 21.2 (19.6–22.9) | 21.6 (20.2–23.2) | .074 |
| Socioeconomic status (tertile), median (IQR) | 2 (1–2) | 2 (1–2) | .59 |
| Gestational age at enrollment, wk, median (IQR) | 23.4 (19.6–27.6) | 23.4 (19.4–27.0) | .76 |
| Previous pregnancies | |||
| 0 | 13 (8.1) | 7 (4.2) | .43 |
| 1 | 15 (9.4) | 20 (12.0) | |
| ≥2 | 132 (82.5) | 139 (83.7) | |
| Hemoglobin level, g/dL, mean ± SD | 11.0 ± 1.3 | 11.0 ± 1.2 | .88 |
| White blood cell count, cells/μL, median (IQR) | 4900 (4100–6100) | 5200 (4300–6400) | .13 |
| Platelet count, × 109/L, mean ± SD | 217.6 ± 60.8 | 208.5 ± 60.4 | .18 |
| CD4+ T-cell count, cells/μL, median (IQR) | 373 (270–496) | 368 (281–505) | .51 |
| HIV RNA load, log10 copies/mL, mean ± SD | 4.2 ± 0.9 | 4.1 ± 0.9 | .42 |
| Outcome characteristics | |||
| Gestational age at delivery, wk, median (IQR) | 39 (37–40) | 38 (37–39) | .061 |
| Birth weight, kg, median (IQR) | 2910 (2680–3240) | 2880 (2650–3210) | .50 |
| Preterm birth | 24 (15.0) | 31 (18.7) | .46 |
| Small for gestational age | 43 (26.9) | 46 (27.7) | .90 |
| Low birth weight | 29 (18.1) | 30 (18.1) | 1.00 |
| Stillbirth | 4 (2.5) | 5 (3.0) | 1.00 |
| Placental malaria | 14 (8.8) | 10 (6.0) | .52 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; EFV, efavirenz; HIV, human immunodeficiency virus; IQR, interquartile range; LPV/r, lopinavir/ritonavir; SD, standard deviation.
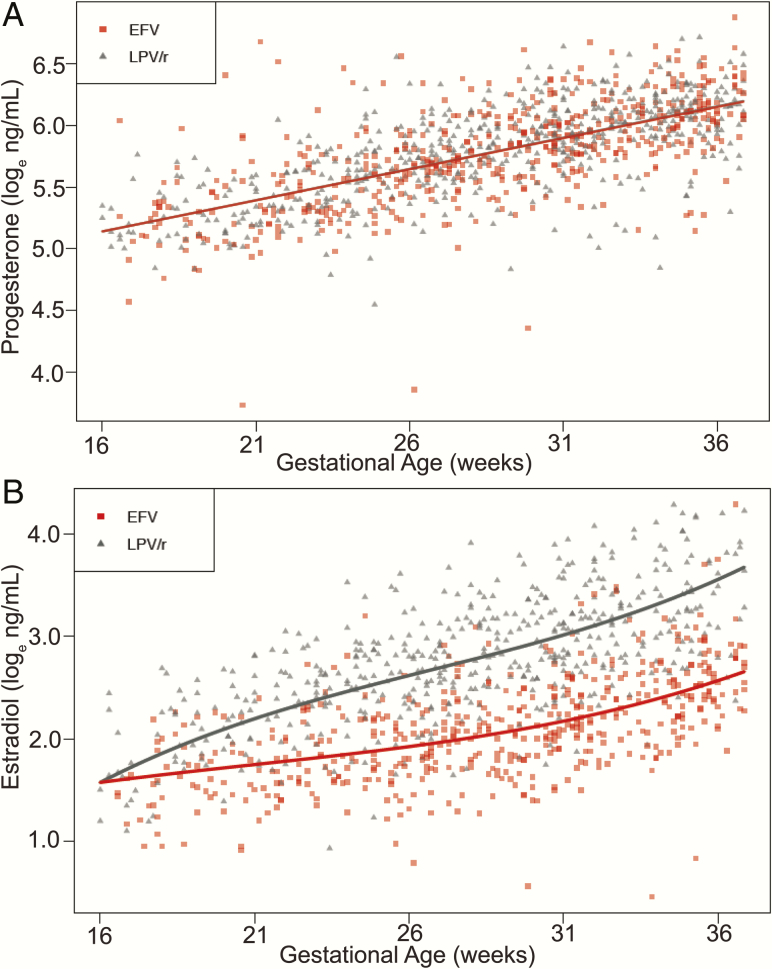
Plasma levels of both hormones increased across pregnancy (Figure 2). Progesterone levels did not differ between treatment arms (P > .05; Figure 2A, Supplementary Table 3) in the LME model. Estradiol was higher in women receiving LPV/r in comparison with women receiving EFV (P < .001; Figure 2B, Supplementary Table 3). In samples collected after GW32, median estradiol was 33.17 (IQR, 21.81–76.0) ng/mL in women receiving LPV/r-based cART and 11.76 (IQR, 9.09–15.48) ng/mL in women receiving EFV-based cART.
Figure 2.
Women receiving lopinavir/ritonavir (LPV/r)–based combination antiretroviral therapy (cART) have higher plasma estradiol in comparison with women receiving efavirenz (EFV)–based cART. Loge-transformed levels of plasma progesterone (A) and estradiol (B) across pregnancy in human immunodeficiency virus–infected women receiving EFV or LPV/r-based cART.
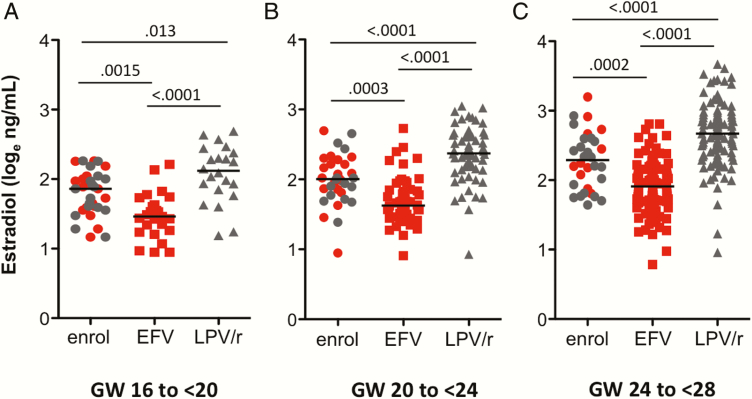
Due to the lack of an HIV– arm, we were not able to compare estradiol levels in our HIV+ women to those of HIV– women. We took advantage of the trial design, which allowed women to enter between GW12 and GW28, to obtain gestational age–matched prerandomization samples that we could use as an HIV+ cART-naive comparator group (Figure 3). Median gestational ages were similar between groups at each gestational window. Compared to gestational age–matched prerandomization women (cART-naive), women exposed to LPV/r had higher estradiol levels at all time points (GW16–GW<20, P = .013; GW20–GW<24, P < .0001; GW24–GW<28, P < .0001), and women exposed to EFV had lower estradiol levels at all time points (GW16–GW<20, P = .0015; GW20–GW<24, P < .0001; GW24<28, P < .0001). We performed a similar analysis of progesterone levels and found no significant differences between levels in the cART-naive and either the LPV/r or EFV groups (Supplementary Figure 2).
Figure 3.
Estradiol levels are higher in lopinavir/ritonavir (LPV/r)–treated women and lower in efavirenz (EFV)–treated women compared with levels in gestational week–matched combination antiretroviral therapy (cART)–naive women (prerandomization). Loge-transformed estradiol levels in plasma collected between gestational week (GW) 16 and <20 (A), 20 and <24 (B), and 24 and <28 (C). Data shown in circles (red for those who went on to be randomized to EFV and gray for those who went on to be randomized to LPV/r) are from prerandomization samples collected prior to cART initiation (enrol), data in red squares are from samples exposed to EFV, and data in gray triangles are from samples exposed to LPV/r. Statistical significance assessed by Kruskal-Wallis test with Dunn posttest.
Consistent with the parent trial [23], prevalence of birth outcomes (PTB, SGA, LBW, stillbirth) did not differ between treatment arms (Table 1). The frequency of PTB in the combined cohort, including both EFV and LPV/r treatment groups, was 16.8% (n = 55), SGA was 27.3% (n = 89), LBW was 18.1% (n = 59), and stillbirth was 2.8% (n = 9). We were interested in examining whether regimen-associated changes in hormone levels were linked to birth outcomes.
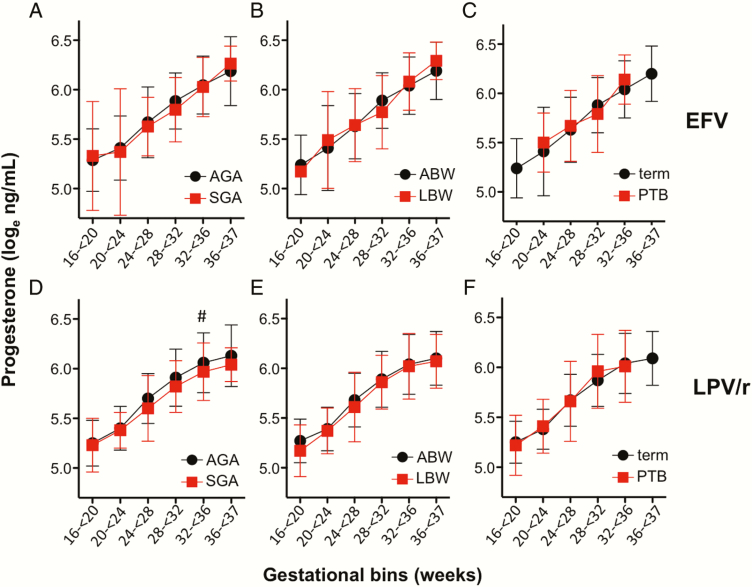
We observed no association between plasma progesterone levels and PTB, SGA, or LBW outcomes in the EFV arm (Figure 4A–C), or PTB or LBW in the LPV/r arm (Figure 4E and 4F). There was an association between lower progesterone levels and SGA in the LPV/r arm (P = .04) (Figure 4D).
Figure 4.
Progesterone levels and adverse birth outcomes in efavirenz (EFV) and lopinavir/ritonavir (LPV/r)–treated women. Loge-transformed plasma progesterone in average for gestational age (AGA) and small for gestational age (SGA) outcomes (A and D), average birth weight (ABW) and low birth weight (LBW) deliveries (B and E), and term and preterm (PTB) deliveries (C and F). Data are from women on EFV-based combination antiretroviral therapy (cART) (A–C) and from women on LPV/r-based cART (D–F). All samples analyzed were from women on treatment. Figures depict mean and standard deviations by gestational age category. Statistical significance assessed by 2-way analysis of variance (ANOVA) with Holm posttest. For (D), P = .04 for SGA by 2-way ANOVA. #P = .10 for posttest.
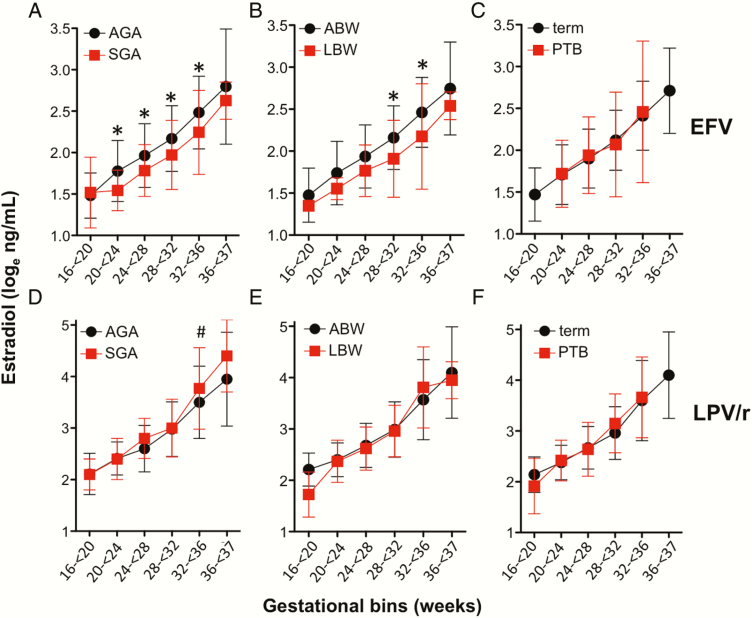
In the EFV arm, lower levels of estradiol were associated with SGA (P = .0019) and LBW (P = .019), but not with PTB (Figure 5A–C). Estradiol levels were lower in EFV-exposed women with SGA from GW20 to GW36, and in women with LBW from GW28 to GW36.
Figure 5.
Estradiol levels and adverse birth outcomes in efavirenz (EFV) and lopinavir/ritonavir (LPV/r)–treated women. Loge-transformed levels of plasma estradiol in average for gestational age (AGA) and small for gestational age (SGA) outcomes (A and D), average birth weight (ABW) and low birth weight (LBW) deliveries (B and E), and term and preterm (PTB) deliveries (C and F). Data are from women on EFV-based combination antiretroviral therapy (cART) (A–C) and from women on LPV/r-based cART (D–F). All samples analyzed were from women on treatment. Figures depict mean and standard deviations by gestational age category. Statistical significance assessed by 2-way analysis of variance (ANOVA) with Holm posttest. For (A), P = .0019 for SGA by 2-way ANOVA and *P < .05 for posttest. For (B), P = .019 for LBW by 2-way ANOVA, *P < .05 for posttest. For (D), P = .027 for SGA by 2-way ANOVA, #P = .07 for posttest.
We did not observe any association between estradiol levels and LBW or PTB in the LPV/r arm (Figure 5E and 5F), but we did observe an association between estradiol and SGA (P = .027), with a trend toward higher estradiol levels in LPV/r-exposed women with SGA at GW32–GW36 (P = .07) (Figure 5D).
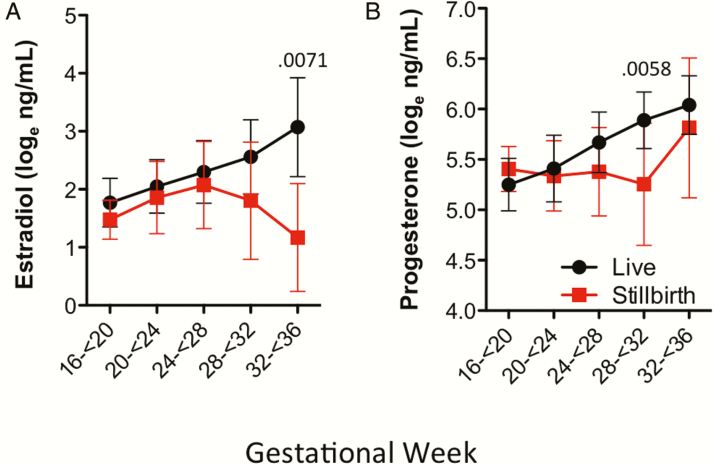
Due to the small number of stillbirths in this cohort, we combined the trial arms to examine levels of progesterone and estradiol in cases of stillbirth. We observed lower plasma estradiol in cases of stillbirth at GW32–GW36 compared with pregnancies in the same gestational age bracket that resulted in subsequent live births (P = .0071; Figure 6A). Plasma progesterone levels were lower in cases of stillbirth at GW28–GW32 (P = .0058; Figure 6B). In 5 of the 8 cases of stillbirth with multiple samples processed, levels of both estradiol and progesterone declined 1 to 9 weeks prior to stillbirth (Supplementary Figure 3).
Figure 6.
Plasma estradiol and progesterone levels in cases of stillbirth. Loge-transformed estradiol (A) and progesterone (B) levels in plasma samples in live birth and cases of stillbirth in women receiving efavirenz or lopinavir/ritonavir–based treatment combined. Figures depict mean and standard deviations by gestational age category. Statistical significance assessed by Wilcoxon rank-sum test.
DISCUSSION
In this study, we examined the longitudinal changes in estradiol and progesterone in HIV+ treatment-naive pregnant women randomized to initiate either EFV-based or LPV/r-based cART. Using plasma samples collected prior to cART initiation, we were able to compare estradiol and progesterone levels between cART-treated and cART-naive women. While levels of progesterone did not differ between the EFV and LPV/r arms, we observed higher levels of estradiol in women receiving LPV/r-based cART compared with women receiving EFV-based cART. This effect was observed beginning early in pregnancy (GW <20) and persisted until term (GW37). By comparing estradiol levels in the EFV and LPV/r arms to those of gestational age–matched prerandomization samples (cART naive), we were able to determine that LPV/r was associated with increased estradiol levels, whereas EFV was associated with decreased estradiol levels. These data suggest that EFV and LPV/r differentially impact circulating levels of estradiol in pregnancy.
The changes in estradiol in both the LPV/r- and EFV-treated women may be the result of reduced or induced metabolism of estrogens resulting from disruptions to cytochrome P450 (CYP) activity [26]. The first step in estradiol metabolism is hydroxylation catalyzed by CYP enzymes, mainly CYP1A2 and CYP3A4 in the liver, where the majority of estrogen metabolism takes place. Both LPV and ritonavir are inhibitors, while EFV is an inducer of CYP-mediated metabolism, specifically CYP3A4 [26, 27]. Inhibition of CYP3A4 would be expected to lead to higher estradiol levels (as observed in the LPV/r arm), whereas induction of CYP3A4 would be expected to lead to lower estradiol levels (as observed in the EFV arm).
Elevated estradiol levels in the LPV/r arm may also result from higher availability of the precursor for placental estradiol synthesis DHEAS. During pregnancy, the fetus is a major source of DHEAS to the placenta. Previous research has reported increased DHEAS levels in newborns exposed to LPV/r in utero [21], which could imply a higher availability of the estradiol precursor during pregnancy. In a separate analysis using samples from a Canadian HIV pregnancy cohort, we observed elevated cord DHEAS levels in HIV+ PI-cART–exposed women compared with HIV– controls, which correlated with higher maternal and cord blood estradiol levels [28].
Dysregulation of estradiol levels may also be the result of disruption of estrogen receptor (ER)–mediated feedback mechanisms involved in the homeostatic control of estradiol during pregnancy. Ritonavir was shown to downregulate ER expression in foam cells and to inhibit ERα translocation to the nucleus [29], whereas EFV was shown to directly activate ER in vitro with an affinity greater than estradiol [30].
Reduced estradiol levels in late gestation have been reported in pregnancies complicated by fetal growth restriction [31]. In agreement with these studies, we found that in the EFV arm, lower estradiol levels were associated with SGA and LBW, but not PTB. Due to the correlative nature of our study, we are not able to establish a direct effect of low estradiol on fetal growth. Experimental data suggest that estradiol plays a role in the development of the feto-placenta vasculature through the regulation of VEGF expression, and in placenta perfusion by acting as a potent vasodilator of uterine arterioles [31]. Failure to establish an optimal placental vasculature and perfusion could lead to poor fetal growth. However, because the placenta is the site of estradiol production in pregnancy, low estradiol may be secondary to placenta insufficiency induced by other mechanisms [32].
In contrast to the low estradiol levels seen in the EFV arm, estradiol levels were elevated with LPV/r exposure. We observed an association between elevated estradiol in late gestation and SGA (but not with LBW or PTB) in the LPV/r arm. In pregnancies with ovarian stimulation and in animal models of estrogen stimulation, high estradiol levels have been associated with an increased risk for LBW [33, 34]. Exposure of the fetus to elevated estradiol could also have significant consequences postnatally. Elevated maternal and cord estradiol levels in patients with ovarian stimulation were associated with dyslipidemia in newborns, a risk factor for metabolic disease later in life [35]. In rodent models, administration of supplementary estrogen was associated with disruptions in the normal development of the reproductive system, and with metabolic changes leading to obesity [36]. Future studies investigating the possible long-term effects of altered estradiol exposure in utero on factors such as genitourinary outcomes (eg, undervirilization in males) and metabolic abnormalities are merited.
Levels of progesterone did not differ between treatment arms. We have previously reported lower progesterone levels in a cohort of Canadian HIV+ women on PI-based cART (ritonavir-boosted lopinavir or atazanavir, most with cART exposure preconception) compared with HIV–women [19, 20]. Due to the lack of an HIV–control group in the current study, we are unable to determine if LPV/r or EFV-based cART is actually lowering progesterone levels below those seen in HIV–pregnancies. However, progesterone levels did not vary significantly by treatment arm after initiation of either LPV/r- or EFV-based cART. One key difference that may influence progesterone levels is the time of cART initiation. In the Canadian cohort, the majority of HIV+ women had initiated cART prior to conception [19, 20], whereas in the current study women initiated cART in the second or third trimester. It is possible that initiating cART in pregnancy vs conceiving while already taking cART may have different consequences on progesterone levels in pregnancy.
In agreement with our previous findings [19], we observed an association between SGA and lower progesterone in the LPV/r arm, but not in the EFV arm. Progesterone levels were not associated with PTB in either arm. Unlike in rodent pregnancy where a decrease in progesterone precedes labor, in human pregnancy functional progesterone withdrawal via changes in progesterone receptor expression, rather than a decline in progesterone levels, regulates initiation of labor [37].
Given the scarcity of data on the mechanistic pathways leading to stillbirth in the context of HIV and cART, we examined hormones in relation to stillbirth outcomes, despite the small number of cases. In the combined cohort, we observed reduced levels of progesterone at 28–32 weeks, and estradiol at 32–36 weeks in cases of stillbirth. When we examined cases of stillbirth individually, we observed a drop in hormones immediately prior to stillbirth. The reduction in progesterone and estradiol observed in cases of stillbirth is consistent with previous literature in the field, providing evidence that both hormones are essential for the maintenance of pregnancy [10]. Because progesterone and estradiol are produced in the placenta, the decline in both hormones prior to stillbirth may be indicative of placenta insufficiency severe enough to lead to stillbirth. Additionally, the reduction in estradiol prior to stillbirth may be an early signal of fetal distress, given that the fetal adrenal is the primary source of the precursor for placental estradiol.
Strengths of this study include the randomized control study design of the parent trial and the collection of repeated plasma samples collected throughout pregnancy. The study has several limitations. Women were enrolled after 12 weeks’ gestation, so it was not possible to examine the association of treatment regimen and obstetric outcomes originating in the first trimester. Additionally, estradiol levels were evaluated to be high or low in comparison to prerandomization specimens drawn from the same women whose hormonal levels were followed longitudinally. All women enrolled in the parent trial were HIV+; therefore, it was not possible to examine the impact of HIV infection on circulating hormone levels in this cohort. However, in agreement with the findings of this study, we have also observed higher maternal and cord plasma estradiol levels in HIV+ PI-cART–exposed women compared with HIV– women [28]. A systematic review reported maternal estradiol levels of approximately 20 ng/mL in the third trimester of uncomplicated pregnancies [38]. This supports our findings of elevated estradiol in the LPV/r group (33 ng/mL) and decreased estradiol levels in the EFV group (12 ng/mL). Finally, several clinical and demographic factors that are associated with adverse birth outcomes (eg, nutrition, socioeconomic factors) are common in HIV+ women and may have influenced our correlation analyses between cART exposure, hormone levels, and birth outcomes.
This study provides evidence of significant alterations of estradiol levels in pregnancy by LPV/r-based and EFV-based cART. We report an increase in estradiol levels associated with LPV/r-based cART, and a decrease in estradiol levels associated with EFV-based cART in pregnancy. We further observed a correlation between estradiol levels and SGA outcomes. Our findings contribute toward a better understanding of cART safety in pregnancy, and demonstrate that the type of cART regimen used in pregnancy uniquely affects the in utero environment. Given the association between hormone levels and obstetrical outcome, future studies could evaluate hormonal interventions to improve obstetrical and neonatal outcomes among HIV+ women receiving cART during pregnancy. Our findings also support the need for future studies to investigate the possible long-term effects of altered in utero estradiol exposure on offspring reproductive and metabolic health.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We acknowledge and thank the mothers, field teams, nurses, midwives, supervisors, laboratory staff, and administrative staff who made this study possible.
Financial support. This work was supported by the Canadian Institutes of Health Research (CIHR) MOP-130398 and PJT-148684 (to L. S.) and CIHR Emerging Team Grant in Maternal Health (to L. S.); the Ontario HIV Treatment Network G655 (to L. S.); the Canadian Foundation for AIDS Research (to L. S.); Global Alliance to Prevent Prematurity and Stillbirth and Grand Challenges in Global Health: Preventing Preterm Birth Initiative Grant No. 12003 (to K. C. K.); CIHR Foundation (to K. C. K.); CIHR Fellowship (to A. L. C. and C. R. M.); and Canada Research Chair (to K. C. K.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, Switzerland: WHO, 2016. [PubMed] [Google Scholar]
- 2. Chen JY, Ribaudo HJ, Souda S et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis 2012; 206:1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koss CA, Natureeba P, Plenty A et al. Risk factors for preterm birth among HIV-infected pregnant Ugandan women randomized to lopinavir/ritonavir- or efavirenz-based antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 67:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li N, Sando MM, Spiegelman D et al. Antiretroviral therapy in relation to birth outcomes among HIV-infected women: a cohort study. J Infect Dis 2016; 213:1057–64. [DOI] [PubMed] [Google Scholar]
- 5. Powis KM, Kitch D, Ogwu A et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis 2011; 204:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS 2007; 21:607–15. [DOI] [PubMed] [Google Scholar]
- 7. Sibiude J, Warszawski J, Tubiana R et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost?Clin Infect Dis 2012; 54:1348–60. [DOI] [PubMed] [Google Scholar]
- 8. Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 2012; 62:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol 2000; 182:432–8. [DOI] [PubMed] [Google Scholar]
- 10. Schindler AE. Endocrinology of pregnancy: consequences for the diagnosis and treatment of pregnancy disorders. J Steroid Biochem Mol Biol 2005; 97:386–8. [DOI] [PubMed] [Google Scholar]
- 11. Quenby SM, Farquharson RG. Predicting recurring miscarriage: what is important?Obstet Gynecol 1993; 82:132–8. [PubMed] [Google Scholar]
- 12. Castracane VD. Endocrinology of preterm labor. Clin Obstet Gynecol 2000; 43:717–26. [DOI] [PubMed] [Google Scholar]
- 13. Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 2000; 21:514–50. [DOI] [PubMed] [Google Scholar]
- 14. Daily CA, Laurent SL, Nunley WC Jr. The prognostic value of serum progesterone and quantitative beta-human chorionic gonadotropin in early human pregnancy. Am J Obstet Gynecol 1994; 171:380–3; discussion 383–4. [DOI] [PubMed] [Google Scholar]
- 15. Johnson MR, Riddle AF, Irvine R et al. Corpus luteum failure in ectopic pregnancy. Hum Reprod 1993; 8:1491–5. [DOI] [PubMed] [Google Scholar]
- 16. Meis PJ, Klebanoff M, Thom E et al. ; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003; 348:2379–85. [DOI] [PubMed] [Google Scholar]
- 17. Morris RK, Oliver EA, Malin G, Khan KS, Meads C. Effectiveness of interventions for the prevention of small-for-gestational age fetuses and perinatal mortality: a review of systematic reviews. Acta Obstet Gynecol Scand 2013; 92:143–51. [DOI] [PubMed] [Google Scholar]
- 18. Lamba H, Goldmeier D, Mackie NE, Scullard G. Antiretroviral therapy is associated with sexual dysfunction and with increased serum oestradiol levels in men. Int J STD AIDS 2004; 15:234–7. [DOI] [PubMed] [Google Scholar]
- 19. Papp E, Mohammadi H, Loutfy MR et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels, suggesting a potential mechanism contributing to fetal growth restriction. J Infect Dis 2015; 211:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papp E, Balogun K, Banko N et al. Low prolactin and high 20-α-hydroxysteroid dehydrogenase levels contribute to lower progesterone levels in HIV-infected pregnant women exposed to protease inhibitor-based combination antiretroviral therapy. J Infect Dis 2016; 213:1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simon A, Warszawski J, Kariyawasam D et al. ; ANRS French Perinatal Cohort Study Group Association of prenatal and postnatal exposure to lopinavir-ritonavir and adrenal dysfunction among uninfected infants of HIV-infected mothers. JAMA 2011; 306:70–8. [DOI] [PubMed] [Google Scholar]
- 22. Cohan D, Natureeba P, Koss CA et al. Efficacy and safety of lopinavir/ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS 2015; 29:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Natureeba P, Ades V, Luwedde F et al. Lopinavir/ritonavir-based antiretroviral treatment (ART) versus efavirenz-based ART for the prevention of malaria among HIV-infected pregnant women. J Infect Dis 2014; 210:1938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brenner WE, Edelman DA, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynecol 1976; 126:555–64. [DOI] [PubMed] [Google Scholar]
- 25. Jiménez-Nácher I, Alvarez E, Morello J, Rodriguez-Nóvoa S, de Andrés S, Soriano V. Approaches for understanding and predicting drug interactions in human immunodeficiency virus-infected patients. Expert Opin Drug Metab Toxicol 2011; 7:457–77. [DOI] [PubMed] [Google Scholar]
- 26. Croxtall JD, Perry CM. Lopinavir/ritonavir: a review of its use in the management of HIV-1 infection. Drugs 2010; 70:1885–915. [DOI] [PubMed] [Google Scholar]
- 27. Xiang J, Wang Y, Su K et al. Ritonavir binds to and downregulates estrogen receptors: molecular mechanism of promoting early atherosclerosis. Exp Cell Res 2014; 327:318–30. [DOI] [PubMed] [Google Scholar]
- 28. Balogun K, Guzman Lenis M, Papp E et al. Elevated levels of estradiol in human immunodeficiency virus-infected pregnant women on protease inhibitor-based regimens 2017; 10.1093/cid/cix761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sikora MJ, Rae JM, Johnson MD, Desta Z. Efavirenz directly modulates the oestrogen receptor and induces breast cancer cell growth. HIV Med 2010; 11:603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salas SP, Marshall G, Gutiérrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 2006; 47:203–8. [DOI] [PubMed] [Google Scholar]
- 31. Thoumsin HJ, Alsat E, Cedard L. In vitro aromatization of androgens into estrogens in placental insufficiency. Gynecol Obstet Invest 1982; 13:37–43. [DOI] [PubMed] [Google Scholar]
- 32. Hu XL, Feng C, Lin XH et al. High maternal serum estradiol environment in the first trimester is associated with the increased risk of small-for-gestational-age birth. J Clin Endocrinol Metab 2014; 99:2217–24. [DOI] [PubMed] [Google Scholar]
- 33. Jin M, Lv PP, Yu TT, Shen JM, Feng C, Huang HF. IGFBP1 involved in the decreased birth weight due to fetal high estrogen exposure in mice. Biol Reprod 2016; 95:96. [DOI] [PubMed] [Google Scholar]
- 34. Meng Y, Lv PP, Ding GL et al. High maternal serum estradiol levels induce dyslipidemia in human newborns via a hepatic HMGCR estrogen response element. Sci Rep 2015; 5:10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 2003; 111:994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Virgo BB, Bellward GD. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology 1974; 95:1486–90. [DOI] [PubMed] [Google Scholar]
- 37. Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab 2012; 97:E719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuijper EA, Ket JC, Caanen MR, Lambalk CB. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online 2013; 27:33–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.