Summary
This study demonstrates that immune responses and side-effect profiles following diethylcarbamazine or ivermectin treatment of loiasis are similar. Changes in eosinophil counts and eosinophil activation posttreatment suggest that eosinophils play a key role in the posttreatment responses to both drugs.
Keywords: Loa loa, ivermectin, diethylcarbamazine, eosinophil, filariasis
Abstract
Background.
Severe adverse reactions have been observed in individuals with Loa loa infection treated with either diethylcarbamazine (DEC), the drug of choice for loiasis, or ivermectin (IVM), which is used in mass drug administration programs for control of onchocerciasis and lymphatic filariasis in Africa. In this study, posttreatment clinical and immunologic reactions were compared following single-dose therapy with DEC or IVM to assess whether these reactions have the same underlying pathophysiology.
Methods.
Twelve patients with loiasis and microfilarial counts <2000 mf/mL were randomized to receive single-dose DEC (8 mg/kg) or IVM (200 µg/kg). Clinical and laboratory assessments were performed at 4, 8, 24, 48, and 72 hours and 5, 7, 9, and 14 days posttreatment.
Results.
Posttreatment adverse events were similar following DEC or IVM, but peaked earlier in subjects who received DEC, consistent with a trend toward more rapid and complete microfilarial clearance in the DEC group. After a transient rise (post-IVM) or fall (post-DEC) in the first 24 hours posttreatment, the eosinophil count rose significantly in both groups, peaking at day 5 in the DEC group and day 9 in the IVM group. Serum interleukin 5 levels and eosinophil activation, as assessed by surface expression of CD69 and serum levels of eosinophil granule proteins, were increased posttreatment in both groups.
Conclusions.
Despite differences in eosinophil and lymphocyte counts during the first 24 hours posttreatment, the overall pattern of hematologic and immunologic changes suggest that posttreatment reactions following DEC and IVM share a common pathophysiology.
Clinical Trials Registration.
Loiasis is a filarial infection that is endemic in Central and West Africa. Infective Loa loa larvae, transmitted during the bite of an infected Chrysops vector, develop over months into adult worms that reside in the subcutaneous tissues. Microfilariae (mf) are released into the bloodstream, where they can reach levels >100000/mL. Although characteristic symptoms include migratory angioedema (Calabar swellings) and subconjunctival migration of an adult worm (eyeworm), most infected residents of endemic areas are asymptomatic despite high numbers of circulating microfilariae [1, 2].
Diethylcarbamazine citrate (DEC), the treatment of choice for loiasis, has activity against both microfilariae and adult worms. Although mild side effects are common, severe post-DEC reactions, including fatal encephalopathy, occur almost exclusively in patients with high microfilarial loads [3]. The anthelmintic drug ivermectin (IVM) is microfilaricidal in loiasis, and posttreatment reactions similar to those seen with DEC have occurred during IVM mass drug administration for onchocerciasis control in Loa-endemic areas of Africa [4]. In a prospective study in Cameroon, Loa loa microfilarial load was the major risk factor for serious reactions following IVM administration (odds ratio >1000 for subjects with >50000 mf/mL blood) [5]. These posttreatment reactions have hampered expansion of filariasis elimination programs to Loa-endemic areas.
The pathophysiology of posttreatment reactions in filariasis is poorly understood. However, because reactions following DEC and ivermectin are similar in nature and are both correlated with microfilarial count, it has been theorized that an immune response to antigen release from dying parasites underlies posttreatment reactions to both drugs. This hypothesis is supported by the demonstration of posttreatment increases in serum interleukin (IL) 5 and absolute eosinophil count (AEC) in patients with lymphatic filariasis and onchocerciasis [6–8]. Whereas increases in inflammatory cytokines, such as IL-6 and tumor necrosis factor alpha (TNF-α), have also been described [9], Loa loa does not harbor the intracellular bacterial endosymbiont, Wolbachia, found in other human filarial parasites and implicated in posttreatment reactions in these settings [10].
The aim of this study was to characterize and compare posttreatment responses following single-dose DEC or IVM in subjects with Loa loa microfilaremia. A better understanding of the pathophysiology of these posttreatment reactions should help in the development of strategies to prevent adverse treatment outcomes.
MATERIALS AND METHODS
Study Site and Enrollment
The study was conducted approximately 150 km from Yaoundé, Cameroon, in the town of Akonolinga and 5 surrounding villages (Abem, Mimbama, Ngole, Mbiele, and Nlobole), which are endemic for loiasis with a prevalence of Loa loa microfilaremia of 28% (Kamgno et al, unpublished data), but not for onchocerciasis or lymphatic filariasis. The study was approved by the Cameroonian National Ethics Committee and the Institutional Review Board of the National Institute of Allergy and Infectious Diseases (NCT 01593722). Individual written consent was obtained from all participants after the study was explained in French.
Otherwise healthy, nonpregnant adult subjects with circulating Loa loa microfilariae (20–2000 mf/mL) were eligible for the study. Subjects were excluded if they had a positive pregnancy test, chronic kidney or liver disease, hemoglobin <10 mg/dL, potential coinfection with Onchocerca volvulus or Wuchereria bancrofti, or had taken DEC or IVM within the past 6 months or immunosuppressive medications within the past month. Based on a prescreening questionnaire, 92 subjects underwent midday calibrated thick smear for quantification of Loa loa microfilariae. Among the 30 subjects with Loa loa microfilaremia, 15 subjects completed the screening evaluation (medical history and physical examination, complete blood count with differential, urinalysis, and measurement of serum creatinine, alanine aminotransferase [ALT], aspartate aminotransferase [AST], and bilirubin), of which 12 were enrolled in the study (Supplementary Figure 1).
Study Objectives
The primary objective of this pilot study was to compare posttreatment responses following administration of single-dose DEC or IVM to subjects with loiasis and low levels of microfilaremia. The secondary objective was to assess the rapidity of microfilarial clearance in response to single-dose DEC or IVM.
Study Design
Eligible subjects were stratified into 2 groups based on the screening AEC before block randomization to single-dose DEC (8 mg/kg) or IVM (200 µg/kg). Subjects were admitted to Akonolinga Health District Hospital for 3 days following study drug administration. Subsequent evaluations were performed at a central village location. A standardized symptom questionnaire, physical examination, routine laboratory testing, and research studies were performed at baseline and at 4, 8, 24, 48, and 72 hours and 5, 7, 9, and 14 days posttreatment. New or worsening symptoms, physical examination findings, and laboratory abnormalities were considered adverse events (AEs) and were scored using a modification of the Common Terminology Criteria for Adverse Events version 4.0 (Supplementary Table 1). At the day 14 visit, subjects in the IVM group were treated with single-dose DEC (8 mg/kg), as only DEC has efficacy against adult worms. Subjects in the DEC arm received IVM (200 µg/kg) at day 14 due to the high prevalence of intestinal helminthiasis in this group.
Clinical Laboratory Assessments
Complete blood counts (CBC), serum chemistries (creatinine, ALT, AST, total bilirubin), and urinalysis were performed in the central laboratory at Akonolinga Hospital or a private laboratory in Yaounde (GT Labo). Absolute counts were calculated from the complete blood count results and the differential count from a Giemsa-stained thin blood smear. Loa loa and Mansonella perstans microfilarial counts were assessed by Nuclepore filtration of 1 mL of anticoagulated blood drawn between 11 am and 2 pm [11]. Wuchereria bancrofti circulating antigen was assessed by immunochromatographic card test (BinaxNOW Filariasis, Binax).
Serum Mediator Analysis
Serum levels of eosinophil granule proteins, cytokines, and chemokines were measured by suspension array multiplex immunoassays, using a previously described assay [12] and Millipore kits (Milliplex MAP Human T-Helper 17 Cytokine Panel and Cytokine/Chemokine Panels I and II, EMD Millipore). Specific mediators measured and minimal detectable levels are given in the Supplementary Data.
Flow Cytometric Analysis
Whole blood flow cytometry was performed at baseline and at 24 and 72 hours posttreatment for quantification of CD3+ T-cell subsets (CD4+, CD8+, αβTCR+, and γδTCR+), B cells (CD19+), natural killer cells (CD3–CD16+CD56+), and monocytes (CD14+) and for assessment of lymphocyte and eosinophil activation (Supplementary Methods).
Statistical Analysis
Unless stated otherwise, geometric means (GMs) were used as measures of central tendency. Nonparametric tests were used for all comparisons (Supplementary Data). For cytokine analyses, values below the limit of detection were replaced by the limit of detection. P < .05 was considered significant for all tests.
RESULTS
Baseline Characteristics of the Study Population
Twelve subjects between 20 and 70 years of age (median, 44 years) with Loa loa microfilaremia were enrolled in the study. Eight of the 12 subjects were male, and 4 were coinfected with Mansonella perstans. Baseline demographics and laboratory parameters did not differ between the 2 groups, with the exception of AEC, which was increased in the DEC group (GM, 3269 vs 1761 × 109/L in the IVM group; P < .01; Table 1).
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | DEC (n = 6) |
IVM (n = 6) |
|---|---|---|
| Sex, M/F | 4/2 | 4/2 |
| Median age, y (range) | 44.5 (20–70) |
38.5 (25–53) |
| Median Loa loa, mf/mL (range) | 860 (200–20380a) |
380 (60–2300) |
| GM AEC × 109 cells/L (range) |
3.27 (2.30–5.76) |
1.76b (1.05–2.35) |
| Intestinal helminths | 5 | 3 |
| Mansonella perstans | 2 | 2 |
Abbreviations: AEC, absolute eosinophil count, DEC, diethylcarbamazine; GM, geometric mean, IVM, ivermectin; mf, microfilariae.
One eligible subject randomized after the screening visit to receive DEC was inadvertently replaced by a different subject (a 70-year-old man) with the same name for the treatment portion of the study. The error was not recognized until day 3 posttreatment when his baseline smear was read and the mf count was determined to be 20380/mL. This subject was also immunochromatographic card test–positive without clinical evidence of Wuchereria bancrofti infection. The remaining clinical and laboratory data for this subject were similar to those of the other subjects who received DEC. Consequently, this subject has been included in the analysis.
Mann-Whitney test, P < .01 as compared to DEC group.
Posttreatment Adverse Events
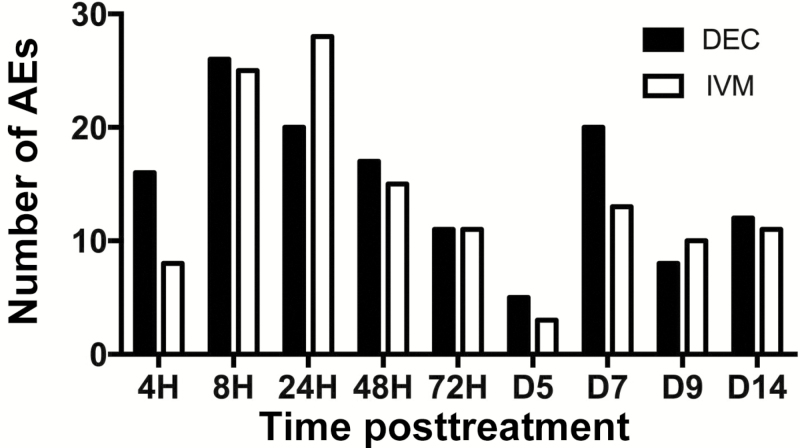
All 12 subjects developed mild to moderate AEs in the week following DEC or IVM administration (Table 2). Overall, headache and pruritus were the most common symptoms, reported in 8 and 7 subjects, respectively. Laboratory abnormalities were uncommon. Transient, mild ALT elevations (44 and 59 IU/L; normal range, 10–40 IU/L) occurred on day 3 post-DEC in 2 subjects, and 1 subject had a minimal transient rise in serum total bilirubin (1.4; normal range, <1.0 mg/dL) at 72 hours post-IVM and proteinuria (1+) on day 7. No severe (grade 3 or 4) or serious AEs were reported in either group. Posttreatment AEs were documented as early as 4 hours posttreatment and peaked at 8 hours in the DEC group and 24 hours in the IVM group (Figure 1).
Table 2.
Adverse Events During the First 7 Days Posttreatment With Diethylcarbamazine or Ivermectin
| Symptom, by Organ System | DEC (n = 6) |
IVM (n = 6) |
|---|---|---|
| Any clinical symptom |
6 |
6 |
| Loa-specific symptoms | 4 | 2 |
| Eyeworm | 1 | 2 |
| Calabar swelling | 3 | 2 |
| Sensation of worm under skin | 1 | 0 |
| Neurologic | ||
| Any | 6 | 5 |
| Blurry vision | 3 | 1 |
| Numbness/tingling | 1 | 1 |
| Dizziness | 4 | 2 |
| Headache | 4 | 4 |
| Musculoskeletal | ||
| Any | 4 | 6 |
| Joint effusion | 1 | 0 |
| Back pain | 2 | 4 |
| Myalgia/arthralgia | 2 | 5 |
| Gastrointestinal | ||
| Any | 5 | 5 |
| Diarrhea | 1 | 3 |
| Increased ALT | 2 | 0 |
| Increased total bilirubin | 0 | 1 |
| Nausea/vomiting | 2 | 2 |
| Abdominal pain | 3 | 2 |
| Cardiovascular | ||
| Any | 4 | 5 |
| Hemodynamic changesa | 4 | 4 |
| Palpitations | 0 | 1 |
| Anemiab | 2 | 4 |
| Respiratory | ||
| Any | 5 | 4 |
| Dyspnea | 2 | 0 |
| Cough | 3 | 1 |
| Chest pain | 3 | 3 |
| Abnormal pulmonary examc | 4 | 2 |
| Dermatologic | ||
| Any | 4 | 4 |
| Rash | 3 | 1 |
| Hives | 0 | 1 |
| Pruritus | 3 | 4 |
| Constitutional | ||
| Any | 2 | 0 |
| Fatigue/malaise | 2 | 0 |
| Subjective fever | 1 | 0 |
| Other | ||
| Conjunctival hemorrhage | 0 | 1 |
| Lymphadenopathy | 1 | 3 |
| Proteinuria | 0 | 1 |
| Leukocytosisd | 2 | 2 |
| Leukopenia | 1 | 1 |
Abbreviations: ALT, alanine aminotransferase; DEC, diethylcarbamazine; IVM, ivermectin.
Defined as a change in blood pressure of 30 mm Hg systolic or 20 mm Hg diastolic compared to the previous recording. One subject in the DEC group developed hypertension; 3 subjects in the DEC group had labile blood pressures with both hypotension and hypertension. Two subjects in the IVM group developed hypotension and 2 developed hypertension. None of these changes required intervention (grade 1).
Defined as development of anemia (<12 g/dL for women and <13 g/dL for men) or, if the subject was anemic at baseline, a drop of >1 g from the previous value.
Rales in 3 subjects who received DEC and 2 who received IVM; pleural rub in 1 subject who received DEC.
Only leukocytosis not attributable to a rise in absolute eosinophil count was included.
Figure 1.
Time course of posttreatment adverse events (AEs). The histogram shows the total number of AEs recorded for subjects in the diethylcarbamazine (DEC; black bar) and ivermectin (IVM; gray bar) groups for each time point.
Microfilarial Clearance
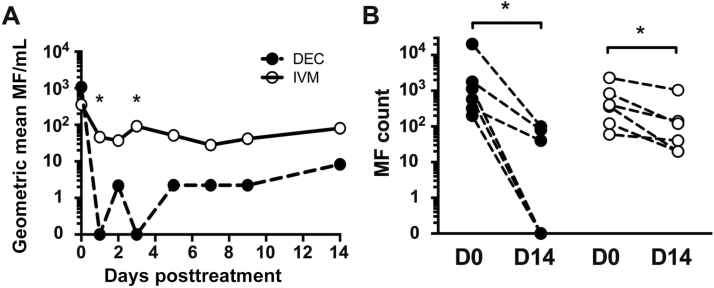
Although microfilarial counts decreased rapidly in all subjects following single-dose therapy, with median times to the minimum microfilarial count of 1 day and 1.5 days in the DEC and IVM groups, respectively (P = .2; Figure 2A), complete microfilarial clearance was observed at 1 day posttreatment in all 6 subjects who received DEC, but only 2 of 6 who received IVM (P = .06). This trend toward greater efficacy of DEC persisted throughout the follow-up period with microfilarial clearance at day 14 in 3 of 6 subjects post-DEC and 0 of 6 subjects post-IVM (Figure 2B). Mansonella perstans microfilarial counts were stable in the 4 coinfected subjects during the same time frame (GM, 87 mf/mL at baseline and 79 mf/mL at day 14; P = not significant).
Figure 2.
Microfilarial clearance following single-dose administration of diethylcarbamazine (DEC) or ivermectin (IVM). A, The geometric mean microfilariae (MF) count per milliliter of blood is shown over time during the first 14 days post-DEC (black circles, dashed line) or post-IVM (open circles, solid line). Geometric mean of 0 indicates that mf were undetectable in all individuals in the group. B, Individual mf counts at baseline and 14 days posttreatment in both groups. *P < .05 between the 2 groups; Mann-Whitney U test (A) and Wilcoxon signed-rank test (B).
Hematologic Evaluation
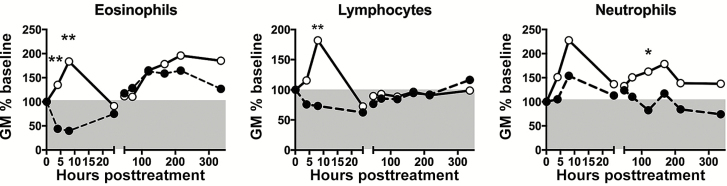
Within 24 hours posttreatment, AEC declined (GM AEC, 3269 to 1139 × 109/L; P = .03) in all subjects in the DEC group; whereas the AEC rose transiently in all subjects who received IVM (GM AEC, 1761 to 4081 × 109/L; P = .03) (Figure 3A). The changes in lymphocyte counts in the first day posttreatment paralleled the changes in AEC for each group (Figure 3B). The absolute neutrophil count (ANC) rose transiently in both groups before returning to baseline at 24 hours posttreatment (Figure 3C).
Figure 3.
Hematologic responses following single-dose administration of diethylcarbamazine (DEC) or ivermectin (IVM). Each symbol represents the geometric mean of the absolute cell count expressed as a percentage of baseline for subjects who received DEC (black circles, dashed line) or IVM (open circles, solid line). The gray area indicates below the 100% baseline value. *P < .05 and **P < .01 between the 2 groups; Mann-Whitney U test. Abbreviation: GM%, geometric mean %.
Beginning at 48 hours, GM AEC rose in both groups from a baseline GM of 3269 to a peak GM of 6373 × 109/L (163% of baseline) at day 5 in the DEC group (P = .03) and from a baseline GM of 1761 to a peak GM of 5069 × 109/L (196% of baseline) at day 9 in the IVM group (P = .03) (Figure 3A). Despite comparable levels at 24 hours posttreatment, GM ANC rose in the IVM group and declined in the DEC group over the subsequent 2 days (Figure 3C).
Eosinophil Activation
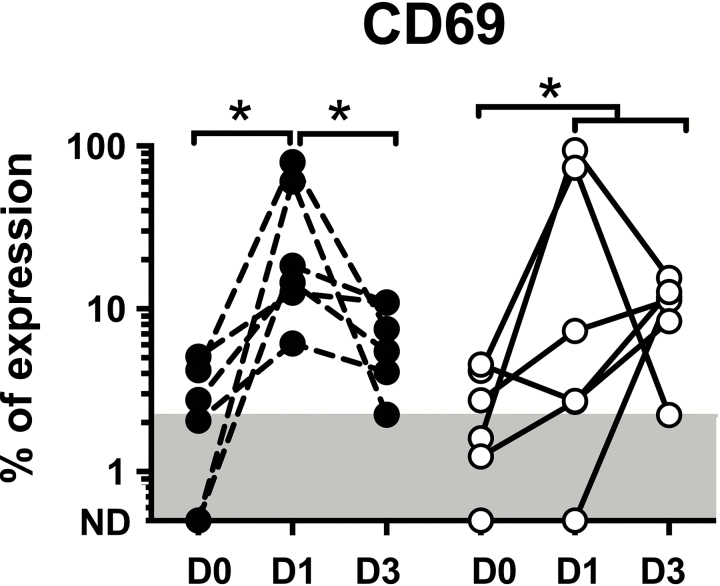
The percentage of eosinophils expressing the early activation surface marker, CD69, increased at 24 hours post-DEC in all 6 subjects (GM% expression of 21.5% vs. 1.0% at baseline; P = .03; Figure 4A) and decreased, albeit not back to baseline levels, in all 6 subjects by day 3 posttreatment (GM % expression of 6.0% at day 3 vs day 1; P = .03). The percentage of CD69+ eosinophils also increased post-IVM in all 6 subjects, peaking at 24 hours in 3 subjects and on day 3 in 3 subjects (GM% peak expression of 21.5% vs 1.5% at baseline; P = .03). Eosinophil surface expression of CD25, CD23, and HLA-DR showed no significant pattern during the same time frame (data not shown).
Figure 4.
Eosinophil surface activation marker expression following single-dose administration of diethylcarbamazine (DEC) or ivermectin (IVM). Percentage of CD69+ eosinophils at day 0, day 1, and day 3 post-DEC (black circles, dashed line) and post-IVM (open circles, solid line). The gray area shows the range of normal values in >125 healthy subjects (≤2.2%). *P < .05 compared to baseline, Wilcoxon signed-rank test. Abbreviation: ND, not detectable.
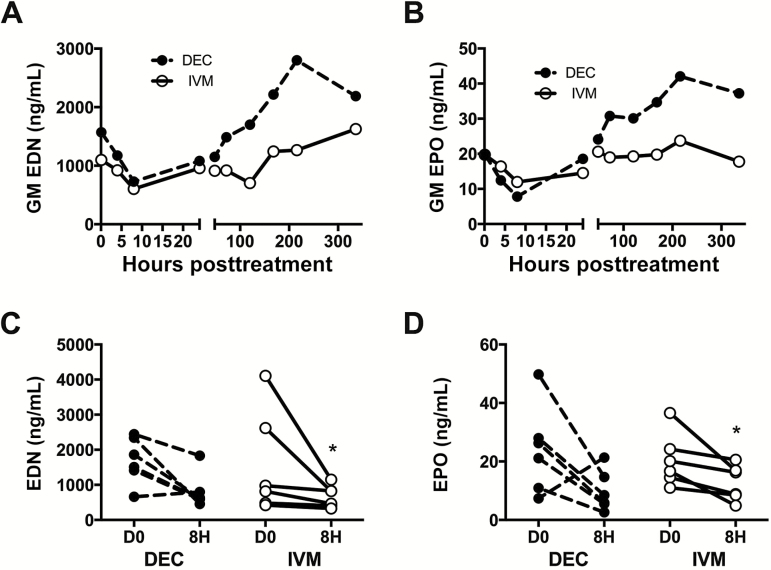
In contrast to the AEC, which showed differing early patterns between the DEC and IVM groups, serum levels of eosinophil-derived neurotoxin (EDN) and eosinophil peroxidase (EPO) decreased acutely in both treatment groups, reaching a nadir at 4 or 8 hours posttreatment in 11 of 12 subjects before slowly rising over the ensuing 14 days (Figure 5). GM serum levels of EDN fell from 1.6 µg/mL at baseline to 0.73 µg/mL at 8 hours following DEC (P = .06) and from 1.1 to 0.60 µg/mL at 8 hours following IVM (P = .03). GM EPO levels showed a similar pattern. Although the GM serum levels of both EDN and EPO were higher in the DEC group at all time points >24 hours, none of these differences achieved statistical significance. Serum levels of eosinophil cationic protein and major basic protein showed a variable pattern posttreatment (data not shown).
Figure 5.
Eosinophil granule protein levels following single-dose administration of diethylcarbamazine (DEC) or ivermectin (IVM). Geometric mean (GM) levels of eosinophil-derived neurotoxin (EDN) and eosinophil peroxidase (EPO) over time posttreatment (A and B, respectively) and EDN and EPO levels in individual subjects at baseline and at 8 hours posttreatment (C and D, respectively) are shown. Subjects who received DEC are indicated by black circles and dashed lines and those who received IVM by open circles and solid lines. *P < .05, Wilcoxon signed-rank test.
Lymphocyte Subset Analyses
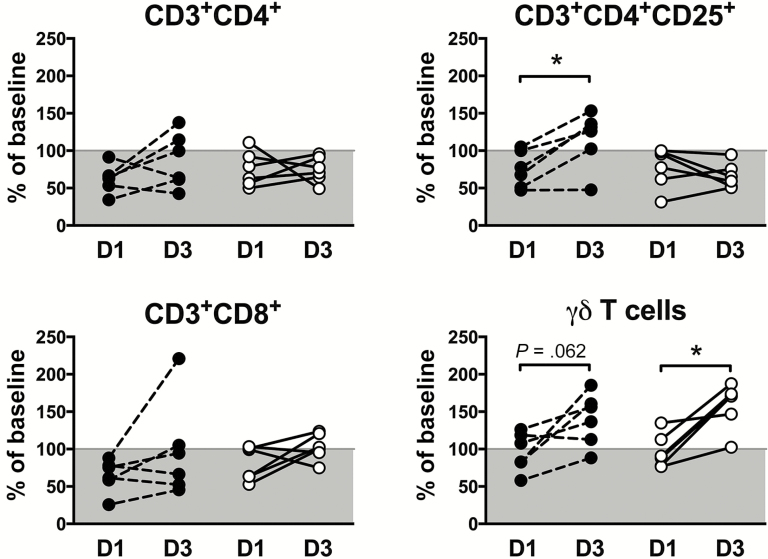
To further explore the role of T lymphocytes in posttreatment reactions, CD3+ lymphocyte subsets (CD4+, CD4+CD25+, CD8+, and γδTCR+) were quantified by flow cytometry at baseline and at days 1 and 3 posttreatment (Figure 6). All CD3+ subsets decreased at day 1 posttreatment in nearly all subjects in both treatment groups. Whereas CD3+CD4+ and CD3+CD8+ cell numbers showed no significant trends at day 3, CD3+CD4+CD25+ cell numbers increased above baseline levels in 5 of 6 subjects who received DEC and 0 of 6 subjects who received IVM (P = .02; Fisher exact test). CD3+ γδ T cells increased between days 1 and 3 posttreatment in 11 of 12 subjects with GM levels increasing from 93% to 136% of baseline in the DEC group and 95% to 156% of baseline in the IVM group (P = .06 and P = .03, respectively; Figure 6).
Figure 6.
Lymphocyte subset analysis following single-dose administration of diethylcarbamazine (DEC) or ivermectin (IVM). Percentages of baseline CD3+CD4+, CD3+CD4+CD25+, CD3+CD8+, and CD3+TCRγδ+ in peripheral blood measured at day 1 and day 3 posttreatment. Data for individual subjects who received DEC are denoted by black circles and dashed lines and those who received IVM by open circles and solid lines. The gray areas indicate a decrease from baseline values. *P < .05, Wilcoxon signed-rank test.
Serum Mediator Profiles
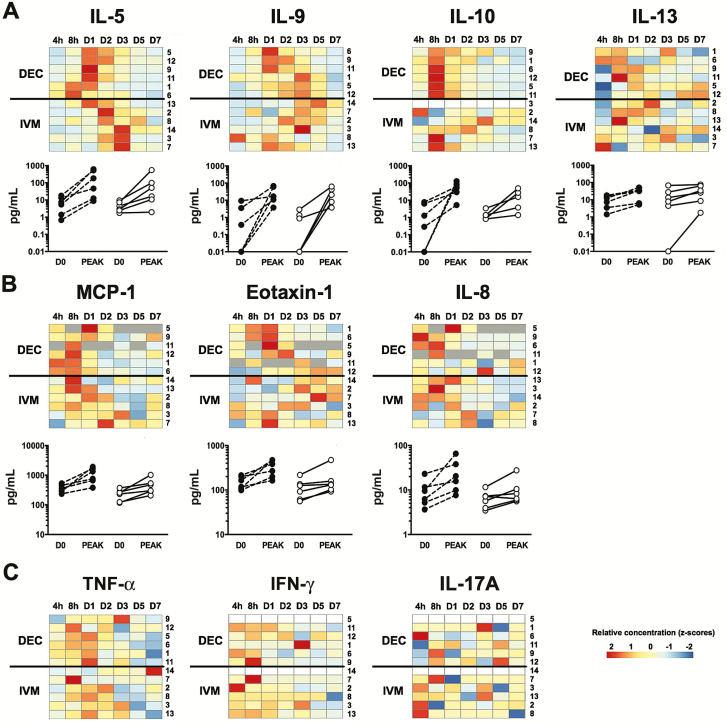
Baseline serum levels were similar between the 2 treatment groups for all mediators tested (Supplementary Figure 2). Although serum IL-5 levels increased significantly posttreatment in both the DEC and IVM groups with GM peak IL-5 levels of 81.7 pg/mL and 31.2 pg/mL, respectively (Figure 7A), levels peaked earlier in subjects who received DEC (median time to peak, 24 vs 60 hours; P = .01). Serum levels of IL-13 and IL-9 also increased in most subjects posttreatment (Figure 7A). Peak levels were comparable between the 2 groups. Interestingly, peak serum IL-5, IL-13, and IL-9 levels were significantly correlated with baseline microfilarial count (r = 0.59; P = .05), but not with peak AEC. Serum IL-4 levels were measurable in only 3 subjects (2 from the DEC group and 1 from the IVM group), precluding analysis (data not shown).
Figure 7.
Serum mediator levels following single-dose administration of diethylcarbamazine (DEC) or ivermectin (IVM). Heat maps represent the range of net values for the first 7 days posttreatment (from low in blue to high in red) measured for interleukin (IL) 5, IL-9, IL-10, IL-13 (A), monocyte chemotactic protein-1 (MCP-1), eotaxin-1, IL-8, Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) (B), and tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), and IL-17A (C). Subjects have been clustered separately for the 2 treatment groups with subject number shown along the right side of the heat map. A gray square indicates a missing value. Individual values measured at baseline (D0) and at peak are represented below the heat maps for mediators showing a clear expression pattern (DEC group, black circle, IVM group, white circle).
Serum levels of IL-10 increased posttreatment in all subjects Figure 7A), but were significantly higher in the DEC group at all time points within the first 48 hours posttreatment (GM levels of 12.7, 41.7, 25.6, and 14.4 pg/mL post-DEC compared with 1.2, 1.7, 1.6, and 1.8 pg/mL post-IVM at 4, 8, 24, and 48 hours, respectively; P ≤ .04 for all comparisons). Peak serum IL-10 levels were correlated with baseline microfilarial levels for subjects who received DEC (r = 0.81; P = .05) and for the group as a whole (r = 0.76; P = .006).
Serum levels of the chemokine monocyte chemotactic protein-1 (MCP1/CCL2) increased in all subjects posttreatment, with peak levels occurring at the 8-hour time point in 7 of 12 subjects (Figure 7B). Peak serum levels of MCP-1/CCL2 were significantly higher in subjects who received DEC (GM peak, 969 pg/mL vs 408 pg/mL post-IVM; P = .04). Serum levels of IL-8 and eotaxin-1 also increased within 24 hours posttreatment in all subjects in the DEC group and 4 of 6 subjects in the IVM group. Peak levels were similar between the 2 groups. Serum levels of RANTES (Regulated on Activation, Normal T Cell Expressed and Secreted) showed no consistent pattern (data not shown).
Serum levels of the inflammatory and type 1 cytokines IL-17A, TNF-α, interferon gamma (IFN-γ) (Figure 7C), IL-21, IL-23, and IL-33 (data not shown), were detectable pre- and posttreatment in some subjects, but showed no clear posttreatment pattern and were not correlated with baseline microfilarial count or peak AEC posttreatment. Serum levels of granulocyte macrophage colony-stimulating factor were undetectable, and serum levels of IL-2, IL-4, IL-6, IL-12p70, IL-17F, IL-22, IL-25, IL-31, and macrophage inflammatory protein (MIP) 1α/CCL3 were detectable in <50% of the subjects and time points tested, precluding further analysis.
DISCUSSION
The association between DEC and severe posttreatment reactions, including encephalopathy, in patients with high levels of Loa loa microfilaremia was first described in 1948 [13]. More recently, similar reactions have been documented following IVM administration in Loa-endemic areas [5]. In fact, of 207 serious AEs reported between 1989 and 2002 following ivermectin mass drug administration, two-thirds were “probable” or “possible” cases of Loa loa–related encephalopathy [14]. In the present study, the number and character of AEs was similar following DEC and IVM. The peak number of events occurred slightly earlier in the DEC group, paralleling earlier peaks in both serum IL-5 and CD69 expression post-DEC. These findings are consistent with the tendency toward more rapid and complete microfilarial killing following single-dose DEC therapy and are supported by the data from prior single drug studies in loiasis and other filarial infections in which the peak of AEs following DEC is typically within 8 hours [15, 16] compared with 24–36 hours post-IVM [17–21] (Supplementary Table 3).
Despite the similarity in AE profiles following DEC and IVM, eosinophil and lymphocyte counts showed opposite patterns during the first 24 hours posttreatment. Although prior studies in filariasis have not directly compared early changes in cell counts following DEC and IVM, data from 2 separate studies in onchocerciasis in Ghana confirm our findings with respect to changes in AEC within the first 8 hours posttreatment [19, 20, 22] (Supplementary Table 3). The drop in AEC following DEC administration has been attributed to eosinophil recruitment to sites of microfilarial sequestration. The reason for the transient increase in eosinophil and lymphocyte counts post-IVM is unclear, but may be related to differences in the site or mechanism of parasite killing between the 2 drugs or, less likely, to the effect of IVM on intestinal helminths.
Irrespective of the early differences, a consistent rise in AEC was seen in both treatment groups after 24 hours. Moreover, markers of eosinophil activation were comparable between the 2 groups. A rise in type 2 cytokines, including IL-5, preceded the posttreatment increase in AEC, as previously reported in onchocerciasis and lymphatic filariasis [6–8, 20], and peak IL-5 levels correlated with baseline microfilarial counts in both groups, consistent with the hypothesis that IL-5–driven eosinophilia is responsible for DEC- and IVM-induced microfilarial killing in loiasis. In keeping with the absence of Wolbachia in Loa loa parasites, proinflammatory cytokine levels (TNF-α, IL-6, and IL-17A) did not change appreciably post-DEC or -IVM treatment.
One notable difference in posttreatment profiles between the groups was the significantly higher serum IL-10 and MCP-1/CCL2 levels post-DEC. Although both mediators can be secreted by numerous cells types, including eosinophils [23, 24], these findings in the context of the observed increased in CD3+CD4+CD25+ cells post-DEC (but not post-IVM) suggest a more pronounced regulatory T-cell response post-DEC. Alternatively, the observed delay in IL-5 response in the subjects who received IVM could have delayed the antigen-specific CD4+CD25+ regulatory T-cell response [25] such that it was not detectable within the 3-day study window.
The chief limitation of the study was the size of the study population. Originally designed to include 20 subjects, the stringent inclusion criteria and logistical requirements resulted in premature termination of enrollment after 12 subjects. The major impediment to recruitment was exclusion of subjects with microfilarial counts ≥2000 mf/mL, a limit set intentionally to minimize the risk of treatment-related serious AEs. Despite the small number of subjects and relatively low microfilarial levels, patterns of response were clearly identified, and all subjects reported posttreatment symptoms.
In summary, AEs following single dose DEC or IVM administration to patients with microfilaremic loiasis are similar in character and associated with IL5-driven eosinophilia and eosinophil activation in the setting of microfilarial killing. The enhanced microfilaricidal efficacy of DEC compared to IVM likely accounts for differences in the rapidity and severity of AEs reported post-DEC. Whether blocking the posttreatment eosinophil response would be sufficient to decrease the severity of posttreatment AEs in loiasis, and the effect this would have on treatment efficacy, are currently being investigated.
Supplementary Material
Notes
Acknowledgments. The authors thank the population of Akonolinga and the staff at the Akonolinga Hospital, without whom this study would not have been possible.
Financial support. The work was supported by the Division of Intramural Research of the National Institute for Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Klion AD, Massougbodji A, Sadeler BC, Ottesen EA, Nutman TB. Loiasis in endemic and nonendemic populations: immunologically mediated differences in clinical presentation. J Infect Dis 1991; 163:1318–25. [DOI] [PubMed] [Google Scholar]
- 2. Herrick JA, Metenou S, Makiya MA, et al. Eosinophil-associated processes underlie differences in clinical presentation of loiasis between temporary residents and those indigenous to Loa-endemic areas. Clin Infect Dis 2015; 60:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carme B, Boulesteix J, Boutes H, Puruehnce MF. Five cases of encephalitis during treatment of loiasis with diethylcarbamazine. Am J Trop Med Hyg 1991; 44:684–90. [DOI] [PubMed] [Google Scholar]
- 4. Scientific Working Group on Serious Adverse Events in Loa Loa Endemic Areas. Report of a scientific working group on serious adverse events following Mectizan treatment of onchocerciasis in Loa loa endemic areas. Filaria J 2004;2:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardon J, Gardon-Wendel N, Demanga-Ngangue, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997; 350:18–22. [DOI] [PubMed] [Google Scholar]
- 6. Limaye AP, Abrams JS, Silver JE, et al. Interleukin-5 and the posttreatment eosinophilia in patients with onchocerciasis. J Clin Invest 1991; 88:1418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Limaye AP, Ottesen EA, Kumaraswami V, et al. Kinetics of serum and cellular interleukin-5 in posttreatment eosinophilia of patients with lymphatic filariasis. J Infect Dis 1993; 167:1396–400. [DOI] [PubMed] [Google Scholar]
- 8. Gopinath R, Hanna LE, Kumaraswami V, et al. Perturbations in eosinophil homeostasis following treatment of lymphatic filariasis. Infect Immun 2000; 68:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner PF, Rockett KA, Ottesen EA, Francis H, Awadzi K, Clark IA. Interleukin-6 and tumor necrosis factor in the pathogenesis of adverse reactions after treatment of lymphatic filariasis and onchocerciasis. J Infect Dis 1994; 169:1071–5. [DOI] [PubMed] [Google Scholar]
- 10. McGarry HF, Pfarr K, Egerton G, et al. Evidence against Wolbachia symbiosis in Loa loa. Filaria J 2003; 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dickerson JW, Eberhard ML, Lammie PJ. A technique for microfilarial detection in preserved blood using Nuclepore filters. J Parasitol 1990; 76:829–33. [PubMed] [Google Scholar]
- 12. Makiya MA, Herrick JA, Khoury P, Prussin CP, Nutman TB, Klion AD. Development of a suspension array assay in multiplex for the simultaneous measurement of serum levels of four eosinophil granule proteins. J Immunol Methods 2014; 411:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Bogaert L, Dubois A, Janssens PG, Radermecker J, Tverdy G, Wanson M. Encephalitis in loa-loa filariasis. J Neurol Neurosurg Psychiatry 1955; 18:103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Twum-Danso NA. Loa loa encephalopathy temporally related to ivermectin administration reported from onchocerciasis mass treatment programs from 1989 to 2001: implications for the future. Filaria J 2003; 2:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Francis H, Awadzi K, Ottesen EA. The Mazzotti reaction following treatment of onchocerciasis with diethylcarbamazine: clinical severity as a function of infection intensity. Am J Trop Med Hyg 1985; 34:529–36. [DOI] [PubMed] [Google Scholar]
- 16. Shookhoff HB, Dwork KG. Treatment of Loa loa infections with Hetrazan. Am J Trop Med Hyg 1949; 29:589–93. [DOI] [PubMed] [Google Scholar]
- 17. Ducorps M, Gardon-Wendel N, Ranque S, et al. Secondary effects of the treatment of hypermicrofilaremic loiasis using ivermectin. Bull Soc Pathol Exot 1995; 88:105–12. [PubMed] [Google Scholar]
- 18. Martin-Prevel Y, Cosnefroy JY, Tshipamba P, Ngari P, Chodakewitz JA, Pinder M. Tolerance and efficacy of single high-dose ivermectin for the treatment of loiasis. Am J Trop Med Hyg 1993; 48:186–92. [DOI] [PubMed] [Google Scholar]
- 19. Cooper PJ, Awadzi K, Ottesen EA, Remick D, Nutman TB. Eosinophil sequestration and activation are associated with the onset and severity of systemic adverse reactions following the treatment of onchocerciasis with ivermectin. J Infect Dis 1999; 179:738–42. [DOI] [PubMed] [Google Scholar]
- 20. Cooper PJ, Schwartz LB, Irani AM, Awadzi K, Guderian RH, Nutman TB. Association of transient dermal mastocytosis and elevated plasma tryptase levels with development of adverse reactions after treatment of onchocerciasis with ivermectin. J Infect Dis 2002; 186:1307–13. [DOI] [PubMed] [Google Scholar]
- 21. Coutinho AD, Dreyer G, Medeiros Z, et al. Ivermectin treatment of bancroftian filariasis in Recife, Brazil. Am J Trop Med Hyg 1994; 50:339–48. [DOI] [PubMed] [Google Scholar]
- 22. Hagan JB, Bartemes KR, Kita H, et al. Elevations in granulocyte-macrophage colony-stimulating factor and interleukin-5 levels precede posttreatment eosinophilia in onchocerciasis. J Infect Dis 1996; 173:1277–80. [DOI] [PubMed] [Google Scholar]
- 23. Lamkhioued B, Gounni AS, Aldebert D, et al. Synthesis of type 1 (IFN gamma) and type 2 (IL-4, IL-5, and IL-10) cytokines by human eosinophils. Ann N Y Acad Sci 1996; 796:203–8. [DOI] [PubMed] [Google Scholar]
- 24. Izumi S, Hirai K, Miyamasu M, et al. Expression and regulation of monocyte chemoattractant protein-1 by human eosinophils. Eur J Immunol 1997; 27:816–24. [DOI] [PubMed] [Google Scholar]
- 25. Tran GT, Hodgkinson SJ, Carter NM, et al. IL-5 promotes induction of antigen-specific CD4+CD25+ T regulatory cells that suppress autoimmunity. Blood 2012; 119:4441–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.