Abstract
Background:
Our previous studies have shown that Tongxinluo (TXL), a compound Chinese medicine, can decrease myocardial ischemia-reperfusion injury, protect capillary endothelium function, and lessen cardiac ventricle reconstitution in animal models. The aim of this study was to illuminate whether TXL can improve hypercholesterolemia-impaired heart function by protecting artery endothelial function and increasing microvascular density (MVD) in heart. Furthermore, we will explore the underlying molecular mechanism of TXL cardiovascular protection.
Methods:
After intragastric administration of TXL (0.1 ml/10 g body weight) to C57BL/6J wild-type mice (n = 8) and ApoE-/- mice (n = 8), total cholesterol, high-density lipoprotein-cholesterol, very-low-density lipoprotein (VLDL)-cholesterol, triglyceride, and blood glucose levels in serum were measured. The parameters of heart rate (HR), left ventricular diastolic end diameter, and left ventricular systolic end diameter were harvested by ultrasonic cardiogram. The left ventricular ejection fraction, stroke volume, cardiac output, and left ventricular fractional shortening were calculated. Meanwhile, aorta peak systolic flow velocity (PSV), end diastolic flow velocity, and mean flow velocity (MFV) were measured. The pulsatility index (PI) and resistant index were calculated in order to evaluate the vascular elasticity and resistance. The endothelium-dependent vasodilatation was evaluated by relaxation of aortic rings in response to acetylcholine. Western blotting and real-time quantitative reverse transcription polymerase chain reaction were performed for protein and gene analyses of vascular endothelial growth factor (VEGF). Immunohistochemical detection was performed for myocardial CD34 expression. Data in this study were compared by one-way analysis of variance between groups. A value of P < 0.05 was considered statistically significant.
Results:
Although there was no significant decrease of cholesterol level (F = 2.300, P = 0.240), TXL inhibited the level of triglyceride and VLDL (F = 9.209, P = 0.024 and F = 9.786, P = 0.020, respectively) in ApoE-/- mice. TXL improved heart function of ApoE-/- mice owing to the elevations of LVEF, SV, CO, and LVFS (all P < 0.05). TXL enhanced aortic PSV and MFV (F = 10.774, P = 0.024 and F = 11.354, P = 0.020, respectively) and reduced PI of ApoE-/- mice (1.41 ± 0.17 vs. 1.60 ± 0.17; P = 0.037). After incubation with 10 μmol/L acetylcholine, the ApoE-/- mice treated with TXL aortic segment relaxed by 44% ± 3%, significantly higher than control group mice (F = 9.280, P = 0.040). TXL also restrain the angiogenesis of ApoE-/- mice aorta (F = 21.223, P = 0.010). Compared with C57BL/6J mice, the MVD was decreased in heart tissue of untreated ApoE-/- mice (54.0 ± 3.0/mm2 vs. 75.0 ± 2.0/mm2; F = 16.054, P = 0.010). However, TXL could significantly enhance MVD (65.0 ± 5.0/mm2 vs. 54.0 ± 3.0/mm2; F = 11.929, P = 0.020) in treated ApoE-/- mice. In addition, TXL obviously increased the expression of VEGF protein determined by Western blot (F = 20.247, P = 0.004).
Conclusions:
TXL obviously improves the ApoE-/- mouse heart function from different pathways, including reduces blood fat to lessen atherosclerosis; enhances aortic impulsivity, blood supply capacity, and vessel elasticity; improves endothelium-dependent vasodilatation; restraines angiogenesis of aorta-contained plaque; and enhances MVD of heart. The molecular mechanism of MVD enhancement maybe relate with increased VEGF expression.
Keywords: Heart Function, Microvascular Protection, Tongxinluo
摘要
背景:
既往众多研究表明,作为一种复方中药,通心络(TXL)可以减少心肌缺血-再灌注损伤,保护毛细血管内皮功能,抑制动物模型心室重构。本研究目的在于阐明通心络是否可以通过内皮功能保护或增加心肌微血管数量,从而改善高胆固醇血症诱发的心功能障碍,并对其潜在的分子机制做深入探讨。
方法:
本实验研究共计8周。实验开始后,通心络组小鼠(n=8)给予0.5%羧甲基纤维素钠(CMC-Na)注悬的通心络超微粉(1.52g 生药/ kg/d)口服灌胃(0.1 ml/10g 体重),每日1次;8只特征匹配的野生型C57BL/6J小鼠作为对照组。模型组(n=8)与对照组小鼠饲以同等剂量的CMC-Na口服灌胃,每日1次。实验结束后测定各组小鼠血清总胆固醇、高密度脂蛋白胆固醇、极低密度脂蛋白胆固醇、甘油三酯和血糖水平;通过超声心动图检查,采集各组小鼠心率(HR)、左室舒张末期内径(LVDED)、左室收缩末期内径(LVSED)等参数,计算左室射血分数(LVEF)、每搏输出量(SV)、心输出量(CO)及左室短轴缩短率(LVFS)等指标以评价心功能、同时测量小鼠胸主动脉收缩期峰值流速(PSV)、舒张末血流速度(EDV)与平均血流速度(MFV),计算搏动指数(PI)与阻力指数(RI)以评价血管弹性与顺应性;采用体外乙酰胆碱诱导主动脉环舒张反应方法评价各组小鼠内皮依赖性血管舒张反应;体外血管新生实验在一周内完成;开展小鼠心肌CD34免疫组织化学检测;分别采用蛋白印迹分析与RT-PCR方法测定各组小鼠心肌组织中血管内皮生长因子(VEGF)蛋白与基因表达表达水平。本研究的数据采用单因素方差分析(ANOVA),P < 0.05为有统计学意义。
结果:
与模型组比较,通心络组小鼠血清胆固醇水平无显著改变 (F = 2.300, P = 0.240),但通心络可以使模型小鼠的甘油三酯和极低密度脂蛋白胆固醇水平降低(F = 9.209, P =0.024;F = 9.786, P = 0.020);通心络可以改善模型小鼠的心脏功能,增加LVEF、SV、CO和LVFS(P < 0.05);通心络可增强模型小鼠主动脉PSV和MFV(F = 10.774, P =0.024; F = 11.354, P = 0.020),减少模型组小鼠主动脉的PI(1.41±0.17 vs 1.60±0.17;F = 14.270, P = 0.037)。本研究中,乙酰胆碱可剂量依赖性(0.1–10 μmol/L)诱导正常组小鼠主动脉环的舒张反应(51% ± 9%),模型组小鼠内皮依赖性血管舒张则明显受损(23% ± 2%);与模型组比较,通心络组小鼠血管舒张率明显改善(44% ± 3%,F=9.280, P=0.040);实验证实通心络可以抑制模型小鼠主动脉血管生成(F = 21.223, P = 0.010);与对照组比较,模型组小鼠心肌组织微血管密度(MVD)显著减少(54.0 ± 3.0/mm2 vs 75.0 ± 2.0/mm2;F=16.054, P=0.010);与模型组比较,通心络小鼠心肌MVD则明显增加(65.0 ± 5.0/mm2 vs 54.0 ± 3.0/mm2; F=11.929, P=0.020)。通心络明显增加模型组小鼠VEGF蛋白表达(F = 20.247, P = 0.004),使VEGF mRNA表达增加4.59倍。
结论:
通心络可多方面改善ApoE-/-模型小鼠心功能,除降低血脂以延缓动脉粥样硬化进程外,对血管尤其是微血管的保护在其作用机制中十分关键,这包括提高主动脉血供能力及弹性、改善内皮依赖性血管舒张功能、抑制含斑块动脉组织血管新生以及增加心肌组织微血管密度,后者深层分子机制可能与其增强VEGF基因与蛋白表达有关。
INTRODUCTION
Coronary artery disease (CAD) is the leading cause of death in industrialized countries.[1] The major CAD risk factors include advancing age, male sex, hypertension, smoking, diabetes, elevated total serum cholesterol, and family history of premature CAD.[2] Atherosclerosis is the main cause and pathological foundation of CAD. As an independent and important risk factor for atherosclerosis, hypercholesterolemia explains <50% of CAD risk.[3]
Endothelium, as the first line of defense in the vessel wall, plays an important role in maintaining normal vascular homeostasis by producing a balance of paracrine factors such as nitric oxide (NO) and angiotensin II, which is the primary target of many atherogenic risk factors. Prior studies indicate that the effects of chronic hyperlipidemia were complex in the condition that results in not only the deposition of lipids in the atheromatous lesions but also the production of the primary endothelial injury that initiates the process of atherosclerosis as well. At the same time, as the clinical outcomes of reperfused myocardial infarction and left ventricular remodeling are strongly related to that of microvascular injury,[4] hypercholesterolemia-induced microvascular injury and changes were linked to left ventricular dysfunction in the nonnarrow coronary heart disease.[5]
Despite the significant progress in the understanding of endothelial dysfunction and vascular disease, no pharmacologically active agent has been developed to therapeutically modulate this connection.[3] On the other hand, traditional medicine has been practiced for thousands of years in some communities, such as American-Indians and Chinese. Twenty years ago, as an extract of compound Chinese medicine, Tongxinluo (TXL) was developed for the treatment of CAD (registered in the State Food and Drug Administration of China). It has been successfully used in thousands of patients with CAD in reducing the occurrence of acute coronary events or sudden death. An adequate number of studies have shown that TXL can decrease myocardial ischemia-reperfusion injury, protect capillary endothelium function, and lessen cardiac ventricle reconstitution in animal models.[6] Clinical trials have shown that TXL is effective in reducing infarct size, recovery time, and improvement in ventricular function in patients with acute coronary syndrome and in improving microcirculation in patients with CAD.[7] However, the mechanisms underlying its effects on the CAD remain unknown.
The major purpose of this study was to illuminate whether TXL can improve hypercholesterolemia-impaired heart functioning through protecting artery endothelial function and increasing microvascular density (MVD) in heart, and also to explore its further molecular mechanism.
METHODS
Ethical approval
The animal care and procedures of this study were conducted according to the principles of Laboratory Animal Care. The local Institutional Review Board approved all animal procedures.
Animal procedures
Sixteen ApoE-/- mice (28–32 g) aged 12 months were purchased from the Center for Laboratory Animal Medicine and Care of medical school located in University of Texas. Eight characteristic-matched C57BL/6J wild-type (WT) mice were used as normal control. Mice were randomly divided into three groups. Intragastric administration of the respective treatments was provided continuously for 8 weeks. During the experimental period, ApoE-/- mice (n = 8) were administrated (0.1 ml/10 g body weight) with TXL (Shijiazhuang Yiling Pharmaceutical CO., Ltd., Shijiazhuang, Hebei, China) which was product by ultra-micro-crushing technology (Yiling Pharmaceutical Co., Ltd., Shijiazhuang, Hebei, China) suspended by 0.5% sodium carboxymethyl cellulose (CMC-Na) one time per day, according to the dosage of 1.52 g crude drug/kg/day. Another set of ApoE-/- mice (n = 8) and WT mice (n = 8) were fed on equivalent volume of CMC-Na one time per day. All the mice were fed on regular diet for 8 weeks.
Blood lipid and blood glucose determination
Peripheral blood was drawn from eye sockets before the mice were sacrificed. Total cholesterol, high-density lipoprotein (HDL)-cholesterol, very-low-density lipoprotein (VLDL)-cholesterol, triglyceride, and blood glucose levels in serum were measured in the Equine Laboratories of Texas Heart Institute.
Echocardiography
After anesthetized by isoflurane (Veterinary Anesthesia Systems, America), all the mice were detected by ultrasonic cardiogram (Visualsonics Vevo 770 system, Canada) with 40 MHz frequency scratching unit before sacrificed. These parameters of heart rate (HR), left ventricular diastolic end diameter, and left ventricular systolic end diameter were harvested. And then, the left ventricular ejection fraction (LVEF), stroke volume (SV), cardiac output (CO), and left ventricular fractional shortening (LVFS) were calculated as previously described so as to evaluate heart function.[8] Meanwhile, aortic peak systolic flow velocity (PSV), end diastolic flow velocity (EDV), and mean flow velocity (MFV) were measured, and subsequently, pulsatility index (PI) and resistant index (RI) were calculated in order to evaluate the vascular elasticity and resistance using the following formula: PI = (PSV − EDV)/MFV; RI = (PSV − EDV)/PSV.
Endothelium-dependent vasodilatation function test
The endothelium-dependent vasodilatation was evaluated by relaxation of aortic rings in response to acetylcholine.[9] Aorta was excised immediately in deep isoflurane anesthesia and placed at 4°C into Kreb's-Henseleit buffer (NaCl 118 mmol/L, KCl 4.7 mmol/L, CaCl2 2.5 mmol/L, MgCl2 1.2 mmol/L, KH2 PO4 1.2 mmol/L, NaHCO3 25 mmol/L, glucose 11 mmol/L, and pH 7.4) and saturated with a mixture of gas including 95% O2 and 5% CO2 in advance. Subsequently, it was divided into four sections about 3 mm long each after peeling adventitial fat and connective tissue carefully. These ex vivo vascular rings were drawn by two stainless steel needles which were located at the bath of DMT myograph (ADinstruments, Australia) to achieve 1 g tension and maintained for 1 h. And then, Kalium chloratum whose final concentration was 80 mmol/L was used to stimulate vascular rings to achieve maximal contract amplitude followed by washing with Kreb's-Henseleit buffer three times to recover its state. After that, making the vascular rings contracted up to 70% maximal amplitude through dropping into ethyl phenylephrine whose final concentration was 10 μmmol/L, different cumulative final concentrations of acetylcholine including 1 nmol/L, 3 nmol/L, 10 nmol/L, 30 nmol/L, 100 nmol/L, 300 nmol/L, 1 μmol/L, 3 μmol/L, and 10 μmol/L were exerted into a bath each 5-min interval to induce vascular rings’ relaxation. The signal was recorded and analyzed by PowerLab 8/30 Data Recording and Analysis System (ADinstruments, Australia). The endothelial function was evaluated through vasodilation rate in different concentrations of acetylcholine.
Angiogenesis experiment in vitro
After mice were sacrificed, a midline incision was made and the thoracic cavity was exposed. The right atria were cut, and phosphate-buffered saline (PBS) with 1.25% penicillin, 1.25% streptomycin, and 10 U/ml heparin was administered by injection into the left ventricle. Perfusion continued until the fiber and fat tissue were removed from the aorta and it was excised. The aorta was cut into equal segments and placed in order (proximal to distal) on a fibrin-based gel prior to full polymerization at three arterial segments per chamber on a 2-chamber slide. A total of six aortas were used for each animal analyzed. Multiple images were taken of microtubules’ outgrowth from aortas cultured in modified fibrin gels for 1 week. Images were stitched together utilizing Photoshop CS4 (Adobe Systems Incorporated, California, USA). From these images, tubule outgrowth was calculated using ImageJ software (National Institute of Health, Bethesda, Maryland, USA). Data represent the average and standard deviation (SD) of the five longest outgrowths.
Myocardial CD34 immunohistochemical detection
Heart tissues were immediately fixed with 4% paraformaldehyde in 0.1 mol/L sodium phosphate buffer (pH 7.4), dehydrated with a graded series of ethanol solutions, and embedded in paraffin. Sections (4 μm thickness) were prepared by using microtome (Leica, Germany) from the paraffin-embedded tissues and mounted on glass slides. After removal of the paraffin with xylene, the sections were blocked with 3% hydrogen dioxide for 10 min, washed, and antigen was repaired with microwave. The sections were exposed to diluted 10% normal goat serum and then incubated overnight at 4°C with rabbit polyclonal antibody to CD34 (Santa Cruz biotechnology, 1:50) which was diluted in 4% nonfat milk and 0.1% Tween 20 in PBS. After that, the sections were incubated with a biotinylated anti-rabbit IgG (Vector Labs, 1:100) for 30 min. Immune complexes were detected with a Vectastain ABC-AP kit (Vector Labs, America). Microvascular profiles identified by CD34 staining were performed.[10] Subsequent to immunohistochemistry, the entire heart section was scanned at low power (original magnification ×100) to identify the hot spots, which represent the areas of highest neovascularization. The individual microvascular profiles were later counted under high power (original magnification ×400) to obtain a vessel count in a defined area. An average vessel count in the five hot spots was calculated as MVD. The results were calculated as heart MVD per square millimeter (vessels/mm2).
Real-time quantitative polymerase chain reaction and Western blotting analysis of vascular endothelial growth factor expression
To detect the vascular endothelial growth factor (VEGF) mRNA level, total RNA from heart tissues was extracted with Trizol (Invitrogen, America) and purified (Qiagen, Germany). The mRNAs were reverse transcribed into cDNAs with a SuperScript cDNA synthesis kit (Invitrogen, America). The iCycler iQ real-time polymerase chain reaction (PCR) detection system (Bio-Rad Laboratories, America) was used for cDNA quantification. Primers were designed through Beacon Designer 2.0 software (Premier Biosoft International, California, USA). Mouse VEGF primer sequences were as follows: forward 5’-AACCATGAACTTTCTGCTCTC-3’ and reverse 5’-GTG ATTTTCTGGCTTTGTTC-3’; GAPDH primer sequences were as follows: forward 5’-CCCTTC ATTGACCTCAACTACAATGGT-3’ and reverse: 5’-GAGGGGCCATCCACAGTCTTCTG-3’. Each quantitative reaction contained iQ SYBR Green I (Bio-Rad, America) 25 μl, forward and reverse primers (200 nmol/L, respectively), cDNA (equivalent to 350 ng total RNA), and water up to a volume of 50 μl. The real-time PCR protocol consisted of 3 min at 95°C followed by 40 cycles of 10 s at 95°C, 30 s at 56°C, and 30 s at 72°C. To assess PCR specificity, melting curves from 55°C in 0.5°C steps of 10 s with measurement of fluorescence were generated at the end of each PCR. A single melting peak and a single band on 2% agarose gel electrophoresis were confirmed. The mRNA levels were acquired from the value of threshold cycle (Ct) of the real-time PCR and normalized against the house-keeping gene GAPDH.
To detect VEGF protein expression level, Western blotting analysis was performed. Heart tissues from mice were lysed in lysis buffer (10 mmol/L Tris, PH 7.4, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% NP40, 1% Txiton X-100) containing a protease inhibitory cocktail (Sigma, USA) using a micro-potter. Protein concentration was determined with BCA Protein Assay (Thermo scientific, America). Samples containing 20 μg were analyzed by electrophoresis on 4–15% precast SDS-polyacrylamide gels (Bio-rad, USA) under reducing conditions and transferred to nitrocellulose membrane and incubated overnight at 4°C with a monoclonal antibody to VEGF (Clone VG1, DakoCytomation, Denmark). Antibodies were diluted (1:400) in 4% nonfat milk and 0.1% Tween 20 in PBS. The membranes were then washed and incubated with a 1:5000 dilution of goat anti-mouse IgG horseradish peroxidase-conjugated antibody (Santa Cruz biotechnology, USA). Bound antibodies were detected by enhanced chemiluminescence (ECL, Thermo scientific and Hyperfilm TM-MP, BioExpress, USA) according to the manufacturer's instruction.
Statistical analysis
Data in this study were representative of at least three different experiments and presented as the mean ± standard deviation (SD) and compared by one-way analysis of variance (ANOVA) between groups. Statistical analysis was performed using SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Tongxinluo reduced serum triglyceride and very-low-density lipoprotein levels
Regardless of fed with regular or high-fat diet, ApoE-/- mice have spontaneous hypercholesterolemia and develop atherosclerotic lesions. Therefore, we first investigated whether TXL reduced the blood fat levels of ApoE-/- mice. The results showed no significant changes in serum glucose of each group's mice; however, the level of blood fat in ApoE-/- mice significantly increased compared with that of C57BL/6J mice, including HDL, triglyceride, and VLDL (all P < 0.01; Figure 1). Although no decreasing trend of cholesterol was observed (F = 2.300, P = 0.240), TXL reduced the level of triglyceride and VLDL in ApoE-/- mice (F = 9.209, P = 0.024 and F = 9.786, P = 0.020, respectively; Figure 1), which was beneficial for lessening atherosclerosis degree.
Figure 1.
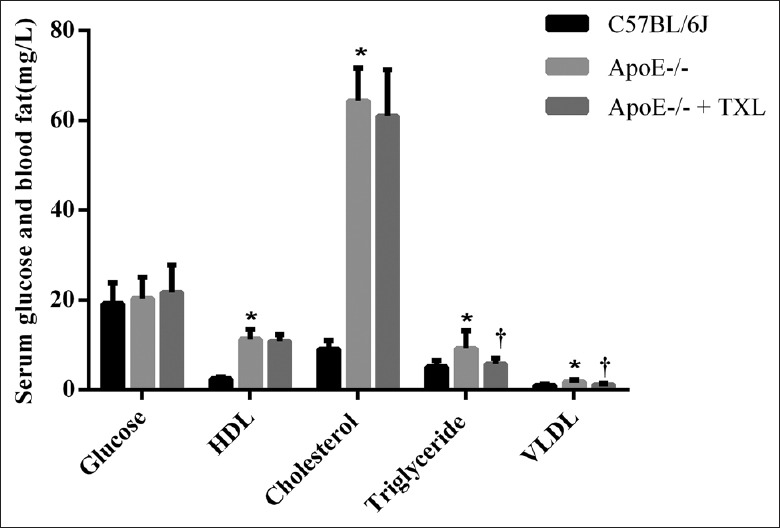
TXL decreased serum triglyceride and VLDL levels in ApoE-/- mice. The contents (mg/L) of glucose, HDL-cholesterol, total cholesterol, triglyceride, and VLDL in serum were detected by automatic biochemistry analyzer (n = 8 for each group). The level of blood fat in ApoE-/- mice, including HDL-cholesterol, triglyceride, and VLDL, increased than those of C57BL/6J mice significantly (all *P < 0.01); TXL can make the level of triglyceride and VLDL in ApoE-/- mice down significantly. (F = 9.209, †P = 0.024 and F = 9.786, †P = 0.020; respectively). TXL: Tongxinluo; VLDL: Very-low-density lipoprotein; HDL: High-density lipoprotein.
Tongxinluo improved heart function of ApoE-/- mice
Serious coronary atherosclerosis is always accompanied with heart hypofunction in clinical CAD patients. In this study, the arterial wall of C57BL/6J mice was smooth, no abnormalities were observed [Figure 2a], many atherosclerotic plaques forming on the aortic walls of ApoE-/- mice were very obvious and extensive [Figure 2b]. At the same time, these items reflecting heart function acquired from ultrasonic cardiogram were all decreased in ApoE-/- mice compared with C57BL/6J mice, including HR, LVEF, SV, CO, and LVFS [Table 1]. However, TXL improved the heart function of ApoE-/- mice owing to the elevations of LVEF, SV, CO, and LVFS (all P < 0.05; Table 1).
Figure 2.
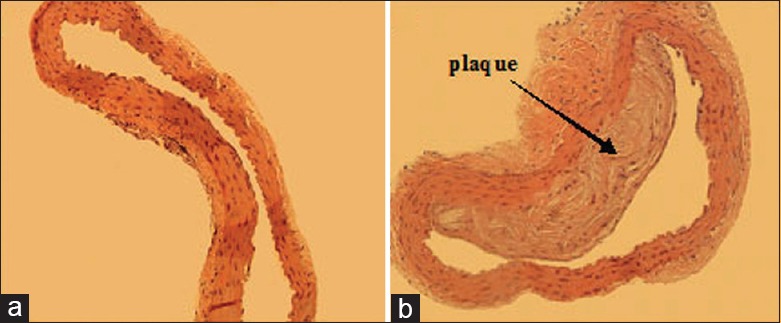
Morphology of aorta (Hematoxylin and Eeosin, original magnification ×100) (a) C57BL/6J mice; (b) ApoE-/- mice.
Table 1.
Comparison of cardiac function indexes in each group
| Groups | HR (beats/min) | LVEF (%) | SV (μl) | CO (ml) | LVFS (%) |
|---|---|---|---|---|---|
| C57BL/6J | 378.37 ± 51.07 | 60.51 ± 10.63 | 37.55 ± 12.21 | 14.13 ± 4.63 | 32.21 ± 7.47 |
| ApoE-/- | 413.87 ± 46.20 | 38.89 ± 14.49* | 26.95 ± 12.13 | 11.12 ± 5.30 | 18.98 ± 7.88* |
| ApoE-/- + TXL | 407.57 ± 41.94 | 60.71 ± 11.50† | 41.60 ± 10.93† | 16.68 ± 3.58† | 32.49 ± 7.80† |
| F | 5.245 | 13.216 | 10.948 | 14.395 | 14.480 |
| P | 0.872 | 0.042 | 0.034 | 0.039 | 0.025 |
Data are presented as mean ± standard deviation. All the mice were anesthetized and then HR, LVDED, and LVSED were collected by ultrasonic cardiogram; LVEF, SV, CO, and LVFS were calculated according to formula (LVEF=SV/LVEDV×100%; SV= LVEDV-LVESV; CO=HR×SV; LVFS=(Dd-Ds)/Dd×100%, n = 8 for each group). *P <0.05 versus C57BL/6J; †P <0.05 versus ApoE-/-. TXL: Tongxinluo; HR: Heart rate; LVDED: Left ventricular diastolic end diameter; LVSED: Left ventricular systolic end diameter; LVEF: Left ventricular ejection fraction; SV: Stroke volume; CO: Cardiac output; LVFS: Left ventricular fractional shortening. LVEDV: Left ventricular end-diastolic volume; LVESV: Left ventricular end-systolic volume; Dd: Diastolic left ventricular short axis; Ds: Systolic left ventricular short axis.
Tongxinluo enhanced vascular blood supply intensity and elasticity
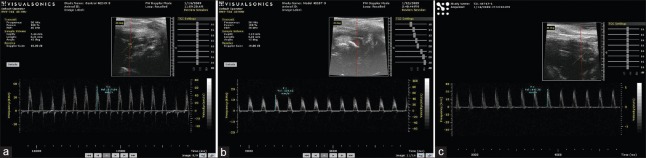
In this experiment, PSV and MFV of aorta in ApoE-/- mice were all decreased compared with that of C57BL/6J mice. Compared with C57BL/6J mice, PI was increased in ApoE-/- mice (1.48 ± 0.13 vs. 1.60 ± 0.17; F = 8.270, P = 0.056), but no statistical significant differences. However, TXL made a conversion in the group of treated ApoE-/- mice. The results showed that TXL enhanced aortic PSV and MFV (F = 10.774, P = 0.024 and F = 11.354, P = 0.020, respectively; Figures 3 and 4) and reduced PI of ApoE-/- mice (1.41 ± 0.17 vs. 1.60 ± 0.17; F = 14.270, P = 0.037).
Figure 3.
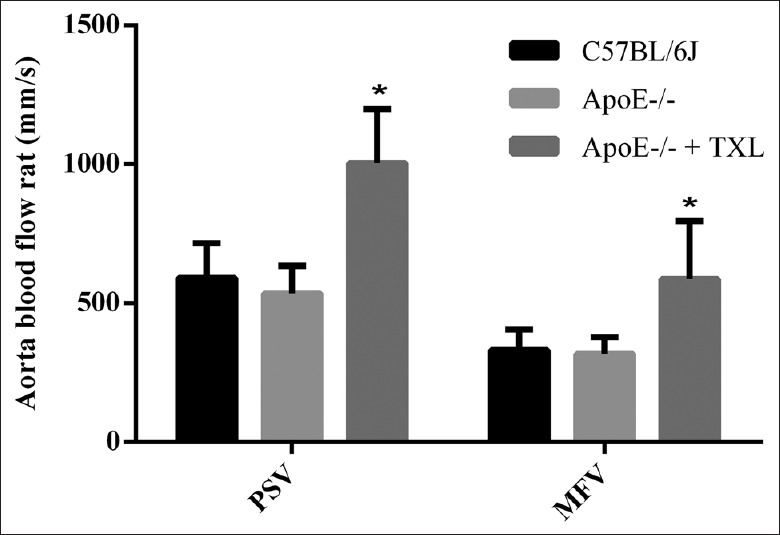
TXL enhanced vascular blood supply intensity. The extent of arterial blood supply can be reflected by aortic PSV and MFV. There was no statistically significant difference between C57BL/6J mice and ApoE-/- mice in PSV (591.97 ± 123.30 mm/s vs. 535.46 ± 98.87 mm/s; F = 2.139, P = 0.084) and in MFV (331.39 ± 74.15 mm/s vs. 318.61 ± 59.16 mm/s; F = 4.274, P = 0.061). However, the corresponding values were 1004.53 ± 293.74 mm/s (F = 10.774, *P = 0.024) and 587.32 ± 207.90 mm/s (F = 11.354, *P = 0.020) after treatment with TXL. TXL: Tongxinluo; PSV: Peak systolic flow velocity; MFV: Mean flow velocity.
Figure 4.
Typical graphs of aortic peak systolic flow velocity (mm/s) which were collected by ultrasonic cardiogram (Visualsonics Vevo 770 system, Canada). (a) C57BL/6J; (b) ApoE-/-; (c) ApoE-/- + Tongxinluo.
Tongxinluo improved endothelium-dependent arterial relaxation
Then, we assessed the TXL potential mechanism of improving vascular elasticity. In this experiment, acetylcholine produced a dose-dependent relaxation in aortic rings from the C57BL/6J mice [Figures 5 and 6a]. However, compared with C57BL/6J mice, the vasorelaxation to acetylcholine (0.1–10 μmol/L) was significantly impaired in ApoE-/- mice (all P < 0.01; Figures 5 and 6b). After incubation with 10 μmol/L acetylcholine, the C57BL/6J mouse aortic segment relaxed by 51% ± 9% and that of the ApoE-/- mice relaxed by 23% ± 2%, whereas the TXL-treated ApoE-/- mouse aortic segment relaxed by 44% ± 3%, obviously more than untreated ApoE-/- mice (F = 9.280, P = 0.040; Figures 5 and 6c). The aortic relaxation rate almost reaches to that of C57BL/6J mice.
Figure 5.
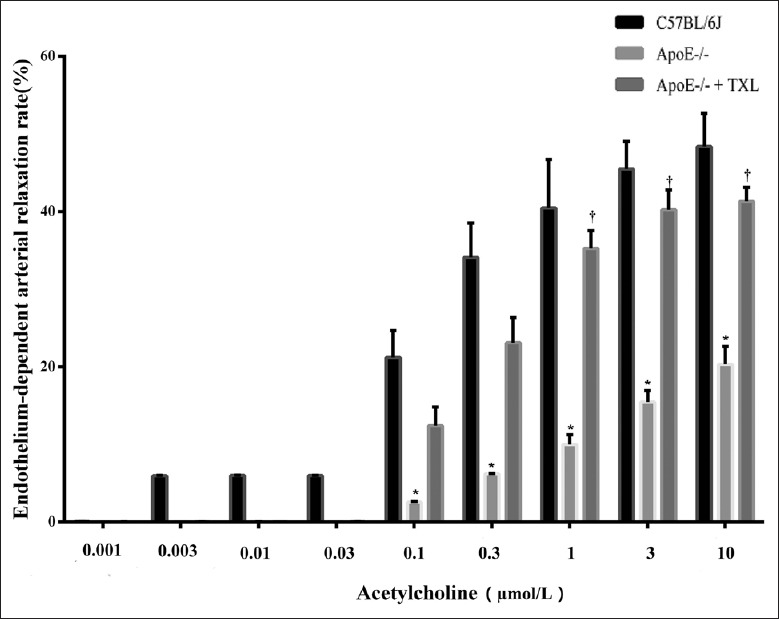
TXL improved endothelium-dependent arterial relaxation. Aortic rings were precontracted with KCL of 80 mmol/L (final concentration) and contracted with phenylephrine of 12 μmol/L (final concentration), 10 min. Dose–response relaxation was measured for cumulative increments of acetylcholine (from 1 nmol/L to 10 μmol/L) at 3-min intervals (n = 7 for each group). In this experiment, acetylcholine produced a dose-dependent relaxation in aortic rings from the C57BL/6J mice. However, vasorelaxation to acetylcholine (0.1–10 μmol/L) was significantly impaired in ApoE-/- mice, compared with C57BL/6J mice (all *P < 0.01). After incubation with 10 μmol/L acetylcholine, the aortic segment relaxed in the ApoE-/- mice by 23% ± 2%, whereas aortic segment relaxed in the ApoE-/- + TXL mice by 44% ± 3% (F = 9.280, †P = 0.040). TXL: Tongxinluo.
Figure 6.
Typical graphs of endothelium-dependent arterial relaxation. The signal were recorded and analyzed by PowerLab 8/30 Data Recording and Analysis System. (a) C57BL/6J; (b) ApoE-/-; (c) ApoE-/- + Tongxinluo.
Tongxinluo restrained atheromatous plaque angiogenesis of ApoE-/- mice
In this study, the microtubules’ outgrowth from ApoE-/- mouse aorta which contained plaque was increased compared with C57BL/6J mice in 1 week (975.27 ± 85.57 μm vs. 639.62 ± 125.85 μm; F = 15.236, P = 0.014, Figures 7 and 8). As expected, TXL could restrain angiogenesis of ApoE-/- mouse aorta (664.09 ± 107.25 μm vs. 975.27 ± 85.57 μm; F = 21.223, P = 0.010, Figures 7 and 8).
Figure 7.
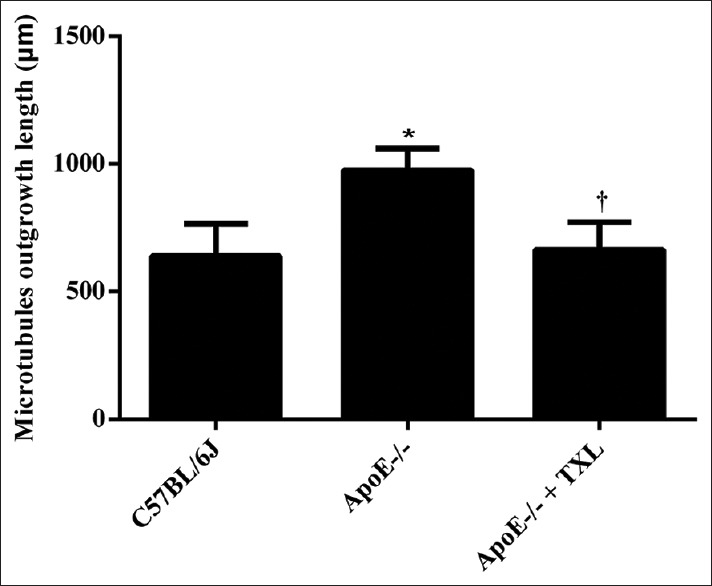
TXL restrained atheromatous plaque angiogenesis of ApoE-/- mice. After mice were sacrificed, the aorta was cut into equal segments and placed in order on a fibrin-based gel prior to full polymerization. After 1 week, multiple images were taken of microtubules’ outgrowth from aortas cultured in modified fibrin gels. Data showed (n = 6 for each group) that angiogenesis from aorta of ApoE-/- mice was faster than C57BL/6J (F = 15.236, *P = 0.014), however, which was restrained by TXL (F = 21.223, †P = 0.010). TXL: Tongxinluo.
Figure 8.
Typical graphs of angiogenesis of aorta. (a) C57BL/6J; (b) ApoE-/-; (c) ApoE-/- + Tongxinluo.
Tongxinluo increased microvascular density in the heart of ApoE-/- mice
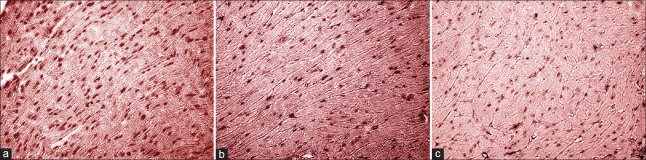
Using the technology of immunohistochemistry, we observed CD34 expression to evaluate MVD in mouse heart. As a new microvascular marker, CD34 is better than other marks in displaying vascular endothelial cell (EC).[11] The CD34 antibody produced positive staining for the endothelial lining of all identifiable blood vessels in heart tissues. The results showed that MVD in ApoE-/- mouse heart tissue significantly decreased compared with C57BL/6J mice (54.0 ± 3.0/mm2 vs. 75.0 ± 2.0/mm2; F = 16.054, P = 0.010, Figure 9). While, TXL increased MVD (65.0 ± 5.0/mm2 vs. 54.0 ± 3.0/mm2; F = 11.929, P = 0.020) in treated ApoE-/- mouse heart tissue.
Figure 9.
CD34 staining of myocardial tissue (original magnification, ×200). (a) C57BL/6J; (b) ApoE-/-; (c) ApoE-/- + Tongxinluo.
Tongxinluo enhanced the vascular endothelial growth factor expression of heart in the levels of protein and gene
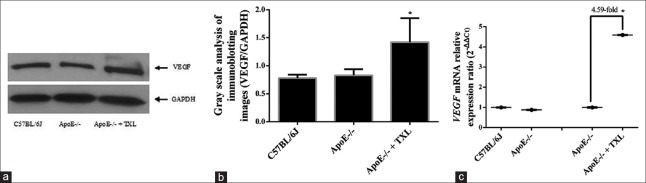
Furthermore, we investigated the molecular mechanism of TXL increasing MVD in the heart of ApoE-/- mice. Compared with C57BL/6J mouse heart tissues, VEGF protein and mRNA expression had no significant changes in hearts of untreated ApoE-/- mice (F = 5.298, P > 0.05; Figure 10). However, TXL still increased the expression level of VEGF protein (F = 20.247, P = 0.004; Figure 10a and 10b) and VEGF mRNA level increased over four times in that of treated ApoE-/- mice (F = 17.489, P = 0.003; Figure 10c).
Figure 10.
The expression of VEGF in heart at the levels of protein and mRNA. (a) Representative gel of four independent experiments. (b) The gray scale analysis of scanned gel images. Data have shown that the VEGF protein expression has no significant changes between ApoE-/- mice and C57BL/6J mice (0.83 ± 0.11 vs. 0.78 ± 0.06; F = 5.298, P > 0.05), but it was enhanced in ApoE-/- + TXL mice (1.42 ± 0.43 vs. 0.83 ± 0.11; F = 20.247, *P = 0.004). (c) TXL enhanced VEGF mRNA expression. VEGF mRNA levels were quantified by real-time PCR and normalized to GAPDH mRNA. As the expression of protein, VEGF mRNA expression has no change between ApoE-/- mice and C57BL/6J mice, but it was enhanced by TXL up to 4.59-fold in ApoE-/- mice (F = 17.489, *P = 0.003). VEGF: Vascular endothelial growth factor; TXL: Tongxinluo.
DISCUSSION
As atherosclerosis is thought to be an age-related disease, older animals develop more atherosclerotic plaques than younger counterparts; even with the same cholesterol burden, we used the old ApoE-/- mice at 1-year age in this study. Results have shown that the level of blood fat in ApoE-/- mice was very high, even seven-fold in cholesterol than WT C57BL/6J mice, indicating the presence of hyperlipidemia. At the same time, many atheromatous plaques were found in the aortic lumens by morphologic inspection, indicating that the atherosclerosis model was definitive and successful.
Hypercholesterolemia or hyperlipidemia plays a vital role in the genesis and development of atherosclerosis. Cholesterol-lowering therapy, especially with statins, has been clearly demonstrated to be the single most effective, cost-effective, and safest method to reduce CVD risk and events, and as such has become the cornerstone of prevention of CVD. The advent of statin class drugs, HMG-CoA reductase inhibitor, has made cholesterol reduction readily achievable, but currently employed pharmaceutical development strategies appear to be stagnant in discovering new drug with efficacy as powerful as the statins.[3] First, we have investigated whether TXL, a kind of traditional Chinese medicine, is effective on hyperlipidemia in ApoE-/- mice. Interestingly, TXL could obviously reduce serum triglyceride and VLDL levels which prompted atherosclerotic process, but less effective on cholesterol.
Serious coronary atherosclerosis is always accompanied with heart hypofunction in clinical CAD patients. We also observed that the heart function significantly descended in ApoE-/- mice, especially LVEF, reflecting left ventricle contractile function, and prognosis was degraded. Surprisingly, TXL improved the ApoE-/- mouse heart function in this study. To determine its mechanism, we start up further research on large vessels and microvascular protection.
We observed the effect of TXL on large vessel function from two aspects. First, the extent of arterial blood supply can be reflected by aortic PSV and MFV. Meanwhile, PI is a good parameter for vessel elasticity, which reflects vascular resistance and compliance take on positive correlation with atherosclerosis. RI was used to judge vascular resistance. In general, PI and RI augmentation prompts that vascular elasticity and compliance is weakening and vascular resistance is increasing. In the present study, TXL could improve atherosclerotic elasticity and reinforce blood supply capability to myocardial tissue, which was probably one of its most important mechanisms of improving heart function in ApoE-/- mice. Second, the endothelium plays an important role in maintaining vascular homeostasis by producing vasoactive factors that regulate the tone of the vascular system in response to cell surface receptor stimulation or mechanical stress. Endothelial dysfunction is considered to be a key event and a hallmark of a number of vascular disorders, such as changes in vascular compliance, increased monocyte adhesion, and atherogenesis. Endothelial dysfunction is believed to be an event that leads to atherosclerosis as well as being a marker of the severity of atherosclerosis. In ApoE-/- mice, the vasomotor function is negatively correlated with the plaque size.[12] Results from several studies indicated that endothelial function in coronary and peripheral arteries is impaired by hyperlipidemia. The vascular dysfunction induced by hyperlipidemia is associated with blunted vasodilator responses to several endothelium-dependent agonists, including acetylcholine. In the functional endothelium, acetylcholine binding to muscarinic receptors on the luminal surface is associated with the generation of NO by endothelial NO synthase (eNOS). The NO subsequently diffuses into the smooth muscle cells and facilitates the relaxation of the vessel. In this study, our finding showed that the aged ApoE-/- mice had lower endothelium-dependent vasorelaxation elicited by acetylcholine in aortic segments mounted in myographs. In contrast, TXL improved endothelium-dependent vasodilatation in ApoE-/- mice, indicating its protective effect on the vascular ECs, which proved the results of other clinic trials,[13] and that mechanism probably correlated with elevating eNOS activity and increasing NO synthesis.[14]
In addition, we observed the effect of TXL on microvascular changes in aorta and heart of ApoE-/- mice. As is known, angiogenesis can occur in a number of pathological conditions, including atherosclerosis, hypertrophy, and infarction. More importantly, neovascularization may play a role in the progression of the atherosclerotic plaque as well in the development of complications such as intraplaque hemorrhage or aneurysms.[15] Neovascularization of the atherosclerotic plaque is responsible for its weakening and consequently for the complications of clinical vascular disease, such as plaque internal hemorrhage, plaque rupture, and unstable angina pectoris. Angiogenesis involves the proliferation and migration of ECs, which form tubes. Vascular smooth muscle cells later migrate and associate with these tubes to form the inherent microvascular. However, the mechanism and stimuli for neovascularization in atherosclerotic plaque remain unknown. Some observations indicate the role of smooth muscle cells through the secretion of VEGF.[16] In this study, the microtubules’ outgrowth from ApoE-/- mouse aorta which contained plaque increased compared with C57BL/6J mice, indicating obviously the presence of angiogenesis. As expected, TXL could restrain angiogenesis of ApoE-/- mouse aorta and gave us a prompt about its function of stabilizing atherosclerotic plaque and inhibiting atherosclerotic process. The mechanism was probably correlated with reducing the VEGF expression in aortic plaque.[17] To acute myocardial infarction, the extent of myocardial salvage is critically dependent on blood flow to the risk area. Thus, the primary objective of reperfusion therapies should be not only to achieve rapid and sustained epicardial patency, but also to restore microvascular flow and myocardial tissue perfusion.
We thought that microvascular amount and function were equally important to CAD. As a new microvascular marker, CD34 is better than other marks in displaying vascular EC.[11] Using the technology of immunohistochemistry, we observed CD34 expression so as to evaluate MVD in the heart. Nevertheless, different results came out in the detection of MVD in heart tissue. We found out that MVD in ApoE-/- mouse heart tissue significantly decreased compared with C57BL/6J mice, while it increased in mice treated with TXL. In other words, the results have manifested that TXL could promote neovascularization of chronic myocardial ischemia induced by atherosclerosis. However, what is the further mechanism?
To answer this question, we investigated the molecular mechanism of TXL increasing MVD in the heart of ApoE-/- mice. VEGF is a multifunctional glycoprotein which is mitogenic for ECs. It has a high affinity with ECs in the macro- and microvascular vessels. It is the most important regulator of pathological or physiological angiogenesis and additionally leads to the increased vascular permeability. VEGF is thought to stimulate EC proliferation and increased permeability by binding to two high-affinity receptors expressed predominantly by ECs: flk-1 and flt-1. During embryonic development, VEGF and its receptors are expressed in cells surrounding the expanding vasculature and VEGF is predominantly produced in tissues acquiring new capillary networks. As an angiogenesis-associated growth factor, VEGF promotes angiogenesis, induces an improvement in regional blood flow, and stimulates endothelial lineage cell survival via VEGF-mediated phosphorylation of Akt and eNOS.[18] Due to the less number of experimental animals or other reasons, VEGF protein and mRNA expression had no significant changes in mouse heart tissues between untreated ApoE-/- and C57BL/6J mice, which needs to be further explained. However, TXL still could increase the VEGF mRNA expression in heart of treated ApoE-/- mice, which was coincident with protein expression. Several groups of investigators have demonstrated that increased VEGF mRNA levels are predominately due to increased gene transcription, so we thought that TXL probably promoted neovascularization in the heart through the pathway of increasing VEGF gene transcription.
One of the major limitations of the current study is that the TXL doses used in the current study were based on previous experiments and dose-dependent effects observed in other laboratory; however, the subgroup design was not carried out, it would be adjusted accordingly in future studies.
In this study, we showed that ApoE-/- mice featured with hyperlipidemia and atherosclerosis had heart hypofunction and, on the other hand, exhibited many changes in the aspect of aorta and microvascular, including the extent of arterial blood supply and vessel elasticity reduced, endothelium-dependent vasodilatation weakened, angiogenesis from aorta contained plaque increased and MVD in heart decreased. The findings indicated that TXL had obviously positive effects on the ApoE-/- mice's heart functioning. It showed beneficial effects on downregulated serum triglyceride and VLDL levels, thus reducing susceptibility to atherosclerosis. In addition, it protected blood vessels, particularly at the microvascular level, which play a very important role in its mechanisms of action.
On considering all factors, our results suggested that TXL could obviously improve the ApoE-/- mouse heart function from different pathways, including reduced blood fat to lessen atherosclerosis; enhanced the extent of arterial blood supply and vessel elasticity; improved endothelium-dependent vasodilatation; restrained angiogenesis of aorta-contained plaque; and enhanced MVD of heart. The molecular mechanism of MVD enhancement maybe related with the increased VEGF expression.
Financial support and sponsorship
This study was supported by a grant from National Basic Research Program of China (973 programs, No. 2012CB518606).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–14. doi: 10.1161/CIRCGENETICS.109.852814. doi: 10.1161/circgenetics.109.852814. [DOI] [PubMed] [Google Scholar]
- 2.März W, Scharnagl H, Winkler K, Tiran A, Nauck M, Boehm BO, et al. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: The Ludwigshafen Risk and cardiovascular health study. Circulation. 2004;110:3068–74. doi: 10.1161/01.CIR.0000146898.06923.80. doi: 10.1161/01.cir.0000146898.06923.80. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Wu Y, Jia Z, Zhang Y, Shen HY, Wang XL, et al. Protective effects of a compound herbal extract (Tong Xin Luo) on free fatty acid induced endothelial injury: Implications of antioxidant system. BMC Complement Altern Med. 2008;8:39. doi: 10.1186/1472-6882-8-39. doi: 10.1186/1472-6882-8- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reffelmann T, Kloner RA. The no-reflow phenomenon: A basic mechanism of myocardial ischemia and reperfusion. Basic Res Cardiol. 2006;101:359–72. doi: 10.1007/s00395-006-0615-2. doi: 10.1007/s00395-006-0615-2. [DOI] [PubMed] [Google Scholar]
- 5.Theilmeier G, Verhamme P, Dymarkowski S, Beck H, Bernar H, Lox M, et al. Hypercholesterolemia in minipigs impairs left ventricular response to stress: Association with decreased coronary flow reserve and reduced capillary density. Circulation. 2002;106:1140–6. doi: 10.1161/01.cir.0000026805.41747.54. doi: 10.1161/01.cir.0000026805.41747.54. [DOI] [PubMed] [Google Scholar]
- 6.Qi K, Li L, Li X, Zhao J, Wang Y, You S, et al. Cardiac microvascular barrier function mediates the protection of tongxinluo against myocardial ischemia/reperfusion injury. PLoS One. 2015;10:e0119846. doi: 10.1371/journal.pone.0119846. doi: 10.1371/journal.pone.0119846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang HT, Jia ZH, Zhang J, Ye ZK, Yang WX, Tian YQ, et al. No-reflow protection and long-term efficacy for acute myocardial infarction with Tongxinluo: A randomized double-blind placebo-controlled multicenter clinical trial (ENLEAT trial) Chin Med J. 2010;123:2858–64. doi: 10.3760/cma.j.issn.0366-6999.2010.20.021. [PubMed] [Google Scholar]
- 8.Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: Comparison with dobutamine stress echocardiography. Circulation. 1999;99:763–70. doi: 10.1161/01.cir.99.6.763. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd EE, Pandit LM, Crossland RF, Marrelli SP, Bryan RM., Jr Endothelium-dependent relaxations in the aorta from K (2p) 6.1 knockout mice. Am J Physiol Regul Integr Comp Physiol. 2013;305:R60–7. doi: 10.1152/ajpregu.00126.2013. doi: 10.1152/ajpregu.00126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–80. doi: 10.1007/BF00666038. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 11.Kimura H, Nakajima T, Kagawa K, Deguchi T, Kakusui M, Katagishi T, et al. Angiogenesis in hepatocellular carcinoma as evaluated by CD34 mmunohistochemistry. Liver. 1998;18:14–9. doi: 10.1111/j.1600-0676.1998.tb00121.x. doi: 10.1111/j.1600-0676.1998.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 12.Crauwels HM, Van Hove CE, Holvoet P, Herman AG, Bult H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovasc Res. 2003;59:189–99. doi: 10.1016/s0008-6363(03)00353-5. doi: 10.1016/s0008-6363(03)00353-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Liu Y, Lu XT, Wu YL, Zhang C, Ji XP, et al. Traditional Chinese medication Tongxinluo dose-dependently enhances stability of vulnerable plaques: A comparison with a high-dose simvastatin therapy. Am J Physiol Heart Circ Physiol. 2009;297:H2004–14. doi: 10.1152/ajpheart.00208.2009. doi: 10.1152/ajpheart.00208.2009. [DOI] [PubMed] [Google Scholar]
- 14.Wang B, Yang Q, Bai WW, Xing YF, Lu XT, Sun YY, et al. Tongxinluo protects against pressure overload-induced heart failure in mice involving VEGF/Akt/eNOS pathway activation. PLoS One. 2014;9:e98047. doi: 10.1371/journal.pone.0098047. doi: 10.1371/journal.pone.0098047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staub D, Patel MB, Tibrewala A, Ludden D, Johnson M, Espinosa P, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke. 2010;41:41–7. doi: 10.1161/STROKEAHA.109.560342. doi: 10.1161/STROKEAHA.109.560342. [DOI] [PubMed] [Google Scholar]
- 16.Kuzuya M, Satake S, Esaki T, Yamada K, Hayashi T, Naito M, et al. Induction of angiogenesis by smooth muscle cell-derived factor: Possible role in neovascularization in atherosclerotic plaque. J Cell Physiol. 1995;164:658–67. doi: 10.1002/jcp.1041640324. doi: 10.1002/jcp.1041640324. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Wu ZG, Liao DN, Pan XM. Effects of Tongxinluo on the expression of vascular endothelial growth factor in rabbit aortic atherosclerotic plaques (in Chinese) Chin J Arterioscler. 2004;2:177–82. doi: 10.3969/j.issn.1007-3949.2004.02.014. [Google Scholar]
- 18.Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000;97:13801–6. doi: 10.1073/pnas.250488097. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]