Abstract
Background:
Chronic kidney disease (CKD) is closely related to the cardiovascular events in vascular calcification (VC). However, little has known about the characteristics of kidney injury caused by VC. Fibroblast growth factor 21 (FGF21) is an endocrine factor, which takes part in various metabolic actions with the potential to alleviate metabolic disorder diseases. Even FGF21 has been regarded as a biomarker in CKD, the role of FGF21 in CKD remains unclear. Therefore, in this study, we evaluate the FGF21 on the kidney injury in VC rats.
Methods:
The male Sprague-Dawley rats were divided into three groups: (1) control group, (2) Vitamin D3 plus nicotine (VDN)-induced VC group, (3) FGF21-treated VDN group. After 4 weeks, the rats were killed and the blood was collected for serum creatinine, urea nitrogen, calcium, and phosphate measurement. Moreover, the renal tissues were homogenized for alkaline phosphatases (ALPs) activity and calcium content. The levels of FGF21 protein were measured by radioimmunoassay. The levels of β-Klotho and FGF receptor 1 (FGFR1) protein were measured by enzyme-linked immunosorbent assay (ELISA). The structural damage and calcifications in aortas were stained by Alizarin-red S. Moreover, the structure of kidney was observed by hematoxylin and eosin staining.
Results:
The renal function impairment caused by VDN modeling was ameliorated by FGF21 treatment, inhibited the elevated serum creatinine and urea level by 20.5% (34.750 ± 4.334 μmol/L vs. 27.630 ± 2.387 μmol/L) and 4.0% (7.038 ± 0.590 mmol/L vs. 6.763 ± 0.374 mmol/L; P < 0.01), respectively, together with the structural damages of glomerular atrophy and renal interstitial fibrosis. FGF21 treatment downregulated the ALP activity, calcium content in the kidney of VC rats by 42.1% (P < 0.01) and 11.7% (P < 0.05) as well as ameliorated the aortic injury and calcification as compared with VDN treatment alone group, indicating an ameliorative effect on VC. ELISA assays showed that the expression of β-Klotho, a component of FGF21 receptor system, was increased in VDN-treated VC rats by 37.4% (6.588 ± 0.957 pg/mg vs. 9.054 ± 0.963 pg/mg; P < 0.01), indicating an FGF21-resistant state. Moreover, FGF21 treatment downregulated the level of β-Klotho in renal tissue by 16.7% (9.054 ± 0.963 pg/mg vs. 7.544 ± 1.362 pg/mg; P < 0.05). However, the level of FGFR1, the receptor of FGF21, kept unchanged under VDN and VDN plus FGF21 administration (0.191 ± 0.0376 ng/mg vs. 0.189 ± 0.032 ng/mg vs. 0.181 ± 0.034 ng/mg; P > 0.05).
Conclusions:
In the present study, FGF21 was observed to ameliorate the kidney injury in VDN-induced VC rats. FGF21 might be a potential therapeutic factor in CKD by cutting off the vicious circle between VC and kidney injury.
Keywords: Chronic, Fibroblast Growth Factor 21, Renal Insufficiency, Vascular Calcification, β-Klotho
摘要
背景:
在血管钙化中,慢性肾脏病与心血管事件发生密切相关。然而,血管钙化对肾脏损伤的作用知之甚少。FGF21是一种内分泌因子,其在多种代谢过程中发挥作用,并有治疗代谢紊乱性疾病的潜能。尽管FGF21已经被视为一种慢性肾脏病的生物标志物,但其在慢性肾脏病中的作用尚不明确。因此,本实验旨在观察血管钙化对大鼠肾脏结构及功能的损伤情况,以及与肾脏组织局部成纤维细胞生长因子21(Fibroblast growth factor 21,FGF21)、β-Klotho及成纤维细胞生长因子受体1(Fibroblast growth factor receptor 1,FGFR1)表达变化关系;同时探讨外源性给予FGF21对血管钙化大鼠肾脏损伤的影响及可能的机制。
方法:
雄性SD大鼠按电脑随机数字表法分为正常组(Con)、钙化组(VDN)及钙化+FGF21组(FGF21),每组8只。钙化组采用维生素D3联合尼古丁诱导大鼠血管钙化模型。钙化+FGF21组在钙化组基础上,予大鼠皮下埋入含有FGF21溶液的渗透泵,微量渗透泵持续给药,剂量为70 ug/kg/d。其余两组大鼠皮下埋入含有等量生理盐水的渗透泵。4周后取动物肾脏组织行碱性磷酸酶及钙含量检测。以肌氨酸氧化酶法检测大鼠血清肌酐浓度,以紫外-谷氨酸脱氢酶法检测大鼠血清尿素氮浓度,以生化法检测大鼠血钙及血磷浓度,以放射免疫法检测大鼠肾脏组织FGF21蛋白含量,以酶联免疫吸附法检测大鼠肾脏组织β-Klotho及FGFR1蛋白含量,以苏木素伊红染色观察肾脏组织结构,以茜素红染色法观察主动脉结构及钙化程度。
结果:
FGF21可改善维生素D加尼古丁诱导的血管钙化导致的肾脏损伤。与单纯血管钙化组相比,其可分别降低20.5%及4%(p < 0.01)的血肌酐和尿素氮。并可减轻肾小球及肾间质的结构损伤。同时相比于单纯血管钙化组,FGF21可分别降低ALP及钙含量42.1% (p < 0.01)及11.7% (p < 0.05),并能减少主动脉损伤及钙化程度。这些结果表明,FGF21可以减轻血管钙化导致的肾损伤。另外,作为FGF21三聚体的组成部分,ELISA结果显示,β-Klotho在血管钙化大鼠中升高了37.4% (p < 0.01),预示机体处于FGF21抵抗状态。而外源性给予FGF21后,大鼠肾脏组织β-Klotho下调16.7% (p < 0.05)。但是FGF21的受体FGFR1在三种大鼠中无显著性差异 (p > 0.05)。
结论:
大剂量维生素D3肌肉注射联合尼古丁灌胃可导致大鼠肾脏组织局部钙超载及微血管钙化形成,并导致肾功能不全,出现早期CKD临床表现。同时病变肾脏组织局部FGF21和β-Klotho表达量上调,而FGFR1在肾脏组织局部未见明显变化。外源性给予FGF21可改善血管钙化情况及肾脏结构与功能,同时下调β-Klotho表达量。FGF21可能通过切断血管钙化与肾脏损伤之间的恶性循环成为治疗血管钙化导致的肾损伤的治疗因子。
INTRODUCTION
Chronic kidney disease (CKD) is a disease characterized by chronic renal structural and functional progressive injury.[1] As the end stage of various kidney diseases, CKD has a wide range of pathogenic factors such as hypertension, diabetes, atherosclerosis, metabolic disorders,[2] and virus infection.[3] Among these risk factors of CKD, vascular calcification (VC) is one of the important factors.[4,5] Recent studies have shown that renal artery calcification is a vital risk factor for CKD and microalbuminuria.[6] Moreover, the correlation between renal artery calcification and renal artery atheromatosis, an important inducement of CKD, has also been confirmed.[7] With the characteristic of calcium and phosphorus depositing in vessel walls, VC is motivated by a phenotypic modulation from vascular cell to osteoblast-like cell, which can be observed in many diseases including diabetes, atherosclerosis, and CKD.[8] In patients with CKD, clinical studies have found the morbidity of VC is much higher than healthy people, which results from the disorder of calcium and phosphorus metabolism caused by renal function impairment.[9,10,11] Meanwhile, hypertension and intraglomerular high pressure due to VC, together with micro-VC in kidney, may accelerate the progress of CKD.[12,13] As VC and CKD are in reciprocal causation,[14] inhibiting the calcification in kidney may be an important aspect to control CKD.
Differing from the traditional view of points that VC is a passive process, the latest study showed that the process of VC was similar to the process of skeleton formation.[6] In fact, VC is an active, preventable, and reversible process regulated by various cytokines.[6,15,16,17] The pathological mechanism of VC mainly related to the increase of intracellular alkaline phosphatase (ALP) activity, the high expression of bone-related proteins, and the transform of vascular smooth muscle cell phenotype.[10,18] Therefore, inhibition of the particular link in the process of VC may be beneficial for reversing the development of calcification-related diseases. Although little research on the kidney injury resulted from VC was reported, there are still evidences which indicate that, in the animal model induced by Vitamin D3 plus nicotine (VDN), not only was there large and middle arterial VC but also the calcium overload and micro-VC could be observed in kidney.[19] Thus, VC may be an important stimulus in the process of CKD.
Fibroblast growth factor 21 (FGF21) is a member of the FGF superfamily, which is predominantly generated in liver, then secreted into circulation, and could affect throughout the body.[20,21] By forming a trimer with the receptor FGF receptor 1 (FGFR1) and its cofactor β-Klotho,[22] FGF21 can activate downstream signaling pathways to exert an antagonistic effect on the apoptosis of vascular endothelial cells,[23] inhibiting atherosclerosis[24] and the calcification of vascular smooth muscle cells.[25] In CKD patients, the increased serum FGF21 level is regarded as a potential biomarker for kidney injury.[26] As renal excretion is regarded as a major route to eliminate FGF21 from circulation,[27] it is reasonable to hypothesize that FGF21 may involve in the regulation of the process of CKD.
Whether VC could cause kidney injury is unknown, and the function of high expression of FGF21 in CKD is still unclear. In this study, we evaluated the changes of the structure and function of kidney in VDN-treated VC rats. In addition, the level of endogenous FGF21, β-Klotho, and its receptor FGFR1 in VDN-induced VC rats was determined. Then, exogenous FGF21 was administrated to clarify whether FGF21 inhibits the process of kidney injury in VC rats.
METHODS
Ethical approval
The study was approved by the Institutional Animal Care and Use Committee of the Capital Medical University. The experimental procedures and protocols performed in this study were in concordance with ethical approval for Animals’ Model Research Committee of the Capital Medical University.
Materials and reagents
FGF21 was from Phoenix Pharmaceuticals (Belmont, CA, USA). Enzyme-linked immunosorbent assay (ELISA) kit for β-Klotho and FGFR1 was purchased from Cloud-Clone Co. (Wuhan, Hubei, China). The ALP kit was from Jiancheng Biological Co. (Nanjing, Jiangsu, China). The calcium assay kit was from Zhongsheng Biosino Bio-technology and Science INC (Beijing, China). The VDN was bought from Sigma-Aldrich (St. Louis, MO, USA). The urea determination kit and creatinine determination kit were purchased from Zhongsheng Biosino Bio-technology and Science INC (Beijing, China). The regents for calcium and phosphate determination were from North Beijing Xinchuangyuan seized Biological Technology Co. Ltd. (Beijing, China). Alzet Mini-Osmotic Pumps, model 2004, were from Durect Corp. (Cupertino, CA, USA). All other reagents were of analytical grade.
Animal and treatments
Eight-week-aged male Sprague-Dawley rats were purchased from the Laboratory Animal Center of Peking University (Beijing, China) and housed in standard laboratory temperature and humidity conditions with a 12 h light/dark cycle. The rats were divided into three groups randomly (n = 8): control group (NC group), model group (VDN group), and FGF21 treatment group (VDN + FGF21 group). The preparation for the VC model was made as previously described.[28] Briefly, rats were intragastric administrated with nicotine (25 mg/kg in 5 ml peanut oil) plus injected with Vitamin D3 intramuscularly (3 × 105 U/kg) at 9 a.m. on day 1. The nicotine administration was repeated at 5 p.m. The modeling continued for 4 weeks. For FGF21 treatment, the rats were anesthetized by pentobarbital, and then an osmotic minipump was implanted subcutaneously. The VDN and VDN + FGF21 group were infused with vehicle or FGF21 (70 μg·kg−1·d−1), respectively, by osmotic minipump for 4 weeks.[23] Rats in the control group received the injection of saline and oral gavage of peanut oil, together with the infusion of saline with minipump.
Measurement of fibroblast growth factor 21 level in renal tissue
Briefly, the renal tissue (100 mg) was excised and immediately acidified with 1.0 mol/L acetic acid and then heated at 100°C for 10 min to inactivate proteases. Then, the tissue was homogenized and centrifuged at 17,000 g for 20 min. Radioimmunoassay (RIA) was performed to measure tissue FGF21 content in supernatants by use of a commercial RIA kit (Phoenix Pharmaceuticals) according to the manufacturer's instructions.
Measurement of serum urea nitrogen and creatinine
Before execution, the rats were anesthetized and the blood was collected by intracardiac puncture. The urea level in serum was measured by a urea determination kit using glutamate dehydrogenase method. Likewise, the serum creatinine level was measured by a creatinine determination kit according to the manufacturer's instructions.
Measurement of serum calcium and phosphate
The levels of serum calcium and phosphate were measured by automatic biochemical analyzer (TOSHIBA TBA-40FR, Japan) with supplementary reagents, respectively.
Hematoxylin and eosin staining for nephritic tissue
The nephritic tissue was fixed in 4% paraformaldehyde overnight, then embedded in paraffin, and sectioned for hematoxylin and eosin (H and E) staining. Briefly, the sections were stained by hematoxylin; after washing and treating with 1% acidic alcohol, the sections were stained by eosin. Then, the sections were washed, dehydrated, and treated by xylene before microscopy. The cross sections were evaluated by Olympus BX 50 microscope (Olympus Optical Tokyo, Japan).
Alizarin red staining for aortic tissues
For Alizarin-red S staining, aortic slides were dehydrated, rinsed rapidly in distilled water, and placed in an Alizarin-red S (Sigma, St. Louis, MO, USA) staining solution at room temperature for 30 min. When a red-orange color appeared, any unbound stain was removed from the tissues, and then the tissues were photographed under an Olympus BX 50 microscope.
Measurement of calcium content in kidney
The renal tissue was first dried at 55°C and weighed, then dissolved in HNO3, dried at 180°C, and re-dissolved with a blank solution (27 nmol/L KCl and 27 μmol/L LaCl3). Calcium levels were determined by colorimetry through a reaction with o-cresolphthalein complexon and normalized to renal tissues dry weight.[29]
Measurement of alkaline phosphatase activity in kidney
The renal tissues were homogenized in ice-cold buffer (20 mmol/L HEPES, 0.2% NP-40, and 20 mmol/L MgCl2, pH 7.4). After centrifugation at 8000 g for 10 min at 4°C, the protein content in supernatant was determined by the Bradford method. ALP activity was measured using the ALP activity assay kit according to the manufacturer's instructions. Briefly, the 1 ml of supernatant was mixed with the reaction buffer and then incubated at 37°C for 15 min. After incubation, 1.5 ml of developer was added, and then the absorbance at 520 nm was recorded. The activity of ALP in each well was calculated from the standard curve. One unit was defined as 1 g tissue protein producing 1 mg phenol for 15 min.[30]
Enzyme-linked immunosorbent assay for fibroblast growth factor receptor 1 and β-Klotho in nephridial tissue
Briefly, 10 mg of nephridial tissue was homogenized at 4°C in PBS, and then the mixture was further treated by ultrasonication. After centrifugation at 5000 g for 5 min at 4°C, the protein content in supernatant was determined by the Bradford method. The level of FGFR1 and β-Klotho in supernatants was measure by ELISA kit according to the manufacturer's instructions.
Statistical analysis
All calculations were performed using GraphPad Prism version 5.0 statistical software (GraphPad Software, San Diego, CA, USA). The data were calculated as the mean ± standard deviation (SD) from at least three independent experiments and analyzed by a one-way ANOVA. The Student's t-test was used when only two groups were compared. Difference was accepted at P < 0.05. A value of P < 0.05 was considered statistically significant, and a P < 0.01 was considered very statistically significant.
RESULTS
Increased expression of fibroblast growth factor 21 in kidney tissue
First, we used RIA to detect the endogenous FGF21 in VDN-induced VC rats. Results showed that the FGF21 expression in kidney tissue of VDN group increased significantly compared to the control group by 2.44-fold (0.248 ± 0.108 vs. 0.607 ± 0.317 ng/mg, P < 0.05), indicating that FGF21 may be involved in the regulation of kidney injury results from VC.
Fibroblast growth factor 21 ameliorated the renal function impairment in vascular calcification rats
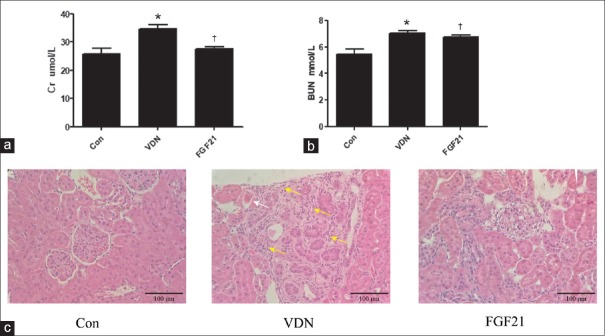
To evaluate the protective effect of exogenous FGF21 on renal function, the serum creatinine and urea were tested [Figure 1a and 1b]. The level of serum creatinine and urea increased dramatically in VDN group compared to the control group by 34.3% and 29.1% (P < 0.01), respectively, indicating that the renal function was damaged. Meanwhile, treatment with FGF21 inhibited the elevated serum creatinine and urea level by 20.5% and 4.0% (P < 0.01), respectively. Likewise, H and E staining showed a normal renal glomerulus structure in the control group. In contrast, VDN-treated rats exhibited pathological changes characterized by glomerular atrophy (white arrow) and prominent renal interstitial fibrosis (yellow arrows) [Figure 1c]. The rats treated with FGF21 showed less structural lesions than VDN group. These results revealed that FGF21 could protect the renal injury in VC rats.
Figure 1.
Effect of exogenous FGF21 on the blood creatinine level in VDN-induced VC rats (n = 8; a); effect of exogenous FGF21 on the urea level in VDN-induced VC rats (n = 8; b); effect of exogenous FGF21 on the histopathology of kidney in VDN-induced VC rats (original magnification ×200). The histological sections of kidney were stained by H and E. The pathologic changes of glomerulus atrophy were marked by white arrows and prominent renal interstitial fibrosis were marked by yellow arrows (c). *P < 0.01 compared with the normal control group. †P < 0.01 compared with the VDN group. FGF21: Fibroblast growth factor 21; VC: Vascular calcification; VDN: Vitamin D3 plus nicotine.
Fibroblast growth factor 21 attenuated calcification in the kidney of Vitamin D3 plus nicotine-treated rats
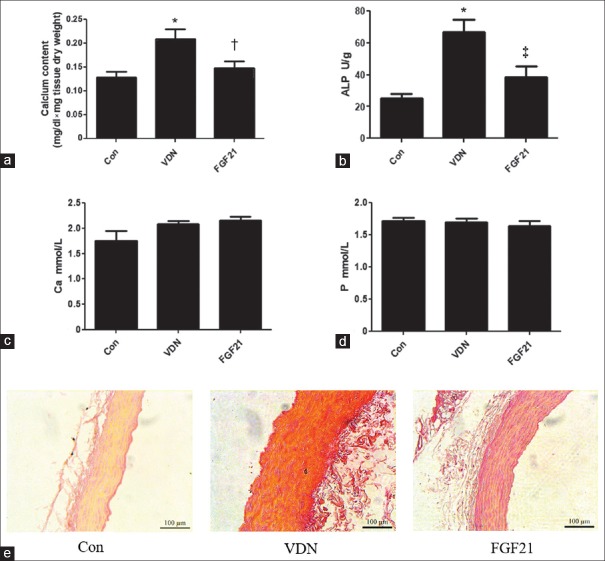
We further investigated whether FGF21 decreased the calcification rate of kidney. Compared with the control group, the calcium content was 36.1% (P < 0.01) higher in VC rats. FGF21 treatment significantly decreased the calcium level in renal tissue by 29.1% (P < 0.05) as compared with VDN treatment alone group [Figure 2a]. Besides VC rats showed increased ALP activity in kidney by 2.63-fold (P < 0.01). FGF21 downregulated the VDN-increased ALP activity in the renal tissue of rats by 42.1% (P < 0.01) [Figure 2b]. In addition, Alizarin-red S staining showed thickened vessel walls, disordered elastic fibers, and widespread calcifications in aortas of VDN group [Figure 2e]. Decreased calcium-phosphate salt deposition in FGF21-treated VC aortas was further confirmed compared with the VDN rats [Figure 2e]. However, the serum calcium and phosphate level did not response neither to VDN nor to FGF21 treatment (P > 0.05) [Figure 2c and 2d].
Figure 2.
Effect of exogenous FGF21 on the renal calcium level in VDN-induced VC rats (n = 6–8; a); effect of exogenous FGF21 on renal activity in VDN-induced VC rats (n = 6–8; b); effect of exogenous FGF21 on serum level of calcium in VDN-induced VC rats (n = 8; c); effect of exogenous FGF21 on serum level of phosphate in VDN-induced VC rats (n = 8; d); effect of exogenous FGF21 in aortas of VDN-induced VC rats by Alizarin-red S staining (original magnification ×200; d). *P < 0.01 compared with the normal control group. †P < 0.05 compared with the VDN group. ‡P < 0.01 compared with the VDN group. FGF21: Fibroblast growth factor 21; VC: Vascular calcification; VDN: Vitamin D3 plus nicotine; ALP: Alkaline phosphatase.
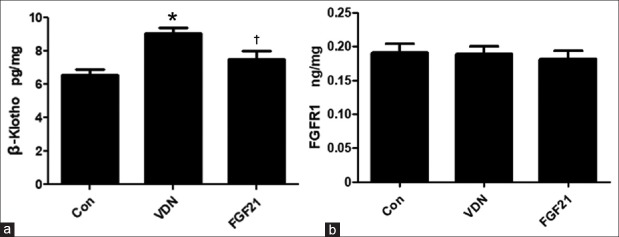
Fibroblast growth factor 21 inhibited the expression of β-Klotho in calcification kidney
FGF21 can only function by forming trimerization with FGFR1 and β-Klotho, so the effect of VDN modeling and FGF21 treatment on the level of renal FGFR1 and β-Klotho was further investigated. In accordance with endogenous FGF21 [Figure 3a], the β-Klotho level in kidney increased significantly in VC rats by 37.4% (P < 0.01). FGF21 administration significantly suppressed the increase of β-Klotho level compared to the VDN group by 16.7% (P < 0.05). However, no change was observed in the level of renal FGFR1 in neither VDN nor VDN + FGF21 treatment (P > 0.05) [Figure 3b]. These results indicated that not FGFR1 but β-Klotho was involved in the regulation of kidney injury in VC model by FGF21.
Figure 3.
Effect of exogenous FGF21 on the levels of renal β-Klotho in the kidney of VDN-induced VC rats (n = 8) by ELISA (a). Effect of exogenous FGF21 on the levels of renal FGFR1 in VDN-induced VC rats (n = 8) by ELISA (b). *P < 0.01 compared with the normal control group. †P < 0.05 compared with the VDN group. FGF21: Fibroblast growth factor 21; VC: Vascular calcification; VDN: Vitamin D3 plus nicotine; ELISA: Enzyme-linked immunosorbent assay.
DISCUSSION
Administration of VDN to rat is a widely used modeling method to cause VC, in which calcium overload generally occurred in many tissues and organs including aorta, heart, and kidney.[28,31,32] In our research, structural and functional injuries in kidney were observed in VDN-treated rat [Figure 1a–1c]. In addition, the VC biomarkers were also measured in renal tissue. ALP, an important marker for the early differentiation of osteoblast, plays a central role in ossification process.[33,34] Being similar with the calcium content, the increased level of ALP activity and calcium content in the renal tissue indicate that calcification is in progress in the kidney [Figure 2a and 2b]. However, no substantial change was observed in the blood calcium and phosphorus between model group and the control group, which indicated that the calcification in kidney was independent from blood calcium and phosphorus [Figure 2c and 2d]. Since the increase of serum calcium and phosphate level could be observed only in 4–5 Stage CKD, these results also demonstrated that, in VDN-treated VC model, the kidney injury happened at early phase.[35]
At present, the change of serum FGF21 in CKD patients has already been observed, but the function of FGF21 in the process of renal damage was still unclear. In our study, an increased expression of endogenous FGF21 in kidney was observed in VDN rats, suggesting that FGF21 may be involved in the regulation of kidney injury and calcification. Therefore, exogenous FGF21 was administrated to VDN modeling rats. As shown in Figure 2a–2c, exogenous FGF21 can downregulate the serum creatinine and blood urea levels of the VC rats and reverse the kidney structural damage, which means FGF21 can alleviate the injury of the renal structure and function in VC rats. At the same time, the renal ALP activity and calcium content were also suppressed by FGF21 [Figure 2a and 2b]. These results demonstrated that FGF21 could protect the kidney from injury and calcification in situ. Meanwhile, the protective effect of FGF21 was independent of regulating serum calcium and phosphate.
As well known, FGF21-FGFR1-β-Klotho tripolymer is essential for FGF21 to function.[22] In our study, different from the level of FGFR1, which kept unchanged under VDN and VDN + FGF21 administration, the level of renal β-Klotho increased significantly in the model group, in accordance with the endogenous renal FGF21 [Figure 3a]. The high expression of renal FGF21 and β-Klotho suggested a high activity of FGF21 signaling pathway in kidney, which is an FGF21-resistant state under VDN administration. While exogenous FGF21 treatment can overcome this resistant state and protect the kidney from injury. The similar result was also reported by Kim et al.[36] in the kidney of db/db mice, which may be partly due to db/db mice and VDN-treated rats all belonging to the metabolic disorders model. These results indicate that β-Klotho plays an important role in the activation of FGF21 signaling, which may be a key target for the treatment of the diseases with the feature of metabolic disorders. In fact, in our study, it is by activating FGF21 signaling pathway that exogenous FGF21 ameliorates the FGF21 resistance and then impair the kidney injury in VDN rats.
In summary, kidney injury and calcification in situ were observed in VDN-induced VC rats. At the same time, the increased level of endogenous FGF21 and β-Klotho in kidney indicates a negative feedback regulation. Exogenous FGF21 treatment can ameliorate the kidney injury and calcification, indicating that FGF21 might be a potential therapeutic target in CKD by cutting off the vicious circle between VC and kidney injury.
Financial support and sponsorship
This study was funded by grants from National Natural Science Fund of China (No. 81570388), Beijing Natural Science Foundation (No. 7142048), and the Major State Basic Research Development Program of China (973 Program, No. 2015CB554404).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Andrassy KM. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney international. 2013;84:622–3. doi: 10.1038/ki.2013.243. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 2.Ye L, Mao W. Metabonomic biomarkers for risk factors of chronic kidney disease. International urology and nephrology. 2016;48:547–52. doi: 10.1007/s11255-016-1239-6. doi: 10.1007/s11255-016-1239-6. [DOI] [PubMed] [Google Scholar]
- 3.Moosa MR, Van der Walt I, Naicker S, Meyers AM. Important causes of chronic kidney disease in South Africa. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2015;105:2681. doi: 10.7196/samj.9535. doi: 10.7196/samj.9535. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus JM, Lowrie EG, Hampers CL, Merrill JP. Cardiovascular disease in uremic patients on hemodialysis. Rhode Island medical journal. 1976;59:57. [PubMed] [Google Scholar]
- 5.Murphey MD, Sartoris DJ, Quale JL, Pathria MN, Martin NL. Musculoskeletal manifestations of chronic renal insufficiency. Radiographics. 1993;13:357–79. doi: 10.1148/radiographics.13.2.8460225. doi: 10.1148/radiographics.13.2.8460225. [DOI] [PubMed] [Google Scholar]
- 6.Roseman DA, Hwang SJ, Manders ES, O’Donnell CJ, Upadhyay A, Hoffmann U, et al. Renal artery calcium, cardiovascular risk factors, and indexes of renal function. Am J Cardiol. 2014;113:156–61. doi: 10.1016/j.amjcard.2013.09.036. doi: 10.1016/j.amjcard.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu YW, Adler S, Budoff M, Takasu J, Ashai J, Mehrotra R, et al. Prevalence and prognostic significance of renal artery calcification in patients with diabetes and proteinuria. Clin J Am Soc Nephrol. 2010;5:2093–100. doi: 10.2215/CJN.03730410. doi: 10.2215/cjn.03730410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodroge A, Trécherel E, Cornu M, Darwiche W, Mansour A, Ait-Mohand K, et al. Oligogalacturonic acid inhibits vascular calcification by two mechanisms: Inhibition of vascular smooth muscle cell osteogenic conversion and interaction with collagen. Arterioscler Thromb Vasc Biol. 2017;37:1391–401. doi: 10.1161/ATVBAHA.117.309513. doi: 10.1161/atvbaha.117.309513. [DOI] [PubMed] [Google Scholar]
- 9.Nasrallah MM, El-Shehaby AR, Salem MM, Osman NA, El Sheikh E, Sharaf El Din UA, et al. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:2679–85. doi: 10.1093/ndt/gfq089. doi: 10.1093/ndt/gfq089. [DOI] [PubMed] [Google Scholar]
- 10.Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P. Alkaline phosphatase: A novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol. 2017;13:429–42. doi: 10.1038/nrneph.2017.60. doi: 10.1038/nrneph.2017.60. [DOI] [PubMed] [Google Scholar]
- 11.Rodenbeck SD, Zarse CA, McKenney-Drake ML, Bruning RS, Sturek M, Chen NX, et al. Intracellular calcium increases in vascular smooth muscle cells with progression of chronic kidney disease in a rat model. Nephrol Dial Transplant. 2017;32:450–8. doi: 10.1093/ndt/gfw274. doi: 10.1093/ndt/gfw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HC, Chou CY, Jheng JS, Chen IR, Liang CC, Wang SM, et al. Loss of Residual Renal Function is Associated With Vascular Calcification in Hemodialysis Patients. Ther Apher Dial. 2016;20:27–30. doi: 10.1111/1744-9987.12376. doi:10.1111/1744-9987.12376. [DOI] [PubMed] [Google Scholar]
- 13.Hill Gallant KM, Spiegel DM. Calcium balance in chronic kidney disease. Curr Osteoporos Rep. 2017;15:214–21. doi: 10.1007/s11914-017-0368-x. doi: 10.1007/s11914-017-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández-Laso V, Sastre C, Valdivielso JM, Betriu A, Fernández E, Egido J, et al. Soluble TWEAK and major adverse cardiovascular events in patients with CKD. Clin J Am Soc Nephrol. 2016;11:413–22. doi: 10.2215/CJN.07900715. doi: 10.2215/cjn.07900715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McRobb LS, McGrath KC, Tsatralis T, Liong EC, Tan JT, Hughes G, et al. Estrogen receptor control of atherosclerotic calcification and smooth muscle cell osteogenic differentiation. Arterioscler Thromb Vasc Biol. 2017;37:1127–37. doi: 10.1161/ATVBAHA.117.309054. doi: 10.1161/atvbaha.117.309054. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Li Y, Du Y, Li G, Wang L, Zhou F, et al. Resveratrol ameliorated vascular calcification by regulating sirt-1 and nrf2. Transplant Proc. 2016;48:3378–86. doi: 10.1016/j.transproceed.2016.10.023. doi: 10.1016/j.transproceed.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Zewinger S, Schumann T, Fliser D, Speer T. Innate immunity in CKD-associated vascular diseases. Nephrol Dial Transplant. 2016;31:1813–21. doi: 10.1093/ndt/gfv358. doi: 10.1093/ndt/gfv358. [DOI] [PubMed] [Google Scholar]
- 18.Fuery MA, Liang L, Kaplan FS, Mohler ER., 3rd Vascular ossification: Pathology, mechanisms, and clinical implications. Bone. 2017 doi: 10.1016/j.bone.2017.07.006. pii: S8756.3282(17)30232-6. doi: 10.1016/j.bone.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XS, Zhou XO, Gao MP, Huang ZJ, Zhu PX, Zhang YG. Injury of kidney in vascular calcification in rat model. South China J Cardiovasc Dis. 2013;19:722–25. doi:10.3969/j.issn.1007-9688.2013.06.019. [Google Scholar]
- 20.Iglesias P, Selgas R, Romero S, Díez JJ. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur J Endocrinol. 2012;167:301–9. doi: 10.1530/EJE-12-0357. doi: 10.1530/eje-12-0357. [DOI] [PubMed] [Google Scholar]
- 21.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35. doi: 10.1172/JCI23606. doi: 10.1172/jci23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degirolamo C, Sabbà C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15:51–69. doi: 10.1038/nrd.2015.9. doi: 10.1038/nrd.2015.9. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Qi YF, Chang JR, Lu WW, Zhang JS, Wang SP, et al. Possible role of fibroblast growth factor 21 on atherosclerosis via amelioration of endoplasmic reticulum stress-mediated apoptosis in apoE(-/-) mice. Heart Vessels. 2015;30:657–68. doi: 10.1007/s00380-014-0557-9. doi: 10.1007/s00380-014-0557-9. [DOI] [PubMed] [Google Scholar]
- 24.Kwok KHM, Lam KSL. Fibroblast Growth Factor 21 Mimetics for Treating Atherosclerosis. Endocrinology and metabolism. 2017;32:145–51. doi: 10.3803/EnM.2017.32.2.145. doi: 10.3803/EnM.2017.32.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y, Lu W, Hou Y, Fu K, Gan F, Liu J. Fibroblast growth factor 21 ameliorates vascular calcification by inhibiting osteogenic transition in vitamin D3 plus nicotine-treated rats. Biochemical and biophysical research communications. 2017;12:pii: S0006. doi: 10.1016/j.bbrc.2017.10.115. doi:10.1016/j.bbrc.2017.10.115. [DOI] [PubMed] [Google Scholar]
- 26.Lin Z, Zhou Z, Liu Y, Gong Q, Yan X, Xiao J, et al. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS One. 2011;6:e18398. doi: 10.1371/journal.pone.0018398. doi: 10.1371/journal.pone.0018398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hindricks J, Ebert T, Bachmann A, Kralisch S, Lössner U, Kratzsch J, et al. Serum levels of fibroblast growth factor-21 are increased in chronic and acute renal dysfunction. Clin Endocrinol (Oxf) 2014;80:918–24. doi: 10.1111/cen.12380. doi: 10.1111/cen.12380. [DOI] [PubMed] [Google Scholar]
- 28.Ren X, Wei Q, Shao H, Sun Z, Liu N. A rat model of diabetic artery calcification. J Endocrinol Invest. 2012;35:497–503. doi: 10.3275/7865. doi: 10.3275/7865. [DOI] [PubMed] [Google Scholar]
- 29.Essalihi R, Dao HH, Yamaguchi N, Moreau P. A new model of isolated systolic hypertension induced by chronic warfarin and Vitamin K1 treatment. Am J Hypertens. 2003;16:103–10. doi: 10.1016/s0895-7061(02)03204-1. [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Wang QQ, Zhao X, Pu XP. Proteome analysis of the left ventricle in the Vitamin D3 and nicotine-induced rat vascular calcification model. J Proteomics. 2011;74:480–9. doi: 10.1016/j.jprot.2010.12.010. doi: 10.1016/j.jprot.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Shi YC, Liu JH. Study on the kidney impairment and expressions of FGF21 from a rat model of vascular calcification. Natl Med J China. 2016;96:3741–4. doi: 10.3760/cma.j.issn.0376-2491.2016.46.010. doi: 10.3760/cma.j.issn.0376-2491.2016.46.010. [DOI] [PubMed] [Google Scholar]
- 33.Narisawa S, Harmey D, Yadav MC, O’Neill WC, Hoylaerts MF, Millán JL, et al. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res. 2007;22:1700–10. doi: 10.1359/jbmr.070714. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- 34.Sheen CR, Kuss P, Narisawa S, Yadav MC, Nigro J, Wang W, et al. Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res. 2015;30:824–36. doi: 10.1002/jbmr.2420. doi: 10.1002/jbmr.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kestenbaum B. Con: Phosphate binders in chronic kidney disease. Nephrol Dial Transplant. 2016;31:189–94. doi: 10.1093/ndt/gfv406. doi: 10.1093/ndt/gfv406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HW, Lee JE, Cha JJ, Hyun YY, Kim JE, Lee MH, et al. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology. 2013;154:3366–76. doi: 10.1210/en.2012-2276. doi: 10.1210/en.2012-2276. [DOI] [PubMed] [Google Scholar]