Abstract
Objective
To compare the 52-week efficacy and safety of SB4 [an etanercept biosimilar] with reference etanercept (ETN) in patients with active RA.
Methods
In a phase 3, randomized, double-blind, multicentre study, patients with moderate to severe RA despite MTX treatment were randomized to receive 50 mg/week of s.c. SB4 or ETN up to week 52. Efficacy assessments included ACR response rates, 28-joint DAS, Simplified and Clinical Disease Activity Indices and changes in the modified total Sharp score (mTSS). Safety and immunogenicity were also evaluated.
Results
A total of 596 patients were randomized to receive either SB4 (n = 299) or ETN (n = 297) and 505 (84.7%) patients completed 52 weeks of the study. At week 52, the ACR20 response rates in the per-protocol set were comparable between SB4 (80.8%) and ETN (81.5%). All efficacy results were comparable between the two groups and they were maintained up to week 52. Radiographic progression was also comparable and the change from baseline in the mTSS was 0.45 for SB4 and 0.74 for ETN. The safety profile of SB4 was similar to that of ETN and the incidence of anti-drug antibody development up to week 52 was 1.0 and 13.2% in the SB4 and ETN groups, respectively.
Conclusion
Efficacy including radiographic progression was comparable between SB4 and ETN up to week 52. SB4 was well tolerated and had a similar safety profile to that of ETN.
Trial registration number
ClinicalTrials.gov NCT01895309, EudraCT 2012-005026-30
Keywords: biosimilar, SB4, Benepali, etanercept, rheumatoid arthritis, biologics
Rheumatology key messages
The efficacy of SB4 and reference etanercept was comparable and maintained up to week 52.
Radiographic progression was comparable between SB4 and reference etanercept.
SB4 was well tolerated and had a similar safety profile to reference etanercept.
Introduction
Targeted biologic therapies such as TNF inhibitors have revolutionized the treatment of RA, AS, psoriasis and other immune-mediated inflammatory diseases [1]. However, the high cost of these treatments places a substantial financial burden on patients and health care systems [2, 3]. Along with the economic burden and imminent patent expiration on many biologic therapies, the interest in biosimilars has increased. In an analysis that assessed the budget impact of introducing an etanercept biosimilar to the five largest European countries for treatment of all licensed reference etanercept (ETN) indications for adults, the substantial cost savings could potentially be used to treat an additional 3100 (UK) to 17 130 (Germany) patients over 5 years, based on a 10 or 25% cost reduction compared with ETN [4].
SB4 is a biosimilar to ETN. Equivalence in pharmacokinetics between SB4 and ETN was demonstrated in a phase 1 study conducted in healthy male subjects [5] and equivalent efficacy and comparable safety between SB4 and ETN up to 24 weeks were demonstrated in a phase 3 study conducted in patients with RA [6]. This report provides data up to 52 weeks from the phase 3 study.
Methods
Patients
Patient eligibility criteria have been described in detail previously [6]. Briefly, patients diagnosed with RA for ⩾6 months and ⩽15 years prior to screening, with a swollen joint count (SJC) ⩾6 and a tender joint count (TJC) ⩾6 and either ESR ⩾28 mm/h or serum CRP ⩾1.0 mg/dl and patients who took MTX for ⩾6 months (stable dose of 10–25 mg/week for ⩾4 weeks prior to screening) were eligible for the study.
Study design
Eligible patients were randomized in a 1:1 ratio to receive 50 mg/week of either SB4 or ETN for up to 52 weeks via self-administered s.c. injection with background MTX (10–25 mg/week) and folic acid (5–10 mg/week). The study was conducted between June 2013 and November 2014 at 73 centres across 10 countries in Europe, Latin America and Asia.
This study was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice Guidelines established by the International Conference Harmonisation. The protocol was reviewed and approved by the institutional review board or the independent ethics committee of each investigational centre. All patients provided written informed consent prior to any study-related procedures.
Assessments
The primary endpoint of this study was the proportion of patients achieving at least 20% improvement in the ACR response criteria (ACR20) at week 24 (results reported previously [6]). Efficacy endpoints up to week 52 included the ACR20, 50 and 70 responses, the numeric index of the ACR response (ACR-N), change in the 28-joint DAS (DAS28) based on ESR and the EULAR response. Major clinical response (ACR70 response for six consecutive months) was assessed at week 52.
Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI) scores, the proportion of patients achieving Boolean-based remission [defined as an SJC ⩽1 and a TJC ⩽1, CRP ⩽1 mg/dl and patient global visual analogue scale (VAS) ⩽1 using a 0–10 scale] and remission based on different indices (DAS28 <2.6, SDAI ⩽3.3, CDAI ⩽2.8) were assessed through post hoc analyses. Physical function and disability were assessed through the HAQ Disability Index (HAQ-DI).
Radiographs of the hands and feet were obtained at baseline and week 52. The images were evaluated by two independent readers who were blinded to patient identity, treatment and the time point taken. When the change score was within the top 5% of cases with the highest differences in score between readers, the radiographs required consensus review by the primary readers. The mean joint erosion and joint space narrowing score of the two readers were used to calculate the van der Heijde modification of the total Sharp score (mTSS) [7]. The proportion of patients with a change in mTSS >0 and mTSS greater than the smallest detectable change (SDC) was calculated post hoc.
Safety assessments included the incidence of adverse events (AEs) and serious AEs (SAEs). Anti-drug antibodies (ADAs) and neutralizing antibodies were measured at weeks 0, 2, 4, 8, 12, 16, 24 and 52. A single-assay approach with SB4 tag was used to assess immunogenicity. ADAs were measured using validated electrochemiluminesence immunoassays and neutralizing antibodies were measured using a competitive ligand-binding assay [MesoScale Discovery (MSD) platform, MesoScale Discovery, Rockville, MD, USA).
Statistical analyses
ACR responses at week 52 were analyzed on the per-protocol set (PPS) in which patients completed the week 52 visit and received 80–120% of both the expected number of study drug administrations and the expected sum of MTX doses without any major protocol deviations affecting the efficacy assessment. The 95% CI of the adjusted treatment difference of ACR responses was estimated using the non-parametric analysis of covariance (ANCOVA) method including baseline CRP as a covariate and the Mantel–Haenszel weight for region. To explore the robustness of the results, the same analysis was repeated for the full analysis set (FAS; all patients who were randomized and received at least one dose of study drug following the intent-to-treat principle) with non-responder imputation for missing data. Other efficacy endpoints at week 52 were summarized descriptively in the FAS without any imputation for missing data.
The radiographic progression between treatment groups was compared with an ANCOVA model for the change in mTSS with treatment group and region as factors and the baseline mTSS as a covariate. In addition, the association of patients with change in mTSS based on SDC (<2.3 vs ⩾2.3) and treatment was analysed post hoc using the chi-square test and the proportion of the progressed patients with a change in mTSS >0 was also compared on the FAS. Safety and immunogenicity endpoints were analysed descriptively on the safety set in which all patients received at least one study drug administration. All statistical analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient disposition and baseline characteristics
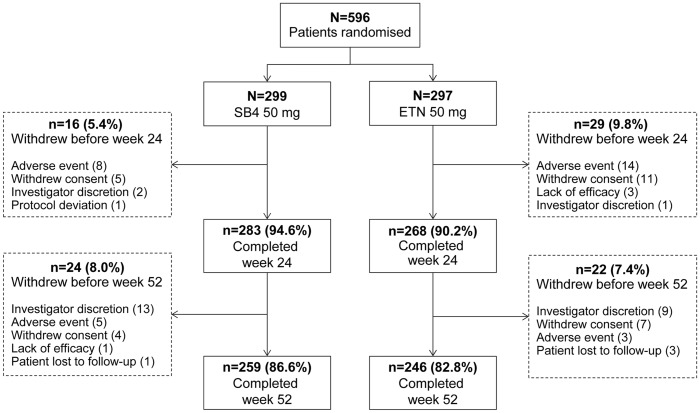
Patient screening started in June 2013 and the 52 week evaluation of the last patient was performed in November 2014. Overall, 596 patients were randomized to receive SB4 (n = 299) or ETN (n = 297) and 505 (84.7%) patients completed 52 weeks of treatment (Fig. 1). The PPS for the 52 week analysis consisted of 224 patients from the SB4 group and 216 patients from the ETN group. Baseline demographic and disease characteristics were comparable between treatment groups (Table 1).
Fig. 1.
Summary of patient disposition
Among the patients who withdrew under investigator discretion, 13 patients in the SB4 group and 8 patients in the ETN group were withdrawn due to the political crisis in Ukraine. ETN: reference etanercept.
Table 1.
Baseline demographics and disease characteristics
| Characteristics | SB4 50 mg (n = 299) | ETN 50 mg (n = 297) | Total (n = 596) |
|---|---|---|---|
| Age, years | 52.1 (11.72) | 51.6 (11.63) | 51.8 (11.67) |
| Age group, n (%) | |||
| <65 years | 253 (84.6) | 262 (88.2) | 515 (86.4) |
| ≥65 years | 46 (15.4) | 35 (11.8) | 81 (13.6) |
| Gender, n (%) | |||
| Male | 50 (16.7) | 44 (14.8) | 94 (15.8) |
| Female | 249 (83.3) | 253 (85.2) | 502 (84.2) |
| Race, n (%) | |||
| White | 279 (93.3) | 273 (91.9) | 552 (92.6) |
| American Indian or Alaskan Native | 5 (1.7) | 7 (2.4) | 12 (2.0) |
| Asian | 11 (3.7) | 13 (4.4) | 24 (4.0) |
| Other | 4 (1.3) | 4 (1.3) | 8 (1.3) |
| Weight, kg | 72.5 (15.93) | 71.0 (14.63) | 71.8 (15.30) |
| Height, cm | 164.4 (8.78) | 164.4 (8.55) | 164.4 (8.66) |
| BMI, kg/m2 | 26.8 (5.51) | 26.3 (5.30) | 26.6 (5.41) |
| Disease duration, years | 6.0 (4.20) | 6.2 (4.41) | 6.1 (4.30) |
| Duration of MTX use, months | 48.2 (39.89) | 47.1 (40.73) | 47.7 (40.28) |
| MTX dose, mg/week | 15.6 (4.52) | 15.5 (4.60) | 15.5 (4.56) |
| CRP, mg/dl | 1.5 (2.00) | 1.3 (1.60) | 1.4 (1.81) |
| ESR, mm/h | 46.5 (22.10) | 46.4 (22.62) | 46.5 (22.34) |
| RF positive, n (%) | 237 (79.3) | 231 (77.8) | 468 (78.5) |
| Swollen joint count (0–66) | 15.4 (7.48) | 15.0 (7.30) | 15.2 (7.39) |
| Tender joint count (0–68) | 23.5 (11.90) | 23.6 (12.64) | 23.5 (12.26) |
| HAQ-DI (0–3) | 1.49 (0.553) | 1.51 (0.560) | 1.50 (0.556) |
| Physician global assessment VAS (0–100) | 62.2 (15.09) | 63.2 (14.76) | 62.7 (14.92) |
| Patient global assessment VAS (0–100) | 61.7 (18.97) | 63.0 (17.70) | 62.4 (18.35) |
| Patient pain assessment VAS (0–100) | 61.8 (20.22) | 62.3 (19.22) | 62.1 (19.71) |
| DAS28-ESR | 6.48 (0.906) | 6.46 (0.885) | 6.47 (0.895) |
| Simplified disease activity index | 39.8 (12.76) | 39.4 (11.81) | 39.6 (12.29) |
| Clinical disease activity index | 38.4 (12.24) | 38.1 (11.57) | 38.2 (11.90) |
| Joint space narrowing scorea | 19.2 (28.83) | 18.4 (26.48) | 18.8 (27.71) |
| Joint erosion scorea | 24.0 (39.63) | 20.5 (28.32) | 22.4 (34.71) |
| Modified total Sharp scorea | 43.3 (67.08) | 38.9 (53.26) | 41.2 (60.86) |
Values are mean (s.d.) unless indicated otherwise.
Based on patients with available radiographic data.
ETN: reference etanercept; VAS: visual analogue scale.
Clinical efficacy
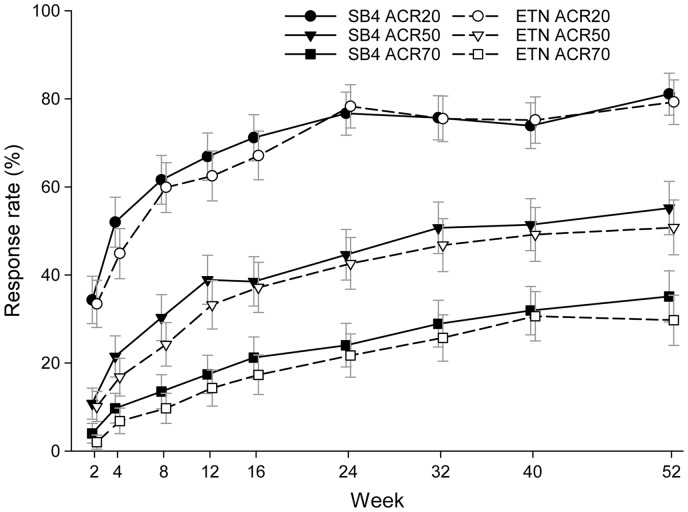
The ACR responses of SB4 were comparable with those of ETN over the time course of the study (Fig. 2). The ACR20 response rate at week 52 in the PPS was 80.8% for SB4 and 81.5% for ETN and the 95% CI of the adjusted difference (SB4 − ETN) was −8.03–6.56%. The ACR50 and ACR70 responses at week 52 in the PPS were 58.5 vs 53.2% and 37.5 vs 31.0% in the SB4 and the ETN groups, respectively. Similar results were shown in the FAS with non-responder imputation with all ACR responses. The results for both PPS and FAS non-responder imputation together with the 95% CIs can be found in supplementary Table S1, available at Rheumatology Online. Subgroup analysis on the ACR20 response rates at week 52 in PPS showed comparable results between the SB4 and ETN group when analysed by the presence of ADA (see supplementary Table S2, available at Rheumatology Online).
Fig. 2.
ACR response rates up to week 52 (full analysis set)
ETN: reference etanercept.
Maintenance of response from week 24 to week 52 was observed (Table 2). Among patients who achieved ACR responses at week 24, a similar proportion of patients in the SB4 and ETN groups maintained the level of responses at week 52 (∼90% for ACR20, 80% for ACR50 and 80% for ACR70). Of the patients who were not ACR responders at week 24, a similar proportion of patients in the SB4 and ETN groups subsequently achieved ACR responses at week 52 (46.6 vs 41.3% for ACR20, 31.9 vs 28.3% for ACR50 and 21.5 vs 15.2% for ACR70, respectively).
Table 2.
Efficacy results at week 52 in FAS with no imputation
| Result | SB4 50 mg (n = 299) | ETN 50 mg (n = 297) |
| ACR response | ||
| ACR20 | 210/259 (81.1) | 195/246 (79.3) |
| Maintenance of response among week 24 responders | 183/201 (91.0) | 176/200 (88.0) |
| Response among week 24 non-responders | 27/58 (46.6) | 19/46 (41.3) |
| ACR50 | 143/259 (55.2) | 125/246 (50.8) |
| Maintenance of response among week 24 responders | 98/118 (83.1) | 86/108 (79.6) |
| Response among week 24 non-responders | 45/141 (31.9) | 39/138 (28.3) |
| ACR70 | 91/259 (35.1) | 73/246 (29.7) |
| Maintenance of response among week 24 responders | 49/64 (76.6) | 44/55 (80.0) |
| Response among week 24 non-responders | 42/195 (21.5) | 29/191 (15.2) |
| ACR-N, mean (s.d.) | 52.08 (30.277) | 49.17 (30.299) |
| Major clinical responsea | 54/259 (20.8) | 45/246 (18.3) |
| DAS28-ESR | ||
| Improvement from baseline, mean (s.d.) | 2.91 (1.360) | 2.80 (1.288) |
| Disease activity | ||
| Low (≤3.2) | 109/260 (41.9) | 86/246 (35.0) |
| Remission (<2.6) | 69/260 (26.5) | 47/246 (19.1) |
| EULAR response | ||
| Good | 108/259 (41.7) | 85/246 (34.6) |
| Moderate | 132/259 (51.0) | 139/246 (56.5) |
| No response | 19/259 (7.3) | 22/246 (8.9) |
| HAQ-DI | ||
| Improvement from baseline, mean (s.d.) | 0.73 (0.582) | 0.70 (0.623) |
| SDAI score | ||
| Improvement from baseline, mean (s.d.) | 28.7 (13.32) | 27.69 (13.740) |
| Disease activity | ||
| Low (>3.3 and ≤11) | 93/260 (35.8) | 92/245 (37.6) |
| Remission (≤3.3) | 62/260 (23.8) | 50/245 (20.4) |
| CDAI score | ||
| Improvement from baseline, mean (s.d.) | 27.9 (12.94) | 26.8 (13.56) |
| Disease activity | ||
| Low (>2.8 and ≤10) | 94/260 (36.2) | 82/246 (33.3) |
| Remission (≤2.8) | 57/260 (21.9) | 48/246 (19.5) |
| Boolean-based remissionb | 40/260 (15.4) | 33/245 (13.5) |
| Radiographic resultsc | ||
| Change from baseline in JSN score, mean (s.d.) | 0.18 (1.142) | 0.43 (2.096) |
| Change from baseline in joint erosion score, mean (s.d.) | 0.26 (1.608) | 0.31 (1.677) |
| Change from baseline in mTSS, mean (s.d.) | 0.45 (2.497) | 0.74 (3.356) |
| Patients with change from baseline in mTSS > 0 | 75/250 (30.0) | 78/228 (34.2) |
| Patients with progression based on the SDCd | 21/250 (8.4) | 32/228 (14.0) |
Values are the number of patients/total number (%) unless indicated otherwise.
ACR70 response for 6 consecutive months.
Defined as SJC ≤1, TJC ≤1, CRP ≤1 mg/dl and patient global VAS ≤1 using a 0–10 scale.
Based on patients with available radiographic results at weeks 0 and 52 (SB4, n = 250; ETN, n = 228).
SDC = 2.3 for change in mTSS.
ETN: reference etanercept; JSN: joint space narrowing.
Other efficacy results at week 52 are presented in Table 2. The mean improvement from baseline in DAS28 was 2.91 for SB4 and 2.80 for ETN and the 95% CI of the difference (SB4 − ETN) in the improvement in DAS28 was −0.092–0.328. Likewise, at week 52 the mean improvement from baseline in SDAI and CDAI were comparable between SB4 and ETN (28.7 vs 27.7 and 27.9 vs 26.8, respectively). The proportion of patients achieving good or moderate EULAR response for SB4 and ETN was 92.7 and 91.1%, respectively. Assessment of patient-reported physical function measured by the HAQ-DI at week 52 showed similar improvements between the two treatment groups. The mean improvement was 0.73 in SB4 and 0.70 in ETN.
In addition, the proportion of patients achieving remission was comparable between the two treatment groups (Table 2). In the SB4 and ETN groups, 15.4 vs 13.5% patients reached Boolean-based remission and 26.5 vs 19.1%, 23.8 vs 20.4% and 21.9 vs 19.5% patients achieved remission by DAS28, SDAI and CDAI, respectively. All efficacy results were comparable throughout the study and a graphical presentation of the mean DAS28, CDAI, SDAI and HAQ-DI scores is shown in supplementary Figs. S1–S4, available at Rheumatology Online.
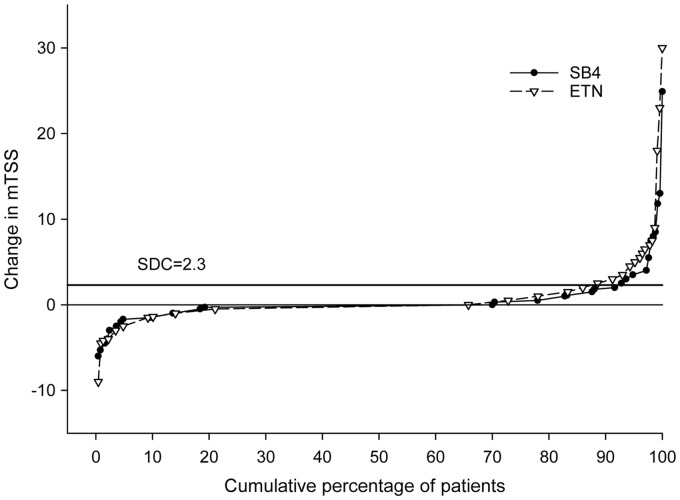
Radiographic progression
The radiographic progression from baseline up to week 52 was comparable between the two treatment groups (SB4, n = 250; ETN, n = 228) (Table 2 and Fig. 3). The mean change from baseline in mTSS was 0.45 and 0.74 in the SB4 and ETN groups, respectively, and the 95% CI of the difference in mTSS was −0.80 to 0.26. Overall 30.0% of patients in SB4 and 34.2% of patients in ETN had a change from baseline in mTSS of >0 (P = 0.325). When evaluated by progression based on the SDC (2.3), 8.4 and 14.0% of patients in the SB4 and the ETN groups, respectively, showed radiographic progression.
Fig. 3.
Change in mTSS at week 52
ETN: reference etanercept.
Safety
Overall, 175 (58.5%) patients in the SB4 group and 179 (60.3%) patients in the ETN group reported at least one treatment-emergent adverse event (TEAE) during the study up to week 52. Frequently occurring TEAEs by preferred term are shown in Table 3. The most frequently reported TEAEs were upper respiratory tract infection (8.0%) and an increase in alanine aminotransferase (ALT; 6.0%) in the SB4 group and injection site erythema (11.1%) and an increase in ALT (5.7%) in the ETN group. Most of the TEAEs were mild to moderate in severity and TEAEs considered related to the study drug were reported in 88 (29.4%) and 109 (36.7%) patients for SB4 and ETN, respectively. Serious TEAEs were reported in 18 (6.0%) patients in the SB4 group and 15 (5.1%) patients in the ETN group and 36 patients discontinued treatment due to TEAEs [16 (5.4%) vs 20 (6.7%) patients in the SB4 and ETN groups, respectively].
Table 3.
TEAEs reported in ≥ 2% of patients by preferred term
| Preferred term | SB4 50 mg (n = 299) | ETN 50 mg (n = 297) |
|---|---|---|
| Upper respiratory tract infection | 24 (8.0) | 16 (5.4) |
| Alanine aminotransferase increased | 18 (6.0) | 17 (5.7) |
| Nasopharyngitis | 15 (5.0) | 16 (5.4) |
| Headache | 13 (4.3) | 8 (2.7) |
| Hypertension | 11 (3.7) | 11 (3.7) |
| Rheumatoid arthritis | 9 (3.0) | 10 (3.4) |
| Aspartate aminotransferase increased | 8 (2.7) | 9 (3.0) |
| Viral infection | 7 (2.3) | 5 (1.7) |
| Injection site erythema | 6 (2.0) | 33 (11.1) |
| Bronchitis | 6 (2.0) | 6 (2.0) |
| Rash | 6 (2.0) | 4 (1.3) |
| Rhinitis | 6 (2.0) | 4 (1.3) |
| Leucopenia | 6 (2.0) | 3 (1.0) |
| Pharyngitis | 5 (1.7) | 8 (2.7) |
| Diarrhoea | 5 (1.7) | 7 (2.4) |
| Urinary tract infection | 5 (1.7) | 7 (2.4) |
| Cough | 4 (1.3) | 10 (3.4) |
| Lymphocyte count decreased | 4 (1.3) | 6 (2.0) |
| Erythema | 2 (0.7) | 10 (3.4) |
| Dizziness | 2 (0.7) | 7 (2.4) |
| Injection site rash | 2 (0.7) | 6 (2.0) |
| Injection site reaction | 1 (0.3) | 8 (2.7) |
Values are n (%).
Patients who had a history of tuberculosis (TB) or who were considered to have latent TB through the QuantiFERON Gold test at the time of screening were included in the study if they completed at least 30 days of treatment for latent TB prior to the first study drug administration. At screening, 12 patients from the SB4 group and 15 patients from the ETN group were considered to have latent TB and no cases of active TB were reported during the study. Serious infections were reported in one (0.3%) patient in the SB4 group (cholecystitis, peritonitis and liver abscess) and five (1.7%) patients in the ETN group (pneumonia, two patients with cellulitis, appendicitis and erysipelas). Malignancies were reported in four (1.3%) patients in the SB4 group (gastric adenocarcinoma, basal cell carcinoma, breast cancer and lung cancer metastatic) and in one (0.3%) patient in the ETN group (invasive ductal breast carcinoma). Breast cancer in the SB4 group and invasive ductal breast carcinoma in the ETN group were considered by the investigator to be related to the study drug, but the other three malignancies were not considered to be related to the study drug.
Injection site reactions (ISRs), counted by the high-level group term of administration site reaction, occurred in significantly fewer patients in the SB4 group compared with the ETN group. One additional patient treated with ETN reported ISRs after the data cut-off point for the 24-week report and the overall incidence of ISRs up to week 52 was 3.7% (22 ISRs reported in 11 patients) in the SB4 group and 17.5% (157 ISRs reported in 52 patients) in the ETN group (P < 0.001). The ISRs reported by preferred term can be found in supplementary Table S3, available at Rheumatology Online. ISRs were also counted as a separate assessment by the investigator at every visit. The evaluation was reported as an ISR when the evaluation result indicated clinically significant abnormality or the abnormality of the ISRs worsened after study drug administration compared with the previous ISR. The incidence of ISR reported in this manner (up to week 52) was lower in the SB4 group than the ETN group [2 (0.7%) vs 17 (5.7%) patients]. Two deaths were reported in the SB4 treatment group due to cardiopulmonary failure and gastric adenocarcinoma and both were reported to be not related to the study drug.
Immunogenicity
The incidence of ADAs was significantly lower in the SB4 group compared with the ETN group up to week 52. After week 24, only one patient from the SB4 group developed ADA and the overall incidence of ADAs up to week 52 was 1.0 (3/299) vs 13.2% (39/296) in the SB4 and ETN groups (P < 0.001), respectively. Among the patients who developed ADAs, only one patient with an antibody titre of 1024 from the ETN group had ADAs with neutralizing capacity. Almost all ADAs were transient and all patients were reported as positive only once throughout the study except for one patient in the ETN group. This patient tested positive for ADA at two visits (weeks 4 and 8). The median peak antibody titre was 4 in the SB4 group (range 2–32) and 16 in the ETN group (range 2–1024).
Discussion
In this randomized, double-blind, parallel-group, multicentre study, 1 year efficacy, safety and immunogenicity of SB4 were compared with those of ETN in patients with moderate to severe RA despite MTX treatment. The 24 week results, including the primary efficacy endpoint (ACR20 response at week 24) have been reported previously and demonstrated equivalent clinical efficacy and comparable safety between SB4 and ETN. This report shows that the comparable improvement in efficacy as well as the similar safety profile is sustained between SB4 and ETN up to week 52. In addition, this report includes radiographic results of a biosimilar and demonstrated that treatment with a biosimilar not only improves clinical and functional outcomes, but also reduces the rate of radiographic progression to a comparable extent to the reference product.
Of the 596 randomized patients, 505 (84.7%) patients completed 52 weeks of treatment. When excluding patients in Ukraine who withdrew from the study due to the political crisis (Fig. 1), the overall retention rate was 90.6% in the SB4 group and 85.1% in the ETN group. The retention rate is higher compared with what has been reported in previous studies or in daily practice with TNF inhibitor biologics [8–11], implying that SB4 and ETN were well tolerated in this study. Additionally, patients were partly recruited from regions where access to targeted biologic therapies is limited other than through participation in clinical studies and access to etanercept in this study may have contributed to the higher retention rate.
As the adjusted treatment difference in ACR20 response rate was within the predefined equivalence margin, therapeutic equivalence between SB4 and ETN was demonstrated. At week 52, ACR20, ACR50 and ACR70 response rates; ACR-N; DAS28 and EULAR response rates were similar between SB4 and ETN, indicating comparable long-term efficacy between SB4 and ETN.
Approximately 80–90% of the ACR20, ACR50 and ACR70 responders at week 24 in the SB4 and ETN groups sustained their responses up to week 52. Among patients who did not achieve ACR responses at week 24, a substantial proportion (20–40%) of patients achieved ACR responses at week 52, which is a similar result to the retrospective analysis from the TEMPO study between weeks 12 and 24 [12]. Furthermore, in a study where patients who responded inadequately to anti-TNF therapy and switched to abatacept as a second-line biologic, 50.4, 20.3 and 10.2% of patients achieved ACR20, ACR50 and ACR70 responses, respectively, after 6 months of treatment [13]. Similarly, 35, 16 and 10% of patients treated with golimumab [14] and 50.0, 28.8 and 12.4% of patients treated with tocilizumab [15] achieved ACR20, ACR50 and ACR70 responses, respectively, after 24 weeks of treatment. Although it is recommended to adjust the treatment when there is no improvement by 3 months, or at most when the target has not been reached by 6 months [16], the above results suggest that maintaining the original treatment could improve clinical response to a similar extent as switching to second-line biologics.
In addition to ACR response rates, various other efficacy responses and self-reported outcomes were analysed not only at specific time points but at every visit during the study. The efficacy endpoints including ACR-N, DAS28, EULAR responses and HAQ-DI were similar over the time course of the study. Demonstration of similar kinetics of the clinical responses in various efficacy endpoints throughout the study provides additional evidence on the biosimilarity of SB4 to ETN [17].
The importance of radiographic inhibition in the evaluation of treatment efficacy has been noted previously [18]. Although radiographic changes cannot be used to determine therapeutic equivalence due to the low power from small differences in radiographic outcomes, it is an objective indicator to compare disease progression. Here we report the results on structural damage assessed on plain films. In both the SB4 and ETN groups, the radiographic progression measured by the change in mTSS was minimal and the numerical values were consistent with previous reports (0.45 in SB4 and 0.74 in ETN) [19]. To further compare the structural damage between the two groups, the proportion of patients showing radiographic progression beyond the SDC (SDC = 2.3 in this study, 8.4% for SB4 and 14.0% for ETN; P = 0.050) and the proportion of patients with mTSS >0 (0.0% for SB4 and 34.2% for ETN; P = 0.325) was assessed. These results suggest that the overall radiographic progression was numerically similar, although the study was not powered to show a difference. Overall, the safety profile of SB4 up to week 52 was comparable to that of ETN and was similar to those observed in the pivotal trials with ETN.
No cases of active TB were reported during the study and only one patient (0.3%) in the SB4 and five patients (1.7%) in the ETN group reported serious infections. The incidence of serious infections observed in the ETN group in this study is lower than that in certain pivotal trials or long-term, open-label extension trials with ETN [10, 11, 20, 21] and similar to that in two pivotal trials with ETN [22, 23].
Malignancies were reported in four (1.3%) patients from the SB4 group and one (0.3%) patient from the ETN group. The incidence of malignancy observed in this study is similar to previously conducted studies [19–25]. No cases of lymphoproliferative disease or lymphoma were reported.
The cumulative percentage of patients with ISRs up to week 52 was 3.7 and 17.5% in the SB4 and ETN groups, respectively. The most common symptom of ISR was redness. Two patients in the SB4 group and seven patients in the ETN group were withdrawn from the study due to AEs of ISRs.
In line with earlier findings on ADAs against ETN, most of the ADAs in this study were transient and detected in the early phase of the treatment (week 4) [6]. There was only one additional patient from the SB4 treatment group who developed ADA after week 24. The overall ADA incidence up to week 52 was 1.0% in the SB4 group and 13.2% in the ETN group. The immunogenicity result was lower in the SB4 group compared with the ETN group when it was reassessed in the pharmacokinetics population with an advanced assay in terms of drug tolerance [26]; additional information on the immunogenicity assessment and results of this study has been published in the correspondences by Emery et al. [27–29]. Despite the difference in immunogenicity profile, there was no impact on clinical efficacy or safety. The ACR20, 50 and 70 response rates at week 52 among patients without ADA were comparable between the SB4 and ETN groups (see supplementary Table S2, available at Rheumatology Online).
The safety and efficacy of SB4 up to week 100 was further assessed in an open-label extension study in a subset of patients who completed the 52 week randomized controlled period of the study [30].
In conclusion, SB4 has shown comparable clinical efficacy, including radiographic progression, to ETN and maintenance of efficacy up to week 52. SB4 was well tolerated with a similar 1 year safety profile to ETN.
Supplementary Material
Acknowledgements
The study was designed by Samsung Bioepis authors in collaboration with the academic authors. The authors thank the patients who were involved in this study, the study personnel who made this work possible and the study investigators: Bulgaria: I. Goranov, M. Geneva-Popova, K. Shimbova, M. Mihaylova, R. Nestorova, R. Stoilov, S. Todorov, E. Dimitrov; Colombia: F. Vargas, J. Londoño; Czech Republic: L. Podrazilova, Z. Mosterova, M. Sedlackova, G. Simkova, H. Brabcova, Z. Urbanova, J. Kopackova, P. Vitek, Z. Stejfova; Hungary: M. Nagy, G. Sulyok, A. Kranicz, A. Sillo, E. Simoncsics; Republic of Korea: J. Choe, S. Lee, S. Lee, S. Kang, S. Bae, J. Kim; Lithuania: V. Lietuvininkiene, R. Kausiene, S. Stropuviene, V. Kriauciuniene; Mexico: I. Garcia de la Torre; Poland: I. Janecka, A. Rychlewska-Hanczewska, D. Lis-Studniarska, A. Zielinska, S. Daniluk, J. Hilt, J. Glogowska-Szelag, A. Kolczewska, B. Sliwowska, M. Rell-Bakalarsk, B. Grabowicz-Waśko, J. Marcinkiewicz, Z. Ruzga, S. Hajduk-Kubacka; Ukraine: G. Ignatenko, M. Vatutin, Y. Gasanov, A. Gnylorybov, S. Shevchuk, D. Rekalov, V. Povoroznyuk, R. Yatsyshyn, A. Petrov, M. Stanislavchuk; UK: R. Haigh. The authors also thank the study team for assistance with the logistic management and reporting of the study, specifically Ilsun Hong (lead clinical study manager) and Evelyn Eubene Hong (Scientific Solutions for writing and editorial support).
Funding: This work was supported by Samsung Bioepis.
Disclosure statement: J.V. has received speaker fees, honoraria and grants/research support from Samsung, UCB, Pfizer, AbbVie and MSD. P.E. has undertaken clinical trials and provided expert advice to Pfizer, MSD, AbbVie, Bristol-Myers Squibb, UCB, Roche, Novartis, Samsung, Sandoz and Lilly. L.P. has received speaker fees and grants/research support from Samsung Bioepis, Roche, MSD, Janssen, Novo-Nordisk, UCB, Pfizer, Novartis, GlaxoSmithKline, Bristol-Myers Squibb and Amgen. S.Y.C. and J.G. are full-time employees of Samsung Bioepis. A.B. has received grants/research support and consulting fees from AbbVie and Samsung Bioepis. R.M. has received royalties, honoraria and grants/research support from Samsung Bioepis. P.W. and A.S. have received research support from Samsung Bioepis. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Kuek A, Hazleman BL, Ostor AJ.. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J 2007;83:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curtis JR, Singh JA.. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther 2011;33:679–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kay J. Biosimilars: a regulatory perspective from America. Arthritis Res Ther 2011;13:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruff L, Rezk MF, Uhlig T, Gommers JW.. Budget impact analysis of an etanercept biosimilar for the treatment of rheumatoid arthritis in Europe. Value Health 2015;18:A639. [Google Scholar]
- 5. Lee YJ, Shin D, Kim Y. et al. A randomized phase l pharmacokinetic study comparing SB4 and etanercept reference product (Enbrel®) in healthy subjects. Br J Clin Pharmacol 2016;82:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emery P, Vencovsky J, Sylwestrzak A. et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2017;76:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 1999;26:743–5. [PubMed] [Google Scholar]
- 8. Duclos M, Gossec L, Ruyssen-Witrand A. et al. Retention rates of tumor necrosis factor blockers in daily practice in 770 rheumatic patients. J Rheumatol 2006;33:2433–8. [PubMed] [Google Scholar]
- 9. Frazier-Mironer A, Dougados M, Mariette X. et al. Retention rates of adalimumab, etanercept and infliximab as first and second-line biotherapy in patients with rheumatoid arthritis in daily practice. Joint Bone Spine 2014;81:352–9. [DOI] [PubMed] [Google Scholar]
- 10. Klareskog L, Gaubitz M, Rodriguez-Valverde V. et al. A long-term, open-label trial of the safety and efficacy of etanercept (Enbrel) in patients with rheumatoid arthritis not treated with other disease-modifying antirheumatic drugs. Ann Rheum Dis 2006;65:1578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klareskog L, Gaubitz M, Rodriguez-Valverde V. et al. Assessment of long-term safety and efficacy of etanercept in a 5-year extension study in patients with rheumatoid arthritis. Clin Exp Rheumatol 2011;29:238–47. [PubMed] [Google Scholar]
- 12. Kavanaugh A, Klareskog L, van der Heijde D. et al. Improvements in clinical response between 12 and 24 weeks in patients with rheumatoid arthritis on etanercept therapy with or without methotrexate. Ann Rheum Dis 2008;67:1444–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Genovese MC, Becker JC, Schiff M. et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005;353:1114–23. [DOI] [PubMed] [Google Scholar]
- 14. Smolen JS, Kay J, Doyle MK. et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 2009;374:210–21. [DOI] [PubMed] [Google Scholar]
- 15. Emery P, Keystone E, Tony HP. et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smolen JS, Landewe R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kay J, Smolen JS.. Biosimilars to treat inflammatory arthritis: the challenge of proving identity. Ann Rheum Dis 2013;72:1589–93. [DOI] [PubMed] [Google Scholar]
- 18. van der Heijde D. Radiographic progression in rheumatoid arthritis: does it reflect outcome? Does it reflect treatment? Ann Rheum Dis 2001;60(Suppl 3):iii47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bathon JM, Martin RW, Fleischmann RM. et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586–93. [DOI] [PubMed] [Google Scholar]
- 20. Klareskog L, van der Heijde D, de Jager JP. et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81. [DOI] [PubMed] [Google Scholar]
- 21. Moreland LW, O’Dell JR, Paulus HE. et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum 2012;64:2824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emery P, Breedveld FC, Hall S. et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82. [DOI] [PubMed] [Google Scholar]
- 23. Genovese MC, Bathon JM, Martin RW. et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum 2002;46:1443–50. [DOI] [PubMed] [Google Scholar]
- 24. Combe B, Codreanu C, Fiocco U. et al. Efficacy, safety and patient-reported outcomes of combination etanercept and sulfasalazine versus etanercept alone in patients with rheumatoid arthritis: a double-blind randomised 2-year study. Ann Rheum Dis 2009;68:1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA. et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA 2012;308:898–908. [DOI] [PubMed] [Google Scholar]
- 26. Emery P, Vencovsky J, Ghil J, Kang JW.. Response to: ‘Comparing the immunogenicity of the etanercept biosimilar SB4 with the innovator etanercept: another consideration’ by Marshall et al.. Ann Rheum Dis 2016;75:e38. [DOI] [PubMed] [Google Scholar]
- 27. Emery P, Vencovsky J, Ghil J.. Response to: ‘Reporting of potential immunogenicity with biologic drugs: clarity and accuracy required’ by Moots et al.. Ann Rheum Dis 2016;75:e25. [DOI] [PubMed] [Google Scholar]
- 28. Emery P, Vencovsky J, Kang JW, Ghil J.. Confirmation on the immunogenicity assay used in the SB4 phase III study: response to the comments by Meacci et al.. Ann Rheum Dis 2016;75:e40. [DOI] [PubMed] [Google Scholar]
- 29. Emery P, Vencovsky J, Ghil J, Kang JW.. Response to: ‘Lower anti-drug antibodies with etanercept biosimilar: can Ctrough explain the differences’ by Shah. Ann Rheum Dis 2016;75:e61. [DOI] [PubMed] [Google Scholar]
- 30. Emery P, Vencovsky J, Sylwestrzak A. et al. Long-term safety and efficacy of SB4 (etanercept biosimilar) in patients with rheumatoid arthritis: comparison between continuing SB4 and switching from etanercept reference product to SB4. Ann Rheum Dis 2016;75(Suppl 2):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.