Abstract
Background:
High platelet reactivity (HPR) during clopidogrel treatment predicts postpercutaneous coronary intervention (PCI) ischemic events strongly and independently. Tongxinluo capsules (TCs) are a traditional Chinese medicine formulation used as antiplatelet treatment. However, its efficacy against HPR is not known. The aim of the present study was to evaluate the effects of TCs in acute coronary syndrome (ACS) patients with HPR.
Methods:
This multicenter, randomized, double-blind, placebo-controlled study prospectively analyzed 136 ACS patients with HPR who underwent PCI. The patients were enrolled from November 2013 to May 2014 and randomized to receive placebo or TCs in addition to standard dual antiplatelet therapy (DAPT) with aspirin and clopidogrel. The primary end points were the prevalence of HPR at 30 days and the mean change in P2Y12 reaction units (PRUs) between baseline and 30 days. Survival curves were constructed with Kaplan-Meier estimates and compared by log-rank tests between the two groups.
Results:
Both groups had a significantly reduced prevalence of HPR at 30 days versus baseline, but the TC group, compared with the placebo group, had greater reduction (15.8% vs. 24.8%, P = 0.013), especially among patients with one cytochrome P450 2C19 loss of function (LOF) allele (χ2= 2.931, P = 0.047). The TC group also had a lower prevalence of HPR (33.3% vs. 54.2%, t = 5.284, P = 0.022) and superior performance in light transmittance aggregometry and higher levels of high-sensitivity C-reactive protein (hsCRP), but the composite prevalence of ischemic events did not differ significantly (χ2= 1.587, P = 0.208).
Conclusions:
In addition to standard DAPT with aspirin and clopidogrel, TCs further reduce PRU and hsCRP levels, especially in patients carrying only one LOF allele. The data suggest that TCs could be used in combination therapy for ACS patients with HPR undergoing PCI.
Keywords: Acute Coronary Syndrome, Platelet Function Testing, Randomized Controlled Trial, Traditional Chinese Medicine
摘要
背景:
血小板高反应性(High platelet reactivity, HPR)与经皮冠状动脉介入治疗(Percutaneous coronary intervention, PCI)术后应用抗血小板药物氯吡格雷出现缺血事件息息相关。作为中药,通心络也是一种抗血小板药物。但是,在HPR患者中的疗效尚不明确。本研究的目的在于评价通心络在血小板高反应性的急性冠脉综合症(Acute coronary syndrome, ACS)患者中的疗效。
方法:
本项多中心、随机、安慰剂对照、双盲研究于2013年11月至2014年5月入选了136例HPR的ACS患者,并在行PCI后接受双联抗血小板治疗并随机加用通心络或安慰剂。主要终点是术后30天P2Y12受体单元的平均变化率(P2Y12 reaction units, PRU)并应用Kaplan-Meier曲线评价两组患者的生存率。
结果:
与基线相比,术后30天两组的HPR发生率均明显下降,但是与对照组相比,通心络组的变化更明显(15.8% vs. 24.8%, P=0.013),尤其是携带一个CYP2C19无功能基因的患者变化更明显(χ2=2.931, P=0.047)。此外,通心络治疗组;患者术后30 天光学比浊法测定的血小板反应性(33.3% vs. 54.2%, t=5.284, P=0.022)以及高敏C反应蛋白均较对照组有明显改善。但是并未在缺血临床不良事件中达到统计学差异(χ2=1.587, P=0.208)。
结论:
在传统的阿司匹林和氯吡格雷基础上加用通心络胶囊可以进一步降低PRU和高敏C反应蛋白水平,尤其是在携带一个CYP2C19无功能基因的患者中。本研究数据提示通心络胶囊可以在HPR的ACS患者行PCI术后与常规双联抗血小板联用。
INTRODUCTION
Each year, more than 5 million people worldwide undergo percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS).[1] Dual antiplatelet therapy (DAPT) with aspirin and a blocker of P2Y12 receptors (e.g., clopidogrel) is the cornerstone strategy to prevent recurrent ischemic events in ACS patients and in those undergoing PCI.[2] However, clopidogrel shows major individual variation in its antiplatelet effect in association with an increased incidence of ischemic events (e.g., stent thrombosis [ST]) in patients with high platelet reactivity (HPR)[3] as defined according to various platelet function test (PFT) methods (e.g., P2Y12 reaction units [PRUs] level ≤235).[4,5] Therefore, updated guidelines from the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA)/Society for Cardiovascular Angiography and Interventions and European Society of Cardiology issued a class-IIb recommendation for PFT to facilitate the choice of P2Y12 receptor blocker in select high-risk patients undergoing PCI.[6,7,8]
In traditional Chinese medicine (TCM), HPR and ischemic events are classified as “collateral stagnation” and “blood stasis syndrome”, respectively. They have been treated for decades with demonstrated efficacy with Tongxinluo capsules (TCs).[9,10] Platelets are activated via multiple pathways, and the activation and aggregation of platelets does not influence ischemic events alone. Hence, a single-antiplatelet treatment strategy (e.g., high-dose clopidogrel) directed against a specific receptor cannot be expected to completely overcome HPR, or to reduce the risk of recurrent ischemic events.[11,12]
Therefore, triple antiplatelet therapy has gained popularity.[13] Here, we sought to evaluate the effects of triple antiplatelet therapy with TCs plus standard DAPT in ACS patients with HPR who underwent PCI.
METHODS
Ethical approval of the study protocol
The study protocol was approved by the Ethics Committee of General Hospital of Shenyang Military Region (No. 2013015). The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All participants provided written informed consent.
Design and population
The primary objective of the present study was to ascertain if TCs were superior to a placebo in ACS patients with HPR. This was a multicenter, randomized, double-blind, placebo-controlled study based on standard DAPT therapy. The target enrolment was 136 patients at three clinical research centers in China. The study population involved ACS patients with HPR who underwent PCI. ST-segment elevation myocardial infarction (STEMI) was defined according to guidelines set by the ACCF/AHA in 2009.[14] Non-STEMI and unstable angina pectoris were defined according to guidelines set by the ACCF/AHA in 2012.[15] The inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria of the study
| Inclusion criteria |
| Patients aged 18–75 years with symptoms of ACS |
| At least one coronary stent implantation |
| VerifyNow assay showed PRU >235 at least 24 h after administration of clopidogrel 300 mg loading dose |
| Exclusion criteria |
| Contraindication to Tongxinluo capsule |
| Unable to give informed consent or a life expectancy of <1 year |
| Malignancy with increase in bleeding risk* |
| Women who are known to be pregnant or who have given birth within the past 90 days or who are breastfeeding |
| Severe renal function impairment needing dialysis |
| Contraindication to anticoagulation or at increased bleeding risk* |
| Cardiogenic shock (SBP ≤80 mmHg for >30 min) or intra-aortic balloon pump placed |
| History of major surgery, severe trauma, fracture, or organ biopsy within 90 days before randomization |
| Clinically significant out-of-range values for platelet count or hemoglobin level* |
*According to the investigator. 1 mmHg = 0.133 kPa. ACS: Acute coronary syndrome; PRU: P2Y12 reaction unit; SBP: Systolic blood pressure.
Study protocol
In this double-blind trial, patients were evaluated based on self-reported history, physical examinations, laboratory screening, electrocardiography, and coronary computed tomography. Samples of venous blood were obtained 24 h after administration of a loading dose of aspirin (300 mg) and clopidogrel (300 mg or 600 mg). A PFT was undertaken within 2 h after the blood sample had been taken. Eligible patients were assigned randomly to two groups that received TCs or a placebo for 1 year (in a 1:1 ratio; the two types of treatments were provided as capsules that were identical in size, color, and shape) in addition to their standard treatment for ACS by the attending physicians. The study medication was labeled with sequential randomization numbers. Each patient was assigned the lowest number available at each site at the visit for which randomization was carried out. The dose used in the present study was 3 TCs or 3 placebo capsules three times daily. Patients attended follow-up appointments at 30 days of treatment. At this visit, blood samples were collected to test platelet function as well as levels of markers of coagulation and inflammation: plasma fibrinogen (FIB), thrombin time (TT), prothrombin time (PT), and high-sensitivity C-reactive protein (hsCRP). In the follow-up telephone call at 180 and 360 days, patients were asked about the occurrence of any clinical event or adverse event (AE).
Laboratory tests
Routine laboratory tests (complete blood count, serum ionized, cardiac enzyme, coagulation, and inflammatory markers) were undertaken in the local laboratories of the participating institutions. PRUs, light transmittance aggregometry (LTA), vasodilator-stimulated phosphoprotein phosphorylation-platelet reactivity index (VASP-PRI), and the cytochrome P450 (CYP) 2C19 genotype were measured in the core laboratory.
PRU was tested by the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA). LTA was analyzed by optical turbidimetric aggregometry using a four-channel platelet aggregation chromogenic kinetic system (Helena Laboratories, Beaumont City, TX, USA). VASP-PRI was analyzed by Platelet VASP kits (Diagnostica Stago, Asnières, France). DNA was extracted from the peripheral blood of enrolled patients using TIANamp Blood DNA kits (Tiangen Biotech, Beijing, China). All 19 selected single-nucleotide polymorphisms were genotyped using standard polymerase chain reaction methods. DNA sequencing was done on an ABI Prism 3730 genetic analyzer (Applied Biosystems, Foster City, CA, USA) using an ABI dye terminator cycle sequencing kit (Applied Biosystems).
End points
The primary end point was HPR prevalence at 30 days. The secondary laboratory end point was the mean change in levels of FIB, TT, PT, and hsCRP between baseline and 30-day follow-up. The composite clinical ischemic end points were all-cause death, MI, revascularization of the target vessel, ST (classified according to the Academic Research Consortium criteria), and stroke.[16] All AEs were adjudicated by a committee looking at clinical end points and who were blinded to the study protocol.
Calculation of sample size
The sample size was calculated based on the expected proportion of patients demonstrating a reduction in PRU to ≤235. According to previous clinical results, we assumed the proportion of patients demonstrating a reduction in PRU to ≤235 in the placebo group would be 45%, 17% lower than that in the TCs group. Therefore, given a prevalence of a Type-I error of alpha = 0.05 and a power of 80% (prevalence of a Type-II error of beta = 0.2), the sample size for one arm needed to be 56, resulting in n = 2 × 56 = 112 patients. Considering a dropout prevalence about 20% for randomized patients, 136 patients (68 per treatment group) needed to be randomized to achieve the required number of patients for efficacy analyses. Under this assumption, we recruited 136 patients to the study, who were subsequently allocated, at a 1:1 ratio, to the TC group or placebo group.
Statistical analyses
All statistical analyses were undertaken with SPSS version 20.0 (IBM, Armonk, NY, USA). Data from all patients who underwent randomization were evaluated according to the “full analysis set” principle. Continuous variables were presented as the mean ± standard deviation (SD). Nonnormally distributed data were presented as n (%) or median (interquartile range). The comparability of the characteristics between the two study groups was assessed using a two-sample Student's t-test for continuous variables and the Chi-square test or Wilcoxon test, if appropriate, for categorical variables. The Wilcoxon paired signed-rank test was used for within-group comparisons. Survival curves were constructed with Kaplan-Meier estimates and compared by log-rank tests. A value of P < 0.05 was considered statistically significant, and all tests were two tailed.
RESULTS
Baseline characteristics
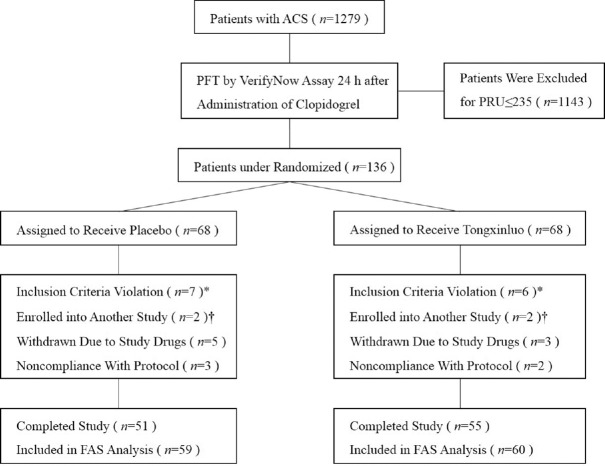
A total of 136 patients underwent randomization at three sites in China, the flow diagram of patients through the study is presented in Figure 1. The baseline characteristics of the study groups are shown in Table 2. The mean age of the total population was 58.4 years, and 63.0% were male. The demographic and clinical characteristics in the TC and placebo groups were well balanced and homogeneous.
Figure 1.
Flow diagram illustrating the number of patients in each group throughout the study. *These patients refused stent implantation or preferred to undergo coronary artery bypass grafting. †These patients enrolled into another study which underwent a different antiplatelet therapy before the administration of Tongxinluo capsules. ACS: Acute coronary syndrome; PFT: Platelet function testing; PRU: P2Y12 reaction unit; PCI: Percutaneous coronary intervention; FAS: Full analysis set.
Table 2.
Baseline characteristics of patients receiving placebo or Tongxinluo capsules
| Characteristics | Placebo (n = 59) | Tongxinluo capsules (n = 60) | Statistics | P |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 58.1 ± 11.6 | 58.7 ± 10.8 | −0.433† | 0.753 |
| Male* | 36 (61.0) | 39 (65.0) | 0.203‡ | 0.653 |
| Measurements | ||||
| BMI (kg/m2) | 24.4 ± 2.8 | 24.8 ± 2.7 | −0.616† | 0.539 |
| SBP (mmHg) | 140.7 ± 22.7 | 142.5 ± 18.6 | −0.471† | 0.639 |
| DBP (mmHg) | 79.8 ± 12.4 | 83.1 ± 12.2 | −1.481† | 0.141 |
| Heart rate (beats/min) | 77.8 ± 13.7 | 76.1 ± 13.4 | 0.688† | 0.493 |
| LVEF (%) | 63.6 ± 8.9 | 63.2 ± 6.9 | 0.218† | 0.828 |
| Medical history | ||||
| Hypertension* | 37 (62.7) | 38 (63.3) | 0.005‡ | 0.944 |
| Diabetes mellitus* | 19 (32.2) | 17 (28.3) | 0.211‡ | 0.646 |
| Smoking history* | 15 (25.4) | 16 (26.7) | 0.024‡ | 0.877 |
| Stroke* | 9 (15.3) | 10 (16.7) | 0.044‡ | 0.833 |
| Medication | ||||
| Stains nonmetabolized by CYP3A4* | 26 (44.1) | 25 (41.7) | 0.070‡ | 0.791 |
| Stains metabolized by CYP3A4* | 28 (47.5) | 28 (46.7) | 0.007‡ | 0.931 |
| Laboratory measurements | ||||
| Creatinine (µmol/L) | 62.7 ± 15.5 | 65.6 ± 17.0 | −0.863† | 0.390 |
| Triglycerides (mmol/L) | 1.6 (0.8) | 1.7 (1.3) | 0.302 | |
| Total cholesterol (mmol/L) | 3.8 ± 1.0 | 3.7 ± 0.8 | 0.184† | 0.856 |
| HDL-C (mmol/L) | 0.9 (0.2) | 0.9 (0.3) | 0.512 | |
| LDL-C (mmol/L) | 1.9 (1.4) | 2.0 (1.2) | 0.807 | |
| Alanine aminotransferase (U/L) | 19.5 (12.8) | 21.0 (15.7) | 0.230 | |
| Aspartate aminotransferase (U/L) | 20.0 (8.0) | 22.0 (7.8) | 0.229 | |
| Angiographic characteristics | ||||
| Numbers of lesions | 2.0 (1.0) | 2.0 (1.75) | 0.243 | |
| Left main coronary artery disease* | 8 (13.6) | 7 (11.7) | 0.097‡ | 0.756 |
| Number of stent implantation | 1.0 (0) | 1.0 (0) | 0.871 |
Data are presented as mean ± SD, *n (%), or median (interquartile range). †t values; ‡χ2 values. BMI: Body mass index; LVEF: Left ventricular ejection fraction; HDL-C: High-density lipoprotein-cholesterol; LDL-C: Low-density lipoprotein-cholesterol; SD: Standard deviation; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
Changes in the primary end point
A favorable effect of TCs was observed on the PFT [Table 3]. After 30 days of treatment, both groups showed a significant reduction in PRU and LTA levels from baseline, but treatment with TCs led to a significantly greater mean percentage reduction than that elicited by the placebo (15.8% vs. 24.8%, P = 0.013). In addition, 33.3% of patients in the TC group continued to have HPR, compared with 54.2% of patients in the placebo group (χ2= 5.284, P = 0.022). The LTA assay yielded similar results to that of the VerifyNow assay (17.9% vs. 28.8%, P = 0.007). With regard to the VASP-PRI, the difference between the TC (7.1%) and placebo (11.3%) groups did not reach significance (P = 0.337).
Table 3.
Change in primary endpoints and other PFTs results from baseline to 30 days follow-up
| Parameters | Placebo (n = 59) | t | P | Tongxinluo capsules (n = 60) | t | P | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | 30 days | Baseline | 30 days | |||||
| Verify now PRU assay | ||||||||
| PRU | 284.7 ± 42.3 | 235.3 ± 41.1 | 6.498 | <0.001 | 295.9 ± 40.1 | 220.9 ± 37.6 | 10.561 | <0.001 |
| LTA assay | ||||||||
| LTA (%) | 63.1 ± 8.4 | 50.3 ± 7.6 | 3.592 | <0.001 | 62.6 ± 7.9 | 44.2 ± 6.2 | 5.973 | <0.001 |
| VASP-PRI assay (30 vs. 30)* | ||||||||
| PRI (%) | 60.7 ± 8.3 | 57.0 ± 10.6 | 1.260 | 0.145 | 60.6 ± 8.6 | 51.7 ± 15.2 | 2.271 | 0.019 |
Data are presented as mean ± standard deviation. *All platelet function tests (PFTs) were free for enrolled patients, so only 30 patients in each group underwent. VASP-PRI assay due to shortage of research funding. PRU: P2Y12 reaction units; LTA: light transmittance aggregometry; VASP-PRI: Vasodilator-stimulated phosphoprotein phosphorylation-platelet reactivity index
Change in secondary laboratory end points
Measurement of levels of FIB, TT, PT, and hsCRP was done at baseline and at 30 days. HsCRP levels did not differ significantly between the two groups at baseline [Table 4]. Greater improvement was observed after treatment with TCs (t = 1.999, P = 0.048) but not with placebo (t = 0.932, P = 0.353), accompanying DAPT at the 30-day visit. In addition, TC treatment led to a significantly greater mean percentage reduction of the hsCRP level than did the placebo (8.7% vs. 15.0%, P = 0.037). No significant differences from baseline to the 30-day visit were observed for levels of FIB (1.0% vs. 4.6%, P = 0.395), TT (3.2% vs. 1.8%, P = 0.954), or PT (0.8% vs. 1.1%, P = 0.967).
Table 4.
Change in secondary laboratory endpoints from baseline to 30 days follow-up
| Parameters | Placebo (n = 59) | t | P | Tongxinluo capsules (n = 60) | t | P | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | 30 days | Baseline | 30 days | |||||
| Plasma fibrinogen (g/L) | 4.2 ± 0.9 | 4.2 ± 0.9 | −0.081 | 0.935 | 4.3 ± 0.8 | 4.4 ± 0.8 | -0.898 | 0.371 |
| Thrombin time (s) | 13.3 ± 1.6 | 13.5 ± 1.1 | −0.797 | 0.427 | 13.3 ± 1.3 | 13.7 ± 1.1 | -1.514 | 0.133 |
| Prothrombin time (s) | 13.1 ± 0.8 | 13.3 ± 1.0 | −1.163 | 0.247 | 13.0 ± 1.1 | 13.2 ± 1.0 | -0.828 | 0.409 |
| hsCRP (mg/L) | 2.0 ± 1.0 | 1.8 ± 0.8 | 0.932 | 0.353 | 2.0 ± 1.2 | 1.4 ± 0.7 | 1.999 | 0.048 |
Data are presented as mean ± standard deviation. hsCRP: High-sensitivity C-reactive protein.
Loss of function alleles of cytochrome P450 2C19
The genotype was tested at baseline. The proportion of patients carrying none, one, or two loss of function (LOF) alleles of CYP2C19 was 31.9%, 49.6%, and 17.6%, respectively [Table 5]. After 30 days of treatment, patients with or without LOF alleles showed a significant reduction in PRU in both groups. However, only patients with one LOF allele in the TC group showed a lower prevalence of HPR (31.0% vs. 56.7%, χ2= −2.931, P = 0.047) than those in the placebo group.
Table 5.
Change in primary endpoints among patients with different genotypes from baseline to 30 days follow-up
| PRU | Placebo | t | P | Tongxinluo capsules | t | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | 30 days | n | Baseline | 30 days | |||||
| No LOF | 17 | 277.7 ± 42.5 | 210.9 ± 39.4 | 4.190 | <0.001 | 21 | 290.6 ± 43.0 | 209.6 ± 46.5 | 5.734 | <0.001 |
| One LOF | 30 | 287.1 ± 39.1 | 243.9 ± 39.9 | 4.250 | <0.001 | 29 | 297.8 ± 31.6 | 222.7 ± 28.2 | 9.255 | <0.001 |
| Two LOF | 11 | 288.7 ± 40.2 | 247.3 ± 34.0 | 2.972 | 0.019 | 10 | 301.7 ± 35.6 | 239.5 ± 36.0 | 3.602 | 0.002 |
Data are presented as mean ± standard deviation LOF: Loss of function; PRU: P2Y12 reaction unit.
Clinical outcomes
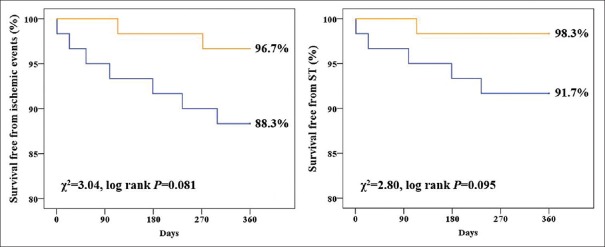
Figure 2 presents the prevalence of ischemic event composites at 1 year for both groups. Overall, 3.3% and 11.9% of patients in the TC and placebo groups experienced ischemic events, respectively (χ2= 1.997, P = 0.158). Figure 2 reveals that the TC group showed higher ischemic- and ST-free survival than that of the placebo group. However, no significant difference was observed between these two groups.
Figure 2.
Ischemic- and ST-free survival rate between placebo group and Tongxinluo capsules group. Orange line represents Tongxinluo capsules group; blue line represents placebo group. ST: Stent thrombosis.
Adverse events
Sixty-six patients in each group were included in the safety set analyses [Table 6]. The total number of AEs was 12 in the TC group versus 18 in the placebo group (χ2= −1.553, P = 0.213). With regard to serious AEs, the total number was 3 in the TC group versus 8 in the placebo group (χ2= 1.587, P = 0.208). Some patients reported more than one AE. There was no report of any serious AEs related to the study drugs. An analysis of drug-induced AEs and withdrawal revealed no significant differences between the two groups.
Table 6.
Summary of adverse events of the study (n = 66)
| Events | Placebo | Tongxinluo capsules | χ2 | P | ||||
|---|---|---|---|---|---|---|---|---|
| AEs | 18 (27.3) | 12 (18.2) | 1.553 | 0.213 | ||||
| AEs related to study drugs | 10 (14.7) | 5 (8.8) | 1.880 | 0.170 | ||||
| SAEs | 8 (11.8) | 3 (4.4) | 1.587 | 0.208 | ||||
| Death | 4 (5.9) | 1 (1.5) | 0.831 | 0.362 | ||||
| Hospitalization | 6 (8.8) | 3 (4.4) | 0.477 | 0.490 | ||||
| MI | 1 (1.5) | 1 (1.5) | 0.508 | 0.476 | ||||
| TVR* | 3 (6.7) | 1 (1.7) | 0.258 | 0.612 | ||||
| ST* | 3 (5.0) | 0 (0) | 1.364 | 0.243 | ||||
| Stroke | 1 (1.5) | 1 (1.5) | 0.508 | 0.476 | ||||
| Recurrent angina | 1 (1.5) | 0 (0) | – | 1.000 | ||||
| Heart failure | 0 (0) | 1 (1.5) | – | 1.000 | ||||
| Withdrew due to study drugs | 5 (7.4) | 3 (4.4) | 0.133 | 0.715 |
Data are presented as n (%). The analysis included all patients who received at least 1 dose of the study medication. Some patients reported >1 event. *One dead patient in the placebo group was not included in the full analysis set analysis for a car accident. AE: Adverse event; SAE: Serious adverse event; MI: Myocardial infarction; TVR: Target vascular revascularization; ST: Stent thrombosis.
DISCUSSION
In this randomized trial of ACS patients with HPR who underwent PCI, addition of TCs to standard DAPT with aspirin and clopidogrel was associated with favorable effects on platelet function relative to placebo addition.
In the present study, the VerifyNow assay was used to test platelet function, and HPR was defined as PRU ≥235, which is consistent with the GRAUITAS and ARCTIC studies[11,12] and the consensus definition of HPR based on various PFT methods.[4,5] It has been demonstrated that HPR during clopidogrel treatment is a strong and independent risk factor for post-PCI ischemic events.[17] Studies have indicated that clopidogrel resistance plays an important part in HPR and that a substantial percentage of patients (>35%) exhibit negligible or no antiplatelet response to clopidogrel.[4,5] Multiple lines of evidence strongly suggest that variable and insufficient active generation of metabolites are the primary reasons for the variability in response and nonresponsiveness of clopidogrel.[4,18,19,20,21,22,23,24,25,26] Given the multiplicity of factors that affect platelet reactivity to adenosine diphosphate, a single-antiplatelet treatment strategy directed against a specific receptor cannot be expected to overcome HPR or to reduce the risk of ischemic events.
Two antiplatelet drugs that were introduced recently, ticagrelor and prasugrel, offer advantages over clopidogrel. Both show a stronger antiplatelet effect with much less interpatient variation, as well as a faster onset of action and reduction in ischemic events, as shown in the PLATO and TRITON-TIMI 38 studies.[1,27] However, they also have drawbacks compared with clopidogrel. First, they have been associated with an increase in the prevalence of noncoronary artery bypass graft-related major bleeding, which is known to correlate with increased mortality.[28,29] Second, uncertainty about dose adjustments for low body weight and older age in patients using prasugrel, the mandated twice-daily dosing of ticagrelor, and the frequently observed side effect of dyspnea in patients using ticagrelor limit the general use of these newer drugs.[30] Third, in many countries, social health insurance does not cover the cost of newer P2Y12 receptor blockers such as ticagrel, which results in a major increase in drug costs compared with the generic clopidogrel.
The advantages and disadvantages of newer antiplatelet medications discussed above justify the search for a multiple-target drug for HPR treatment. TCM provides one such alternative in the form of TCs. TCM theory holds that HPR is caused by stagnation of collateral qi. After oral administration of clopidogrel, active metabolites fail to selectively and irreversibly combine with adenosine diphosphate receptors on the surface of platelet membranes. Hence, P-selectin expression decreases, which cannot inhibit activation of platelets, and causes stasis of channels, thrombosis, and vascular stenosis. This phenomenon can be analogized to a traffic-signal fault that induces a traffic jam. Stagnation of collateral qi is like a traffic-signal fault, and stasis of channels can cause a traffic jam at a crossroad.
Ginseng in the formulation of TCs can enrich heart qi, and abundant blood qi can promote the blood circulation, and channels become clear; this phenomenon can be analogized to a commander solving a traffic jam and who has a coordinating role. Sandalwood, rosewood, and frankincense can smooth collateral qi; this phenomenon can be analogized to stopping the traffic jam by solving the traffic-signal fault. Leeches and eupolyphaga can remove vascular stasis; this phenomenon can be analogized to immediate dispersion of the traffic jam caused by a traffic-signal fault, leading to traffic proceeding normally.
A recent study demonstrated TCs to be associated with a reduction in levels of hsCRP and low-density lipoprotein cholesterol, with similar protective effects to that of simvastatin in terms of lowering lipid levels, inhibiting inflammation, and anti-oxidation effects.[31] The effect on reduction in the hsCRP level was also found in the present study [Table 4]. Studies have demonstrated that patients with lower serum levels of hsCRP have better clinical outcomes than those with higher levels of hsCRP.[32,33,34] Based on the aforementioned effects and the results of the present study, TCs show promise as an antiplatelet drug that can be used routinely with standard DAPT for ACS patients with HPR.
Genetic variations, such as the CYP2C19 *2 and *3 LOF alleles, account (at least in part) for the variation in response to clopidogrel. Patients who carry a LOF allele of CYP2C19 have a higher thrombotic risk after PCI compared with noncarriers.[35,36,37,38] The utility of genetic tests to identify differences in CYP2C19 function has been highlighted by the “boxed warning” issued by the US Food and Drug Administration advising health-care professionals to consider the use of other antiplatelet medications or alternative dosing strategies for clopidogrel in these patients.[39] In the present study, TCs were found to reduce PRU regardless of genotype. In addition, patients with one LOF allele in the TC group had significantly greater mean percentage reduction and lower prevalence of HPR than those in the placebo group after 30 days of treatment.
The effects of a drug are meaningful only if they are associated with better clinical outcomes. Although differences in clinical end points between the TC and placebo groups did not reach significance in the present study, the composite prevalence of ischemic events and ischemic event- and ST-free survival was numerically better in the TC group.
The present study was a pilot study and not designed to show differences in clinical end points, so an appropriately powered study is needed. Despite the high hopes for use of the results of PFT and CYP2C19 genotype testing for prognostication, recent prospective, randomized trials have failed to demonstrate that personalized antiplatelet therapy based on platelet function is effective in reducing the prevalence of ischemic events.[9,10,40] Since all PFTs were free for the enrolled patients, only thirty patients in each group underwent VASP-PRI assay due to shortage of research funding and it might lead to no significant difference between the two groups in PRI levels. In summary, combination therapy of standard DAPT with aspirin and clopidogrel plus TCs seems to be a promising choice for ACS patients undergoing PCI.
Financial support and sponsorship
This study was supported by a grant from the National Key Technology R&D Program in the 13th 5-Year Plan of China (No. 2016YFC1301303).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to express sincere thanks to Dr. Roberto Partaca and all investigators who involved in this study.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 2.Gurbel PA, Tantry US. Combination antithrombotic therapies. Circulation. 2010;121:569–83. doi: 10.1161/CIRCULATIONAHA.109.853085. doi: 10.1161/CIRCULATIONAHA. 109.853085. [DOI] [PubMed] [Google Scholar]
- 3.Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: Response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–13. doi: 10.1161/01.CIR.0000072771.11429.83. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 4.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–33. doi: 10.1016/j.jacc.2010.04.047. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 5.Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–73. doi: 10.1016/j.jacc.2013.07.101. doi: 10.1016/j.jacc. 2013.07.101. [DOI] [PubMed] [Google Scholar]
- 6.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol. 2011;58:e44–122. doi: 10.1016/j.jacc.2011.08.007. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:645–81. doi: 10.1016/j.jacc.2012.06.004. doi: 10.1016/j.jacc. 2012.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 9.Liu K, Wang XJ, Li YN, Li B, Qi JS, Zhang J, et al. Tongxinluo reverses the hypoxia-suppressed claudin-9 in cardiac microvascular endothelial cells. Chin Med J. 2016;129:442–7. doi: 10.4103/0366-6999.176076. doi: 10.4103/0366-6999.176076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YN, Wang XJ, Li B, Liu K, Qi JS, Liu BH, et al. Tongxinluo inhibits cyclooxygenase-2, inducible nitric oxide synthase, hypoxia-inducible factor-2α/vascular endothelial growth factor to antagonize injury in hypoxia-stimulated cardiac microvascular endothelial cells. Chin Med J. 2015;128:1114–20. doi: 10.4103/0366-6999.155119. doi: 10.4103/0366-6999.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al. Standard- vs.high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: The GRAVITAS randomized trial. JAMA. 2011;305:1097–105. doi: 10.1001/jama.2011.290. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 12.Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367:2100–9. doi: 10.1056/NEJMoa1209979. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- 13.Han YL, Zhang QY, Li Y, Guan SY, Jing QM, Wang ZL, et al. Clinical presentations, antiplatelet strategies and prognosis of patients with stent thrombosis: An observational study of 140 patients. PLoS One. 2012;7:e48520. doi: 10.1371/journal.pone.0048520. doi: 10.1371/journal.pone.0048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 15.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:645–81. doi: 10.1016/j.jacc.2012.06.004. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 17.Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet. 2013;382:614–23. doi: 10.1016/S0140-6736(13)61170-8. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 18.Lau WC, Gurbel PA, Watkins PB, Neer CJ, Hopp AS, Carville DG, et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation. 2004;109:166–71. doi: 10.1161/01.CIR.0000112378.09325.F9. doi: 10.1161/01.CIR.0000112378.09325.F9. [DOI] [PubMed] [Google Scholar]
- 19.Lau WC, Welch TD, Shields T, Rubenfire M, Tantry US, Gurbel PA, et al. The effect of St. John's Wort on the pharmacodynamic response of clopidogrel in hyporesponsive volunteers and patients: Increased platelet inhibition by enhancement of CYP3A4 metabolic activity. J Cardiovasc Pharmacol. 2011;57:86–93. doi: 10.1097/FJC.0b013e3181ffe8d0. doi: 10.1097/FJC.0b013e3181ffe8d0. [DOI] [PubMed] [Google Scholar]
- 20.Bliden KP, Dichiara J, Lawal L, Singla A, Antonino MJ, Baker BA, et al. The association of cigarette smoking with enhanced platelet inhibition by clopidogrel. J Am Coll Cardiol. 2008;52:531–3. doi: 10.1016/j.jacc.2008.04.045. doi: 10.1016/j.jacc.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: The randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256–60. doi: 10.1016/j.jacc.2007.06.064. doi: 10.1016/j.jacc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 22.Gurbel PA, Bliden KP, Fort J, Zhang Y, Plachetka JR, Antonino M, et al. Pharmacodynamic evaluation of clopidogrel plus PA32540: The Spaced PA32540 with Clopidogrel Interaction Gauging (SPACING) study. Clin Pharmacol Ther. 2011;90:860–6. doi: 10.1038/clpt.2011.201. doi: 10.1038/clpt. 2011.201. [DOI] [PubMed] [Google Scholar]
- 23.Gurbel PA, Ohman EM, Jeong YH, Tantry US. Toward a therapeutic window for antiplatelet therapy in the elderly. Eur Heart J. 2012;33:1187–9. doi: 10.1093/eurheartj/ehr458. doi: 10.1093/eurheartj/ehr458. [DOI] [PubMed] [Google Scholar]
- 24.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Sabaté M, Jimenez-Quevedo P, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–5. doi: 10.2337/diabetes.54.8.2430. doi:10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 25.Sibbing D, von Beckerath O, Schömig A, Kastrati A, von Beckerath N. Platelet function in clopidogrel-treated patients with acute coronary syndrome. Blood Coagul Fibrinolysis. 2007;18:335–9. doi: 10.1097/MBC.0b013e3280d21aed. doi: 10.1097/MBC.0b013e3280d21aed. [DOI] [PubMed] [Google Scholar]
- 26.Angiolillo DJ, Fernández-Ortiz A, Bernardo E, Barrera Ramírez C, Sabaté M, Fernandez C, et al. Platelet aggregation according to body mass index in patients undergoing coronary stenting: Should clopidogrel loading-dose be weight adjusted? J Invasive Cardiol. 2004;16:169–74. [PubMed] [Google Scholar]
- 27.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 28.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S, et al. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 29.Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking Angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4:654–64. doi: 10.1016/j.jcin.2011.02.011. doi: 10.1016/j.jcin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Storey RF, Becker RC, Harrington RA, Husted S, James SK, Cools F, et al. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011;32:2945–53. doi: 10.1093/eurheartj/ehr231. doi: 10.1093/eurheartj/ehr231. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Liu Y, Lu XT, Wu YL, Zhang C, Ji XP, et al. Traditional Chinese medication Tongxinluo dose-dependently enhances stability of vulnerable plaques: A comparison with a high-dose simvastatin therapy. Am J Physiol Heart Circ Physiol. 2009;297:H2004–14. doi: 10.1152/ajpheart.00208.2009. doi: 10.1152/ajpheart.00208.2009. [DOI] [PubMed] [Google Scholar]
- 32.Albert MA, Danielson E, Rifai N, Ridker PM PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Rifai N, Lowenthal SP. Rapid reduction in C-reactive protein with cerivastatin among 785 patients with primary hypercholesterolemia. Circulation. 2001;103:1191–3. doi: 10.1161/01.cir.103.9.1191. doi: 10.1161/01.CIR.103.9.1191. [DOI] [PubMed] [Google Scholar]
- 35.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: A cohort study. Lancet. 2009;373:309–17. doi: 10.1016/S0140-6736(08)61845-0. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 36.Harmsze AM, van Werkum JW, Ten Berg JM, Zwart B, Bouman HJ, Breet NJ, et al. CYP2C19*2 and CYP2C9*3 alleles are associated with stent thrombosis: A case-control study. Eur Heart J. 2010;31:3046–53. doi: 10.1093/eurheartj/ehq321. doi: 10.1093/eurheartj/ehq321. [DOI] [PubMed] [Google Scholar]
- 37.Kubica A, Kozinski M, Grzesk G, Fabiszak T, Navarese EP, Goch A, et al. Genetic determinants of platelet response to clopidogrel. J Thromb Thrombolysis. 2011;32:459–66. doi: 10.1007/s11239-011-0611-8. doi: 10.1007/s11239-011-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A meta-analysis. JAMA. 2010;304:1821–30. doi: 10.1001/jama.2010.1543. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.FDA Drug Safety Communication: reduced Effectiveness of Plavix (clopidogrel) in Patients who are Poor Metabolizers of the Drug. [Last accessed on 2011 Apr 28]. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm .
- 40.Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: Results of the TRIGGER-PCI (Testing platelet reactivity in patients undergoing elective stent placement on clopidogrel to guide alternative therapy with prasugrel) study. J Am Coll Cardiol. 2012;59:2159–64. doi: 10.1016/j.jacc.2012.02.026. doi: 10.1016/j.jacc.2012.02.026. [DOI] [PubMed] [Google Scholar]