HIV infection increases the risk of developing tuberculosis (TB), but our understanding of HIV’s impact on risk of mortality for children treated for TB is limited. We aimed to identify predictors of mortality in children treated for drug-susceptible TB.
Keywords: tuberculosis, childhood, mortality, outcomes
Abstract
Background
Tuberculosis (TB) remains a leading cause of death in children globally. It is recognized that human immunodeficiency virus (HIV) infection increases the risk of developing TB, but our understanding of the impact of HIV on risk of mortality for children treated for TB is limited. We aimed to identify predictors of mortality in children treated for drug-susceptible TB.
Methods
A retrospective analysis of all children (<15 years of age) routinely treated between 2005 and 2012 for drug-susceptible TB in Cape Town was conducted using the programmatic electronic TB treatment database. Survival analysis using Cox regression was used to estimate hazard ratios for death. Logistic regression was used to estimate the odds of unfavorable outcomes.
Results
Of 29519 children treated for and notified with TB over the study period, <1% died during TB treatment and 89.5% were cured or completed treatment. The proportion of children with known HIV status increased from 13% in 2005 to 95% in 2012. Children aged <2 years had an increased hazard of death (adjusted hazard ratio [aHR], 3.13; 95% confidence interval [CI], 1.78–5.52) and greater odds of unfavorable outcome (adjusted odds ratio [aOR], 1.44; 95% CI, 1.24–1.66) compared with children aged 10–14 years. HIV-infected children had increased mortality compared to HIV-negative children (aHR, 6.85; 95% CI, 4.60–10.19) and increased odds of unfavorable outcome (aOR, 2.01; 95% CI, 1.81–2.23). Later year of TB treatment was a protective predictor for both mortality and unfavorable outcome.
Conclusions
We demonstrate a dramatic improvement in HIV testing in children with TB over time and excellent overall treatment outcomes. HIV infection and young age were associated with increased risk of death and unfavorable outcome.
The World Health Organization (WHO) estimated that 1 million children (<15 years of age) developed tuberculosis (TB) in 2015 with a mortality of 210000 [1]. This makes TB one of the most significant global causes of death in children. As only a third of the children estimated to have TB are identified, diagnosed, and notified [2], it is likely that a large proportion of the mortality is due to untreated TB. Although far more can be done to improve case detection, many children do not survive even when started on appropriate TB treatment. Understanding risk factors for death in children treated for TB would allow more focused interventions to support these children once diagnosed.
In high-TB-burden settings, the majority of children demonstrate evidence of Mycobacterium tuberculosis infection before reaching adulthood [3]. However, once infected with M. tuberculosis, children <2 years of age are at the highest risk of progressing to TB disease, with severe and disseminated forms of disease, including TB meningitis, also more frequently seen in this age group [4]. These forms of disease are associated with high rates of mortality, even if diagnosed and appropriately treated [5].
The WHO estimates that nearly 100000 children die from TB in Africa each year, and that about one-third are human immunodeficiency virus (HIV) infected. It is well established that HIV increases the risk of a child developing TB [6, 7], but our understanding of the impact of HIV on the risk of mortality for children who are treated for TB disease is incomplete. There are few studies documenting outcomes for children with drug-susceptible TB, and age-disaggregated mortality data are not easily available. Operational studies in Africa and Asia have identified HIV infection and young age as risk factors for mortality in children [8, 9], but these are limited by small numbers and short study durations. This study aimed to review TB treatment outcomes and identify predictors of mortality among all children routinely treated for drug-susceptible TB at the community level, in Cape Town, South Africa, between 2005 and 2012.
METHODS
Setting
This retrospective cohort study was conducted in the City of Cape Town, Western Cape, South Africa. According to a 2011 population estimate, 25% of the total population of 3.7 million people in Cape Town was <15 years of age [10]. In 2012, South Africa reported the highest notification rate of TB in the world at 993 per 100000 population, and Cape Town reported a TB incidence of 741 per 100000 and an overall HIV prevalence of 19.8% (estimated using antenatal survey) [11]. Children (<15 years) accounted for 13.3% of notified TB cases in Cape Town between 2009 and 2012 [12]. TB care in Cape Town is decentralized, with 103 primary healthcare facilities offering outpatient TB treatment. Clinical history taking, examination, tuberculin skin test, and chest radiographs are implemented according to national guidelines for the diagnosis of TB in children [13]. Sputum testing, including smear microscopy and mycobacteria growth indicator tube culture techniques, were available but not routinely conducted for children, and the use of the Xpert MTB/RIF test (Cepheid, Sunnyvale, California) was not available during the study period [13].
Study Population and Data Sources
An electronic TB register (ETR.net) is routinely completed at a subdistrict level from collated facility-based paper TB treatment registers. Fields captured include patient-specific details (age, sex, address), disease-specific details (site of disease, sputum smear results, treatment regimen, HIV status, and CD4 count), and TB treatment outcome (treatment completed, cured, loss to follow-up, died, failed). All patients treated for TB should be recorded in the facility-based register even if the diagnosis and treatment initiation took place at a hospital; hospitals generally do not function as TB reporting units in this setting. A separate register is maintained for drug-resistant TB reporting (EDRWeb). All children (<15 years) who started treatment for presumed or confirmed drug-susceptible TB between 1 January 2005 and 30 June 2012 and who were recorded in ETR.net for the City of Cape Town District were included. The cutoff of 15 years was used to be consistent with the age category used for notification data nationally and by WHO.
Definitions
Retreatment cases were defined as children having previously received >4 weeks of TB treatment, regardless of the time since their previous episode or outcome. Patients were recorded as sputum smear positive if a sputum specimen, taken prior to TB treatment, was noted as 1+, 2+, or 3+ for acid-fast bacilli on microscopy. Children were classified as having either pulmonary TB (PTB) alone or having any extrapulmonary TB (EPTB), which may have included isolated EPTB or a combination of PTB and EPTB. Primary TB referred to first infection with M. tuberculosis, as typically seen in children with nonsevere disease, classified by a treating clinician. This usually included children with documented exposure and minimal disease seen on chest radiograph. Children were considered HIV infected if they had any of the following recorded: a positive HIV result, a CD4 result, or were receiving either antiretroviral medication or cotrimoxazole prophylaxis. TB treatment outcomes included cured, completed, died, loss to follow-up, failed, moved, or transferred out (outcome 1). “Death” referred to mortality due to any cause before the end of TB treatment. Two additional outcome classifications were created for analysis. A binary mortality indicator (outcome 2) classified all nonsurviving children as “died,” whereas children who were cured, completed treatment, or failed treatment but were alive at the end of treatment were classified as “alive.” This definition excluded children with unknown outcomes or those who moved, transferred, or were lost to follow-up. A binary classification (outcome 3) was created defining children who were cured or completed treatment as “favorable” and all other outcomes as “unfavorable.”
Data Collection
Data were exported from ETR.net per subdistrict and combined into a single database. Personal identifiers were included for matching and exclusion of duplicate entries, and subsequently removed. The data was analyzed using SAS software (SAS Institute, Cary, North Carolina). Ethical approval and a waiver of individual informed consent were received from the Stellenbosch University Health Research Ethics Committee (S12/01/018), and permission was obtained from the City of Cape Town Health Directorate.
Statistical Analysis
Descriptive statistics for demographic and clinical variables were calculated and analyzed by HIV status. Missing data were excluded from analysis except for HIV, where unknown HIV status was included as a separate category. All variables were analyzed categorically using frequencies and percentages. Age was stratified into the following bands: <2 years, 2–4 years, 5–9 years, and 10–14 years. Time to death was calculated as the time in days between TB treatment initiation and documented date of death. Patients were censored from analysis at 273 days after TB treatment initiation (9 months) or at the time of their death, whichever occurred first. Kaplan-Meier survival curves were generated for time to death by HIV status and age category. Graphical testing of the proportional hazards assumption was conducted using the log likelihood of survival and person years, across the strata of HIV categories (positive, negative, and unknown). A Cox proportional hazards model was used to determine the unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for epidemiological and clinical predictors of death. Odds ratios (ORs) and 95% CIs for predicting unfavorable outcomes were estimated using generalized estimating equations with logit link. Predictors were added incrementally with attention to the change in significance of each model to produce a final multivariable model, which allowed the reporting of adjusted HRs and ORs.
RESULTS
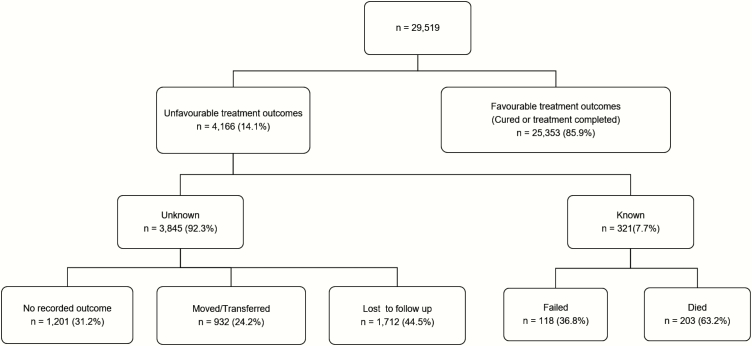
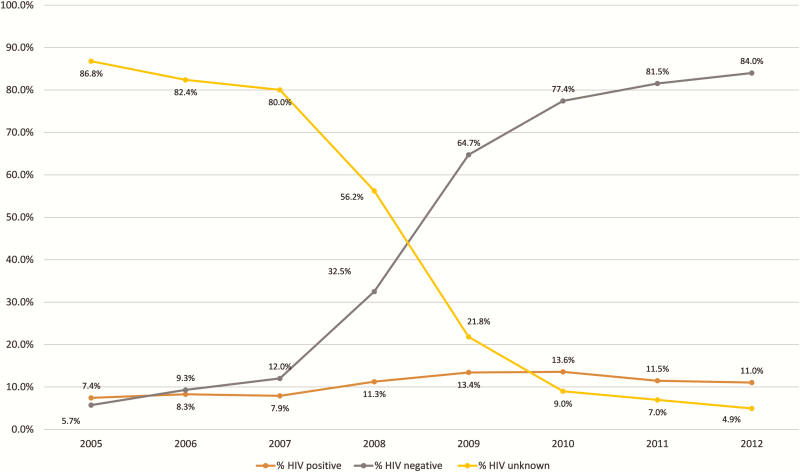
Between 1 January 2005 and 30 June 2012, 29519 children aged <15 years were recorded in the ETR.net (Figure 1). Sex proportions were approximately equal; 70% of children were <5 years of age (Table 1). Less than 14% of children had sputum smear results but of those who did, almost 60% were sputum smear positive (Table 1). The majority of children treated (92.5%) did not have any EPTB and almost 80% had primary TB (Table 1). Overall, only 55% of children had known HIV status (Table 1). However, when disaggregated by year of treatment, >90% of children had a recorded HIV status after 2010, with 95.1% having known HIV status in 2012 (Table 2 and Figure 2). Treatment outcomes were recorded in 95.9% of children, although this included children who were lost to follow-up, moved, transferred, or failed TB treatment in which the survival outcome was unknown (Figure 1). Less than 1% of children with recorded treatment outcomes died during TB treatment and 89.5% were cured or completed TB treatment (Table 1).
Figure 1.
Overview of treatment outcomes of children 0–14 years of age routinely treated for tuberculosis between 1 January 2005 and 30 June 2012 in Cape Town, South Africa.
Table 1.
Demographic Characteristics of Children Treated for Tuberculosis Between 1 January 2005 and 30 June 2012 in Cape Town, South Africa (N = 29519)
| Characteristic | Variable | Total, No. (%) |
|---|---|---|
| Age (n = 29519) | <2 y | 10100 (34.2) |
| 2–4 y | 10575 (35.8) | |
| 5–9 y | 5477 (18.6) | |
| 10–14 y | 3367 (11.4) | |
| Sex (n = 29519) | Male | 14885 (50.4) |
| Female | 14634 (49.6) | |
| Type of treatment (n = 29519) | New | 28701 (97.2) |
| Re-treatment | 818 (2.8) | |
| Smear status (n = 3839) | Positive | 2243 (58.4) |
| Negative | 1596 (41.6) | |
| Site of TB disease (n = 29518) | PTB alone | 27229 (92.3) |
| Any EPTB | 2289 (7.8) | |
| Disease type (n = 29487) | Primary | 23437 (79.5) |
| Nonprimary | 6050 (20.5) | |
| HIV status (n = 29519) | Positive | 3143 (10.7) |
| Negative | 13162 (44.6) | |
| Unknowna | 13214 (44.8) | |
| Year of TB treatment (n = 29519) | 2005 | 3519 (11.9) |
| 2006 | 3825 (13) | |
| 2007 | 3784 (12.8) | |
| 2008 | 4132 (14) | |
| 2009 | 4110 (13.9) | |
| 2010 | 4319 (14.6) | |
| 2011 | 3928 (13.3) | |
| 2012b | 1902 (6.4) | |
| Treatment outcome 1 (n = 28318) | Cured/completed | 25353 (89.5) |
| Died | 203 (0.7) | |
| Moved/transferred | 932 (3.3) | |
| Lost to follow-up | 1712 (6.1) | |
| Failed | 118 (0.4) | |
| Treatment outcome 2c (n = 25674) | Alived | 25471 (99.2) |
| Died | 203 (0.8) | |
| Treatment outcome 3 (n = 29519) | Unfavorablee | 4166 (14.1) |
| Favorablef | 25353 (85.9) |
Abbreviations: EPTB, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; PTB, pulmonary tuberculosis; TB, tuberculosis.
aMissing data were recorded as unknown and excluded from analysis except for HIV, where HIV unknown is included as a separate category.
bYear of treatment includes only 6 months of data for 2012.
cExcludes children with unknown outcomes or those who moved, transferred, or were lost to follow-up during the course of treatment where survival status was unknown.
dAlive includes all children who were cured, completed, or failed treatment.
eAll children who failed, died, moved, transferred, were lost to follow-up, or not evaluated.
fAll children who were cured or completed treatment.
Table 2.
Demographic Characteristics of Children Treated for Tuberculosis Between 1 January 2005 and 30 June 2012 in Cape Town, South Africa, Stratified by Human Immunodeficiency Virus status (N = 29519)
| Characteristic | Variable | HIV Infected | HIV Uninfected | HIV Status Unknowna |
|---|---|---|---|---|
| Total | 3143 (10.7) | 13162 (44.6) | 13214 (44.8) | |
| Age (n = 29519) | <2 y | 983 (31.3) | 4414 (33.5) | 4703 (35.6) |
| 2–4 y | 846 (26.9) | 5018 (38.1) | 4711 (35.7) | |
| 5–9 y | 839 (26.7) | 2134 (16.2) | 2504 (19) | |
| 10–14 y | 475 (15.1) | 1596 (12.1) | 1296 (9.8) | |
| Sex (n = 29519) | Male | 1529 (48.7) | 6626 (50.3) | 6730 (50.9) |
| Female | 1614 (51.4) | 6536 (49.7) | 6484 (49.1) | |
| Type of TB treatment (n = 29519) | New | 2892 (92) | 12880 (97.9) | 12929 (97.8) |
| Re-treatment | 251 (8) | 282 (2.1) | 285 (2.2) | |
| Smear status (n = 3839) | Positive | 193 (27.8) | 855 (44.7) | 548 (44.5) |
| Negative | 502 (72.2) | 1058 (55.3) | 683 (55.5) | |
| Site of TB disease (n= 29518) | PTB alone | 2838 (90.3) | 12273 (93.3) | 12118 (91.7) |
| Any EPTB | 305 (9.7) | 888 (6.8) | 1096 (8.3) | |
| TB disease type (n = 29487) | Primary | 2265 (72.1) | 10550 (80.3) | 10622 (80.5) |
| Nonprimary | 876 (27.9) | 2596 (19.8) | 2578 (19.5) | |
| Outcome 1 (n = 28318) | Cured/completed | 2454 (83.4) | 11699 (92.1) | 11200 (88.4) |
| Died | 78 (2.7) | 41 (0.3) | 84 (0.7) | |
| Moved/transferred | 123 (4.2) | 342 (2.7) | 467 (3.7) | |
| Lost to follow-up | 269 (9.1) | 577 (4.5) | 866 (6.8) | |
| Failed | 19 (0.7) | 51 (0.4) | 48 (0.4) | |
| Outcome 2b (n = 25674) | Alivec | 2865 (97.4) | 12669 (99.7) | 12581 (99.3) |
| Died | 78 (2.7) | 41 (0.3) | 84 (0.7) | |
| Outcome 3 (n = 29519) | Unfavorabled | 689 (21.9) | 1463 (11.1) | 2014 (15.2) |
| Favorablee | 2454 (78.1) | 11699 (88.9) | 11200 (84.8) | |
| Yearf (n = 29519) | 2005 | 262 (7.4) | 202 (5.7) | 3055 (86.8) |
| 2006 | 317 (8.3) | 356 (9.3) | 3152 (82.4) | |
| 2007 | 300 (7.9) | 455 (12.0) | 3029 (80.0) | |
| 2008 | 465 (11.3) | 1343 (32.5) | 2324 (56.2) | |
| 2009 | 552 (13.4) | 2661 (64.7) | 897 (21.8) | |
| 2010 | 586 (13.6) | 3344 (77.4) | 389 (9.0) | |
| 2011 | 451 (11.5) | 3203 (81.5) | 274 (7.0) | |
| 2012 | 210 (11.0) | 1598 (84.0) | 94 (4.9) |
Data are presented as No. (%).
Abbreviations: EPTB, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; PTB, pulmonary tuberculosis; TB, tuberculosis.
aMissing data were recorded as unknown and excluded from analysis except for HIV, where HIV unknown is included as a separate category.
bExcludes children with unknown outcomes or those who moved, transferred, or were lost to follow-up during the course of treatment where survival status was unknown.
cAlive includes all children who were cured, completed, or failed treatment.
dAll children who failed, died, moved, transferred, were lost to follow-up, or not evaluated.
eAll children who were cured or completed treatment.
fFor year of treatment, the percentages are calculated and shown per year to demonstrate the change in HIV status over time.
Figure 2.
Changes in human immunodeficiency virus (HIV) testing and recording of children routinely treated for tuberculosis between 1 January 2005 and 30 June 2012 in Cape Town, South Africa.
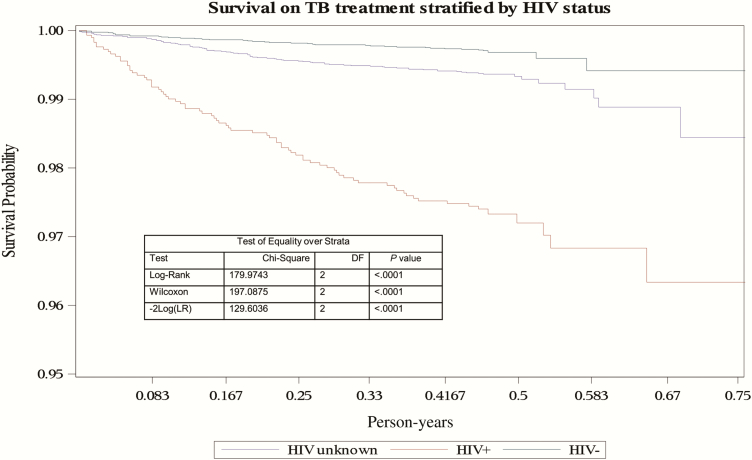
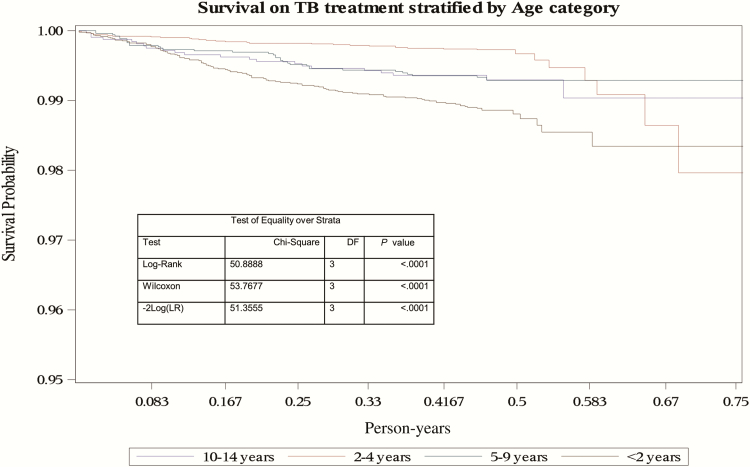
In multivariable analysis for predictors of death, age, HIV status, the presence of disseminated TB, type of TB disease, and year of treatment were included. Children aged <2 years had a higher rate of death (aHR, 3.13; 95% CI, 1.78–5.52) than children aged 10–14 years (Table 3). HIV-infected children had an increased death rate compared with HIV-uninfected children (aHR, 6.85; 95% CI, 4.60–10.19), and treatment from 2007 onward was associated with a lower rate of death, regardless of HIV status (Table 3). Figure 3 demonstrates the rapid reduction in the probability of survival in HIV-infected children, with children with unknown HIV status also showing a more rapid decline in survival compared with the HIV-uninfected children after the first month of treatment. Figure 4 demonstrates the more rapid decline in survival probability in children <2 years of age throughout the 6-month period of treatment.
Table 3.
Crude and Adjusted Model for Predicting Hazard Ratio of Death for Children Treated for Tuberculosis Between 1 January 2005 and 30 June 2012 in Cape Town, South Africa, Using Cox Regression
| Characteristic | Variable | HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|---|
| aAge | <.001 | <.001 | |||
| <2 y | 1.80 (1.14–2.85) | .01 | 3.13 (1.78–5.52) | <.001 | |
| 2–4 y | 0.51 (.30–.86) | .01 | 0.94 (.50–1.76) | .84 | |
| 5–9 y | 0.99 (.58–1.68) | .96 | 1.25 (.71–2.22) | .44 | |
| 10–14 y | Ref | ||||
| Sex | Male | Ref | |||
| Female | 1.07 (.81–1.41) | .64 | |||
| Type of TB treatmentb | New | Ref | |||
| Re-treatment | 2.49 (1.44–4.33) | <.01 | |||
| aHIV status | <.001 | <.001 | |||
| Unknown | 2.09 (1.44–3.03) | <.001 | 1.43 (.91–2.24) | .12 | |
| Positive | 8.31 (5.69–12.15) | <.001 | 6.85 (4.60–10.19) | <.001 | |
| Negative | Ref | ||||
| Smear statusc | Positive | Ref | |||
| Negative | 2.45 (1.11–5.39) | .03 | |||
| aSite of TB diseasec | PTB alone | Ref | |||
| Any EPTB | 1.96 (1.32–2.91) | <.001 | 1.39 (.82–2.35) | .22 | |
| aTB disease typeb | Primary | Ref | |||
| Nonprimary | 1.52 (1.12–2.06) | <.01 | 1.63 (1.00–2.66) | .05 | |
| aYear | <.001 | <.01 | |||
| 2005 | Ref | ||||
| 2006 | 0.87 (.56–1.35) | .53 | 0.86 (.56–1.33) | .50 | |
| 2007 | 0.45 (.26–.77) | <.01 | 0.47 (.27–.80) | <.01 | |
| 2008 | 0.51 (.31–.84) | <.01 | 0.49 (.30–.81) | <.01 | |
| 2009 | 0.55 (.34–.89) | .02 | 0.52 (.31–.88) | .01 | |
| 2010 | 0.57 (.35–.92) | .02 | 0.59 (.35–.99) | .05 | |
| 2011 | 0.34 (.19–.61) | <.001 | 0.39 (.21–.72) | <.01 | |
| 2012 | 0.22 (.09–.56) | <.01 | 0.25 (.10–.66) | <.01 |
Abbreviations: CI, confidence interval; EPTB, extrapulmonary tuberculosis; HR, hazard ratio; HIV, human immunodeficiency virus; PTB, pulmonary tuberculosis; TB, tuberculosis.
aVariable used in final/adjusted multivariable Cox regression model.
b,cDue to the likelihood of collinearity, only 1 of each of these variables was included in the final model.
Figure 3.
Kaplan-Meier curve of survival on tuberculosis (TB) treatment stratified by human immunodeficiency virus status of children routinely treated for TB between 1 January 2005 and 30 June 2012 in Cape Town, South Africa. Abbreviations: DF, degrees of freedom; HIV, human immunodeficiency virus; LR, likelihood ratio; TB, tuberculosis.
Figure 4.
Kaplan-Meier curve of survival on tuberculosis (TB) treatment stratified by age category of children routinely treated for TB between 1 January 2005 and 30 June 2012 in Cape Town, South Africa. Abbreviations: DF, degrees of freedom; LR, likelihood ratio; TB, tuberculosis.
In analysis for predictors of unfavorable treatment outcome, age, HIV status, the presence of disseminated TB, type of disease, and year of treatment were included. Children <2 years had an increased odds of unfavorable outcome (aOR, 1.44; 95% CI, 1.24–1.66) compared to children aged 10–14 years (Table 4). The presence of any EPTB (aOR, 1.38; 95% CI, 1.20–1.59), treatment for nonprimary TB (aOR, 1.15; 95% CI, 1.01–1.31) and HIV-infected status (aOR, 2.01; 95% CI, 1.81–2.23) were also associated with unfavorable outcome (Table 4). Later year of treatment was associated with lower odds of unfavorable outcome (Table 4).
Table 4.
Crude and Adjusted Model of Unfavorable Outcomes for Children Treated for Tuberculosis Between 1 January 2005 and 30 June 2012 in Cape Town, South Africa, Using Logistic Regression
| Characteristic | Variable | OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|---|
| aAge | <.001 | <.001 | |||
| <2 y | 1.14 (1.03–1.28) | .02 | 1.44 (1.24–1.66) | <.001 | |
| 2–4 y | 0.83 (.74–.93) | <.01 | 1.06 (.92–1.23) | .43 | |
| 5–9 y | 0.88 (.78–1.00) | .04 | 0.99 (.86–1.14) | .88 | |
| 10–14 y | Ref | ||||
| Sex | Male | Ref | |||
| Female | 1.00 (.93–1.07) | .94 | |||
| Type of TB treatmentb | New | Ref | |||
| Re-treatment | 2.40 (2.05–2.81) | <.001 | |||
| aHIV status | <.001 | <.001 | |||
| Unknown | 1.44 (1.34–1.55) | <.001 | 1.06 (.97–1.17) | .22 | |
| Positive | 2.25 (2.03–2.48) | <.001 | 2.01 (1.81–2.23) | <.001 | |
| Negative | Ref | ||||
| Smear statusc | Positive | Ref | |||
| Negative | 1.14 (.94–1.37) | .18 | |||
| aSite of TB diseasec | PTB alone | Ref | |||
| Any EPTB | 1.54 (1.38–1.71) | <.001 | 1.38 (1.20–1.59) | <.001 | |
| aTB disease typeb | Primary | Ref | |||
| Nonprimary | 1.21 (1.12–1.31) | <.001 | 1.15 (1.01–1.31) | .03 | |
| aYear | <.001 | <.001 | |||
| 2005 | Ref | ||||
| 2006 | 0.83 (.74–.94) | <.01 | 0.83 (.74–.94) | <.01 | |
| 2007 | 0.78 (.69–.88) | <.001 | 0.78 (.69–.88) | <.001 | |
| 2008 | 0.74 (.66–.83) | <.001 | 0.74 (.65–.83) | <.001 | |
| 2009 | 0.60 (.53–.68) | <.001 | 0.59 (.52–.68) | <.001 | |
| 2010 | 0.58 (.51–.66) | <.001 | 0.58 (.51–.67) | <.001 | |
| 2011 | 0.52 (.46–.59) | <.001 | 0.53 (.46–.62) | <.001 | |
| 2012 | 0.52 (.44–.61) | <.001 | 0.54 (.45–.64) | <.001 |
Abbreviations: CI, confidence interval; EPTB, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; OR, odds ratio; PTB, pulmonary tuberculosis; TB, tuberculosis.
aVariable used in final/adjusted multivariable Cox regression model.
b,cDue to the likelihood of collinearity, only 1 of each of these variables was included in the final model.
DISCUSSION
The evaluation and interpretation of routine data was identified in 2009 as a priority for South Africa’s public health response to TB and HIV [14]. This analysis represents by far the largest single reported cohort of children treated for TB, with almost 30000 children included over a period of >7 years. Completeness of documentation was excellent.
In low-TB-incidence settings, 30%–40% of childhood TB has been reported to occur in children aged <5 years [15–17]. In this study, approximately 70% of children with TB were <5 years of age. Although it is possible that this may reflect some degree of overtreatment [18], it is likely that this high proportion reflects the vulnerability of this age group to TB disease progression. The proportion of children with any EPTB was 7.6%, much lower than is seen in other cohorts [19]. A previous study from Cape Town showed at least 40% underreporting of culture-confirmed TB in children. This included many children with severe forms of disease, such as TB meningitis, that are commonly referred to hospitals for investigations and treatment [20]. Another explanation for the low proportion of EPTB in this cohort includes the classification of intrathoracic lymph node TB as PTB rather than EPTB. It may also reflect healthcare workers diagnosing and treating TB at an earlier stage in the disease pathogenesis, prior to more severe (and extrapulmonary) forms of disease developing.
Overall, excellent treatment outcomes were demonstrated; 85.9% of children had a favorable outcome, a high proportion considering that all unknown outcomes (including those who moved or transferred) were classified as unfavorable. We found a mortality rate in children treated for TB of <1% (0.32% among HIV-uninfected children and 2.7% among HIV-infected children). This is similar to death rates reported in low-TB-incidence settings [15, 17] as well as a pooled estimate from a recent meta-analysis from low-HIV-prevalence settings [21]. In regions of high TB and HIV prevalence, mortality for children on TB treatment has varied from 3.3% to 17% [8, 9, 16, 19, 21–23]. This wide range is reflective of differences in setting (inpatient and outpatient care) as well as date of study, with some taking place prior to widespread HIV treatment. Loss to follow-up during TB treatment was 6.1% in our study. This is consistent with previous reports of children with TB in other countries in Africa [9, 22].
In our cohort, HIV positivity and age <2 years were independent risk factors for death. This finding is similar to previous studies where HIV positivity [21, 24, 25] and age <5 years [3, 23] were associated with an increased likelihood of death. Unknown HIV status, TB meningitis, and sputum smear–positive PTB have been reported in previous studies as risk factors for death in children on TB treatment [8, 9]. Although the presence of EPTB and unknown HIV status were associated with an increased risk of death in univariate analysis, they did not remain significant in multivariable analysis. For predictors of unfavorable outcomes, age <2 years, HIV positivity, the presence of EPTB, and nonprimary TB were associated with unfavorable outcome.
The proportion of children tested for HIV increased over the study period. By 2012, >95% of children treated for TB had an HIV test result recorded (Figure 2). Although the overall proportion of children with HIV has remained relatively unchanged, the proportion of positive tests among children with known HIV status has fallen. This is likely to be a function of a decrease in HIV prevalence among children in the Western Cape as a result of effective prevention of mother-to-child transmission (PMTCT) as well as only high-risk children being tested for HIV in the earlier years of the study period. Later year of treatment was found to be protective for death and unfavorable outcome while on TB treatment. This is likely to be due in large part to the change in government response to the interlinked HIV and TB epidemics. Over the study period, funding significantly increased for the expansion of antiretroviral therapy (ART), scaling up of PMTCT, the promotion of HIV and TB treatment integration, and increased investments in HIV prevention [26]. This scale-up specifically improved access for children with TB and HIV coinfection by expanding the CD4 count threshold at which children could receive ART and by making ART available to all those with TB as well as all children <1 year of age [27]. The improved TB outcomes seen with each incremental year in our study are most likely attributable to the impact of these changes, as during this period the TB management of children had remained unchanged, including diagnostic strategies and treatment regimens [13].
Data completion was excellent for most fields, with missing data in some. This included tuberculin skin test usage, chest radiograph results, or the basis for a clinical diagnosis. Details of HIV treatment or the provision of cotrimoxazole prophylaxis was not available in the electronic TB register, highlighting an opportunity for further improvement in the integration of TB/HIV services. Pilot programs have been initiated in the Western Cape to implement a 3-tier monitoring system at the country level for pre-ART wellness, ART, TB, and mother and child health services to ensure harmonization and accurate monitoring of services [28]. It is expected that integrated monitoring systems will mitigate the limitations we have seen in this study. The overall number of children with unknown HIV status was high, and as exclusion may have led to bias, these children were included in all analyses as a separate group. We used the date of death in the register for analysis; linkage to a vital statistics or mortality register was not done to verify these dates. Additional variables such as nutritional status, vaccination records, or opportunistic infections are not recorded in ETR.net. Although the protective effect of BCG vaccination against disseminated disease is well documented [29–31], we had no information on BCG vaccination status. However, BCG vaccination is routinely given at birth to all infants and coverage was estimated to be 84% across South Africa in 2012 [32]. Our study defined death as a child who died before the end of TB therapy and did not differentiate death due to TB from other causes. Records reviews and/or postmortem studies to more accurately document the causes of death would be required. This study only included outcomes for children recorded in TB treatment registers, and might underestimate mortality. Children with severe and disseminated forms of TB admitted to hospital may die before diagnosis or after diagnosis but prior to recording in ETR.net. Further research linking multiple data sources is therefore needed to estimate overall mortality for pediatric TB. Children who moved, transferred, or were lost to follow-up during treatment represented a large proportion of children with unfavorable outcomes. Classifying these children as having unfavorable outcomes will mean that favorable outcomes were underestimated. Finally, this study was restricted to 1 city in South Africa; it may not be possible to generalize findings to other settings.
Our study reports a large cohort of children treated for TB over a 7-year period in a setting with a high burden of TB and HIV. It has demonstrated significant improvement in HIV testing over time and excellent TB treatment outcomes among those reported to the TB program. The predominance of children treated for primary TB highlights the early diagnosis currently taking place in a high-burden setting. Specific higher-risk groups for mortality and unfavorable outcomes have been identified for further study and interventions.
Notes
Author contributions. M. O., K. L., A. C. H., and J. A. S. contributed to conception and design of the study. M. O. and R. D. undertook data extraction and matching. M. O. carried out statistical analysis. K. D. P. contributed toward data interpretation and setting-specific contextualization. M. O. and J. A. S. wrote a first draft of the article with subsequent critical input from all authors. A. C. H. and J. A. S. were co–senior authors. All authors approved the final version.
Acknowledgments. The authors acknowledge the City of Cape Town for access to the electronic databases.
Disclaimer. The contents are the responsibility of the author(s) and do not necessarily reflect the views of the US Agency for International Development (USAID).
Financial support. This work was supported by USAID (TREAT TB; cooperative agreement number GHN‐A‐00‐08‐00004‐00). M. O. was supported by the Columbia University–Southern African Fogarty AIDS International Training and Research Program, Implementation Science Scholarship Program funded by the US President’s Emergency Plan for AIDS Relief through the Fogarty International Center, National Institutes of Health (grant number D43 TW000231). The PhD from which this study emanated was funded by the Medical Research Council of South Africa in terms of the National Health Scholars Programme from funds provided for this purpose by the National Department of Health Public health Enhancement Fund.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report (2016). Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis 2016; 16:1193–201. [DOI] [PubMed] [Google Scholar]
- 3. Wood R, Liang H, Wu H et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis 2010; 14:406–12. [PMC free article] [PubMed] [Google Scholar]
- 4. Marais BJ, Gie RP, Schaaf HS et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 5. Chiang SS, Khan FA, Milstein MB et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:947–57. [DOI] [PubMed] [Google Scholar]
- 6. Hesseling AC, Cotton MF, Jennings T et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis 2009; 48:108–14. [DOI] [PubMed] [Google Scholar]
- 7. Dodd PJ, Prendergast AJ, Beecroft C, Kampmann B, Seddon JA. The impact of HIV and antiretroviral therapy on TB risk in children: a systematic review and meta-analysis. Thorax 2017; 72:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Russell GK, Merle CS, Cooke GS, Casas EC, Silveira da Fonseca M, du Cros P. Towards the WHO target of zero childhood tuberculosis deaths: an analysis of mortality in 13 locations in Africa and Asia. Int J Tuberc Lung Dis 2013; 17:1518–23. [DOI] [PubMed] [Google Scholar]
- 9. Hailu D, Abegaz WE, Belay M. Childhood tuberculosis and its treatment outcomes in Addis Ababa: a 5-years retrospective study. BMC Pediatr 2014; 14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. STASSA. Metropolitan municipality South Africa: Statistics. Available at: http://www.statssa.gov.za/?page_id=1021&id=city-of-cape-town-municipality. Accessed 2 February 2017.
- 11. Massyn N, Day C, Dombo M, Barron P, English R, Padarath A. District health barometer 2012/13. Durban, South Africa: Health Systems Trust, 2013. [Google Scholar]
- 12. Osman M, Hesseling AC, Beyers N et al. Routine programmatic delivery of isoniazid preventive therapy to children in Cape Town, South Africa. Public Health Action 2013; 3:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Department of Health. National tuberculosis management guidelines 2009. Pretoria, South Africa: Department of Health, 2009. [Google Scholar]
- 14. Abdool Karim SS, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet 2009; 374:921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dendup T, Dorji T, Edgnton ME et al. Childhood tuberculosis in Bhutan: profile and treatment outcomes. Public Health Action 2013; 3:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ade S, Harries AD, Trébucq A et al. The burden and outcomes of childhood tuberculosis in Cotonou, Benin. Public Health Action 2013; 3:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abubakar I, Laundy MT, French CE, Shingadia D. Epidemiology and treatment outcome of childhood tuberculosis in England and Wales: 1999-2006. Arch Dis Child 2008; 93:1017–21. [DOI] [PubMed] [Google Scholar]
- 18. Seddon JA, Jenkins HE, Liu L et al. Counting children with tuberculosis: why numbers matter. Int J Tuberc Lung Dis 2015; 19suppl 1:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harries AD, Hargreaves NJ, Graham SM et al. Childhood tuberculosis in Malawi: nationwide case-finding and treatment outcomes. Int J Tuberc Lung Dis 2002; 6:424–31. [PubMed] [Google Scholar]
- 20. du Preez K, Schaaf HS, Dunbar R et al. Incomplete registration and reporting of culture-confirmed childhood tuberculosis diagnosed in hospital. Public Health Action 2011; 1:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jenkins HE, Yuen CM, Rodriguez CA et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adejumo OA, Daniel OJ, Adebayo BI et al. Treatment outcomes of childhood TB in Lagos, Nigeria. J Trop Pediatr 2016; 62:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drobac PC, Shin SS, Huamani P et al. Risk factors for in-hospital mortality among children with tuberculosis: the 25-year experience in Peru. Pediatrics 2012; 130:e373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soeters M, de Vries AM, Kimpen JL, Donald PR, Schaaf HS. Clinical features and outcome in children admitted to a TB hospital in the Western Cape—the influence of HIV infection and drug resistance. S Afr Med J 2005; 95:602–6. [PubMed] [Google Scholar]
- 25. Mukadi YD, Wiktor SZ, Coulibaly IM et al. Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Côte d’Ivoire. AIDS 1997; 11:1151–8. [DOI] [PubMed] [Google Scholar]
- 26. Mayosi BM, Lawn JE, van Niekerk A, Bradshaw D, Abdool Karim SS, Coovadia HM; Lancet South Africa Team Health in South Africa: changes and challenges since 2009. Lancet 2012; 380:2029–43. [DOI] [PubMed] [Google Scholar]
- 27. Simelela NP, Venter WD. A brief history of South Africa’s response to AIDS. S Afr Med J 2014; 104:249–51. [DOI] [PubMed] [Google Scholar]
- 28. Osler M, Hilderbrand K, Hennessey C et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. J Int AIDS Soc 2014; 17:18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006; 367:1173–80. [DOI] [PubMed] [Google Scholar]
- 30. Mangtani P, Abubakar I, Ariti C et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014; 58:470–80. [DOI] [PubMed] [Google Scholar]
- 31. Roy A, Eisenhut M, Harris RJ et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ 2014; 349:g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. South Africa: WHO and UNICEF estimates of immunization coverage: 2015 revision. Geneva, Switzerland: WHO, 2016. [Google Scholar]