Abstract
The Rhipicephalus sanguineus (Latreille) complex (Acari:Ixodidae) is composed of species with intra- and interspecific morphological variation that make their diagnosis difficult. In the present study, male specimens of the R. sanguineus complex were collected from dogs in six districts of three regions of Brazil and submitted to molecular and scanning electron microscopy (SEM) analyses. Analysis of COX1 gene, 12S rDNA, and D-loop rDNA shows that ticks classified as R. sanguineus form two different clades. Morphological comparisons using SEM found adult males to exhibit morphological differences in Haller’s organ, festoons, and adanal, spiracular, and genital plates, with the last having potential usefulness in distinguishing male specimens of the complex.
Keywords: Acari, Ixodidae, molecular phylogeny, scanning electron microscopy, taxonomy
Rhipicephalus sanguineus (Latreille), also known as the “brown dog tick,” is the most widespread ectoparasite of dogs, and probably has the largest zoogeographic range among ticks. This species exhibits great intraspecific morphological diversity, as noted by several authors (Pegram et al.1987a; Estrada-Peña and Sanchez 1988; Ribeiro et al. 1995; Oliveira et al. 2005; Rosa et al. 2006, 2010). The species is also recognized as a vector of numerous pathogens (Walker et al. 2005, Dantas-Torres 2008, Gray et al. 2013), and is responsible for considerable public health issues and animal deaths worldwide (Dantas-Torres 2008).
This tick is an introduced species in South America, with multiple routes involved in its colonization (Szabó et al. 2005), and was first recorded in Brazil early in the 20th century (Rohr 1909). The process of human migration can generate, for the people involved, severe health consequences, including the spread of endemic diseases and the outbreak of diseases initiated by human penetration into natural environments. Changes in nosology in different regions and in the epidemiological characteristics of various diseases have resulted from such human movements (Marques 1983).
Burlini et al. (2010) believed that R. sanguineus sensu stricto (s. s.) was the only species of this genus in Latin America; however, several studies evaluating morphology, biology, and genetics have shown that there are at least two species (Oliveira et al. 2005, Szabó et al. 2005, Moraes-Filho et al. 2011, Levin et al. 2012, Nava et al. 2012, Labruna et al. 2017). Based on these findings, the application of the term “R. sanguineus species group” or “R. sanguineus species sensu lato (s. l.)” was suggested instead of “R. sanguineus sensu strictu” (Nava et al. 2015, Labruna et al. 2017). The morphological similarity of these two species, and the minor variation among individual ticks, makes the identification of taxa of the R. sanguineus group difficult (Pegram et al. 1987a,b; Dantas-Torres et al. 2013). However, based on morphological and genetic variation, Dantas-Torres et al. (2013) divided the world population of R. sanguineus into tropical species (R. sanguineus s. l.) and temperate species (R. sp. II). Zemtsova et al. (2016) also observed a similar pattern of distribution between temperate and tropical regions.
Therefore, in this paper we will refer to these entities as the “tropical species” and the “temperate species,” although it needs to be kept in mind that these entities might not actually represent individual species.
Due to the relevance of this “species complex” as a parasite of dogs and its capacity to serve as a vector in Babesia vogeli (Costa-Júnior et al. 2009, O’Dwyer et al. 2009, Ramos et al. 2010), Ehrlichia canis (Vieira et al. 2011, Tanikawa et al. 2013), and Rickettsia rickettsii (Moraes-Filho et al. 2009, Cunha et al. 2009, Gehrke et al. 2009) transmission, it has been the focus of numerous studies. Analysis of morphological and molecular variation among populations and species is valuable information and could contribute to a better understanding of regional differences in the epidemiology of diseases transmitted by R. sanguineus s. l.
Therefore, the aim of this study was to make morphological and molecular comparisons of male R. sanguineus s. l. from different geographical regions of Brazil, using scanning electron microscopy (SEM) and mitochondrial genes COX1, 12S rDNA, and D-loop rDNA.
Materials and Methods
Male specimens of ticks classified as Rhipicephalus sanguineus were collected from dogs in six states of three regions of Brazil: Roraima (RR) and Rondônia (RO) from the North, Pernambuco (PE) and Rio Grande do Norte (RN) from the Northeast, and Paraná (PR) and Rio Grande do Sul (RS) from the South. Some of the ticks collected in each state were used for morphological analysis with SEM, while the remainder were used for molecular analysis. Tick collections were undertaken as a partnership between the Laboratório de Referência Nacional em Vetores das Riquetsioses (LIRN)—IOC/FIOCRUZ and state Health Departments in Brazil.
The identification of ticks followed Aragão and Fonseca (1961), Walker et al. (2005), and Guglielmone et al. (2006).
For morphological analysis, 30 specimens from each state were examined under a stereomicroscope (Leika), with those having the best physical integrity being chosen for SEM processing. These ticks were cleaned with distilled water, dehydrated in an increasing alcohol series with solutions of 50, 60, 70, 80, 90, and 100%, placed on metallic supports, coated with a thin layer of gold (20–30 nm), and examined with a JEOL 6390LV scanning electron microscope (SEM) (Akishima, Tokyo, Japan).
The morphological structures analyzed included the capitulum, Haller’s organ, adanal plates, genital opening, spiracles, festoons, and other aspects of general morphology. The terminology used followed Walker et al. (2005).
Molecular analysis was performed on tick samples from all the states analyzed in the morphological study with the exception of Pernambuco because it had an insufficient number of specimens. Ticks were submitted to DNA extraction as described elsewhere (Aljanabi and Martinez 1997; method with NaCl). The final number of processed ticks was 11 from RN, 10 from RO, 10 from RR, 14 from PR, and 23 from RS. Polymerase chain reaction (PCR) was executed according to the methodology proposed by Bitencourth et al. (2016), using the GeneAmp PCR System 9700 (Applied Biosystems, CA). The primers used were T1B and T2A for 12S rDNA (≈360 bp; Beati and Keirans 2001) and D-loop 3-1x and D-loop 4-1x, for D-loop rDNA (≈440 bp; Burkman 2009). The COX1 gene (≈820 bp) was amplified with primers Cox1F and Cox1R under the temperature/time cycle 95 °C 5’; [95 °C 30”, 55 °C 1’, 72 °C 1’/Kb] 40X; 72 °C 5’; 20 °C ∞ (Chitimia et al. 2010). The PCR products were submitted to electrophoresis on a 2% agarose gel stained with ethidium bromide. The amplicons with the expected size were purified using the NucleoSpin Extract II Kit (Macherey Nagel, KG, Germany), following the manufacture’s protocol. Sequencing reactions were performed using a BigDye Terminator Version 3.1 Cycle Sequencing Kit (Applied Biosystems), with the same PCR primers, and sequenced on an ABI 3730 DNA Analyzer (Applied Biosystems).
The sequences were edited in ChromasPro 1.5 (Technelysium Pty Ltd, Qld, Australia) and deposited in GenBank with the accession numbers KX383796–KX383820 for COX1 gene, KX383865–KX383911 for 12S rDNA, and KX383821–KX383864 for D-loop (Supp. Table 1 [online only]). A total of 47 individual sequences were generated for 12S rRNA (10 from RN, 7 from RO, 6 from RR, 14 from PR, and 10 from RS), 44 individual sequences for D-loop rDNA (9 from RN, 7 from RR, 11 from PR, and 17 from RS), and 25 individual sequences for the gene COX1 (3 from RN, 7 from RO, 1 from RR, 3 from PR, and 11 from RS). Only eight specimens had sequences for all three mtDNA portions (LIC5958B and LIC5958C from RN, 6084B from RR, 5584D and 6664A from PR, and 5424B, 5533B, and 5554A from RS).
In a previous study done by our group, specimens collected in other states were subjected to the same molecular analyses and the generated sequences were deposited in GenBank with the accession numbers KX714591–KX714601.
After alignment with MAFFT online server (Kuraku et al. 2013), PhyML with Smart Model Selection (Guindon et al. 2010) was used to identify the optimal evolutionary model that best described our sequence dataset and to construct maximum-likelihood phylogenetic trees. Akaike information criterion (AIC) indicated the models that best fit each sequence dataset and the results were HKY85 + G + I+F for the concatenated dataset (12S + COX1) and TN93 + G + F for 12S rDNA, COX1 gene, and D-loop rDNA. As a measure of the robustness of each node, bootstrapping was applied with 1,000 replicates. The pairwise analysis of COX1 sequences was conducted using the Kimura 2-parameter (K2P; Kimura 1980) method in MEGA 5.2 (Tamura et al. 2011). This analysis used the sequence KC243890, with 472 bp as the boundary length.
Results
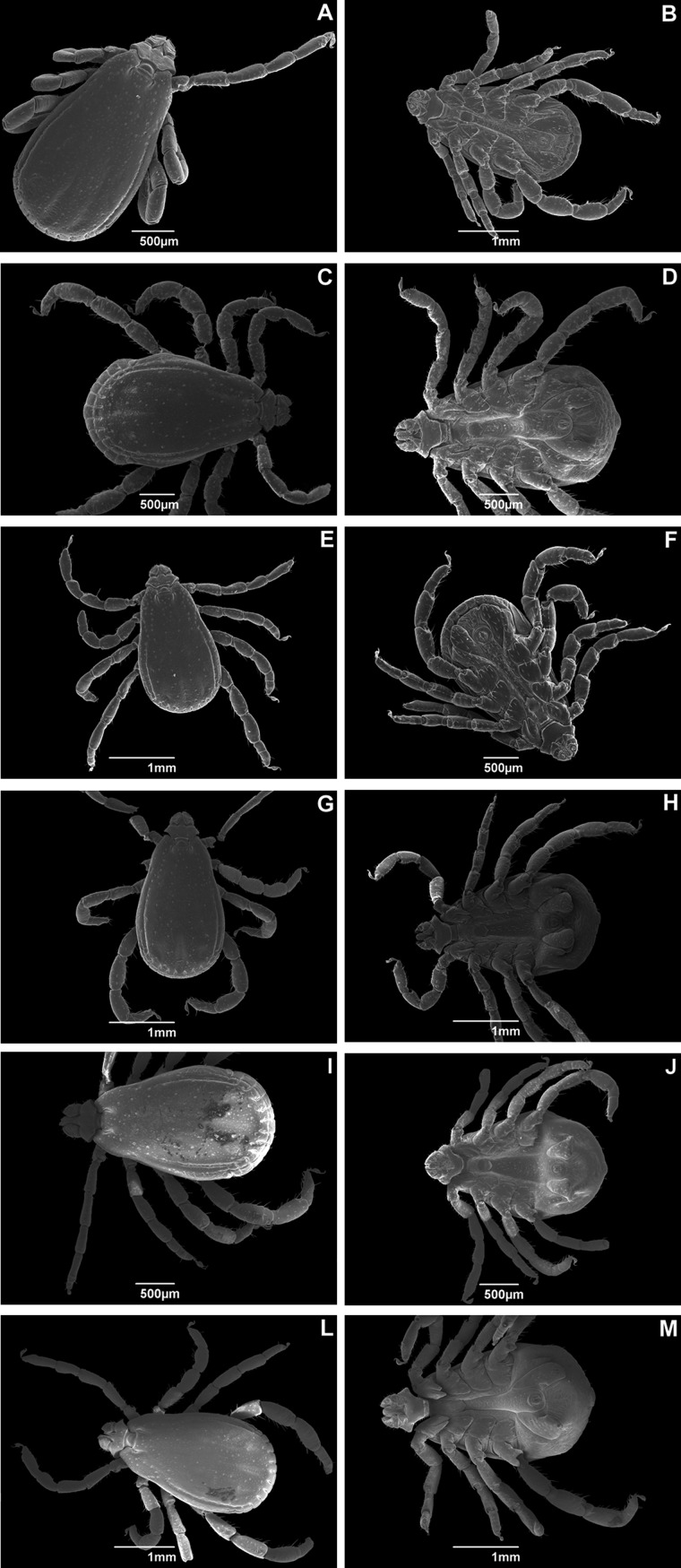
Analysis of the dorsal region of the specimens revealed inter- and intraspecific variation in the distribution of setae (Fig. 1).
Fig. 1.
Scanning electron micrographs of adult male Rhipicephalus sanguineus (Acari: Ixodidae) collected in Brazil. Dorsal and ventral view of ticks from (A) Rondônia (× 33); (B) Rondônia (× 23); (C) Roraima (× 27); (D) Roraima (× 30); (E) Pernambuco (× 25); (F) Pernambuco (× 27); (G) Rio Grande do Norte (× 25); (H) Rio Grande do Norte (× 25); (I) Paraná (× 23); (J) Paraná (× 25); (L) Rio Grande do Sul (× 23); and (M) Rio Grande do Sul (× 25).
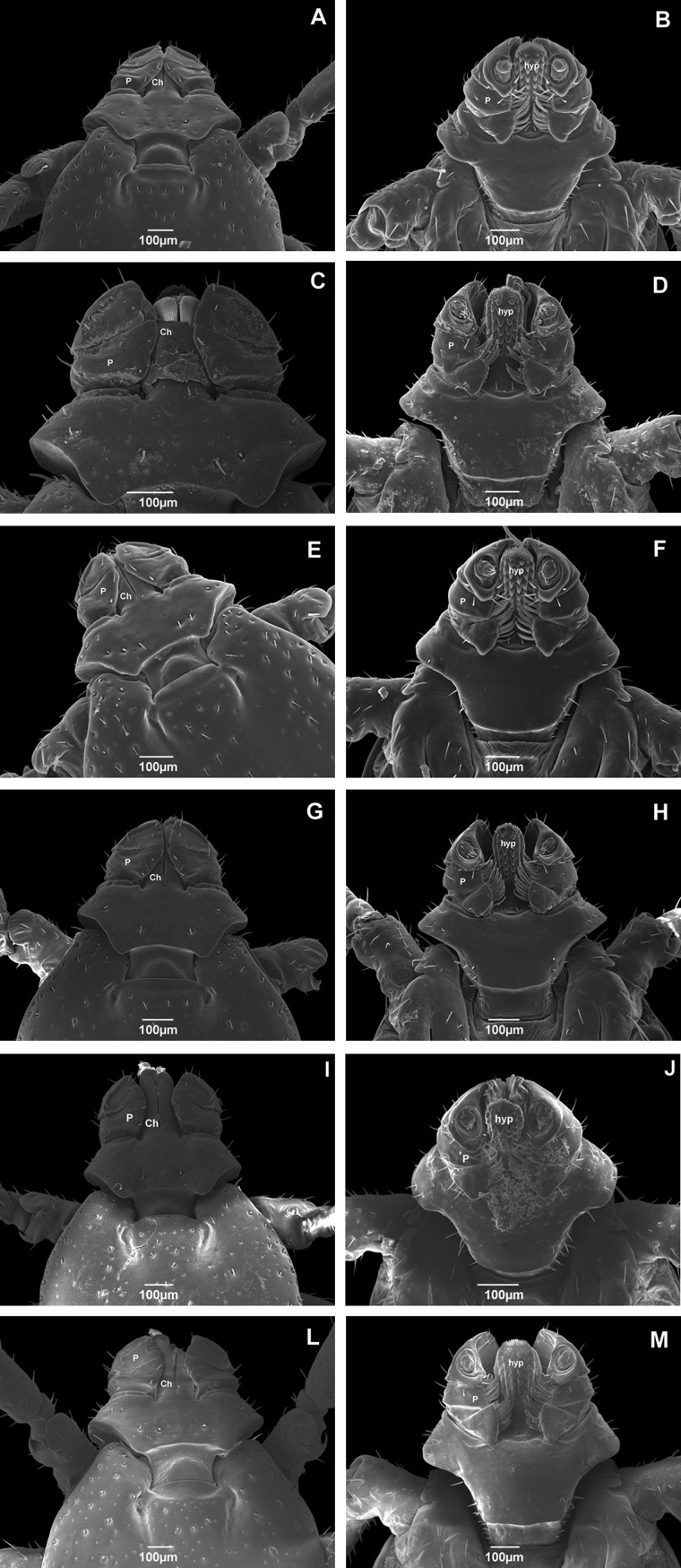
The capitulum is the movable anterior portion of the body and is composed of a basis capitulum, a hypostome (hyp), two palps (P), and two chelicerae (Ch; Walker et al. 2005; Fig. 2). The basis capitulum is the basal portion of the capitulum where mouthparts are attached. In the genus Rhipicephalus, the basis capitulum is hexagonal in shape and, in all the specimens analyzed, was found to possess setae and sensilla without a clear pattern of distribution (Fig. 2).
Fig. 2.
Scanning electron micrographs of the capitulum of adult male Rhipicephalus sanguineus (Acari: Ixodidae) collected in Brazil. Dorsal and ventral view of ticks from (A) Rondônia (× 100); (B) Rondônia (× 130); (C) Roraima (× 180); (D) Roraima (× 130); (E) Pernambuco (× 130); (F) Pernambuco (× 130); (G) Rio Grande do Norte (× 120); (H) Rio Grande do Norte (× 120); (I) Paraná (× 170); (J) Paraná (× 120); (L) Rio Grande do Sul (× 100); and (M) Rio Grande do Sul (× 110). P, palps; Ch, chelicerae; hyp, hypostome.
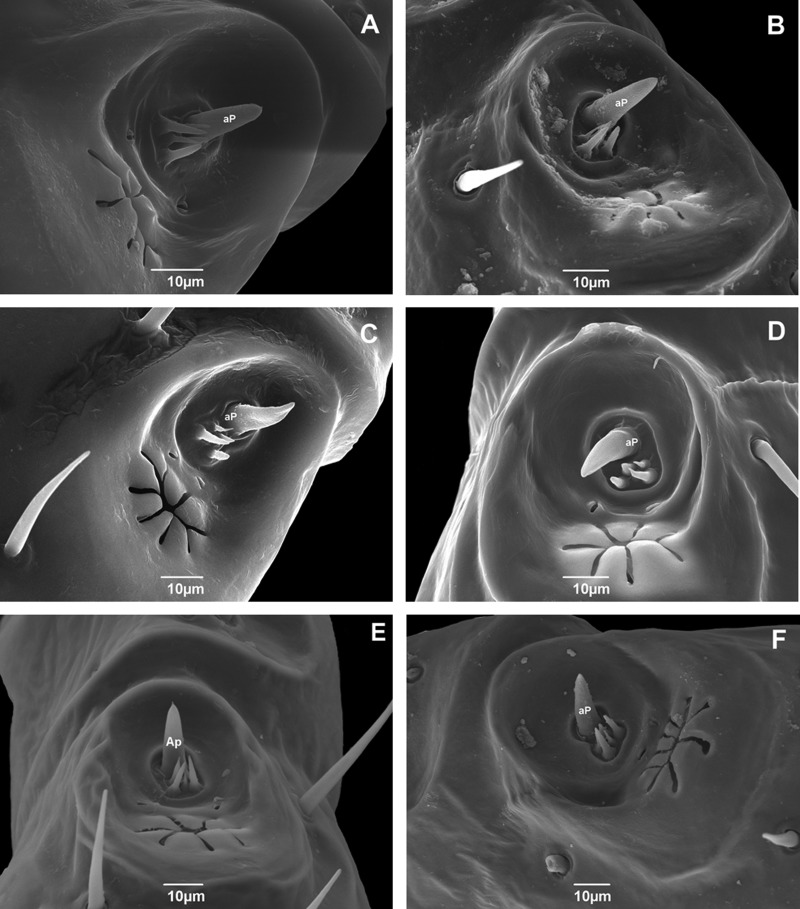
The anterior pit of Haller’s organ possesses a set of sensilla that varies in form and number among the studied populations. This intraspecific variation includes specimens possessing five (Fig. 3B—Roraima, 3C—Pernambuco, 3D—Rio Grande do Norte, 3E—Paraná, 3F—Rio Grande do Sul), or six sensilla (Fig. 3A—Rondônia).
Fig. 3.
Scanning electron micrographs of the Haller’s organ of adult male Rhipicephalus sanguineus (Acari: Ixodidae) collected in Brazil. (A) Rondônia (× 2000); (B) Roraima (× 2000); (C) Pernambuco (× 1000); (D) Rio Grande do Norte (× 2000); (E) Paraná (× 1000); and (F) Rio Grande do Sul (× 1000). aP, anterior pit.
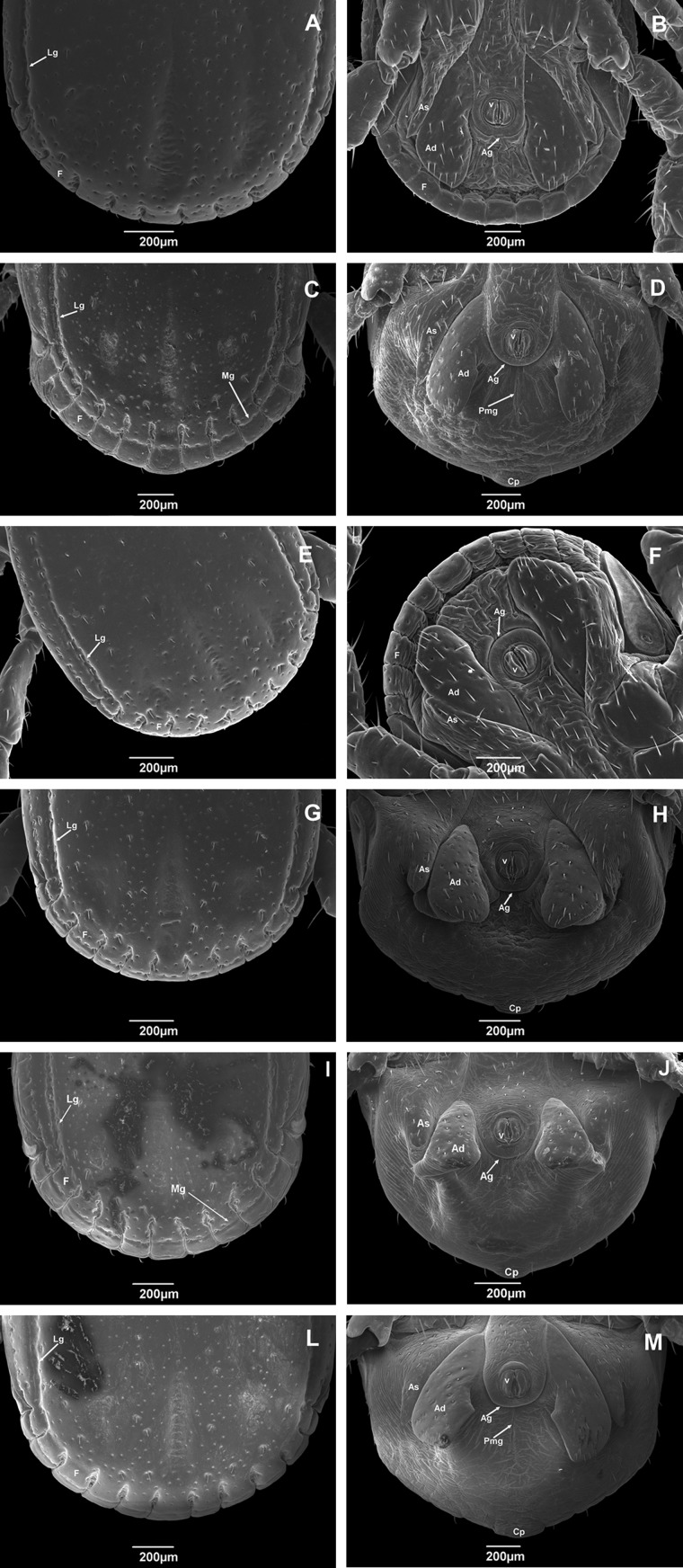
Festoons (F) also exhibited intraspecific variation with specimens from four states, including Rio Grande do Sul, possessing festoons divided into 11 distinct rectangular portions that are delimited by only deep lateral grooves (Fig. 4A, E, G, and L). The festoons of specimens collected in Roraima and Paraná are delimited by lateral grooves (Lg) and by the marginal groove (Mg; Fig. 4C and I). The caudal process (Cp) is a protrusion of the central festoon in fed males (Bristol University Tick ID), and exhibited variation in size among the studied specimens (Fig. 4D, H, J, and M).
Fig. 4.
Scanning electron micrographs of posterior part of body of adult male Rhipicephalus sanguineus (Acari: Ixodidae) collected in Brazil. Dorsal and ventral view of ticks from (A) Rondônia (× 95); (B) Rondônia (× 70); (C) Roraima (× 70); (D Roraima (× 75); (E) Pernambuco (× 85); (F) Pernambuco (× 85); (G) Rio Grande do Norte (× 85); (H) Rio Grande do Norte (× 80); (I) Paraná (× 75); (J) Paraná (× 65);(L) Rio Grande do Sul (× 80); and (M) Rio Grande do Sul (× 65). F, festoon; Lg, lateral groove; Mg, marginal groove; Pmg, postanal median groove; Ag, anal groove; v, valves; Ad, adanal plates; As, accessory shields.
In all the specimens studied, the anal orifice is composed of two valves (v) and anal groove (Ag) in all specimens collected compose the anal orifice. The valves are articulated and possess four setae arranged symmetrically on each part, for a total of four pairs (Fig. 4). A postanal median groove (pmg) was observed in specimens from Roraima and Rio Grande do Sul (Fig. 4D and M).
Adanal plates (Ad) and accessory shields (As) form the anogenital region and are a ventral pair of large sclerotized structures on the side of the anus in males (Walker et al. 2005, Krantz and Walter 2009; Fig. 4). The two pairs of adanal plates of the studied specimens were parallel, long, and varying in form with some having a sharp posterior margin (Fig. 4B) while others were rounded (Fig. 4F). The accessory shields are located outside the adanal plates and vary in form (Fig. 4). No regular pattern of distribution of setae could be observed for these structures (Fig. 4).
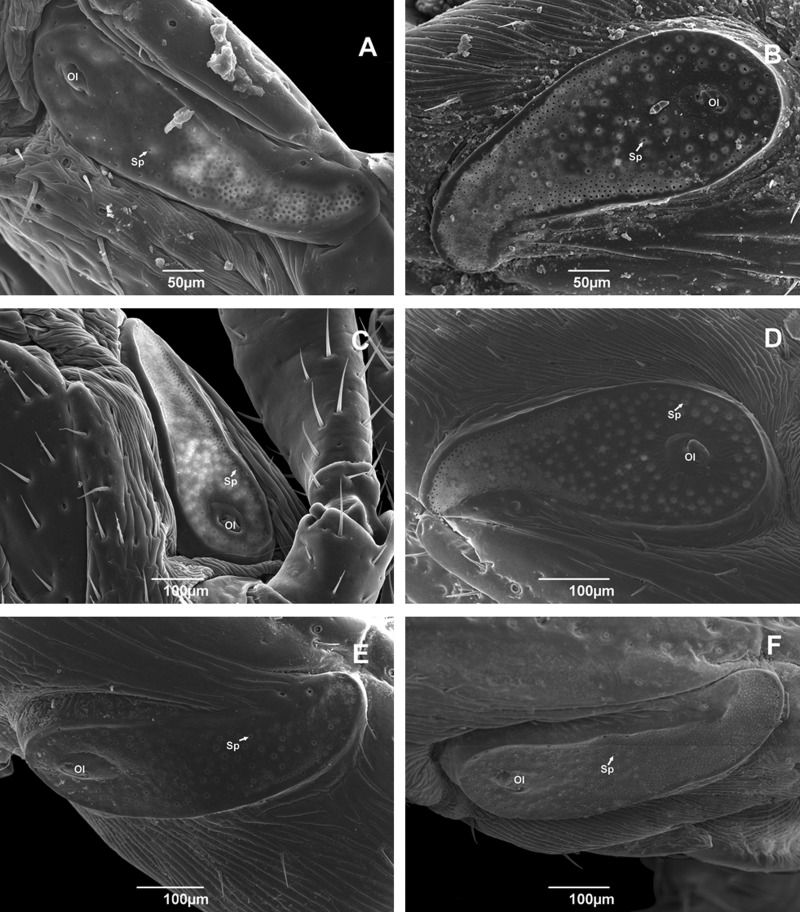
Spiracular plates vary in form, with some being narrow (Fig. 5A—Rondônia, C—Pernambuco, E—Paraná, and F—Rio Grande do Sul), while others are wide (Fig. 5B—Roraima and D—Rio Grande do Norte). The portion called the ostial lip (OI) also varies in size and shape, and possesses a random distribution of surface pores (sp; Fig. 5).
Fig. 5.
Scanning electron micrographs of spiracular plates of adult male Rhipicephalus sanguineus (Acari: Ixodidae) collected in Brazil. (A) Rondônia (× 270); (B) Roraima (× 270); (C) Pernambuco (× 170); (D) Rio Grande do Norte (×230); (E) Paraná (× 220); and (F) Rio Grande do Sul (× 200). Ol, ostial lips; Sp, surface pore.
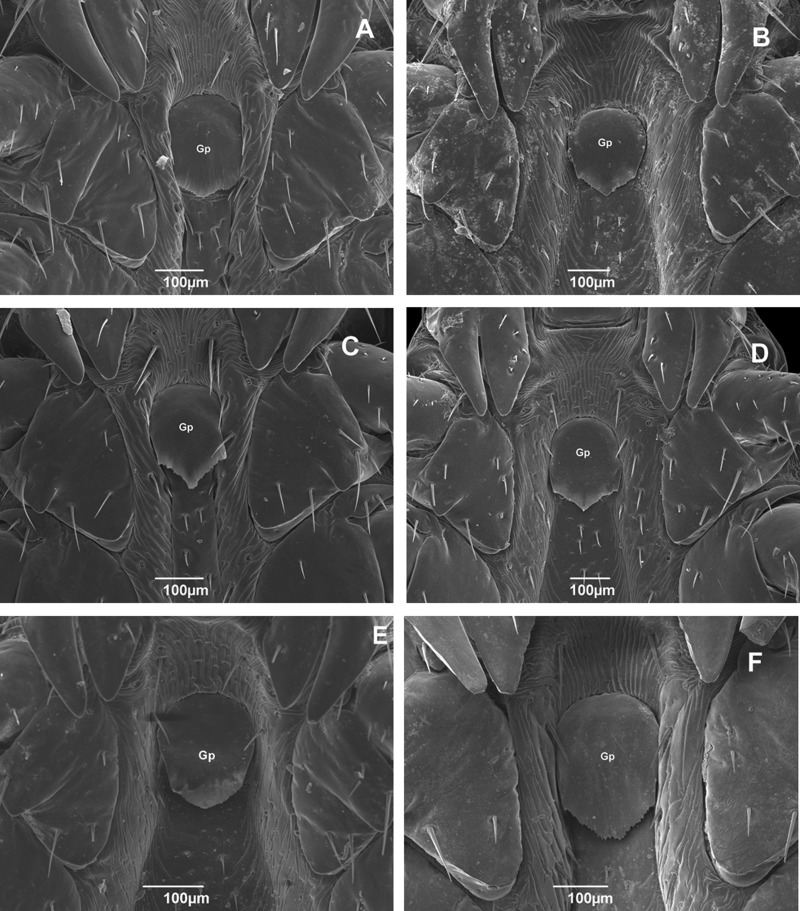
The genital plate exhibits intraspecific variation in angle, but all specimens of the tropical species have a pointed end (Fig. 6A–E). On the other hand, the specimen from Rio Grande do Sul, the representative specimen of the temperate species, exhibited a small recess in the end of the structure that forms two pointed ends rather than one (Fig. 6F).
Fig. 6.
Scanning electron micrographs of genital plates of adult male Rhipicephalus sanguineus (Acari: Ixodidae) collected in Brazil. (A) Rondônia (× 160); (B) Roraima (× 140); (C) Pernambuco (× 160); (D) Rio Grande do Norte (× 130); (E) Paraná (× 130); and (F) Rio Grande do Sul (× 180). Gp, genital plate.
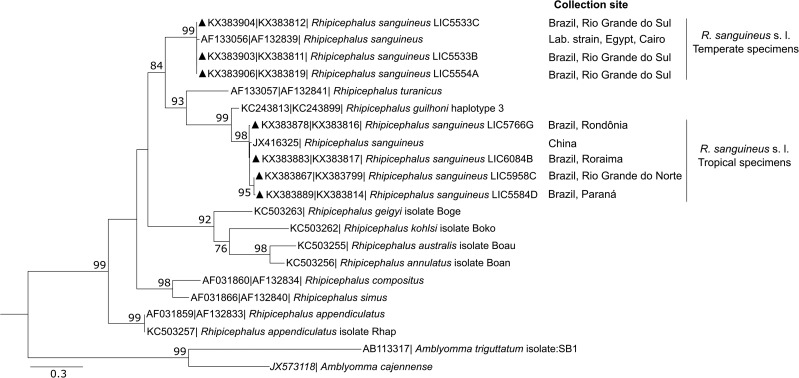
Sequences of 12S rDNA (238 bp) and COX1 (472 bp) genes were concatenated and used for phylogenetic analysis (Fig. 7). The resulting tree shows a clear separation between specimens from Rio Grande do Sul and those from states in the North, Northeast, and South regions of the country.
Fig. 7.
Phylogenetic analysis of Rhipicephalus sanguineus specimens from Brazil. Maximum likelihood phylogenetic tree of Rhipicephalus species using a concatenated sequence of 12S rDNA (238 bp) and COX1 (472 bp) sequences. Scale bar represents genetic distance. Numbers at the branches show bootstrap values (100 = 1,000). Genbank accession numbers are indicated on the left. The triangles represent sequences generated in this study. Other sequences are from Murrell et al. (2000), Dantas-Torres et al. (2013), Liu et al. (2013), and Burger et al. (2014).
Analyses of the individual sequences of 12S rDNA (273 bp), COX1 (453 bp), and D-loop (322 bp) corroborate the results obtained with the concatenated sequence. Phylogenetic analyses found that the sequences for ticks collected in Rio Grande do Sul form a clade separate from a clade composed of ticks from the other 14 states (Supp. Figs. 1–3 [online only]), with the exception of ticks collected in Santa Catarina, which, in the analysis of CO1X gene, were found to belong to the same clade as the ticks from Rio Grande do Sul (Supp. Fig. 2 [online only]).
Several publications have pointed out that R. sanguineus is a complex of at least two, one with a tropical distribution and another with a temperate distribution (Szabó et al. 2005, Moraes-Filho et al. 2011, Nava et al. 2012, Levin et al. 2012, Dantas-Torres et al. 2013, Zemtsova et al. 2016, Labruna et al. 2017). The present study follows the classification of R sanguineus s. l., as proposed by Labruna et al. 2017, and will refer to species as the tropical species (tropical specimens) and the temperate species (temperate specimens).
The molecular results presented here show that sequences obtained from ticks collected in the states of Rio Grande do Sul and Santa Catarina form a clade with sequences of the temperate species, while sequences of ticks from other states form a clade with sequences of the tropical species (Fig. 7, Supp. Figs. 1–3 [online only]).
Supp. Table 2 (online only) provides the pairwise distances observed between the sequences of the different species of the genus Rhipicephalus. Supp. Table 2 (online only) shows the minimum and maximum distances observed when more than one sequence of a species was used. The intraspecific distance of the tropical species and the temperate species do not exceed 3%. The sequence KX383797, which refers to a tick of the tropical species collected in the state of Paraná, shows the greatest intraspecific distance with 2.5% divergence.
A specimen classified as R. turanicus, collected in Turkey (AF132841; Murrell et al. 2000), has a divergence value of 4% when compared with three other sequences of specimens also classified as R. turanicus and that were collected in Italy (KC243917; KC243919) and Greece (KC243912; Supp. Table 2 [online only]). The divergence values among these three sequences are <3%.
All other comparisons between different species exceed 4%, with the least difference existing between the tropical species and R. guilhoni (5.8–7.3%). The two species found in Brazil, the tropical species and the temperate species, have divergence values of 12.3–13.4%. The comparisons R. annulatus X R. australis (8.5%), R. compositus X R. simus (9.3%), and R. guilhoni X temperate species (11.2–11.7%) have values lower than the values for the comparison between the tropical species and the temperate species.
Discussion
Rhipicephalus sanguineus s.s. was first described by Latreille (1806) as “blood-colored, punctuate, with three linear posteriorly depressions, with no distinct spot on the anterior part of the dorsal surface.” Gallia was given as the collection site, a name that would, in 1806, refer to a place now considered to be in France (Nava et al. 2015).
The limited original description and the loss of the type specimen are probably the main sources of problems associated with the name R. sanguineus s.s. Some authors have suggested that the true R. sanguineus s.s. is the species found in tropical regions (Nava et al. 2012), although this association still needs more study in order to be supported.
Currently, R. sanguineus refers to a complex of species (R. sanguineus s. l.) that possess morphological variation, well-supported molecular divergence, and reproductive incompatibility (Szabó et al. 2005, Moraes-Filho et al. 2011, Nava et al. 2012, Levin et al. 2012, Dantas-Torres et al. 2013, Zemtsova et al. 2016, Labruna et al. 2017).
Ticks of the genus Rhipicephalus were considered difficult to classify because they possess a high level of intrageneric morphological uniformity and intraspecific variability. In addition, some authors mention that females are even more difficult to identify since most of the useful characters are found only in males (Warburton 1912).
Despite the difficulties in classifying ticks of this genus, morphological differences were observed between female ticks classified as R. sanguineus collected in São Paulo, Brazil, and Santa Fé, Argentina (Oliveira et al. 2005). Since this study had already addressed female morphology, we choose to perform genetic and morphological analyses using male ticks.
The analyzed sequences of the mitochondrial genes COX1, D-Loop, and 12S rDNA all show that ticks from Rio Grande do Sul, belong to a different well-supported clade separate from a clade of ticks from the other 14 states representing all five geographical regions of the country. The regions and their states are North (Amazonas, Roraima, Rondônia, Tocantins, and Pará), Northeast (Ceará and Rio Grande do Norte), Central-west (Mato Grosso, Mato Grosso do Sul, and Goiás), Southeast (Rio de Janeiro, São Paulo, Espírito Santo), and South (Paraná). Furthermore, analysis of COX1 gene alone provides evidence that the species found in the state of Rio Grande do Sul is the same as the one found in the state of Santa Catarina.
Hebert et al. (2003) developed a COI-based identification system that calculates the nucleotide divergence of 617 pb of the COX1 gene using the Kimura-2-parameter as an evolution model. When applied to the study of lepidopterans they observed that divergence values between species were usually greater than 3%. If the same value is applied as a threshold to guide Rhipicephalus species diagnosis, we find that the sequences of the four ticks classified as R. turanicus have nucleotide divergence values of 4%. Since these ticks were collected from nearby locations and taxonomically classified as the same species, and a reanalysis is not possible, we used 4% as the threshold for classify specimens as belonging to the same species. As result, we observed at least two comparisons with sequences of different species that have nucleotide divergence values higher than 4%, yet smaller than that observed between tropical species and temperate species.
Studying ticks that have already morphologically differentiated (Oliveira et al. 2005), Szabó et al. (2005) tested whether ticks from Argentina and Brazil were able to generate viable progeny. Their results show that in addition to differences in size and other biological characters, these crossings produced sterile hybrids. Another study analyzed three geographically distant populations and showed that ticks from Reunion are reproductively incompatible when crossed with populations from Israel and the United States (Levin et al. 2012). Reproductive compatibility is expected among different populations of a single species, while incompatibility is indicative of crosses between different species. Regarding molecular data, both studies analyzed mitochondrial 12S rDNA, and the resulting phylogenetic trees support the separation of ticks from Argentina and Brazil (Szabó et al. 2005), and from Reunion and Israel and the United States (Levin et al. 2012). Szabó et al. (2005) came to consider that there are at least two species of the R. sanguineus complex in South America, and Levin et al. (2012) considered ticks from Reunion to belong to a different species than those from the United States and Israel, which were distinct populations of the same species. The two species observed by these studies are what we are referring to as the tropical species and the temperate species.
As with the molecular results, the morphological analysis performed here shows differences between species, and confirmed that there is intraspecific variation. The first difference observed was the absence of a pattern in the distribution of the setae located on the dorsum. Barnes and Ruppert (1996) affirmed that the number, shape, and location of setae can vary from one stage to another and among different species.
According to Sonenshine (1991), mechanosensory and chemosensory receptors provide information regarding host fluids and are often found on chelicerae (Ch). However, these structures were not observed in the analysis presented here or by Oliveira et al. (2005).
Haller's organ is a chemoreceptor and hygrosensor located dorsally on tarsi l (Colon et al. 1981). The arrangement of this organ consists of an anterior pit (aP) and a proximal capsule, with several sensilla that respond to different stimuli (Krantz and Walter 2009). According Foelix and Axtell (1972), the Haller’s organ does not differ between larval and adult forms; however, other authors showed that there might be intra- and interspecific variation (Bruce 1971, Foelix and Axtell 1972).
We observed intraspecific variation in the set of festoons between specimens collected in Roraima and Paraná and those from the other states where the tropical species can be found. This specimen has its festoons delimited by the lateral grooves and by the marginal groove. All the other specimens, including the temperate specimen, have their festoons only delimited by deep lateral grooves. Oliveira et al. (2005) also observed that females collected in São Paulo had their festoons delimited by lateral and marginal grooves.
Comparison of the size of palps and chelicerae provides an important character for distinguishing between males and females (Walker et al. 2005). Oliveira et al. (2005) compared female ticks and observed that the temperate species from Argentina possesses palps slightly longer than its chelicerae, whereas the tropical species from Brazil possesses palps much longer than the chelicerae. We observed tropical specimens collected in Pernambuco (Fig. 2E) and Rio Grande do Norte (Fig. 2G), and found them to possess palps which could be considered to be slightly longer than chelicerae.
The anal orifice was found to be composed of two valves and an anal groove in all specimens analyzed. The valves are articulated and possess four symmetrically arranged setae on each part. Oliveira et al. (2005) observed the pattern of four setae on each valve for temperate species; however, the specimen from São Paulo, the tropical species, possessed 3 + 1 setae. A postanal median groove was observed in both species (Fig. 4D and M), and is known to occur in other species of the genus (Walker et al. 2005, Horak et al. 2013).
Rhipicephalus is the tick genus in which the caudal process is most commonly observed (Bristol University Tick ID). Here we found it to vary in size, although some seemed to not have the structure; however, this may be because they were not fed.
It is important to note that direct comparisons between the morphological data observed by Oliveira et al. (2005) and our data cannot be done, as they analyzed females while we analyzed males. Nevertheless, some morphological characteristics that they observed are not sexually dimorphic.
Previous studies have discussed the function of the spiracle (Woolley 1972, Roshdy and Hefnawy 1973, Evans 1992, Dantas-Torres et al. 2013). Hinton (1967) considered the pores of the spiracular plate to be functional openings for gaseous exchange, and the ostium as a nonfunctional, collapsed ecdysial tube formed during the nymphal–adult molt. On the other hand, Hefnawy (1970) suggested that the spiracles are responsible for the physiological control of water loss. The variation in the spiracular plates observed in this study is also present in other species of the genus Rhipicephalus; whereas spiracles of R. walkerae Horak, Apanaskevich et Kariuki, 2013 are long and narrow dorsally (Horak et al. 2013), those of R. turanicus are enlarged (Pegram et al. 1987a). The present study agrees with Dantas-Torres et al. (2013) that the perforation pattern of the spiracular plates can vary among populations.
In summary, the morphological data presented here reinforce the idea that Rhipicephalus exhibits a high level of intraspecific variability and intrageneric morphological uniformity, while other structures are arranged randomly.
According to Walker et al. (2005) only the female genital aperture has taxonomic value for the genus Rhipicephalus. However, the present study provided information about the shape of the genital plate (Gp) of male specimens collected in different localities and showed the potential of this structure to be used as a character to distinguish between the tropical species and the temperate species. It is important to note that there is considerable intraspecific variation in the shape this structure when we compare all the specimens of the tropical species and, because of this, analysis of different specimens of temperate species need to be done to confirm the taxonomic value of this structure.
Our molecular results (12S rDNA, COI gene, and D-loop) and those presented by Nava et al. 2012 (12S rDNA and 16S rDNA), Moraes-Filho et al. 2011 (16S rDNA), and Zemtsova et al. 2016 (12S rDNA) show that the tropical species is found in 19 Brazilian states, the South American countries Argentina, Paraguay, Peru, Colombia, and Venezuela, and in at least 26 other countries. On the other hand, the temperate species is found in at least two Brazilian states (Santa Catarina and Rio Grande do Sul), 11 provinces of Argentina, 3 Departments of Uruguay, and in Chile, Italy, Israel, the United States, South Africa, France, Spain, and Portugal (Supp. Figs. 1–4 [online only]).
Two specimens analyzed here (KX714599.1 and KX714600.1), and another analyzed by Moraes-Filho at al. (2011) (GU553075.1), showed that both the tropical species and the temperate species are found in the state of Santa Catarina. Some specimens are from Garopaba, a seaside town with an altitude of 18 m above sea level, a mesothermal humid climate with hot summers and average annual temperature of 19.9°C, while others came from Lages, a city located in a high region, average of 884 m above sea level, and a subtropical temperate climate with an average annual temperature of 16°C. The characteristics of these two cities may explain why the temperate species is found in the city of Lages, while the tropical species is found in the city of Garopaba. In fact, the species distribution in these cities corroborates the results of Zemtsova et al. (2016) who observed that the temperate species was confined to areas with an annual mean temperature between 10° and 20°C, while the tropical species occupies localities with an annual mean temperature above 20°C.
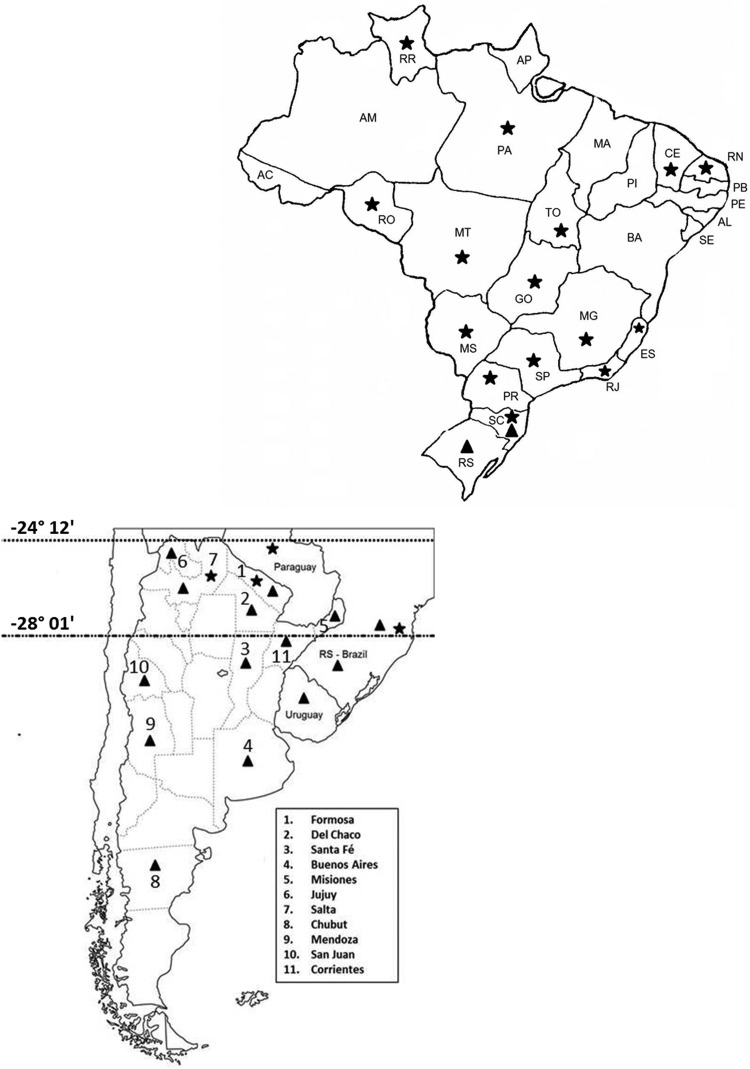
According to the locations of the cities where the samples analyzed and discussed throughout this article were collected, a zone of overlap (sympatry) in the distributions of the two species can be observed starting at GPS coordinates 24° 12' S–65° 19' W (San Salvador de Jujuy—Argentina—JX195167.1), the most northern location for the temperate species, and ending at 28° 01' S–48° 36' W (Garopaba—Brazil), the most southern location of the tropical species (Fig. 8). Indeed, Szabó et al. (2005) showed that the temperate species was able to live at a constant temperature of 20 °C, a relative humidity of 80 ± 5%, and a photoperiod of 14:10 (L:D) h for 2 mo and after this the tick yield of engorged females increased to 100%.
Fig. 8.
Brazilian and Southern Cone maps based on Rhipicephalus saguineus specimens analyzed and discussed throughout this study, showing the zone of overlap between the distributions of the tropical and the temperate species. Triangle—temperate species, Star—tropical species.
The 10 specimens from the state of Paraná, which were molecularly classified as the tropical species, were collected in five cities; Santa Cruz de Monte Castelo (22° 57' S–53° 17' W) was the most northern city, while União da Vitória (26° 13' S–51° 05' W) was the most southern city. If we consider that the average annual temperature of União da Vitória is 17.9 °C, the results of Zemtsova et al. (2016) do not explain the presence of the tropical species in this city. It is possible that, just as there is spatial overlap, there may be overlap in the average annual temperature preferred by these two species, and the fixed value of 20°C may not be the temperature that determines their location.
Regarding the temperate species, since more than half of the territory of Paraná is located south of the city of San Salvador de Jujuy and some cities in the south of the state have annual average temperatures below 20 °C, such as Guarapuava (17 °C) and Curitiba (16.5 °C), it is possible that this species can also be found in the state of Paraná. Despite these possibilities, further studies are needed to examine whether both species are in fact found in this state.
Environmental (Zemtsova et al. 2016) and biological factors appear to influence the dispersion pattern of R. sanguineus s. l. Indeed, Labruna et al. (2017) present results indicating that the absence of the temperate species in tropical regions of Brazil is correlated to a state of inactivity or dormancy (diapause) presented by adult ticks right after molting from nymphs. In contrast, the absence of the tropical species in temperate regions of Brazil is correlated to the fact that this tick species does not enter diapause, and thus is more sensitive to the lethal effects of winter.
Identifying which species is present in a given territory is also important for public health, as vector competence apparently differs between the two species. Tick populations from two Brazilian states (São Paulo and Rio Grande do Sul), one population from Argentina, and another from Uruguay were analyzed and only ticks from São Paulo were shown to be competent vectors of E. canis. The molecular results show that of these, only ticks from São Paulo belong to the tropical species (Szabó et al. 2005, Moraes-Filho et al. 2015).
Acknowledgments
We are grateful to Paulo Vander Ferreira Santana (a teacher from municipality of Rio the Janeiro) and Erik Wild for their help with reviewing the English language part of the manuscript. We would like to thank the Electron Microscopy Platform Rudolf Barth of Instituto Oswaldo Cruz (FIOCRUZ) for the use of the scanning electron microscope and the Genomic Platform-DNA Sequencing (PDTIS-FIOCRUZ) for the assistance with the sequencing of the samples in this study. We would also like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for grants and scholarships.
References Cited
- Aljanabi S. M., Martinez I.. 1997. Universal and rapid salt-extraction of high quality genomic DNA for PCR- based techniques. Nucl. Acids Res. 15: 4692–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragão H., Fonseca F.. 1961. Notas de ixodologia: VIII. Lista e chave para os representantes da fauna ixodológica brasileira: notas de ixolodologia. Mem. Inst. Oswaldo Cruz 59: 115–129.13861962 [Google Scholar]
- Barnes R. D., Ruppert E. E.. 1996. Zoologia dos Invertebrados. Ed. Ltda Roca, São Paulo, p. 651. [Google Scholar]
- Beati L., Keirans J. E.. 2001. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J. Parasitol. 87: 32–48. [DOI] [PubMed] [Google Scholar]
- Bernasconi M. V., Casati S., Peter O., Piffaretti J. C.. 2002. Rhipicephalus ticks infected with Rickettsia and Coxiella in Southern Switzerland (Canton Ticino). Infect. Genet. Evol. 2: 111–120. [DOI] [PubMed] [Google Scholar]
- Bitencourth K., Voloch C. M., Serra-Freire N. M., Machado-Ferreira E., Amorim M., Gazêta G. S.. 2016. Analysis of Amblyomma sculptum haplotypes in an area endemic for Brazilian spotted fever. Med. Vet. Entomol. 30: 342–350. [DOI] [PubMed] [Google Scholar]
- Bristol University Tick ID. Bristol University Tick ID: Online Photographic Guide to Ticks. (http://bristoltickid.blogs.ilrt.org) (accessed 15 January 2016).
- Bruce W. A. 1971. Posterior capsule of Haller’s organ in the lone star ticks Amblyomma americanum (Acari: Ixodidae). Fla. Entomol. 54: 75–72. [Google Scholar]
- Burger T. D., Shao R., Barker S. C.. 2013. Phylogenetic analysis of the mitochondrial genomes and nuclear rRNA genes of ticks reveals a deep phylogenetic structure within the genus Haemaphysalis and further elucidates the polyphyly of the genus Amblyomma with respect to Amblyomma sphenodonti and Amblyomma elaphense. Ticks Tick Borne Dis. 4: 265–274. [DOI] [PubMed] [Google Scholar]
- Burger T. D., Shao R., Labruna M. B., Barker S. C.. 2014. Molecular phylogeny of soft ticks (Ixodida: Argasidae) inferred from mitochondrial genome and nuclear rRNA sequences. Ticks Tick Borne Dis. 5: 195–207. [DOI] [PubMed] [Google Scholar]
- Burkman E. J. 2009. Genetic structure of Amblyomma cajennense (Acari: Ixodidae) populations based on mitochondrial gene sequences. Ph.D. dissertation, Florida International University, Florida.
- Burlini L., Teixeira K. R., Szabó M. P., Famadas K. M.. 2010. Molecular dissimilarities of Rhipicephalus sanguineus (Acari: Ixodidae) in Brazil and its relation with samples throughout the world: Is there a geographical pattern? Exp. Appl. Acarol. 50: 361–374. [DOI] [PubMed] [Google Scholar]
- Chitimia L., Lin R. Q., Cosoroaba I., Wu X. Y., Song H. Q., Yuan Z. G., Zhu X. Q.. 2010. Genetic characterization of ticks from southwestern Romania by sequences of mitochondrial cox1 and nad5 genes. Exp. App. Acarol. 52: 305–311. [DOI] [PubMed] [Google Scholar]
- Colon J. M., Hume D., Rockett C. L.. 1981. Scanning electron microscopy of Haller’s organ in immature stages of Dermacentor variabilis (Say) and Ornithodoros tartakovskyi Olenev (Acari: Ixodida). Micron (1969) 12: 201–202. [Google Scholar]
- Costa-Júnior L. M., Ribeiro M.F.B., Rembeck K., Rabelo E.M.L., Zahler-Rinder M., Hirzmann J., Pfister K., Passos L.M.F.. 2009. Factors associated with seroprevalence of canine babesiosis caused by Babesia vogeli in rural areas of the State of Minas Gerais, Brazil. Res. Vet. Sci. 86: 257–260. [DOI] [PubMed] [Google Scholar]
- Cunha N. C., Fonseca A. H., Rezende J., Rozental T., Favacho A.R.M., Barreira J. D., Massard C. L., Lemos E.R.S.. 2009. First identification of natural infection of Rickettsia rickettsii in the Rhipicephalus sanguineus tick, in the State of Rio de Janeiro. Pesqui. Vet. Bras. 29: 105–108. [Google Scholar]
- Dantas-Torres F. 2008. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): From taxonomy to control. Vet. Parasitol. 152: 173–185. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Latrofa M. S., Annoscia G., Giannelli A., Parísi A., Otranto D.. 2013. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and the Old Words. Parasit. Vectors 6: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peña A., Sanchez C.. 1988. Morfología comparada de Rhipicephalus sanguineus y R. turanicus (Acarina: Ixodidae). Rev. Ib. Parasitol. 48: 51–62. [Google Scholar]
- Evans D. E. 1992. Tick infestation of livestock and tick control methods in Brazil: a situation report. Int. J. Trop. Insect. Sci. 13: 629–643. [Google Scholar]
- Foelix R. F., Axtell R. C.. 1972. Utrastructure of Haller,s Organ in the tick Amblyomma americanum (L.). Z. Zellforsch. Mikrosk. Anat. 124: 275–292. [DOI] [PubMed] [Google Scholar]
- Gehrke F. S., Gazeta G. S., Souza E. R., Ribeiro A., Marrelli M. T., Schumaker T. T.. 2009. Rickettsia rickettsii, Rickettsia felis and Rickettsia sp. TwKM03 infecting Rhipicephalus sanguineus and Ctenocephalides felis collected from dogs in a Brazilian spotted fever focus in the state of Rio de Janeiro/Brazil. Clin. Microbiol. Infect. 2: 267–268. [DOI] [PubMed] [Google Scholar]
- Gray J., Dantas-Torres F., Estrada-Peña A., Levin M.. 2013. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick-Borne Dis. 4: 171–180. [DOI] [PubMed] [Google Scholar]
- Guglielmone A. A., Beati L., Barros-Battesti D. M., Labruna M. B., Nava S., Venzal J. M., Estrada-Peña A.. 2006. Ticks (Ixodidae) on humans in South America. Exp. Appl. Acarol. 40: 83–100. [DOI] [PubMed] [Google Scholar]
- Guindon S., Dufayard J. F., Lefort V., Anisimova M., Hordijk W., Gascuel O.. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59: 307–321. [DOI] [PubMed] [Google Scholar]
- Hebert P. D., Ratnasingham S., de Waard J. R.. 2003. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Biol. Sci. 270(Suppl 1): S96–S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefnawy T. 1970. Biochemical and physiological studies of certain ticks (Ixodoidea). Water loss from the spiracles of Hyalomma (H.) dromedarii Koch (Ixodidae) and Ornithodoros (O.) savignyi (Audouin)(Argasidae). J. Parasitol. 362–366. [PubMed] [Google Scholar]
- Hinton H. E. 1967. The structure of the spiracles of the cattle tick, Boophilus microplus. Aust. J. Zool. 15: 941–945. [Google Scholar]
- Horak I. G., Apanaskevich D. A., Kariuki E. K.. 2013. A new species of Rhipicephalus (Acari: Ixodidae), a parasite of giraffes in Kenya. J. Med. Entomol. 50: 685–690. [DOI] [PubMed] [Google Scholar]
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16: 111–120. [DOI] [PubMed] [Google Scholar]
- Krantz G. W., Walter D. E.. 2009. A Manual of Acarology. Texas Tech University Press, Lubbock, TX, p. 807. [Google Scholar]
- Kuraku S., Zmasek C. M., Nishimura O., Katoh K.. 2013. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 41: W22–W28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna M. B., Gerardi M., Krawczak F. S., Moraes-Filho J.. 2017. Comparative biology of the tropical and temperate species of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) under different laboratory conditions. Ticks Tick Borne Dis. 8: 146–156. [DOI] [PubMed] [Google Scholar]
- Lalzar I., Harrus S., Mumcuoglu K. Y., Gottlieb Y.. 2012. Composition and seasonal variation of Rhipicephalus turanicus and Rhipicephalus sanguineus bacterial communities. Appl. Environ. Microbiol. 78: 4110–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. L., Killmaster L., Zemtsova G., Grant D., Mumcuoglu K. Y., Eremeeva M. E., Dasch G. A.. 2009. Incongruent effects of two isolates of Rickettsia conorii on the survival of Rhipicephalus sanguineus ticks. Exp. Appl. Acarol. 49: 347–359. [DOI] [PubMed] [Google Scholar]
- Levin M. L., Studer E., Killmaster L., Zemtsova G., Mumcuoglu K. Y.. 2012. Crossbreeding between different geographical populations of the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae). Exp. Appl. Acarol. 58: 51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. H., Chen F., Chen Y. Z., Song H. Q., Lin R. Q., Zhou D. H., Zhu X. Q.. 2013. Complete mitochondrial genome sequence data provides genetic evidence that the brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae) represents a species complex. Int. J. Biol. Sci. 9: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Lin X. D., Wang J. B., Qin X. C., Tian J. H., Guo W. P., Fan F. N., Shao R., Xu J., Zhang Y. Z.. 2013. Molecular survey of hard ticks in endemic areas of tick-borne diseases in China. Ticks Tick Borne Dis. 4: 288–296. [DOI] [PubMed] [Google Scholar]
- Marques A. C. 1983. Doenças e Migração Humana. Ver. Soc. Bras. Med. Trop. 16: 125–125. [Google Scholar]
- Moraes-Filho J., Pinter A., Pacheco R. C., Gutmann T. B., Barbosa S. O., Gonzáles M.A.R.M., Muraro M. A., Cecílio S.R.M., Labruna M. B.. 2009. New epidemiological data on Brazilian spotted fever in an endemic area of the State of São Paulo, Brazil. Vector-Borne Zoonotic Dis. 9: 73–78. [DOI] [PubMed] [Google Scholar]
- Moraes-Filho J., Marcili A., Nieri-Bastos F. A., Richtzenhain L. J., Labruna M. B.. 2011. Genetic analysis of ticks belonging to the Rhipicephalus sanguineus group in Latin America. Acta Trop. 117: 51–55. [DOI] [PubMed] [Google Scholar]
- Moraes-Filho J., Krawczak F. S., Costa F. B., Soares J. F., Labruna M. B.. 2015. Comparative evaluation of the vector competence of four South American populations of the Rhipicephalus sanguineus group for the bacterium Ehrlichia canis, the agent of canine monocytic ehrlichiosis. PLoS ONE 10: e0139386.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell A., Campbell N. J., Barker S. C.. 2000. Phylogenetic analyses of the rhipicephaline ticks indicate that the genus Rhipicephalus is paraphyletic. Mol. Phylogenet. Evol. 16: 1–7. [DOI] [PubMed] [Google Scholar]
- Murrell A., Campbell N. J., Barker S. C.. 2003. The value of idiosyncratic markers and changes to conserved tRNA sequences from the mitochondrial genome of hard ticks (Acari: Ixodida: Ixodidae) for phylogenetic inference. Syst. Biol. 52: 296–310. [DOI] [PubMed] [Google Scholar]
- Nava S., Mastropaolo M., Venzal J. M., Mangold A. J., Guglielmone A. A.. 2012. Mitochondrial DNA analysis of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) in the Southern Cone of South America. Vet. Parasitol. 190: 547–555. [DOI] [PubMed] [Google Scholar]
- Nava S., Estrada-Peña A., Petney T., Beati L., Labruna M. B., Szabó M. P., Guglielmone A. A.. 2015. The taxonomic status of Rhipicephalus sanguineus (Latreille, 1806). Vet. Parasitol. 208: 2–8. [DOI] [PubMed] [Google Scholar]
- Norris D. E., Klompen J.S.H., Black W. C.. 1999. Comparison of the mitochondrial 12S and 16S ribosomal DNA genes in resolving phylogenetic relationships among hard ticks (Acari: Ixodidae). Ann. Entomol. Soc. Am. 92: 117–129. [Google Scholar]
- O’dwyer L. H., Lopes V.V.A., Rubini A. S., Paduan K. S., Ribolla P.E.M.. 2009. Babesia spp. infection in dogs from rural areas of São Paulo State, Brazil, Rev. Bras. Parasitol. Vet. 18: 23–26. [DOI] [PubMed] [Google Scholar]
- Oliveira P. R., Bechara G. H., Denardi S. E., Saito K. C., Nunes E., Szabó M. P., Mathias M.. 2005. Comparison of the external morphology of Rhipicephalus sanguineus (Latreille, 1806) ticks from Brazil and Argentina. Vet. Parasitol. 129: 139–147. [DOI] [PubMed] [Google Scholar]
- Pegram R. G., Clifford C. M., Walker J. B., Keirans J. E.. 1987a. Clarification of the Rhipicephalus sanguineus group (Acari, Ixodoidea, Ixodidae). I. R. sulcatus Neumann, 1908 and R. turanicus Pomerantsev, 1936. Syst. Parasitol. 10: 3–26. [Google Scholar]
- Pegram R. G., Clifford C. M., Walker J. B., Keirans J. E.. 1987b. Clarification of the Rhipicephalus sanguineus group (Acari, Ixodoidea, Ixodidae). II. R. sanguineus (Latreille, 1806) and related species. Syst. Parasitol. 10: 27–44. [Google Scholar]
- Ramos R., Ramos C., Araújo F., Oliveira I., Pimentel D., Galindo M., Santana M., Rosas E., Faustino M., Alves L.. 2010. Molecular survey and genetic characterization of tick-borne pathogens in dogs in metropolitan Recife (north-eastern Brazil). Parasitol. Res. 107: 1115–1120. [DOI] [PubMed] [Google Scholar]
- Ribeiro A. L., Faccini J.L.H., Daemon E.. 1995. Estudo das variações morfológicas de Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) no Brasil. Rev. Univ. Rural – Série Ciências da Vida 18: 25–33. [Google Scholar]
- Rosa F., Crespo M. V., Ferreirinha D., Morgado M., Madeira M., Santos-Silva M., Santos A., Sousa R.. 2006. Ticks on dogs and its role as vectors/intermediate hosts Ribatejo and Oeste/Vale do Tejo, Portugal. In: Proccidings of International Congress of Parasitology, Glasgow, Scotland, Medimond S.r.l. pp. 567–570.
- Rosa F., Crespo M. V., Almeida J. P.. 2010. Ixodídeos em cães do concelho de Óbidos. XIV Congresso Ibérico de Entomologia, Lugo, Espanha, 2-5 Setembro. (http://hdl.handle.net/10400.15/179) (accessed 20 January 2016).
- Rohr C. J. 1909. Estudos sobre ixódidas do Brasil. Gomes Irmão, Rio de Janeiro. p. 220. [Google Scholar]
- Roshdy M. A., Hefnawy T.. 1973. The functional morphology of Haemaphysalis spiracles (Ixodoidea: Ixodidae). Zschr Parasitenk 42: 1–10. [DOI] [PubMed] [Google Scholar]
- Sonenshine D. E. 1991. Tick life cycles. Biol. Ticks 1: 51–66. [Google Scholar]
- Szabó M.P.J., Mangold A. J., João C. F., Bechara G. H., Guglielmone A. A.. 2005. Biological and DNA evidence of two dissimilar populations of the Rhipicephalus sanguineus tick group (Acari: Ixodidae) in South America. Vet. Parasitol. 130: 131–140. [DOI] [PubMed] [Google Scholar]
- Tanikawa A., Labruna M. B., Costa A., Aguiar D. M., Justiniano S. V., Mendes R. S., Melo A.L.T., Alves S. J., Azevedo S. S.. 2013. Ehrlichia canis in dogs in a semiarid region of Northeastern Brazil: Serology, molecular detection and associated factors. Res. Vet. Sci. 94: 474–477. [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S.. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira R. F., Biondo A. W., Guimarães A. M., Dos Santos A. P., Dos Santos R. P., Dutra L. H., Diniz P. P., de Morais H. A., Messick J. B., Labruna M. B., et al. 2011. Ehrlichiosis in Brazil. Rev. Bras. Parasitol. Vet. 20: 1–12. [DOI] [PubMed] [Google Scholar]
- Walker J. B., Keirans J. E., Horak I. G.. 2005. The genus Rhipicephalus (Acari, Ixodidae): A guide to the brown ticks of the world. Cambridge University Press, Cambridge, p. 643. [Google Scholar]
- Warburton C. 1912. Notes on the genus Rhipicephalus, with the description of new species, and the consideration of some species hitherto described. Parasitology 5: 1–20. [Google Scholar]
- Woolley T. A. 1972. Scanning electron microscopy of the respiratory apparatus of ticks. Trans. Am. Microsc. Soc. 91: 348–363. [PubMed] [Google Scholar]
- Zemtsova G. E., Apanaskevich D. A., Reeves W. K., Hahn M., Snellgrove A., Levin M. L.. 2016. Phylogeography of Rhipicephalus sanguineus sensu lato and its relationships with climatic factors. Exp Appl Acarol. 69: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]