Abstract
Social, ecological, and climatic factors interact creating a heterogeneous matrix that determines the spatiotemporal distribution of mosquitoes and human risks of exposure to the diseases they transmit. We explore linkages between the social and institutional processes behind residential abandonment, urban ecology, and the interactions of socio-ecological processes with abiotic drivers of mosquito production. Specifically, we test the relative roles of infrastructure degradation and vegetation for explaining the presence of Aedes albopictus Skuse 1894 to better predict spatial heterogeneity in mosquito exposure risk within urban environments. We further examine how precipitation interacts with these socially underpinned biophysical variables. We use a hierarchical statistical modeling approach to assess how environmental and climatic conditions over 3 years influence mosquito ecology across a socioeconomic gradient in Baltimore, MD. We show that decaying infrastructure and vegetation are important determinants of Ae. albopictus infestation. We demonstrate that both precipitation and vegetation influence mosquito production in ways that are mediated by the level of infrastructural decay on a given block. Mosquitoes were more common on blocks with greater abandonment, but when precipitation was low, mosquitoes were more likely to be found in higher-income neighborhoods with managed container habitat. Likewise, although increased vegetation was a negative predictor of mosquito infestation, more vegetation on blocks with high abandonment was associated with the largest mosquito populations. These findings indicate that fine spatial scale modeling of mosquito habitat within urban areas is needed to more accurately target vector control.
Keywords: Aedes albopictus, urban, socio-ecological, vector, northeastern United States
Aedes albopictus (Skuse 1894), commonly known as the Asian tiger mosquito, is an invasive mosquito species of considerable ecological, economic, and human health importance, especially in temperate areas (Juliano and Lounibos 2005, Leisnham and Juliano 2012). Aedes albopictus is a successful invasive species that has spread from southeast Asia throughout the world on the heels of human activity in just three decades (Paupy et al. 2009). Aedes albopictus was first observed in the United States in 1985; since then it has been found in 36 states (Moore and Mitchell 1997, Rochlin et al. 2013a, Kraemer et al. 2015). It has invaded temperate environments by exploiting a variety of larval habitats from natural containers (e.g., tree holes) to anthropogenic containers (e.g., discarded tires). This species can survive extreme weather in microhabitats that buffer these conditions, and lays diapausing eggs that can survive drought and winter (Becker et al. 2012, Waldock et al. 2013). Not only has it successfully invaded temperate North America, there is evidence that it outcompetes many of the resident mosquito species it encounters (Juliano and Lounibos 2005, Costanzo et al. 2011, Rochlin et al. 2013b).
Although the importance of Ae. albopictus in arboviral transmission in the United States remains unclear, multiple endemic arboviruses have been isolated from Ae. albopictus collected in the field from locations across the globe, including Cache Valley, eastern equine encephalitis, Jamestown canyon, La Crosse, dengue, chikungunya, Zika, and West Nile viruses (Ibáñez-Bernal et al. 1997, Moore and Mitchell 1997, Gerhardt et al. 2001, Turell et al. 2005, Pan American Health Organization/World Health Organization 2016). Local transmission of dengue and chikungunya viruses by established Ae. albopictus populations has already occurred in the temperate areas of Europe (Chretien and Linthicum 2007, Rezza et al. 2007) and Asia (Tsuda et al. 2016, Quam et al. 2016).
Even in the absence of disease transmission, infestation with Ae. albopictus may accrue negative health outcomes. It has become the most common nuisance mosquito in the eastern United States, aggressively biting humans during the day, so much so that it is reported as a significant deterrent of outdoor recreation in northeastern U.S. cities (Dowling et al. 2013a, Worobey et al. 2013, Halasa et al. 2014). In a survey of residents across six neighborhoods in Washington, DC, 61% (n = 247) of those surveyed said they change their behavior (did not spend time outside, take walks, or garden) in response to Ae. albopictus biting pressure (Dowling et al. 2013a).
Limiting disease transmission and nuisance biting hinges on reducing vector populations and human–vector contact rates. Principal vector control methods usually include larval source reduction to reduce standing water or the use of night-time insecticides, but these methods are less effective for Aedes, in general, because these mosquitoes mature in small containers, are active during the day, and have evolved resistance to many commonly used insecticides (Bartlett-Healy et al. 2012, Leisnham and Juliano 2012, Marcombe et al. 2014). Instead, effective control depends on the removal or regular maintenance of smaller container habitats, which requires concerted involvement of vector control officials and the community at large.
Although the risk of disease transmission and nuisance biting is linked to adult populations (Gratz 2004, Andreadis et al. 2004), vector population growth is directly influenced by ecological processes, including climatic conditions and resource quality, at the aquatic juvenile stages (e.g., eggs, larvae, and pupae; Kraus and Vonesh 2012, LaDeau et al. 2015). Two key climatic conditions that directly impact Ae. albopictus populations are temperature and precipitation. Temperature can have both direct and indirect influences on adult and juvenile survival, juvenile development, and adult female biting behaviors (Alto and Juliano 2001b, Costa et al. 2010, Roiz et al. 2010, Carrington et al. 2013, Couret et al. 2014). Likewise, precipitation is necessary to fill container habitats and maintain water resources necessary for juvenile mosquito development (Alto and Juliano 2001a, Bartlett-Healy et al. 2011, Unlu et al. 2014).
In its native range, Ae. albopictus habitat includes tree holes and other small, naturally occurring container habitat (Hawley 1988). However, the successful invasion of Ae. albopictus is tied to its ability to take advantage of artificial container habitats that pervade in human-dominated landscapes (Hawley 1988). Urban environments usually provide ample container habitat for larval mosquitoes; however, the quality and volume of container habitats has been shown to vary across fine spatial scales (Leisnham and Slaney 2009, Yee et al. 2012). Infrastructural decay manifested as abandoned buildings and semipermanent dumping grounds (when people dispose of garbage within residential areas [Hotez et al. 2014]) may increase critical habitat for Ae. albopictus (Ferwerda 2009, Becker et al. 2012, Dowling et al. 2013b). Lower socioeconomic status neighborhoods, where histories of public and private disinvestment have increased the likelihood of abandonment and dumping, have repeatedly been shown to have more discarded containers and more containers infested with juvenile Ae. albopictus (Chambers et al. 1986, Joshi et al. 2006, Unlu et al. 2011, Bartlett-Healy et al. 2012, Dowling et al. 2013b, LaDeau et al. 2013, Rochlin et al. 2013a). However, other research has found that permanent containers that are either more closely linked to human water storage or retain water for longer are more suitable larval habitats for Ae. albopictus (Unlu et al. 2011, Becker et al. 2014). These different conclusions are likely derived from differences in the precipitation context during each study, as the amount of precipitation may influence what types of containers are most important at different times. In times of low precipitation, smaller discarded containers are quick to dry out and may be too transient to allow subadult development, and Ae. albopictus may persist in places where people irrigate and unintentionally maintain juvenile mosquito habitat (Bartlett-Healy et al. 2012, Becker et al. 2012).
Vegetation can also alter habitat suitability for juvenile Ae. albopictus. The most well-documented effects of vegetation on container-mosquito ecology are the alteration of detrital resource inputs into containers (Kling et al. 2007, Bartlett-Healy et al. 2012, Yee et al. 2012). Additionally, shade may lower water temperature and the rate of evaporation such that water remains standing longer in containers (Beier et al. 1983). Urban vegetation patterns are also linked to underlying demographic and social processes of cities. For instance, the number of overall and exotic plant species has been related to the number of people and the age and affluence of neighborhoods (Pickett et al. 2011, Johnson et al. 2014). Differences in vegetation cover owing to building removal and municipal- or community-based management practices may further influence the quality of urban juvenile mosquito habitat.
The local habitat conditions that influence mosquito life history often vary at spatial scales significantly finer than the land use and census tract boundaries that inform many social and ecological variables (Rey et al. 2006, Leisnham and Juliano 2009, Leisnham et al. 2014). Aedes albopictus is likely influenced by local biophysical conditions that support larval development, resting survivorship, and host access all within the hundred meter flight range (Marini et al. 2010). Although there is consensus regarding the distribution of Ae. albopictus on large spatial scales, understanding the habitat use and biting behavior variation at fine spatial scales is required to guide effective vector control. Accordingly, we aim to understand the influence of infrastructure degradation, vegetation, and how precipitation interacts with these biophysical variables on the presence of Ae. albopictus to better predict spatial heterogeneity in mosquito exposure risk within urban environments. The findings of this study are based on primary, field-collected data across three active mosquito seasons and five neighborhoods in West Baltimore, MD, that represent a stark socioeconomic gradient. The findings provide novel inference on the interactive influences of precipitation and key socio-ecological variables that vary within urban environments.
Materials and Methods
Neighborhood Selection
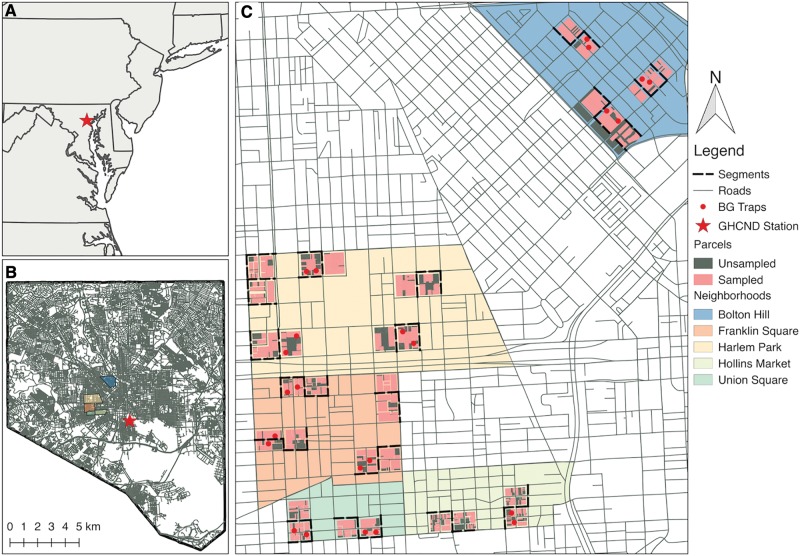
We collected data in five neighborhoods that spanned low, medium, and high socioeconomic status (SES) categorization. These neighborhoods were identified a priori using online data (http://bniajfi.org/and google maps, accessed 11 May 2017) and ground surveys to ensure that selected neighborhoods were predominantly residential and included neighborhoods with median household incomes from below, at and above the City’s 2014 median household income estimate of US$41,819 (United States Census Bureau/American FactFinder 2014). We further examined educational attainment and housing quality extracted from census data to categorize our five focal neighborhoods along an SES gradient (see Supplemental Material [online only]). From the five study neighborhoods, we randomly selected 33 blocks identified as predominantly residential housing (avoiding blocks with schools, large apartment complexes, and businesses). Blocks were selected in clusters of two adjacent, with at least one block between each of the two to five cluster pairs per neighborhood (Fig. 1).
Fig. 1.
Overview maps: (A) Location of Baltimore, MD; (B) Location of West Baltimore neighborhoods and GHCN weather station within the city limits of Baltimore; and (C) Close-up view of the neighborhoods showing the spatial distribution of block clusters within neighborhoods, street segments surveyed for neighborhood attributes, parcels sampled for juvenile mosquitoes, and location of BG-Sentinel traps for adult surveillance.
Surveillance
We conducted comprehensive, block-scale surveillance of larval habitat three times during each season (June, July, and September) during the summers of 2013, 2014, and 2015. One block per cluster was designated as a “focal” block (16 total), and all accessible containers were sampled and categorized as structural, functional, or trash as in previous work described by (Dowling et al. 2013b, LaDeau et al. 2013); structural containers were permanent artificial containers; functional containers were moveable and used for yard work, storage, or recreation; and litter or other trash were categorized as discarded containers. Collected mosquitoes were enumerated and identified to species.
During the same time period, we conducted adult mosquito sampling for 3 d every 3 wk using BG-Sentinel (Biogents, Regensburg, Germany) traps baited with CO2 and octenol lures in each study year. Octenol lures contain a compound found in cattle odors and human sweat and are widely used to trap mosquitoes and other blood-feeding animals (Dekel et al. 2016). Traps were deployed in pairs on 12 of the 16 focal blocks, three blocks each in high and medium SES locations and six in low SES sites (Fig. 1). Traps were deployed and operational for 72 h every 3 wk between May and September in 2013, 2014, and 2015. Mean female mosquito densities per trap night were calculated for each focal block and sampling period corresponding to the three juvenile mosquito sampling periods.
Climate
We used NOAA GHCN-daily climate data from the Maryland Science Center (GHCND: USW00093784; Menne et al. 2015). The station is located on an average 3 km (SD = 0.44 km) from the focal blocks (Fig. 1). We calculated the total precipitation (tenths of mm) for the 2 wk preceding each juvenile sampling date, corresponding to juvenile development timing (Waldock et al. 2013). We also recorded if it rained in the 2 d prior to sampling as a binary indicator variable, as this would inflate the number of rain-filled containers present but not provide sufficient time for larval development. We calculated a long-term average (2005–2015) for each sample period to provide an index for comparison (Supp. Fig. 1 [online only]).
Infrastructural Degradation
In 2014, we performed a neighborhood mapping survey in which street segments (mean = 3.4, SD = 0.9) in each block cluster were surveyed to enumerate specific conditions that might promote Ae. albopictus population growth. Two researchers walked the length of each tract and independently counted the number of trees, abandoned buildings (officially condemned or with boarded-up entry), parks, grass lots, garbage piles, and litter items. Data were entered into a geographic information system (GIS) using the program QGIS (Quantum GIS Development Team 2017), and segment length was calculated in meters. The total segment length surveyed per block cluster ranged from 281 to 695 m (mean = 421, SD = 103). Enumerated characteristics were then divided by the total length surveyed in meters per block cluster to standardize across all block clusters.
Vegetation
Landsat level 2 surface reflectance normalized difference vegetation index (NDVI) data (30 m2) were used to measure vegetation for each year of surveillance (2013–2015). All images were acquired in April (21 April 2013, 24 April 2014, and 11 April 2015). Normalized difference vegetation index ranges from 0 to 1; values from 0.2 to 0.5 are considered to represent sparse vegetation, whereas values from 0.6 to 0.9 indicate dense vegetation (United States Geological Survey 2015). Normalized difference vegetation index (mean and standard deviation) was calculated for each of the 16 block clusters, and values were standardized for each year by subtracting the mean and dividing by the standard deviation to enable comparisons between years.
Dependent Variable
To best understand how SES characteristics and precipitation influence mosquito production in this landscape, we built of a commonly used metric of immature mosquito infestation, the container index. The container index measures the percent of water-holding containers positive for immature mosquitoes and has been linked to adult mosquito densities in Baltimore previously (Bodner 2014). Underlying our data, we find an association between the percent of containers positive and the number of water-holding containers and differences in the number of containers sampled by block reflective of the underlying total area. Based on these relationships, we deem it necessary to incorporate the sampled area in the calculation of the container index. Therefore, we used block-level data for each year to estimate the percent of positive containers per square meter by multiplying the average number of containers per square meter by the percent of positive containers sampled, henceforth called the standardized container index (SCI).
Explanatory Variables
To evaluate colinearity among the suite of variables enumerated, Pearson product-moment correlation coefficients were computed comparing median household income, infrastructure condition metrics, and vegetation. Colinearity of explanatory variables were assessed with a cut-off value of r = 0.5. We explore the power of explanatory variables at both neighborhood and block scales.
Analysis
We used a generalized linear (Poisson) mixed model to evaluate how socio-ecological indicators, infrastructural decline, and vegetation influence SCI at the block scale. We used mixed-model regression to accommodate temporal and spatial sampling structure (sample periods within years). Model Akaike information criterion (AIC) scores were used to compare a baseline model with precipitation, a model that included both climatic and socio-ecological predictors, and a model that included two-way interaction terms among predictors (Burnham and Anderson 2003). Model AIC scores were compared by measuring Delta, which is the difference in AIC score between models (ΔAIC). All analyses were done in the statistical software R with the mixed model regression package glmmADMB (Fournier et al. 2012, Skaug et al. 2012).
Results
Neighborhood Condition
There were differences in the total number of standing abandoned buildings by SES category and minor fluctuations across years: In high SES blocks, there were three apparently abandoned structures out of 509 (1%); in median SES neighborhoods, there were on an average 56.33 (SD = 8.97) out of 1,035 (5%); and in low SES, there were on an average 403.67 (SD = 5.69) out of 1,554 (26%). We repeated the enumeration of abandoned buildings each year of the study. In the middle and low SES neighborhoods, there was a net upward trend in the number of abandoned properties with an additional 16 in medium and 11 in low SES neighborhoods over the 3 yr of surveillance. The number of abandoned buildings on a block describes the state of infrastructural degradation and covaries with garbage (r = 0.83; P value < 0.001) and semipermanent dumping grounds (r = 65; P < 0.001). The number of abandoned buildings, amount of litter, and semipermanent dumping grounds represent proximal measurements for potential breeding habitat. Because these metrics covary, we chose to include only the number of abandoned buildings in our model.
Although NDVI varies across neighborhoods, it does not vary linearly with income (Supp. Fig. 2 [online only]). The relative greenness of a neighborhood is high for both low- and high-income neighborhoods and lowest in medium-income ones. Because it does not vary linearly by our SES categorization as abandoned buildings do, including a vegetation measurement characterizes something other than what abandoned buildings represents. Furthermore, including a measurement of vegetation is important because it influences the duration water remains in containers, water temperature, and the availability of nutrients for developing mosquitoes (Tun-Lin et al. 1996). Its interactive effect with precipitation is therefore of interest.
The median household income was negatively associated with the quantity of garbage (r = −0.22; P-value = 0.006), the number of semipermanent dumping sites (r = −0.36; P < 0.001), and abandoned buildings (r = −0.27; P-value < 0.001) but not NDVI (r = −0.05; P-value = 0.56) in a neighborhood. Owing to the intraneighborhood variability in median household income (see Supplemental Material [online only]: SES Categorization), we decided to use more direct measurements of block cluster socio-ecological variables as described by abandonment and vegetation. The number of abandoned buildings, the quantity of garbage, and semipermanent dumping sites support our a priori designation of a socioeconomic gradient across the selected neighborhoods (Supp. Fig. 2 [online only]).
Mosquito Ecology
The 16 focal blocks across the five neighborhoods consisted of 2,287 total land parcels, of which we visited 75.5% across the duration of the study. Sampling access was highest in low SES blocks, although coverage ranged from 73.0–77.1% across all neighborhoods. Water samples were collected from 1,342 containers. Of these, 72.8% were positive for Ae. albopictus larvae and 24.2% positive for Ae. albopictus pupae, regardless of SES category. Nearly 60% of all positive container habitat contained juvenile Ae. albopictus. Other species collected include Culex (pipiens and restuans, 32% of positive containers) and Aedes japonicus (Theobald) (10% of positive containers). Aedes triseriatus (Say), Culex territans Walker, and Anopheles spp. occurred in <1% of all positive habitats. Aedes albopictus was the predominant species recovered from adult samples, making up 75% of all females collected. Culex spp. (21%), Ae. japonicus (3%), Aedes vexans (Meigen), and Anopholes spp. (<1% each) were also collected in BG-Sentinel traps.
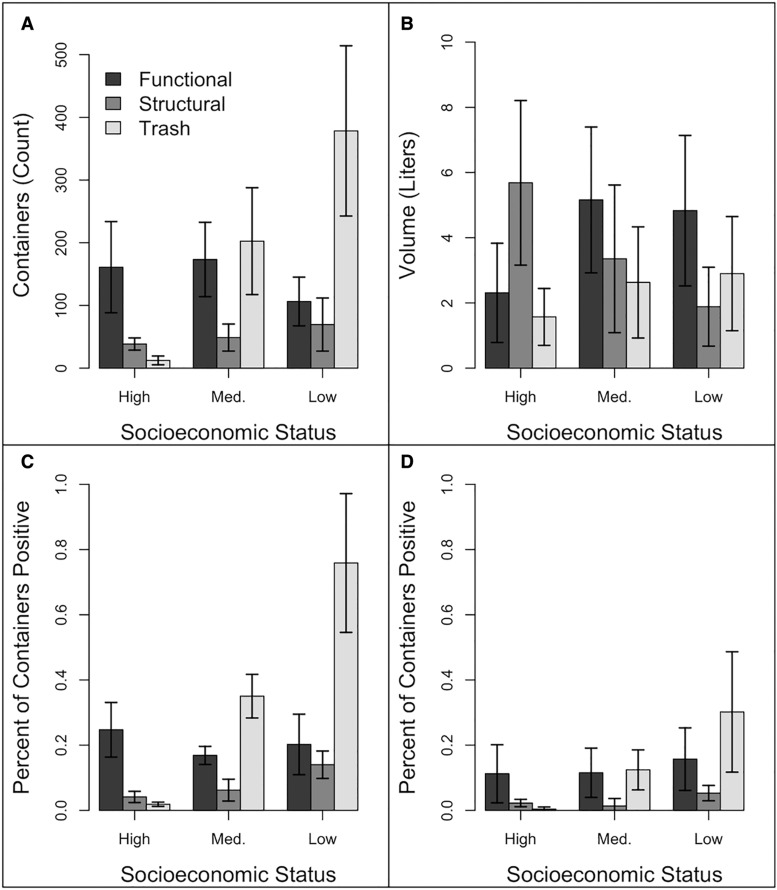
Discarded, trash container habitat was the main type of container habitat in median and low SES blocks, whereas functional and structural container habitat predominated in high SES blocks (Fig. 2, Panel A). Even within a container type category, the mean volume of containers sampled varied across the different neighborhoods, with smaller discarded containers and larger structural containers in high SES blocks compared with those found in lower SES neighborhoods (Fig. 2, Panel B). Consistently, the percentages of positive containers for larvae and pupae were higher in trash containers, and the highest percent of trash containers positive for Ae. albopictus was in low SES blocks (Fig. 2, Panel C).
Fig. 2.
(A) Mean containers per square kilometer; (B) Mean container size in liters; (C) Mean percent of containers positive for larvae; and (D) Mean percent of containers positive for pupae for each socioeconomic status and container type (functional, structural, or trash).
The final model included precipitation, number of abandoned buildings, and vegetation for each block and with interaction terms (AIC = 1998.2; Table 1). This model showed improvements over a baseline model with only the temporally varying climate parameters (previous 2 wk accumulated precipitation and the 2-d indicator; ΔAIC= 550), a model with only spatially predictive variables, abandoned buildings, and vegetation (ΔAIC = 259), and a model incorporating both climatic and spatially explicit variables but no interaction terms (ΔAIC = 193.6).
Table 1.
Poisson generalized linear mixed model results (coefficients and 95% confidence intervals) for larval and pupal SCI and mean adult abundance
| Larvae SCI | Pupae SCI | Mean adults | |
|---|---|---|---|
| (Intercept) | 4.03 (3.54, 4.52) | 1.09 (0.67, 1.51) | 2.3 (1.98, 2.61) |
| PPT2WK | 0.1 (0.08, 0.13) | 0.08 (0.05, 0.11) | NA |
| PPT2DY | −0.47 (−0.56, −0.38) | −0.55 (−0.72, −0.39) | NA |
| NDVI | −0.17 (−0.22, −0.12) | −0.36 (−0.44, −0.29) | −0.08 (−0.16, 0.00) |
| AB | 0.14 (0.08, 0.20) | 0.20 (0.12, 0.28) | 0.19 (0.12, 0.27) |
| NDVI: AB | 0.14 (0.11, 0.18) | 0.15 (0.09, 0.21) | 0.36 (0.28, 0.43) |
| PPT2WK: NDVI | 0.03 (0.01, 0.04) | 0.08 (0.05, 0.11) | NA |
| PPT2WK: AB | 0.09 (0.07, 0.11) | 0.09 (0.06, 0.12) | NA |
SCI, standardized container index; PPT2WK, sum of precipitation in the 2 wk prior to sampling; PPT2DY, indicator variable (0 or 1) if it rained in neither, one, or both of the 2 d prior to sampling; AB, abandoned buildings; NDVI, normalized difference vegetation index.
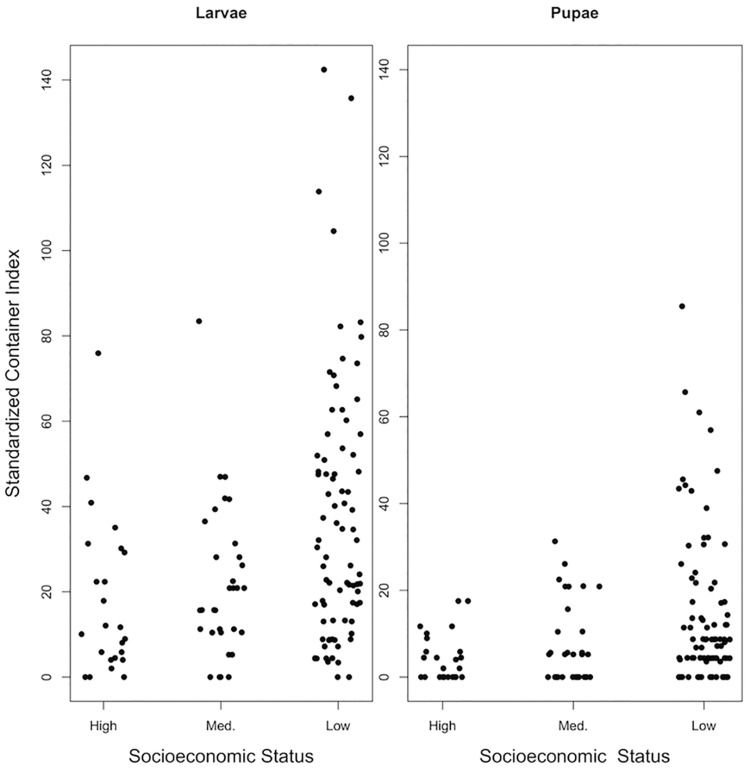
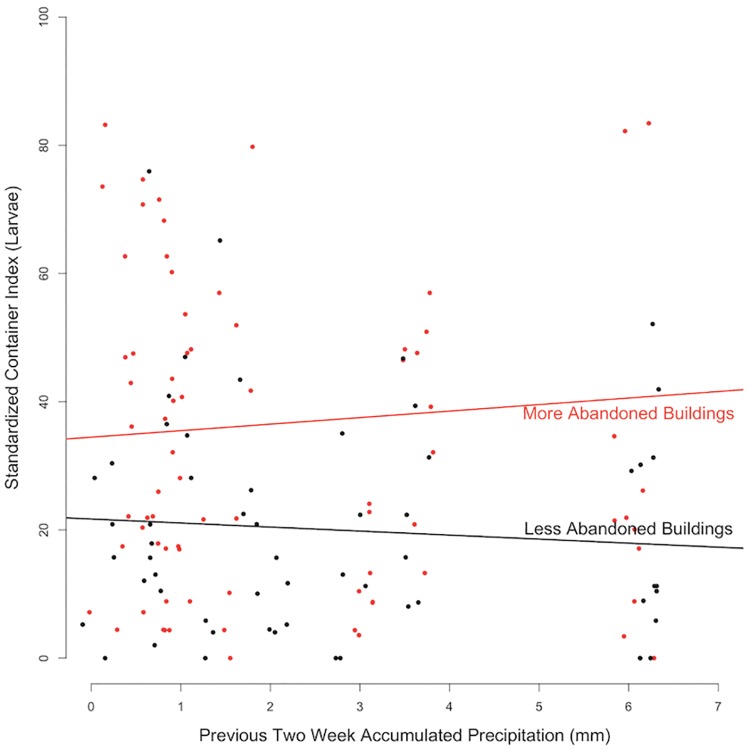
The SCI for both pupae and larvae (Fig. 3) was positively associated with precipitation, numbers of abandoned buildings, and with reduced vegetation (Table 1). In addition, we found significant interactions that support a mediating effect of abandoned buildings on the influence of precipitation (Fig. 4) and vegetation (Table 1). Although areas with more abandoned buildings had higher SCI overall, the effect of precipitation differed based on abandonment. Blocks with fewer abandoned buildings showed a negative relationship between increasing precipitation and SCI, whereas blocks with more abandoned buildings showed a positive relationship, indicating that the regulating effects of precipitation on mosquito productivity varies with infrastructure condition. Likewise, while NDVI was negatively associated with SCI, it was a positive predictor on blocks with more abandoned buildings (Table 1).
Fig. 3.
Standardized container index (SCI) used to account for differences in underlying block cluster areas and therefore differences in the number of containers present for sampling.
Fig. 4.
Mediating effect of abandoned buildings on the influence of precipitation (mm) on SCI larvae.
Additionally, we modeled the influence of these socio-ecological factors, abandonment, and vegetation on adult Ae. albopictus populations. The influence of precipitation was left out of this model, as the link between precipitation and adult numbers is more tenuous owing to success of juvenile development and conditions suitable to adult breeding and biting behaviors. We find that predictors of juvenile habitat production—abandoned buildings and vegetation—are also predictive of spatial-temporal variability of adult abundance (Table 1; Supp. Fig. 3 [online only]). Adult abundance was positively associated with the number of abandoned buildings and, while vegetation on its own was not significant, areas of high abandonment and high vegetation indicate even greater adult abundance than high abandonment alone (Table 1). Across the 3 yr of analysis, we found significant positive relationships between mean adult abundance and larval SCI (r = 0.3, P = 0.004) and mean adult abundance and pupal SCI (r = 0.29, P = 0.005), indicating that knowing larval or pupal SCI on a given block in a given year is predictive of adult abundance at that same spatiotemporal scale.
Discussion
We found that infrastructure decay and vegetation both play important roles in determining the distribution of Ae. albopictus in the urban landscape, with higher rates of abandonment and reduced vegetation increasing the likelihood of Ae. albopictus infestation. We further show that the level of abandonment mediates the influence of both precipitation and vegetation on mosquito abundances. Infrastructural barriers prevalent in many neighborhoods in the United States limit human–mosquito exposure, including regular garbage collection, which limits juvenile habitat, and the use of screens and air conditioning, which reduce vector–host contact rates (Reiter et al. 2003).
Areas with histories of disinvestment in housing and infrastructure, however, are potential footholds for Ae. albopictus populations and vector-borne disease transmission (Hotez et al. 2014). The number of abandoned buildings characterizes the current state of infrastructural degradation, reflects a history of disinvestment, and covaries with median household income, garbage, and semipermanent dumping grounds at the block scale. The co-occurrence of semipermanent dumping sites with abandoned buildings feeds into a negative feedback loop: neither the city nor its residents can maintain these areas, so more garbage accumulates, pests proliferate, housing values may continue to decline, and those residents who do not choose or cannot afford to leave are less able to enjoy the outdoors and are at higher risk for vectors of disease such as Ae. albopictus (Biehler 2013). It is important to note how these drivers and outcomes are coupled. The distribution of abandoned buildings and urban vegetation is rooted in historical social processes (e.g., the legacy of redlining; Biehler 2013) and influenced by current issues (e.g., investment or disinvestment in housing rehabilitation, planting of trees for climate change mitigation; Pickett et al. 2011).
Aedes albopictus biting may influence changes in human behavior, for example, by discouraging residents from spending time outdoors—including time during which they might manage vegetation and trash (Dowling et al. 2013a). On the other hand, emergence of mosquito-borne diseases may influence future urban management decisions. Finally, in areas with high rates of housing abandonment, infrastructural integrity and vegetation are linked when abandoned buildings collapse or are demolished and vegetation returns—under either managed or unmanaged conditions. We demonstrate that at least one metric of vegetation condition, NDVI, is an important indicator of juvenile mosquito occurrence, and when high NDVI occurs on a block with abandoned buildings, it is associated with even greater numbers of adult mosquitoes. Areas with high abandonment and vegetation may be sources of Ae. albopictus production and targeting these “hot spots” for vector control interventions could be effective (Unlu et al. 2015). The negative effect of NDVI on SCI metrics is likely owing to increased NDVI in high SES neighborhoods, although its mechanistic significance is not well understood. Juvenile and adult mosquitoes need vegetation, but the expected positive association with vegetation was only evident on blocks with high abandonment. More work is needed to understand whether this is because high SES habitat has greater human-derived source reduction, limiting juvenile development regardless of vegetation resource availability or microhabitat influence. Future research needs to better describe and quantify differences in vegetation communities between areas of low versus high abandonment. Few studies have undertaken rigorous assessments of vegetation communities in private backyards across different histories of disinvestment let alone relate differences (if any) to adult mosquito ecology (Pickett et al. 2011). Variation in vegetation can affect adult mosquito ecology by providing differences in microsite temperature and humidity, resting sites, and sugar food resources (Gu et al. 2011, Buckner et al. 2016).
Further, we found that more precipitation during the 2 wk prior to sampling led to an increase in the presence of juvenile Ae. albopictus. Significant interactive effects between precipitation, vegetation, and abandoned buildings suggest that fine spatial variability in juvenile habitat productivity responds to precipitation in ways that are mediated by local socio-ecological conditions. The significant interactive effect between abandoned buildings and precipitation within the previous 2 wk indicates that abandoned buildings, and associated discarded container habitat, are good habitat when it rains but are not as good for juvenile development when precipitation is low. In contrast, areas with few abandoned buildings and more managed versus discarded containers are less tightly controlled by weather because people are likely to water and manage these containers. The projected changes in climate for the northeastern United States, especially increased temperatures and precipitation (Pachauri et al. 2014), portend conditions that may increase vector abundance and disease transmission. Climate change may influence Ae. albopictus distribution, abundance, and ability to spread arboviruses; however, the influences of climate and climate change cannot be disentangled from urban socio-ecological influences that contribute to mosquito habitat and production. The interactive effects between precipitation, abandonment, and vegetation in our models indicate that precipitation is not a good predictor unless we account for mechanisms driven by human practices at the local level.
The potential ecosystem benefits of urban trees for human health—decreased temperatures, reduced air pollution, and increased physical activity (Taylor and Hochuli 2015)—may be offset by the proliferation of Ae. albopictus that preferentially seek shady habitat within urban areas (Leisnham and Slaney 2009). Areas with high abandonment and infrastructure demolition are easy targets for increasing urban tree canopy and meeting urban “greening” goals (e.g., http://treebaltimore.org, accessed 11 May 2017). The consequences of increasing tree canopy or vegetation greenness across a city are not clear and unlikely to be linear. Our data show that increasing NDVI without removing and limiting discarded container habitat may exacerbate mosquito infestations. Likewise, under drier precipitation regimes, the value of increased canopy for minimizing heat may have the unintended effects that allow for greater production and survival of Ae. albopictus in urban areas. Other researchers have found differences in Ae. albopictus response to tree canopy in urban areas, implying that while individual trees promote greater abundance, larger patches of trees may reduce Ae. albopictus abundance (Bartlett-Healy et al. 2012, Rochlin et al. 2013b). A more complete understanding of the relationship between vegetation and Ae. albopictus is essential for informing future urban greening efforts that aim to reduce environmental health disparities and combat climate change. Future research should investigate the influence of different configuration and types of vegetation by comparing vacant lots to gardens, investigating patch size of vegetation, and even characterize plant communities. Currently a large “natural experiment” is underway across many urban landscapes, where regreening follows demolition of abandoned buildings and repurposing of vacant lots. These efforts are largely advancing without rigorous examination of either intended or unintended consequences for human health.
Although Ae. albopictus has been observed to take bloodmeals from many different vertebrate species (Niebylski et al. 1994), it has been shown to preferentially bite humans in areas of high population density (Faraji et al. 2014). Although risk to some may be elevated, fewer people live in areas with high rates of building abandonment. Even with associated higher Ae. albopictus infestation rates, the ratio of mosquitoes to humans may be lower in areas with fewer people. More research on Ae. albopictus biting behavior within urban areas is needed to understand where risk to humans is greatest.
This research used data collected at a fine spatial scale, across an SES gradient in West Baltimore, MD, over three mosquito seasons with different climatic conditions. With this spatiotemporal resolution, we have been able to quantify fine-scale differences in container habitat across a steep SES gradient, both within and across neighborhoods. Based on the links between SES, container habitat, and infrastructural integrity, SES profiles emerge. We find that low SES blocks with higher rates of abandonment are also disproportionately represented by low volume, unmanaged containers. Regardless of SES, trash containers are more likely to be infested with Ae. albopictus than other container types and the highest infestation rates are in low SES blocks. These vary by neighborhood condition in ways that are in accordance with others (Dowling et al. 2013a,b; LaDeau et al. 2013) and suggest that Ae. albopictus reduction should be targeted and site-specific across an SES gradient to be most effective. Removing garbage and permanent dumping sites in these areas may greatly reduce the total number of unmanaged containers, the number positive for juvenile Ae. albopictus, and the number of biting adults.
In conclusion, Ae. albopictus populations remain unchecked in temperate North America. Urban centers are particularly at risk of local mosquito-borne disease transmission due to endemic Ae. albopictus populations, connectivity to regions with epidemics, and the density of susceptible people who live in cities (Manore et al. 2017). Areas of endemic poverty and urban decay embedded within northeastern U.S. cities may provide footholds for pestiferous Ae. albopictus populations. Climatic changes and associated urban greening initiatives are likely to influence Ae. albopictus populations in ways that are directly affected by neighborhood condition and human behavior. Although promising vaccines for arboviruses are under development, the introduction of other, emergent arboviruses (e.g., Mayaro; Moore and Mitchell 1997) is possible. Reducing critical habitat for Ae. albopictus mosquitoes in the northeastern United States is essential to reduce nuisance biting as well as transmission risk for present and future arboviruses. The findings highlight the tremendous fine-scale spatial heterogeneities in mosquito habitats within urban environments. By acknowledging and describing driving factors of this urban heterogeneity, we can achieve more effective and specific mosquito and mosquito-borne disease control. Sustainable management decisions will need to embrace the interlocking issues of poverty, urban decay, climate change, urban greening initiatives, and Ae. albopictus infestation.
Acknowledgments
We would like to thank Heather Goodman, Danielle Bodner, Megan Saunders, and the many student field researchers that participated in data collection, the Parks & People Foundation, and the citizens of West Baltimore. This project was supported by a National Science Foundation – Coupled Natural Human Systems award to S.L.L. (DEB 1211797) and the Baltimore Ecosystem Study (National Science Foundation – Long Term Ecological Research (DEB 1027188). Support for P.T.L. was also funded in part by MD-ENST-1443 United States Department of Agriculture Multistate project. Field and data logistics were further supported by the Baltimore Ecosystem Study (NSF-LTER DEB 1027188). E.A.H.L. was supported by two National Institutes of Health grants—ES009089 and 5 T32 ES 23770-3.
References Cited
- Alto B. W., Juliano S. A.. 2001a. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): Implications for range expansion. J. Med. Entomol. 38: 646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto B. W., Juliano S. A.. 2001b. Temperature effects on the dynamics of Aedes albopictus (Diptera: Culicidae) populations in the laboratory. J. Med. Entomol. 38: 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis T. G., Anderson J. F., Vossbrinck C. R., Main A. J.. 2004. Epidemiology of West Nile virus in Connecticut: A five-year analysis of mosquito data 1999–2003. Vector Borne Zoonotic Dis. 4: 360–378. [DOI] [PubMed] [Google Scholar]
- Bartlett-Healy K., Healy S. P., Hamilton G. C.. 2011. A model to predict evaporation rates in habitats used by container-dwelling mosquitoes. J. Med. Entomol. 48: 712–716. [DOI] [PubMed] [Google Scholar]
- Bartlett-Healy K., Unlu I., Obenauer P., Hughes T., Healy S., Crepeau T., Farajollahi A., Ke- Savaraju B., Fonseca D., Schoeler G., et al. 2012. Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae). J. Med. Entomol. 49: 813–824. [DOI] [PubMed] [Google Scholar]
- Becker N., Pluskota B., Kaiser A., Schaffner F.. 2012. Exotic mosquitoes conquer the world, pp. 31–60. InMehlhorn H. (Ed.), Arthropods as vectors of emerging diseases, volume 3 of parasitology research monographs. Springer, Berlin, Germany. [Google Scholar]
- Becker B., Leisnham P. T., LaDeau S. L.. 2014. A tale of two city blocks: Differences in immature and adult mosquito abundances between socioeconomically different urban blocks in Baltimore (Maryland, USA). Int. J. Environ. Res. Public Health 11: 3256–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier J., Patricoski C., Travis M., Kranzfelder J.. 1983. Influence of water chemical and environmental parameters on larval mosquito dynamics in tires. Environ. Entomol. 12: 434–438. [Google Scholar]
- Biehler D. D. 2013. Pests in the city: Flies, bedbugs, cockroaches, and rats. University of Washington Press, Seattle, WA. [Google Scholar]
- Bodner D. 2014. The effectiveness of resident-based mosquito control through changes in knowledge and behavior along a socioeconomic gradient. M.S. thesis, University of Maryland, College Park, MD.
- Buckner E. A., Alto B. W., Lounibos L. P.. 2016. Larval temperature-food effects on adult mosquito infection and vertical transmission of dengue-1 virus. J. Med. Entomol. 53: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham K. P., Anderson D. R.. 2003. Model selection and multimodel inference: A practical information-theoretic approach. Springer Science & Business Media. [Google Scholar]
- Carrington L. B., Seifert S. N., Willits N. H., Lambrechts L., Scott T. W.. 2013. Large diurnal temperature fluctuations negatively influence Aedes aegypti (Diptera: Culicidae) life history traits. J. Med. Entomol. 50: 43–51. [DOI] [PubMed] [Google Scholar]
- Chambers D. M., Young L. F., Hill H. S. Jr.. 1986. Backyard mosquito larval habitat availability and use as influenced by census tract determined resident income levels. J. Am. Mosq. Control Assoc. 2: 539–544. [PubMed] [Google Scholar]
- Chretien J.-P., Linthicum K. J.. 2007. Chikungunya in Europe: What’s next? Lancet 370: 1805–1806. [DOI] [PubMed] [Google Scholar]
- Costa E. A., Santos E. M., Correia J. C., Albuquerque C. M.. 2010. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae). Rev. Brasil. Entomol. 54: 488–493. [Google Scholar]
- Costanzo K. S., Muturi E. J., Lampman R. L., Alto B. W.. 2011. The effects of resource type and ratio on competition with Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 48: 29–38. [DOI] [PubMed] [Google Scholar]
- Couret J., Dotson E., Benedict M. Q.. 2014. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE 9: e87468.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekel A., Pitts R. J., Yakir E., Bohbot J. D.. 2016. Evolutionarily conserved odorant receptor function questions ecological context of octenol role in mosquitoes. Sci. Rep. 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling Z., Armbruster P., LaDeau S. L., DeCotiis M., Mottley J., Leisnham P. T.. 2013a. Linking mosquito infestation to resident socioeconomic status, knowledge, and source reduction practices in suburban Washington, DC. Ecohealth 10: 36–47. [DOI] [PubMed] [Google Scholar]
- Dowling Z., Ladeau S. L., Armbruster P., Biehler D., Leisnham P. T.. 2013b. Socioeconomic status affects mosquito (Diptera: Culicidae) larval habitat type availability and infestation level. J. Med. Entomol. 50: 764–772. [DOI] [PubMed] [Google Scholar]
- Faraji A., Egizi A., Fonseca D. M., Unlu I., Crepeau T., Healy S. P., Gaugler R.. 2014. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban northeastern USA and implications for disease transmission. PLoS Negl. Trop. Dis. 8: e3037.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda C. 2009. Characterizing the relationship between Asian tiger mosquito abundance and habitat in urban New Jersey. M.S. thesis, Rutgers The State University of New Jersey, New Brunswick, NJ.
- Fournier D. A., Skaug H. J., Ancheta J., Ianelli J., Magnusson A., Maunder M. N., Nielsen A., Sibert J.. 2012. Ad model builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optimization Methods Softw. 27: 233–249. [Google Scholar]
- Gerhardt R. R., Gottfried K. L., Apperson C. S., Davis B. S., Erwin P. C., Smith A. B., Panella N. A., Powell E. E., Nasci R. S.. 2001. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg. Infect. Dis. 7: 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz N. G. 2004. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18: 215–227. [DOI] [PubMed] [Google Scholar]
- Gu W., Müller G., Schlein Y., Novak R. J., Beier J. C.. 2011. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS ONE 6: e15996.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasa Y. A., Shepard D. S., Fonseca D. M., Farajollahi A., Healy S., Gaugler R., Bartlett- Healy K., Strickman D. A., Clark G. G.. 2014. Quantifying the impact of mosquitoes on quality of life and enjoyment of yard and porch activities in New Jersey. PLoS ONE 9: e89221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley W. A. 1988. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Supplement 1: 1–39. [PubMed] [Google Scholar]
- Hotez P. J., Murray K. O., Buekens P.. 2014. The gulf coast: A new American underbelly of tropical diseases and poverty. PLoS Negl. Trop. Dis. 8: e2760.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Bernal S., Briseño B., Mutebi J. P., Argot E., Rodríguez G., Martínez-Campos C., Paz R., de la Fuente-San Román P., Tapia-Conyer R., Flisser A.. 1997. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med. Vet. Entomol. 11: 305–309. [DOI] [PubMed] [Google Scholar]
- Johnson A., Tauzer E., Swan C.. 2014. Human legacies differentially organize functional and phylogenetic diversity of urban herbaceous plant communities at multiple spatial scales. Appl. Veg. Sci. 18: 513–527. [Google Scholar]
- Joshi V., Sharma R. C., Sharma Y., Adha S., Sharma K., Singh H., Purohit A., Singhi M.. 2006. Importance of socioeconomic status and tree holes in distribution of Aedes mosquitoes (Diptera: Culicidae) in Jodhpur, Rajasthan, India. J. Med. Entomol. 43: 330–336. [DOI] [PubMed] [Google Scholar]
- Juliano S. A., Lounibos L. P.. 2005. Ecology of invasive mosquitoes: Effects on resident species and on human health. Ecol. Lett. 8: 558–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling L. J., Juliano S. A., Yee D. A.. 2007. Larval mosquito communities in discarded vehicle tires in a forested and unforested site: detritus type, amount, and water nutrient differences. J. Vector Ecol. 32: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer M.U.G., Sinka M. E., Duda K. A., Mylne A., Shearer F. M., Brady O. J., Messina J. P., Barker C. M., Moore C. G., Carvalho R. G., et al. 2015. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data 2: 150035.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. M., Vonesh J. R.. 2012. Fluxes of terrestrial and aquatic carbon by emergent mosquitoes: A test of controls and implications for cross-ecosystem linkages. Oecologia 170: 1111–1122. [DOI] [PubMed] [Google Scholar]
- LaDeau S. L., Leisnham P. T., Biehler D., Bodner D.. 2013. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: Understanding ecological drivers and mosquito-borne disease risk in temperate cities. Int. J. Environ. Res. Public Health 10: 1505–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDeau S. L., Allan B. F., Leisnham P. T., Levy M. Z.. 2015. The ecological foundations of transmission potential and vector-borne disease in urban landscapes. Funct. Ecol. 29: 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham P. T., Juliano S. A.. 2009. Spatial and temporal patterns of coexistence between competing Aedes mosquitoes in urban Florida. Oecologia 160: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham P. T., Slaney D.. 2009. Urbanization and the increasing risk from mosquito-borne diseases: Linking human well-being with ecosystem health, pp. 47–82. InDe Smet L. M. (ed.), Focus on urbanization trends. Nova Science Publishers Inc, Hauppauge, NY. [Google Scholar]
- Leisnham P. T., Juliano S. A.. 2012. Impacts of climate, land use, and biological invasion on the ecology of immature Aedes mosquitoes: Implications for la Crosse emergence. Ecohealth 9: 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham P. T., LaDeau S. L., Juliano S. A.. 2014. Spatial and temporal habitat segregation of mosquitoes in urban Florida. PLoS ONE 9: e91655.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manore C. A., Ostfeld R. S., Agusto F. B., Gaff H., LaDeau S. L.. 2017. Defining the risk of Zika and chikungunya virus transmission in human population centers of the eastern United States. PLoS Negl. Trop. Dis. 11: e0005255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcombe S., Farajollahi A., Healy S. P., Clark G. G., Fonseca D. M.. 2014. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE 9: e101992.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini F., Caputo B., Pombi M., Tarsitani G., della Torre A.. 2010. Study of Aedes albopictus dispersal in Rome, Italy, using sticky traps in mark-release-recapture experiments. Med. Vet. Entomol. 24: 361–368. [DOI] [PubMed] [Google Scholar]
- Menne M., Durre I., Korzeniewski B., McNeal S., Thomas K., Yin X., Anthony S., Ray R., Vose R., Gleason B.. 2015. Global historical climatology network – Daily (GHCN-daily), version 3. NOAA National Climate Data Center. (http://doi.org/10.7289/V5D21VHZ) (accessed 11 May 2017).
- Moore C. G., Mitchell C. J.. 1997. Aedes albopictus in the United States: Ten-year presence and public health implications. Emerg. Infect. Dis. 3: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebylski M. L., Savage H. M., Nasci R. S., Craig G. B. Jr.. 1994. Blood hosts of Aedes albopictus in the United States. J. Am. Mosq. Control Assoc. 10: 447–450. [PubMed] [Google Scholar]
- Pachauri R. K., Allen M. R., Barros V. R., Broome J., Cramer W., Christ R., Church J. A., Clarke L., Dahe Q., Dasgupta P., et al. 2014. Climate change 2014: Synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. IPCC.
- Pan American Health Organization/World Health Organization. 2016. Zika - Epidemiological Update – 21 April 2016. PAHO/WHO, Washington, DC.
- Paupy C., Delatte H., Bagny L., Corbel V., Fontenille D.. 2009. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 11: 1177–1185. [DOI] [PubMed] [Google Scholar]
- Pickett S., Cadenasso M., Grove J., Boone C., Groffman P., Irwin E., Kaushal S., Marshall V., McGrath B., Nilon C., et al. 2011. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Biol. 92: 331–362. [DOI] [PubMed] [Google Scholar]
- Quam M. B., Sessions O., Kamaraj U. S., Rocklöv J., Wilder-Smith A.. 2016. Dissecting Japan’s dengue outbreak in 2014. Am. J. Trop. Med. Hyg. 94: 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quantum GIS Development Team. 2017. Quantum GIS geographic information system. Open Source Geospatial Foundation Project (http://qgis.osgeo.org) (accessed 11 May 2017).
- Reiter P., Lathrop S., Bunning M., Biggerstaff B., Singer D., Tiwari T., Baber L., Amador M., Thirion J., Hayes J., et al. 2003. Texas lifestyle limits transmission of dengue virus. Emerg. Infect. Dis. 9: 86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey J. R., Nishimura N., Wagner B., Braks M.A.H., O’Connell S. M., Lounibos L. P.. 2006. Habitat segregation of mosquito arbovirus vectors in south Florida. J. Med. Entomol. 43: 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza G., Nicoletti L., Angelini R., Romi R., Finarelli A., Panning M., Cordioli P., Fortuna C., Boros S., Magurano F., et al. 2007. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 370: 1840–1846. [DOI] [PubMed] [Google Scholar]
- Rochlin I., Gaugler R., Williges E., Farajollahi A.. 2013a. The rise of the invasives and decline of the natives: Insights revealed from adult populations of container-inhabiting Aedes mosquitoes (Diptera: Culicidae) in temperate North America. Biol. Invasions 15: 991–1003. [Google Scholar]
- Rochlin I., Ninivaggi D. V., Hutchinson M. L., Farajollahi A.. 2013b. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in northeastern USA: Implications for public health practitioners. PloS ONE 8: e60874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiz D., Rosa R., Arnoldi D., Rizzoli A.. 2010. Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector-Borne Zoonotic Dis. 10: 811–816. [DOI] [PubMed] [Google Scholar]
- Skaug H., Fournier D., Nielsen A., Magnusson A., Bolker B.. 2012. Generalized Linear Mixed Models using AD Model Builder. R package version 0.8.0.
- Taylor L., Hochuli D. F.. 2015. Creating better cities: How biodiversity and ecosystem functioning enhance urban residents’ wellbeing. Urban Ecosyst. 18: 747–762. [Google Scholar]
- Tsuda Y., Maekawa Y., Ogawa K., Itokawa K., Komagata O., Sasaki T., Isawa H., Tomita T., Sawabe K.. 2016. Biting density and distribution of Aedes albopictus during the September 2014 outbreak of dengue fever in Yoyogi Park and the vicinity of Tokyo metropolis, Japan. Jpn. J. Infect. Dis. 69: 1–5. [DOI] [PubMed] [Google Scholar]
- Tun-Lin W., Kay B. H., Barnes A., Forsyth S.. 1996. Critical examination of Aedes aegypti indices: Correlations with abundance. Am. J. Trop .Med Hyg. 54: 543–547. [DOI] [PubMed] [Google Scholar]
- Turell M. J., Dohm D. J., Sardelis M. R., Oguinn M. L., Andreadis T. G., Blow J. A.. 2005. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit west nile virus. J. Med. Entomol. 42: 57–62. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau/American FactFinder. 2014. Income in the past 12 months (in 2014 inflation-adjusted dollars). U.S. Census Bureau, 2010-2014 American Community Survey 5-year estimates. (https://factifinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=CF) (accessed 11 May 2017).
- Unlu I., Farajollahi A., Healy S. P., Crepeau T., Bartlett-Healy K., Williges E., Strickman D., Clark G. G., Gaugler R., Fonseca D. M.. 2011. Area-wide management of Aedes albopictus: Choice of study sites based on geospatial characteristics, socioeconomic factors and mosquito populations. Pest Manag. Sci. 67: 965–974. [DOI] [PubMed] [Google Scholar]
- Unlu I., Faraji A., Indelicato N., Fonseca D. M.. 2014. The hidden world of Asian tiger mosquitoes: Immature Aedes albopictus (Skuse) dominate in rainwater corrugated extension spouts. Trans. R. Soc. Trop. Med. Hyg. 108: 699–705. doi:10.1093/trstmh/tru139. [DOI] [PubMed] [Google Scholar]
- Unlu I., Klingler K., Indelicato N., Faraji A., Strickman D.. 2015. Suppression of Aedes albopictus, the Asian tiger mosquito, using a ‘hot spot’ approach. Pest Manag. Sci. 72: 1427–1432. [DOI] [PubMed] [Google Scholar]
- United States Geological Survey 2015. NVI, the foundation for remote sensing phenology. (http://phenology.cr.usgs.gov/ndvi_foundation.php) (accessed 11 May 2017).
- Waldock J., Chandra N. L., Lelieveld J., Proestos Y., Michael E., Christophides G., Parham P. E.. 2013. The role of environmental variables on Aedes albopictus biology and chikungunya epidemiology. Pathog. Glob. Health 107: 224–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobey J., Fonseca D. M., Espinosa C., Healy S., Gaugler R.. 2013. Child outdoor physical activity is reduced by prevalence of the Asian tiger mosquito, Aedes albopictus. J. Am. Mosq. Control Assoc. 29: 78–80. [DOI] [PubMed] [Google Scholar]
- Yee D. A., Allgood D., Kneitel J. M., Kuehn K. A.. 2012. Constitutive differences between natural and artificial container mosquito habitats: Vector communities, resources, microorganisms, and habitat parameters. J. Med. Entomol. 49: 482–491. [DOI] [PubMed] [Google Scholar]