Abstract
In a market undergoing constant evolution, the production of chicken meat that consumers would perceive as “natural” and “animal friendly” is crucial. The use of probiotics in rurally reared chickens could represent a major opportunity to achieve mutual benefit for both the industry and consumers. A total of 264 male Kabir chicks were randomly distributed to one of 2 dietary treatments: the L group received a commercial feed supplemented with 2.0 g/100 kg of Lactobacillus acidophilus D2/CSL, while the C group received the same basal diet without the additive. To assess the effects of probiotic supplementation in the chickens’ diet, productive performance was evaluated at d 21 and 42, whereas microbiological analyses of the intestinal content and intestinal histology and morphometry were performed at the end of the trial (d 42). At d 21 and 42, L birds showed better (P < 0.001) performance in terms of body weight, average daily gain, and feed conversion ratio. Enterococci, staphylococci, and Escherichia coli populations were not influenced by dietary treatment. On the contrary, Lactobacillus population increased (P = 0.032) in the L group. Furthermore, a tendency (P = 0.069) was observed for the coliforms to be influenced by diet, with lower values in the L group in comparison to the C group. Histological techniques revealed that the number of goblet cell containing neutral mucins was lower in the C group. Morphometric evaluations demonstrated that the probiotic supplementation increased the height of the mucosal layer by improving (P = 0.040) villus height, while crypt depth was unaffected. In conclusion, the results obtained in this study demonstrate that it is possible to use Lactobacillus acidophilus D2/CSL (CECT 4529) in rurally reared chicken breeds with positive effects on performance and gut health.
Keywords: Feed Additive, Intestinal Histology, Microbiota, Probiotics, Slow-growing Chicken Genotype
INTRODUCTION
The chicken meat market is undergoing constant evolution. Consumers are now showing a greater awareness and paying more attention to products that are perceived to be obtained in a “more natural” way (Samant and Seo, 2016). Various slow-growing chicken genotypes are available in Europe, and researchers have suggested that the quality of their meat is appropriate for a market characterized by an increased consumer awareness regarding animal husbandry and welfare (Castellini et al., 2002; Gordon and Charles, 2002).
In Italy, 85% of the total poultry industry is managed solely by a few integrated companies that cover the entire food chain of chicken meat, from the egg to the consumer (Nomisma, 2016). No statistics are available on the exact numbers of alternative broiler genotypes (slow-growing or certified broilers) in the EU; however, industry experts estimate the market share to be between 5 and 10% of the total production (EU Commission, 2016). Since the total economic balance of the poultry industry (2,850 million euro) gained more than 31% in 2015, in comparison to 2009, the importance of conducting studies in this field could be crucial.
Considering the economic relevance of the poultry meat industry and the increasing consumer demand for “natural” products, the use of probiotics could represent a major opportunity to achieve mutual benefit for both the industry and consumers. Different kinds of microorganisms can exert a probiotic effect when included in poultry diets. In the literature, data are available for Lactobacillus spp., Streptococcus spp., Bacillus spp., Bifidobacterium spp., Enterococcus spp., Aspergillus spp., Candida spp., Saccharomyces spp., and other microbial species. It is claimed that these strains positively affect growth performance (Smith, 2014), egg production and quality (Forte et al., 2016a), modulation of intestinal microflora and pathogen inhibition (Patterson and Burkholder, 2003), immunomodulation, and chicken meat quality (Mountzouris et al., 2007).
Lactobacilli are often considered in the formulation of probiotics. Lactobacillus is one of the predominant bacterial genera in the gastrointestinal tract of both humans and animals (Amit-Romach et al., 2004). Lactobacilli can be roughly divided into 2 metabolic groups: homofermentative, converting glucose to lactic acid, and heterofermentative, converting glucose to lactic acid, acetic acid, ethanol, and CO2. These metabolites reduce intestinal lumen pH, creating an unfavorable environment for potential pathogenic bacteria (Axelsson, 2004; Menconi et al., 2011). Lactobacillus has been proven to exert a competitive exclusion effect on enterobacteria such as Salmonella enterica serovar Enteritidis in chickens (Penha Filho et al., 2015). Moreover, it positively affects the equilibrium of the gastrointestinal microbiota, increasing the presence of beneficial bacteria such as Bifidobacterium spp., and reducing potentially harmful bacteria such as Escherichia coli, clostridia, and staphylococci (Forte et al., 2016b).
The Lactobacillus genus includes about 200 species (Foschi et al., 2017) and is continuously evolving. Among these, Lactobacillus acidophilus D2/CSL is a bacterium isolated from the intestinal content of broilers (De Cesare et al., 2017), which is currently used as a probiotic in the egg production industry. Studies have demonstrated the efficacy of this particular probiotic in increasing antibody production against viruses such as Newcastle disease (Forte et al., 2016b). In broilers treated with Lactobacillus acidophilus D2/CSL, a positive effect was observed on productive performance and metabolic function, implying improved animal health (De Cesare et al., 2017).
To our knowledge, no studies have been previously performed to investigate the effects of Lactobacillus acidophilus D2/CSL on rurally reared chickens. The purpose of this study was to evaluate the effects of the dietary supplementation of Lactobacillus acidophilus D2/CSL (CECT 4529) on the productive performance of male chickens reared in conditions simulating small rural farming systems.
MATERIALS AND METHODS
Experimental Design
The experiment was conducted in a small farm of Umbria, Central Italy. A total of 264 day-old male Kabir chicks, obtained from the same hatching session, were used. At housing, all chicks were individually weighed and randomly distributed to one of the 2 dietary treatments. The chickens belonging to the L group received a commercial feed supplemented with 2.0 g/100 kg (20 g/ton) of Lactobacillus acidophilus D2/CSL (CECT 4529 - freeze-dried live cells), corresponding to a calculated dose of 1*109 CFU*kg−1. The animals of the C group received the same basal diet without the additive. A starter diet (Diet 1) was administered until the chicks were 21 d old, whereas a grower-finisher diet (Diet 2) was given from 22 to 42 d of age. The 2 treatment groups (C and L), which consisted of 132 individuals per group, were divided into 6 replicates (pens-experimental units), each housing 22 birds. Only birds without any sign of illness and with a normal behavioral pattern were included in the trial. All the birds were vaccinated against IBV, Marek's and Newcastle diseases and coccidiosis in the hatchery. Pens (n = 12) were equipped with one plastic feeder and one plastic waterer each of identical manufacture, type, size, color, and any other notable physical feature. Fresh wood shavings were used as litter. Feed and water were provided ad libitum.
Observations for general flock condition, temperature, lighting, water, feed and mortality were recorded twice daily. The experimental protocol was in accordance with Guide for the Care and Use of Agricultural Animals in Research and Teaching (McGlone, 2010) and EU Directive 2010/63 (European Commission, 2010), and was approved by the Ethical Council of Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche.
Feed Analyses and Productive Performance
Samples of feed were collected twice per week for each dietary treatment (n = 12) and analyzed for chemical determination (Table 1) according to A.O.A.C. methods (1990), while EN 15787:2009 (Standards Centre, 2009) methods were applied for lactobacilli counts. The suggested dosage of the probiotic is 1 × 109 CFU/kg feed and the commercial product contains 5 × 1010 CFU/g. The dosage that was used in this trial is 20 g/ton feed. The feed was supplied in mash form and ad libitum throughout the experiment.
Table 1.
Ingredients and composition of the experimental diets.
| Starter | Grower-finisher | |
|---|---|---|
| (0–21 d) | (21–42 d) | |
| INGREDIENTS (g/100g) | ||
| Corn | 36.00 | 46.90 |
| Soybean Meal | 27.00 | 25.00 |
| Corn Gluten Meal | 19.60 | 15.10 |
| Rice Middlings | – | 5.10 |
| Maize Distillers | 9.00 | – |
| Bran | – | 4.00 |
| Rice Bran | 4.20 | – |
| Soybean Oil | 1.30 | 1.5 |
| Calcium Carbonate | 1.29 | 1.37 |
| Dicalcium Phosphate | 0.40 | – |
| Salt | 0.30 | 0.30 |
| Sodium Bicarbonate | 0.15 | – |
| Vit-Min Premix1 | 0.76 | 0.73 |
| CHEMICAL COMPOSITION (AS FED) | ||
| Analyzed (%) | ||
| Dry Matter | 85.87 | 85.87 |
| Protein | 22.00 | 18.00 |
| Lipid | 5.00 | 5.00 |
| Fiber | 4.00 | 4.50 |
| Ash | 6.40 | 6.20 |
| Calcium | 1.10 | 1.00 |
| Phosphorus | 0.77 | 0.67 |
| Lysine | 1.16 | 0.79 |
| Methionine | 0.50 | 0.35 |
| Calculated | ||
| Metabolizable Energy (kcal/kg) | 2837 | 2771 |
1Supplied per kilogram of diet: vitamin A, 12,500 I.U. (retinol); vitamin D3, 3,000 I.U.; vitamin E (tocopheryl acetate), 50 mg; vitamin K3, 2 mg; thiamine, 2 mg; riboflavin, 4 mg; pyridoxine, 1 mg; cyanocobalamin, 0.015 mg; pantothenic acid 15 mg; folic acid, 50 mg; biotin, 10 mg; choline chloride, 60; iodine, 3 mg; selenium, 20 mg; iron, 3 mg; manganese, 12, mg; copper, 1,5 mg; zinc, 5 mg.
Chicks were weighed at housing (d 1) and at d 21 and 42. Productive performance, average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) were calculated for each period (d 1 to 21 and d 21 to 42) as well as for the overall experiment (d 1 to slaughter), and were corrected for mortality.
Microbiological Analyses
At the end of the trial, samples of intestinal content (ileum, from Merkel's diverticulum to a point 40 mm proximal to the ileocecal junction) were collected from 3 different subjects for each replicate (n = 36). Analyses were conducted according to Forte et al. (2016b) with some modifications. Replicate associated pool samples (n = 12) were obtained by mixing the samples collected from subjects belonging to the same replicate. An aliquot of 1 g was collected from each pool and diluted in tubes containing 2 mL of 0.9% sterile saline solution. Tubes were brought to volume (10 mL) with the same solution. Specimens were 10-fold serially diluted in 0.9% sterile saline solution, thus obtaining dilutions from 10−1 to 10−10 according to UNI EN ISO 6887–1. Chromogen Rapid’E.coli2/Agar (Bio-Rad Laboratories, Redmond, WA) and Slanetz-Bartley Agar (Oxoid Basingstoke, UK) were used for the enumeration of coliforms and enterococci, respectively. Mannitol salt agar (Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche, Perugia, Italy) was used for the enumeration of staphylococci. Plates were incubated aerobically at 37°C for 24–48 h and the colonies were then counted.
For the enumeration of sulfate-reducing anaerobic bacteria, TSC Agar (Tryptose Sulfite Cycloserine Agar—Oxoid Basingstoke, UK) enriched with egg yolk was used. Plates were incubated at 37°C for 24–48 h in anaerobic condition. Enumeration of lactobacilli was performed using Man Rogosa Sharpe (MRS) agar (Thermo Fisher Scientific, Cleveland, OH). Plates were incubated in microaerophilic conditions at 35°C for 72 hours. Results were expressed as log10 cfu/g.
Intestinal Histology and Morphometry
Samples of ileum (as described above, from Merkel's diverticulum to a point 40 mm proximal to the ileocecal junction) collected from 3 subjects per replicate (n = 36) were fixed in 10% neutral buffered formalin solution and processed for histology in accordance with standard procedures. Serial histological sections measuring 4 to 5 μm were subjected to hematoxylin and eosin (HE) and periodic acid-Schiff (PAS) stains. The criterion for villus selection was based on the presence of intact lamina propria; for each sample 5 intact villi, selected in triplicate for each intestinal cross-section, were included in the study. For the histochemical analysis, goblet cells taken from 5 randomly selected fields (40×) were counted. For morphometric evaluations, HE stained intestinal sections were used to evaluate villus height (VH, measured from the tip of the villus to the crypt-villus junction) and crypt depth (CD, measured from its base up to the crypt-villus transition region) as reported by Aliakbarpour et al. (2012). Muscular wall thickness was also recorded and analyzed. Images were digitalized using a Nikon DS-Fi1 digital camera (Nikon Corporation, Tokyo, Japan) connected to a Leica DMR microscope (Leica Microsystems, Milan, Italy), using NIS-Elements Br-2 as software as reported in Forte et al. (2016b).
Statistical Analysis
Data were analyzed using the General Lineal Model (GLM) procedure of SAS (JMP 9; SAS Institute, 2010). An analysis of variance (ANOVA) model was used with diet (C and L) as the fixed factor. The replicate effect did not prove to be statistically significant and was thus removed from the model. The differences of the means were defined using the Tukey test and considered significant when P < 0.05. Tendencies were discussed for P values between 0.090 and 0.051.
RESULTS
Lactobacilli count in feed was in line with expectations, resulting 1 × 106 CFU/g in L feed and 4.2 × 102 CFU/g in C feed. Mortality for the overall duration of the trial was always below values of 0.5% per pen. Post mortem examination did not evidence any significant result, as no pathological signs were found.
Results regarding performance are reported in Table 2. At 21 d of age L birds showed higher BW (P < 0.001) than C birds and showed better (P < 0.05) ADG and FCR (Table 2). Consistently with the initial results, at the end of trial (42 d) L birds showed higher (P < 0.001) BW in comparison to the C birds, as well as better (P < 0.05) ADG and FCR. The overall productive performance from 0 to 42 d (Table 2) revealed that the probiotic supplementation positively affected BW, average daily gain and feed conversion ratio of the birds (P < 0.05).
Table 2.
Effect of dietary treatment on body weight (BW), average daily gain (ADG) and feed conversion efficiency (FCE).
| Item | Sampling time | C | L | SEM | P |
|---|---|---|---|---|---|
| T1 | 45.18 | 45.13 | 0.396 | 0.930 | |
| BW | T2 | 511.67 b | 550.66 a | 8.159 | <0.001 |
| T3 | 1444.03 b | 1636.40 a | 18.845 | <0.001 | |
| T1-T2 | 22.21 b | 24.07 a | 0.549 | 0.038 | |
| ADG | T2-T3 | 44.39 b | 51.70 a | 1.082 | <0.001 |
| OVERALL | 34.97 b | 39.78 a | 0.0591 | <0.001 | |
| T1-T2 | 2.57 a | 2.31 b | 0.052 | 0.018 | |
| FCE | T2-T3 | 3.13 a | 2.68 b | 0.118 | 0.028 |
| OVERALL | 2.94 a | 2.56 b | 0.079 | <0.001 |
T1: d1; T2: d21; T3: d42
C: basal diet; L: basal diet supplemented with 2.0 g/100 kg (20 g/ton) of Lactobacillus acidophilus D2/CSL.
Different letters in the same row denote significant difference.
As for the microbiological evaluations, results are as shown in Table 3. Enterococci, staphylococci and Escherichia coli were not influenced by dietary treatment. On the contrary, Lactobacillus population increased (P = 0.032) in the L group. A tendency (P = 0.069) was observed for the coliforms to be influenced by diet, with lower values in the L group in comparison to the C group.
Table 3.
Effect of Lactobacillus acidophilus supplemented diet on intestinal Enterococcus spp., Staphylococcus spp., Escherichia coli, Coliforms and Lactobacillus spp. populations (log10 cfu/g).
| Enterococcus spp. | Staphylococcus spp. | Escherichia coli | Coliforms | Lactobacillus spp. | |
|---|---|---|---|---|---|
| C | 6.310 | 5.923 | 6.330 | 5.783 | 5.820 b |
| L | 6.005 | 5.657 | 6.447 | 5.080 | 6.438 a |
| SEM | 0.183 | 0.231 | 0.274 | 0.244 | 0.176 |
| P | 0.255 | 0.434 | 0.769 | 0.069 | 0.032 |
C: basal diet; L: basal diet supplemented with 2.0 g/100 kg (20 g/ton) of Lactobacillus acidophilus D2/CSL.
Samples (n = 36) were collected from Merkel's diverticulum to a point 40 mm proximal to the ileocecal junction.
Different letters in the same column denote significant difference.
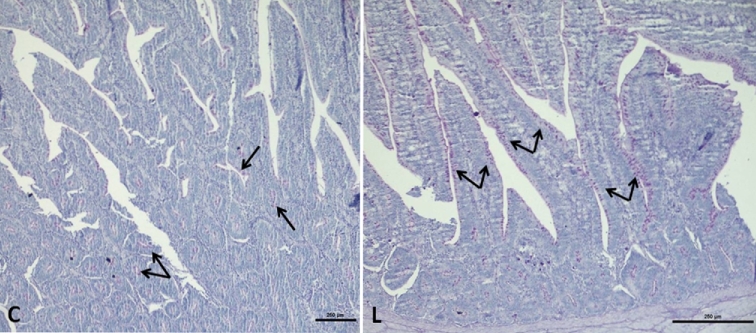
The PAS-stained ileal sections were assessed with histochemical methods, and the number of goblet cells containing neutral mucins was evaluated both in crypts and columnar epithelium on the ileum tract (Fig. 1). The number of these cells resulted lower in the control group (P = 0.005) compared to L group, as reported in Table 4. Morphometric evaluations of the ileum (Table 4) revealed that that the probiotic supplementation increased (P = 0.041) the height of the mucosal layer by improving (P = 0.040) villus height, while crypt depth was unaffected.
Figure 1.
Duodenal sections from control (C) and treated (L) group. Goblet cells (arrows) appear as fuchsia spots (PAS stain. Bars 250 μm).
Table 4.
Effect of Lactobacillus acidophilus supplemented diets on intestinal histology and morphometry.
| Muscular wall thickness | Villus height | Crypt depth | Mucosal layer height | Goblet Cells Pas+ | |
|---|---|---|---|---|---|
| C | 433.27 | 2021.98 b | 588.28 | 2610.27 b | 66.62 b |
| L | 491.54 | 2471.52 a | 777.58 | 3249.11 a | 84.18 a |
| SEM | 48.801 | 146.61 | 80.087 | 209.25 | 4.044 |
| P | 0.406 | 0.040 | 0.107 | 0.041 | 0.005 |
C: basal diet; L: basal diet supplemented with 2.0 g/100 kg (20 g/ton) of Lactobacillus acidophilus D2/CSL.
Samples (n = 36) were collected from Merkel's diverticulum to a point 40 mm proximal to the ileocecal junction.
Pas+: Periodic Acid Schiff-stain positive goblet cells.
Different letters in the same column denote significant difference.
DISCUSSION
The supplementation of Lactobacillus acidophilus D2/CSL (CECT 4529) at the recommended dietary dosage of 1.0 × 109 CFU/kg of feed in rurally reared chickens significantly improved performance (BW, weight increments and FCR) for the full length of the trial. The results are in line with those obtained in other studies that used Lactobacillus acidophilus alone (Salarmoiniand and Fooladi, 2011) or in combination with other Lactobacillus strains (Kalavathy et al., 2003; Smirnov et al., 2005; Pour and Kermanshahi, 2010; Shim et al., 2012; Zhang and Kim, 2014; Hossain et al., 2015). In particular, results acquired in the present study are in accordance with those obtained by Khan et al. (2007) using Lactobacillus strains in Kabir chickens. The positive effects observed in the performance of chickens included in the study are supported by the results obtained from microbiological, histological, and histochemical evaluations.
In 2017, De Cesare et al. observed comparable amounts of Lactobacillus acidophilus in the cecal contents of broilers fed either a control diet or a diet supplemented with the same probiotic strain used in the present study. The authors investigated the cecal microbiome, hypothesizing a probiotic effect resulting from changes in the environmental conditions of the intestinal lumen and a cross-feeding mechanism. Lu et al. (2003) demonstrated that chickens’ gut microbiome varies from one intestinal segment to another; in particular, Lactobacillus species are abundant in the ileum, while in the ceca Clostridiaceae species are the most represented. Lactobacillus acidophilus D2/CSL is a probiotic isolated from the gastro-intestinal tract of healthy chickens (Bianchi Salvadori et al., 1985), therefore it has effective adhesive and multiplicative capacities in the chickens’ enteric environment. It is possible to assume that the increase of the lactobacilli population observed in the present study, which takes into account all the Lactobacillus species of the ileum tract, could be the combined result of both an increase of the chickens’ native intestinal lactobacilli and of the colonization of Lactobacillus acidophilus D2/CSL supplemented in feed.
When comparing studies regarding probiotics, it is essential to consider that mechanisms of action and beneficial effects are suggested to be specific for genus, species, and strain of the examined microorganisms (Timmerman et al., 2004). Furthermore, the variation of a probiotic's efficacy could be due to external experimental conditions, other than to the differences in the preparation itself (Bomba et al., 2002). Studies that employed Lactobacillus acidophilus as a monostrain probiotic are not abundant in literature. Results acquired from the microbiological evaluations of the present study are in accordance with Li et al. (2014). These authors, as well as Mookiah et al. (2014), hypothesize that, being part of the chickens’ healthy gut microflora, Lactobacillus acidophilus supplemented via feed efficiently colonises the intestinal tract and exerts a competitive exclusion effect on pathogenic bacteria. It also creates a lower pH gut environment that inhibits the growth of pathogenic bacteria, yeast, and fungi. However, other studies conducted solely on Lactobacillus acidophilus did not report any significant effect on the lactobacilli population, and only partial effects on coliforms (Jin et al., 1998; Salarmoini and Fooladi, 2011). This demonstrates the complexity of probiotic mechanisms of action and interactions with the host. Olnood et al. (2015) investigated the effects of 4 different strains of Lactobacillus on the intestinal tract of broilers. They observed an increase in the lactobacilli population and a reduction of the population of Enterobacteria in the ileum and ceca of the treated subjects, which the authors attribute to the lactobacilli's production of antimicrobial substances such as volatile fatty acids, other organic acids, and bacteriocins. Watkins and Kratzer (1983; 1984) performed similar studies with Lactobacillus spp. based multistrain probiotics, but did not report significant variations in the lactobacilli and coliform populations. Likewise, studies employing multistrain probiotics containing Lactobacillus acidophilus and other microorganisms, showed mixed results. Some are in accordance with the present study (Smirnov et al., 2005; Shim et al., 2012, Zhang and Kim, 2014; Hossain et al., 2015), and others show no significant results regarding lactobacilli and coliform intestinal populations (Daskiran et al., 2012). The reason for the discrepancy between the results observed in the aforementioned studies could be related to the diversity of probiotic formulations (mono-species/mono-strain, or mono-species/multistrains, or multispecies, or even multigenera), administration methods (specific dosages in feed and/or in water), chicken genotypes and rearing systems taken into consideration, all of which may affect and thus make it difficult to compare the efficacy of different probiotic products (Mountzouris et al., 2007). Further studies characterized by a systematic approach and the use of advanced technologies will be needed in order to fully comprehend the mechanisms of action of different probiotic strains and to better assess their use in poultry nutrition.
Histological methods demonstrated how Lactobacillus acidophilus supplementation can influence villus height, thus inducing small intestinal goblet cell hyperplasia. The present study shows changes in the mucosal architecture in terms of increased duodenal villus height and goblet cell number in the L group. The increase of villus height suggests the development of an increased surface area, capable of greater absorption of available nutrients (Chichlowski et al., 2007). Furthermore, probiotic supplementation induced goblet cell hyperplasia and mucin production. Mucins are synthetized in goblet cells and are then packaged into granules, transported to the cell surface and secreted into the lumen, hence contributing to the production of the mucous intestinal barrier, which acts as a dynamic protective surface (Kim and Khan, 2013). The results obtained in the present research are in line with studies in which intestinal morphology and mucin dynamics were evaluated (Smirnov et al., 2005; Awad et al., 2010; Wang et al., 2012), thus confirming the positive effects of Lactobacillus based probiotics on the intestinal ecosystem. It is important to take into consideration the fact that mucin synthesis might be challenging for the chickens in terms of amino acids consumption, but it is possible to hypothesize that the increased nutrient absorbent surface of the ileum may contribute to an increase of amino acids absorption. This would justify the increase of both mucin production and BW of chickens fed the Lactobacillus acidophilus D2/CSL supplemented diet.
In conclusion, the results obtained in this study demonstrate that it is possible to successfully use Lactobacillus acidophilus D2/CSL (CECT 4529) in rurally reared chicken breeds. The adaptability of Lactobacillus acidophilus D2/CSL to alternative rearing systems could provide the means to offer an innovative product that meets the demands of the new generation of environmentally aware consumers.
ACKNOWLEDGEMENTS
Authors would like to thank PROGEO società cooperativa agricola and Dr. Roberto Bernardi for the technical advice and feed supply; F. Lucignani for help in the field trial and S. O’Leary for help in revising the manuscript.
REFERENCES
- A.O.A.C. , 1990. Official Methods of Analysis, 14th ed Association of Official Analytical Chemists, Washington, D.C. [Google Scholar]
- Aliakbarpour H. R., Chamani M., Rahimi G., Sadeghi A. A., Qujeq D.. 2012. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australas. J. Anim. Sci. 25:1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit-Romach E., Sklan D., Uni Z.. 2004. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult. Sci. 83:1093–1098. [DOI] [PubMed] [Google Scholar]
- Awad W. A., Ghareeb K., Böhm J. 2010. Effect of addition of a probiotic micro-organism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. J. Anim. Physiol. Anim. Nutr. 94:486–494. [DOI] [PubMed] [Google Scholar]
- Axelsson L. 2004. Lactic acid bacteria: classification and physiology. Pages 1–66 in: Lactic Acid Bacteria, 3rd edition, Salminen S, von Wright A., Ouwehand A., eds. Marcel Dekker Inc, New York. [Google Scholar]
- Bianchi Salvadori B., Camaschella P., Lavezzari D.. 1985. Les lactobacilles specifiques du poulet. Leur influence sur la microflore du tube digestif. Microbiol. Aliment. Nutr. 3:73–82. [Google Scholar]
- Bomba A., Nemcová R., Mudruñová D., Guba P.. 2002. The possibilities of potentiating the efficacy of probiotics. Trends Food Sci. Technol. 13:121–126. [Google Scholar]
- Castellini C., Mugnai C., Dal Bosco A.. 2002. Meat quality of three chicken genotypes reared according to the organic system. Ital. J. Food Sci. 14:411–412. [Google Scholar]
- Chichlowski M., Croom W. J., Edens F. W., MacBride B. W., Qiu R., Chiang C. C., Daniel L. R., Havenstein G. B., Koci M. D.. 2007. Microarchitecture and spatial relationship between bacteria and ileal, cecal and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac, and salinomycin. Poult. Sci. 86:1121–1132. [DOI] [PubMed] [Google Scholar]
- Daskiran M., Onol A. G., Cengiz O., Unsal H., Turkyilmaz S., Tatli O., Sevim O.. 2012. Influence of dietary probiotic inclusion on growth performance, blood parameters, and intestinal microflora of male broiler chickens exposed to posthatch holding time. J. Appl. Poult. Res. 21:612–622. [Google Scholar]
- De Cesare A., Sirri F., Manfreda G., Moniaci P., Giardini A., Zampiga M., Meluzzi A.. 2017. Effect of dietary supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) on caecum microbioma and productive performance in broiler chickens. PLoS One. 12:e0176309 https://doi.org/10.1371/journal.pone.0176309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission 2010. Directive 2010 /63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Brussels, European Parliament. [Google Scholar]
- European Commission 2016. Report from the commission to the European Parliament and the council on the impact of genetic selection on the welfare of chickens kept for meat production. Brussels, European Parliament. [Google Scholar]
- Forte C., Moscati L., Acuti G., Mugnai C., Franciosini M. P., Costarelli S., Cobellis G., Trabalza‐Marinucci M.. 2016a. Effects of dietary Lactobacillus acidophilus and Bacillus subtilis on laying performance, egg quality, blood biochemistry and immune response of organic laying hens. J. Anim. Physiol. Anim. Nutr. 100:977–987. [DOI] [PubMed] [Google Scholar]
- Forte C., Acuti G., Manuali E., Casagrande Proietti P., Pavone S., Trabalza-Marinucci M., Moscati L., Onofri A., Lorenzetti C., Franciosini M. P.. 2016b. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult. Sci. 95:2528–2535. [DOI] [PubMed] [Google Scholar]
- Foschi C., Laghi L., Parolin C., Giordani B., Compri M., Cevenini R., Marngoni A., Vitali B.. 2017. Novel approaches for the taxonomic and metabolic characterization of lactobacilli: Integration of 16S rRNA gene sequencing with MALDI-TOF MS and 1H-NMR. PLoS One. 12:e0172483.https://doi.org/10.1371/journal.pone.0172483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. H., Charles D. R.. 2002. Niche and Organic Chicken Products. Nottingham University Press, Nottingham, UK. [Google Scholar]
- Hossain M. M., Begum M., Kim I. H.. 2015. Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Vet. Med-Czech. 60:77–86. [Google Scholar]
- Jin L. Z., Ho Y. W., Abdullah N., Ali M. A., Jalaludin S.. 1998. Effects of adherent Lactobacillus cultures on growth, weight of organs and intestinal microflora and volatile fatty acids in broilers. Anim. Feed. Sci. Tech. 70:197–209. [Google Scholar]
- Kalavathy R., Abdullah N., Jalaludin S., Ho Y. W.. 2003. Effects of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Br. Poult. Sci. 44:139–144. [DOI] [PubMed] [Google Scholar]
- Khan M., Raoult D., Richet H., Lepidi H., La Scola B.. 2007. Growth-promoting effects of single-dose intragastrically administered probiotics in chickens. Br. Poult. Sci. 48:732–735. [DOI] [PubMed] [Google Scholar]
- Kim J. J., Khan W. I.. 2013. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens. 2:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu Q., Yang C., Yang X., Lv L., Yin C., Liu X., Yan H.. 2014. Effects of probiotics on the growth performance and intestinal microflora of broiler chickens. Pakistan J. Pharm. Sci. 27:713–717. [PubMed] [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J. J., Lee M. D.. 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 69:6816–6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone J. 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed www.Fass.org ISBN: 978-1-884706-11-0. [Google Scholar]
- Menconi A., Wolfenden A. D., Shivaramaiah S., Terraes J. C., Urbano T., Kuttel J., Kremer J. C., Hargis B. M., Tellez G.. 2011. Effect of lactic acid bacteria probiotic culture for the treatment of Salmonella enterica serovar Heidelberg in neonatal broiler chickens and turkey poults. Poult. Sci. 90:561–565. [DOI] [PubMed] [Google Scholar]
- Mookiah S., Sieo C. C., Ramasamy K., Abdullah N., Ho Y. W.. 2014. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 94:341–348. [DOI] [PubMed] [Google Scholar]
- Mountzouris K. C., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K.. 2007. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 86:309–317. [DOI] [PubMed] [Google Scholar]
- NOMISMA 2016. L’avicoltura italiana: un modello sostenibile e di integrazione di filiera nel settore delle carni. www.nomisma.it. [Google Scholar]
- Olnood C. G., Beski S. S. M., Choct M., Iji P. A.. 2015. Novel probiotics: their effects on growth performance, gut development, microbial community and activity of broiler chickens. Anim. Nutr. 1:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. A., Burkholder K. M.. 2003. Application of prebiotics and probiotics in poultry production. Poult. Sci. 82:627–631. [DOI] [PubMed] [Google Scholar]
- Penha Filho R. A. C., Díaz S. J. A., Fernando F. S., Chang Y. F., Andreatti Filho R. L., Junior A. B.. 2015. Immunomodulatory activity and control of Salmonella Enteritidis colonization in the intestinal tract of chickens by Lactobacillus based probiotic. Vet. Immunol. Immunopathol. 167:64–69. [DOI] [PubMed] [Google Scholar]
- Pour J. B., Kermanshahi H.. 2010. Effects of cecal cultures and a commercial probiotic (PremaLac®) on performance and serum lipids of broiler chickens. J. Anim. Vet. Adv. 9:1506–1509. [Google Scholar]
- Salarmoini M., Fooladi M. H.. 2011. Efficacy of Lactobacillus acidophilus as probiotic to improve broiler chicks performance. J. Agric. Sci. Tech. 13:165–172. [Google Scholar]
- Samant S. S., Seo H. S.. 2016. Quality perception and acceptability of chicken breast meat labeled with sustainability claims vary as a function of consumers’ label-understanding level. Food Qual. Prefer. 49:151–160. [Google Scholar]
- SAS Institute Inc 2010.JMP® 9 Basic Analysis and Graphing; SAS Institute Inc; Cary, NC. [Google Scholar]
- Shim Y., Ingale S., Kim J., Kim K., Seo D., Lee S., Chae B., Kwon I.. 2012. A multi-microbe probiotic formulation processed at low and high drying temperatures: effects on growth performance, nutrient retention and caecal microbiology of broilers. Br. Poult. Sci. 53:482–490. [DOI] [PubMed] [Google Scholar]
- Smirnov A., Perez R., Amit-Romach E., Sklan D., Uni Z.. 2005. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J. Nutr. 135:187–192. [DOI] [PubMed] [Google Scholar]
- Smith J. M. 2014. A review of avian probiotics. J. Avian Med. Surg. 28:87–94. [DOI] [PubMed] [Google Scholar]
- Standards Centre. 2009.. British Standards Institute, Animal Feeding Stuffs – Isolation and Enumerations of Lactobacillus spp. EN 15787 London, British Standards Institute. 2009 [Google Scholar]
- Timmerman H. M., Koning C. J., Mulder L., Rombouts F. M., Beynen A. C.. 2004. Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy. Int. J. Food Microbiol. 96:219–233. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zeng L., Wang C., Lin H., Jia H., Zhang W.. 2012. Effect of Lactobacillin on the mucosal immune function in duodenum of young broiler. J. Anim. Vet. Adv. 11:3843–3848. [Google Scholar]
- Watkins B. A., Kratzer F. H.. 1983. Effect of oral dosing of Lactobacillus strains on gut colonization and liver biotin in broiler chicks. Poult. Sci. 62:2088–2094. [DOI] [PubMed] [Google Scholar]
- Watkins B. A., Kratzer F. H.. 1984. Drinking water treatment with a commercial preparation of a concentrated Lactobacillus culture for broiler chickens. Poult. Sci. 63:1671–1673. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Kim I.. 2014. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, caecal microbial shedding, and excreta odor contents in broilers. Poult. Sci. 93:364–370. [DOI] [PubMed] [Google Scholar]