Abstract
Background:
The sigma receptors are a relatively novel receptor group with respect to knowledge of their effect on health. Although the sigma-1 receptor agonist PRE-084 exhibits a cardioprotective effect in some studies, the benefits in cases of myocardial ischemia/reperfusion (I/R) are not clear. The aim of this study was to explore the mechanism of action and assess the effect of PRE-084 on myocardial I/R injury in rats.
Methods:
In this study, rats were assigned randomly to three groups with computer (n = 14 for each group): a sham group, an I/R group, and a PRE-084 group. In the PRE-084 group, rats were administered PRE-084 1 h before operation. In the myocardial I/R model, the left anterior descending branch of rats was ligated and opened half an hour later. Cardiac function was assessed, and the apoptosis index was evaluated. The mechanisms of the cardioprotective effects of PRE-084 were explored.
Results:
PRE-084 pretreatment preserved cardiac function and reduced myocardial apoptosis (F = 86.0, P < 0.01) with Western blotting analysis, showing significantly reduced expression of Bax (F = 75.7, P < 0.01) and cleaved-caspase 3 (F = 44.7, P < 0.01), along with increased expression of the Bcl-2 protein (P < 0.01) and phosphorylated protein kinase B (p-Akt) (P < 0.01) and phosphorylated-endothelial nitric oxide synthase (p-eNOS; P < 0.01).
Conclusion:
PRE-084 preserved cardiac function and reduced myocardial apoptosis through the activation of Akt and eNOS.
Keywords: Myocardial Ischemia/Reperfusion, PRE-084, Sigma-1 Receptor
摘要
背景:
Sigma受体是近年来新发现的受体,有研究表明sigma受体激动剂具有心肌保护作用,但其对心肌缺血再灌注损伤的影响目前还不清楚。本研究的目的为研究sigma受体激动剂对大鼠心肌缺血再灌注的影响及探讨其机理。
方法:
42只大鼠随机分为三组:假手术组、缺血再灌注组和PRE-084组。PRE-084组术前1小时给予PRE-084,再灌注组结扎前降支半小时后松开。检测各组心功能和心肌凋亡指数,并研究其可能的机制。
结果:
PRE-084预处理组心功能显著改善,凋亡指数降低, P-Akt,p-eNOS, Bcl2表达明显增强,Bax(P<0.01)和cleaved-caspase3(P<0.01)表达明显下降。
结论:
PRE-084降低大鼠心肌缺血再灌注损伤,其机理可能与激活Akt / eNOS信号通路有关。
INTRODUCTION
According to the World Health Organization, a large number of people worldwide die from cardiovascular disorders each year than from any other cause.[1,2,3] Each year, more than 3.0 million people experience acute ST-elevation myocardial infarction (MI).[4] Currently, myocardial reperfusion is the first option for treatment to reduce the area impacted by the acute MI and improve prognosis. However, the return of blood flow to the ischemic myocardium can also induce injury;[5] this is referred to as myocardial ischemia/reperfusion (I/R) injury and associated with poorer functional recovery and adverse outcomes.[6]
Considerable effort has gone toward finding ways to reduce the degree of injury from reperfusion;[7,8] however, few effective treatments exist, and further research is needed to seek new approaches.
The sigma-1 receptors (σ1Rs) are a relatively novel receptor group and are a ubiquitously expressed, unique binding site in the central nervous system and other peripheral tissues.[9] These receptors are currently expected to be a potential target for drugs that treat neurodegenerative disorders, depression, idiopathic pain, and cancer.[10] Recent studies have suggested that σ1R agonists have potent cardioprotective effects in mice. Intracerebroventricular infusion of the σ1R agonist PRE-084 for 1 month improved both mental and cardiac functioning in mice in which MI was induced.[11] Some selective serotonin reuptake inhibitors (antidepressant drugs), such as sertraline and fluvoxamine, are potent agonists of σ1R. Fluvoxamine attenuated cardiac hypertrophy and restored contractility in transverse aortic constriction mice after being delivered for a month by stimulating σ1R; co-administration of the σ1R antagonist NE-100 eliminated the cardioprotective effect.[12] However, these studies assessed σ1R over a relatively long time; whether PRE-084 is effective right after administration (i.e., in acute treatment directly after MI) is unknown.
To date, no studies have been performed regarding the effects of σ1R agonists on myocardial I/R injury. The aim of this study was to explore the mechanism of action and assess the effect of PRE-084 on myocardial I/R injury in rats.
METHODS
Ethical approval
The experiments were conducted according to the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Wuhan University Ethics Committee on Animal Care.
Animals
Forty-two healthy adult male Sprague-Dawley rats (220–250 g) were purchased from the animal center at Wuhan University, Hubei, China.
Myocardial infarction model preparation
The rats were injected intraperitoneally with PRE-084 or saline (Santa Technology Co., Ltd., USA; dissolved in normal saline 1 mg/kg). One hour later, ischemia was induced by ligation at the left anterior descending coronary artery. Rats were subjected to 30 min of myocardial ischemia and 24 h of reperfusion. Successful reperfusion was defined as recovery of the elevated ST segment. Rats were randomized into three groups with computer (n = 14 for each group): (1) the sham group, in which rats underwent identical surgical procedures but without occlusion of the coronary artery; (2) the I/R group, ischemia was induced in the rats for a period of 30 min; and (3) the PRE-084 group (I/R + PRE-084 1 mg/kg).
Hemodynamics
The maximal velocity of left ventricular pressure development (LV ± dp/dt), left ventricular systolic pressure (LVSP), and left ventricular end-diastolic pressure (LVEDP) were measured through a polyethylene-50 tube that was advanced into the left ventricle through the right carotid artery and connected to a multichannel physiological monitoring system (Biopac MP150) 24 h after reperfusion.
Apoptotic assessment
The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was used to analyze the degree of myocardial apoptosis according to the manufacturer's protocol;[13] there were five rats in each group. To briefly summarize, the TUNEL reaction mixture was added to each sample. The slides were then incubated and washed with phosphate-buffered saline followed by fixation in freshly prepared 4% paraformaldehyde (pH 7.4).[14] 3-3’ diaminobenzidine staining and hematoxylin and eosin restaining were performed. Stained slides were viewed under a light microscope. The apoptotic index was defined as the percentage of apoptotic cells out of the total number of myocardial cells.
Western blotting analyses
Western blotting analyses were performed in five randomly selected rats from each group according to routine protocols. Briefly, the cardiac tissue from the left ventricle was homogenized in tris-buffered saline and then centrifuged at 12,000 bpm for 5 min at 4°C. Protein concentrations were determined using the bicichoninic acid assay (Pierce Chemical, USA). Equal amounts of protein were loaded onto sodium dodecyl sulfate (SDS)-polyacrylamide gel, underwent electrophoresis, and were finally transferred to polyvinylidene difluoride membranes (Merck Millipore, Burlington, Massachusetts, USA). The membranes were blocked with a 5% bovine serum albumin buffer at room temperature for 1 h and incubated with primary antibodies at 4°C overnight. After washing three times with Tris buffered saline Tween 20, the membranes were incubated with secondary antibodies (Santa Cruz, California, USA) for 30 min at 37°C.
Protein signals were detected with a chemiluminescent system; expression levels of the proteins were compared to the control based on the relative intensities of the bands, which were determined using an image analyzer (AlphaEaseFC; Genetic Technologies, Inc.; Miami, Florida, USA).
Statistical analysis
The statistical analysis was performed using SPSS 19.0 software (SPSS Inc., USA). Data were described as means ± standard deviations (SD). The basis of homogeneity of variance was evaluated by a one-way analysis of variance (ANOVA); multiple comparisons between the groups were performed using the Student-Newman-Keuls method, witha P < 0.05 considered statistically significant.
RESULTS
Effect of PRE-084 on cardiac function
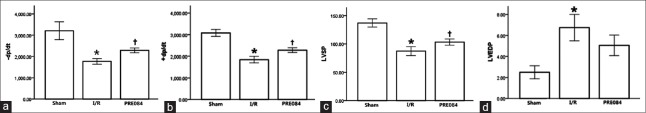
To assess the effect of PRE-084 on cardiac function, hemodynamic parameters such as LVEDP, LVSP, and LV ± dp/dt were measured. Compared with those in I/R group, LV ± dp/dt (+F = 92.4, −F = 36.2, P < 0.01) and LVSP (F = 62.6, P < 0.01) significantly increased in the PRE-084 group. LVEDP (P > 0.05) was not significantly changed between the two groups [Figure 1a–1d].
Figure 1.
Effect of PRE084 on hemodynamic and left ventricular functions after I/R injury in rats. (a) Maximal positive rate of the left ventricular pressure (+LV dp/dt); (b) maximal negative rate of the left ventricular pressure (−LV dp/dt); (c) LVSP; and (d) LVEDP. The values are expressed as mean ± standard error; n = 14 in each group; *P < 0.01 versus sham group; †P < 0.01 versus I/R group. I/R: Ischemia/reperfusion; LV: Left ventricular; LVEDP: Left ventricular end-diastolic pressure.
Effect of PRE-084 on myocardial cell apoptosis
The TUNEL assay was used to assess the effect of PRE-084 on myocardial apoptosis during I/R injury. PRE-084 treatment significantly decreased the degree of myocardial apoptosis in the PRE-084 group (as measured by percentage of apoptotic cells) compared with that of the I/R group (F = 86.0, P < 0.01; Figure 2).
Figure 2.
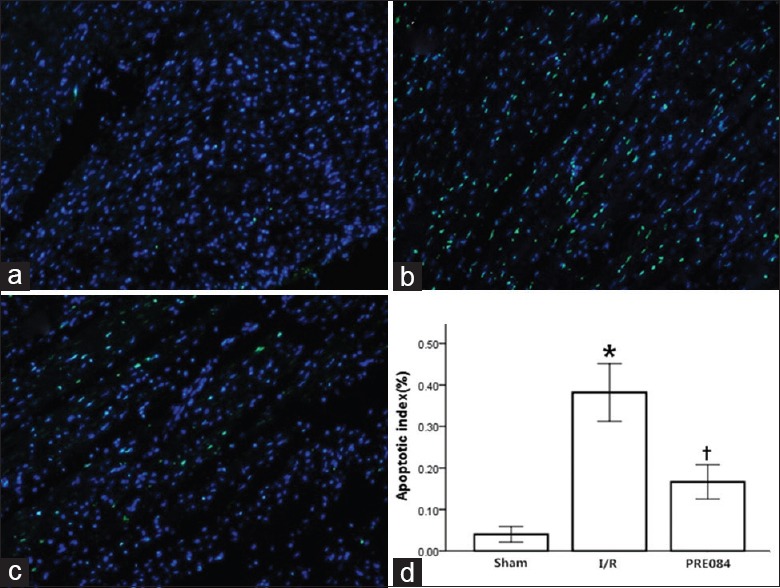
PRE084 reduced apoptosis following myocardial I/R in rats. (a) Sham group; (b) I/R group; (c) I/R + PRE084 group. (d) Values presented as mean ± standard error; n = 5 in each group; *P < 0.01 versus sham group; †P < 0.01 versus I/R group. I/R: Ischemia/reperfusion.
Effect of PRE-084 on Akt and endothelial nitric oxide synthase
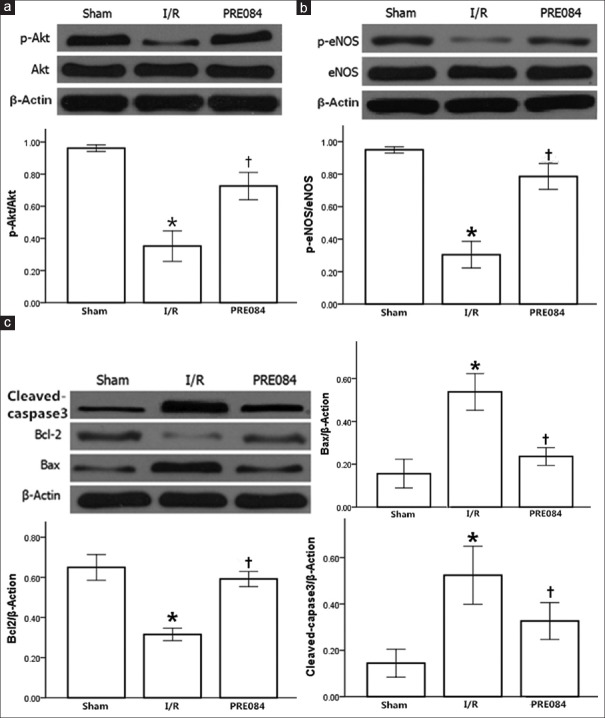
The expression of phosphorylated protein kinase B (p-Akt) (Ser473) and endothelial nitric oxide synthase (p-eNOS) (Ser1177) was assessed to explore the mechanisms underlying the cardioprotective effects of PRE-084. Phosphorylation levels of Akt and eNOS decreased significantly in I/R group compared with the sham group (P < 0.01). PRE-084 treatment led to significantly increased expression of p-Akt (F = 131.7, P < 0.01) and p-eNOS (F = 196.4, P < 0.01) in the PRE-084 group compared with those in the I/R group [Figure 3a and 3b].
Figure 3.
The effect of PRE-084 on protein expression in heart in different experimental groups. (a) Akt, p-Akt; (b) eNOS, p-eNOS; and (c) Bcl-2, Bax, cleaved-caspase3. Protein expressions are normalized with β-actin. All the values are expressed as mean ± standard error; n = 5 per group; *P < 0.01 versus sham group; †P < 0.01 versus I/R group. I/R: Ischemia/reperfusion; eNOS: Endothelial nitric oxide synthase; p-eNOS: Phosphorylated eNOS.
Effect of PRE-084 on cleaved caspase-3 and Bax and Bcl-2 protein expression
Caspase-3, Bcl-2, and Bax are the most important factors in the regulation of apoptosis. We assessed their expression levels to determine the potential mechanism. Compared with the sham group, Bcl-2 protein expression was significantly decreased (F = 37.2, P < 0.01) and Bax protein expression was significantly increased (F = 75.7, P < 0.01), with the Bcl-2/Bax ratio significantly reduced in the I/R group (P < 0.01); PRE-084 pretreatment restored most of the changes as illustrated in Figure 3c (P < 0.01).
Compared with the sham group, the expression of cleaved caspase-3 significantly increased in the I/R group (P < 0.01) and significantly decreased in the PRE-084 group compared with the I/R group (F = 44.7, P < 0.01).
DISCUSSION
Our study showed that PRE-084 protected the heart by reducing myocardial I/R injury in rats, as proved by preserved cardiac function and reduced myocardial apoptosis. Our data suggest that this occurs via changing the expression of apoptosis-related proteins (Bcl-2 and Bax) after activating the Akt-eNOS pathway.
Apoptosis is the major pathogenic mechanism of myocardial I/R injury.[15,16] Since myocardial cells are nonregenerative, apoptosis may cause myocardial dysfunction, which surfaces during ischemia and then is aggravated after reperfusion.[17] In the present study, the TUNEL assay clearly demonstrated that myocardial apoptosis was increased after IR injury, and that PRE-084 reduced its extent.
The mechanism through which IR injury increases myocardial cell apoptosis is not clear. The Bcl-2 gene family is recognized as containing the most important set of genes with respect to mediation of apoptosis.[18,19] Bcl-2 is a protein that inhibits cell apoptosis, while Bax is a protein that promotes cell apoptosis. Accordingly, increases in the Bcl-2/Bax ratio decrease the level of apoptosis. Caspase-3 is a major factor in the activity of the apoptotic pathway and is involved in the final step of the apoptotic process; it is also responsible for the cleavage of many other apoptotic proteins.[20] The important role of caspases in the apoptotic process makes them a potential target for antiapoptotic treatments.[21]
In the present study, PRE-084 upregulated the expression of Bcl-2 protein while downregulating the expression of Bax and caspase-3 proteins, thereby increasing the ratio of Bcl-2 to Bax. These findings suggest that PRE-084 may decrease IR-induced apoptosis of myocardial cells by upregulating Bcl-2 expression and downregulating Bax and caspase-3 expression.
We further investigated the molecular mechanisms of the cardioprotective effects of PRE-084. Studies have shown that some signaling molecules have the function of upstream apoptotic regulation during myocardial reperfusion injury.[22,23] The PI3K/Akt/eNOS pathway is the most important pathway for apoptotic regulation.[14,24,25] Activation of Akt restrains GSK-3β, which inhibits mitochondrial permeability transition pore opening, a primary step for apoptotic and necrotic cell death.[26,27] On the other hand, activation of eNOS by Akt generates NO and activates mitochondrial KATP channels in a cGMP-dependent manner, leading to acute cardioprotection.[27] It has been shown in a previous study that stimulating σ1R can activate Akt and eNOS in the heart, while ERK1/2 and PKC-α phosphorylations were not obviously changed.[12] In the present study, levels of p-Akt and p-eNOS were significantly reduced after IR injury. PRE-084 treatment significantly increased the expression of p-Akt and p-eNOS, suggesting that PRE-084 protects the heart by activating the Akt-eNOS pathway.
Study limitations
Our study has limitations. First, in our study, the animals were pretreated with PRE-084 before MI, which did not mimic the clinical setting, in which drugs would be administered after MI (in most cases, just before reperfusion). Second, previous studies have shown that PRE-084 can protect the heart by stimulating the σ1R in brain, which may also contribute to a certain extent to the cardioprotective effects shown in our study. Finally, σ1R interactions with ion channels, including Ca2+ channels, potassium channels, sodium channels, and chloride channels. These molecular mechanisms may also contribute to its myocardial protective effects.
In conclusion, these findings suggest that PRE-084 reduces apoptosis and improves cardiac function following reperfusion via activation of the Akt-eNOS signaling pathway. The results of this study reveal that PRE-084 may be a promising candidate for the treatment of myocardial I/R injury in ischemic heart diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Santulli G. Epidemiology of cardiovascular disease in the 21st century: Updated numbers and updated facts. J Cardiovasc Dis Res. 2013;1:1–2. [Google Scholar]
- 2.Sidney S, Quesenberry CP, Jr, Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1:594–9. doi: 10.1001/jamacardio.2016.1326. doi: 10.1001/jamacardio.2016.1326. [DOI] [PubMed] [Google Scholar]
- 3.Gao Q, Yang B, Guo Y, Zheng F. Efficacy of adenosine in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: A PRISMA-compliant meta-analysis. Medicine (Baltimore) 2015;94:e1279. doi: 10.1097/MD.0000000000001279. doi: 10.1097/MD.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 5.Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–71. doi: 10.1016/j.jacc.2015.02.032. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 7.Heusch G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–99. doi: 10.1161/CIRCRESAHA.116.305348. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 8.Hernández-Reséndiz S, Palma-Flores C, De Los Santos S, Román-Anguiano NG, Flores M, de la Peña A, et al. Reduction of no-reflow and reperfusion injury with the synthetic 17β-aminoestrogen compound prolame is associated with PI3K/Akt/eNOS signaling cascade. Basic Res Cardiol. 2015;110:1. doi: 10.1007/s00395-015-0464-y. doi: 10.1007/s00395-015-0464-y. [DOI] [PubMed] [Google Scholar]
- 9.Rousseaux CG, Greene SF. Sigma receptors [σRs]: Biology in normal and diseased states. J Recept Signal Transduct Res. 2015;36:327–88. doi: 10.3109/10799893.2015.1015737. doi: 10.3109/10799893.2015.1015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi T. Sigma-1 receptor: The novel intracellular target of neuropsychotherapeutic drugs. J Pharmacol Sci. 2015;127:2–5. doi: 10.1016/j.jphs.2014.07.001. doi: 10.1016/j.jphs.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Hirooka Y, Sunagawa K. Brain sigma-1 receptor stimulation improves mental disorder and cardiac function in mice with myocardial infarction. J Cardiovasc Pharmacol. 2013;62:222–8. doi: 10.1097/FJC.0b013e3182970b15. doi: 10.1097/FJC.0b013e3182970b15. [DOI] [PubMed] [Google Scholar]
- 12.Tagashira H, Bhuiyan S, Shioda N, Hasegawa H, Kanai H, Fukunaga K, et al. Sigma1-receptor stimulation with fluvoxamine ameliorates transverse aortic constriction-induced myocardial hypertrophy and dysfunction in mice. Am J Physiol Heart Circ Physiol. 2010;299:H1535–45. doi: 10.1152/ajpheart.00198.2010. doi: 10.1152/ajpheart.00198.2010. [DOI] [PubMed] [Google Scholar]
- 13.Salas MA, Valverde CA, Sánchez G, Said M, Rodriguez JS, Portiansky EL, et al. The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;48:1298–306. doi: 10.1016/j.yjmcc.2009.12.015. doi: 10.1016/j.yjmcc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He F, Xu BL, Chen C, Jia HJ, Wu JX, Wang XC, et al. Methylophiopogonanone A suppresses ischemia/reperfusion-induced myocardial apoptosis in mice via activating PI3K/Akt/eNOS signaling pathway. Acta Pharmacol Sin. 2016;37:763–71. doi: 10.1038/aps.2016.14. doi: 10.1038/aps.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, et al. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011;2:e244. doi: 10.1038/cddis.2011.130. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner C, Tillack D, Simonis G, Strasser RH, Weinbrenner C. Ischemic post-conditioning reduces infarct size of the in vivo rat heart: Role of PI3-K, mTOR, GSK-3beta, and apoptosis. Mol Cell Biochem. 2010;339:135–47. doi: 10.1007/s11010-009-0377-x. doi: 10.1007/s11010-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 17.McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H1923–35. doi: 10.1152/ajpheart.00935.2003. doi: 10.1152/ajpheart.00935.2003. [DOI] [PubMed] [Google Scholar]
- 18.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–5. doi: 10.1101/gad.10.1.1. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Hatok J, Racay P. Bcl-2 family proteins: Master regulators of cell survival. Biomol Concepts. 2016;7:259–70. doi: 10.1515/bmc-2016-0015. doi: 10.1515/bmc-2016-0015. [DOI] [PubMed] [Google Scholar]
- 20.Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–73. doi: 10.1093/cvr/cvn243. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faubel S, Edelstein CL. Caspases as drug targets in ischemic organ injury. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:269–87. doi: 10.2174/1568008054863754. doi: 10.2174/1568008054863754. [DOI] [PubMed] [Google Scholar]
- 22.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: Targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–60. doi: 10.1016/j.cardiores.2003.09.024. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Zhao MM, Yang JY, Wang XB, Tang CS, Du JB, Jin HF, et al. The PI3K/Akt pathway mediates the protection of SO(2) preconditioning against myocardial ischemia/reperfusion injury in rats. Acta Pharmacol Sin. 2013;34:501–6. doi: 10.1038/aps.2012.204. doi: 10.1038/aps.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H, Ye M, Yang J, Ding J, Yang J, Dong W, et al. Nicorandil protects the heart from ischemia/reperfusion injury by attenuating endoplasmic reticulum response-induced apoptosis through PI3K/Akt signaling pathway. Cell Physiol Biochem. 2015;35:2320–32. doi: 10.1159/000374035. doi: 10.1159/000374035. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Jiang C, Jiang J, Qiu L. Dexmedetomidine protects mice against myocardium ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS pathway. Clin Exp Pharmacol Physiol. 2017;44:946–53. doi: 10.1111/1440-1681.12791. doi: 10.1111/1440-1681.12791. [DOI] [PubMed] [Google Scholar]
- 26.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–49. doi: 10.1172/JCI19906. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L, Pant R, et al. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol. 2007;42:508–16. doi: 10.1016/j.yjmcc.2006.10.018. doi: 10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]