Acentralized hemovigilance program was launched in the country on December 10, 2012, to assure patient safety and promote public health through a well-structured program for monitoring of adverse reactions associated with blood and blood product transfusions. The National Institute of Biologicals (NIB), Noida, is the National Co-ordinating Centre. The Haemovigilance Programme of India (HvPI) was started under the broad ambit of Pharmacovigilance Programme of India. The key objectives outlined in the HvPI are (i) monitor transfusion reactions, (ii) create awareness among health-care professionals, (iii) generate evidence-based recommendations, (iv) advise the Central Drugs Standard Control Organization (CDSCO) for safety-related regulatory decisions, (v) communicate findings to all key stakeholders, and (vi) create national and international linkages. A core group, a national executive committee, and a national advisory committee were constituted for operationalization of the programme. The Transfusion Reaction Reporting Form (TRRF), Guidance document, standardized definitions of transfusion reactions, and the Haemo-Vigil software for online reporting of transfusion reactions were approved. Three expert panels were also constituted for report analysis and training purposes.[1,2]
Setting up the Reporting Structure
The Transfusion Reaction Reporting Form
The TRRF was a simple one-page form divided into six sections as follows:
Patient information
Transfusion product(s) details
Nature of adverse reaction(s)
Outcome of adverse reaction(s)
Reporter
Causalty assessment.
The definitions of adverse events and reactions were adopted from the document published by the International Society of Blood Transfusion Working Party on Haemovigilance as on June 2013 and endorsed by the International Haemovigilance Network.[3]
Haemo-Vigil software
Development of indigenous software for collection and collation of transfusion reaction data from various reporting centers was a major challenge. It required software validation, verification, operationalization, and hands-on training to end users. This software was developed indigenously by the information technology division at the NIB. Security audit and compliance audit were obtained from the National Informatics Centre (NIC), Government of India. The software, Haemo-Vigil, was hosted on the NIB website on January 24, 2013. The adverse transfusion reaction data collected from the reporting centers are secured in the NIC server.
Guidance document
These guidelines are intended for reporting the serious adverse reactions related to blood transfusion by the centers under the HvPI. The document contents are listed below:
HvPI – organogram
Enrollment of centers (medical colleges/institutes/hospitals/blood banks) under the HvPI
Objectives of reporting adverse reactions associated with blood transfusion
Haemo-Vigil software
Privacy and security of data
Documentation and reporting of serious adverse reactions associated with blood and blood product transfusions
Responsibilities of the medical colleges/institutes/hospitals/blood banks under the HvPI
Responsibilities of medical and nursing staff of the centers under the HvPI
Responsibilities of Hospital Transfusion Committee
Responsibilities of NIB, National Co-ordinating Center – HvPI
Responsibilities of CDSCO
Imputability levels
Definitions
Protocol for the investigation of acute transfusion reaction
Forms.
Generating awareness about the programme
Awareness about the haemovigilance programme and its objectives has been generated through hemovigilance newsletters, scientific publications,[2] and organizations of continuing medical education (CME) programs across the country. Twenty-six CMEs have been organized in different centers during this period. The CME content was developed by the core training panel and consisted of the following PowerPoint presentations:
Planning, conceptualization, and implementation of HvPI
Definitions, terms, and scope of hemovigilance
Hospital hemovigilance and role of hospital transfusion committees
Case illustrations and group discussions
Hands-on training for use of Haemo-Vigil software.
Enrollment and Participation of Centers in Haemovigilance Programme of India
The total number of centers enrolled during this period was 368. Year-wise enrollment is shown in Figure 1. Transfusion reaction reports were submitted by 104 centers.
Figure 1.
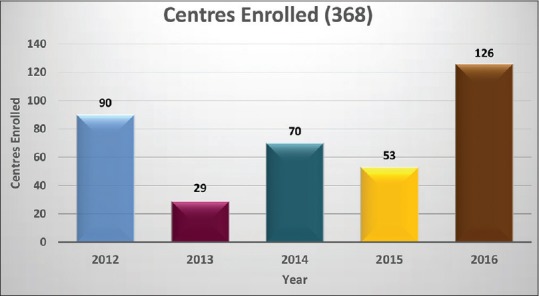
Year-wise enrollment of centers
Analysis of Transfusion Reaction Reports
The transfusion reactions as reported through the TRRF, HvPI, were analyzed with regard to the following parameters:
Type of transfusion reactions
Clinical diagnosis of the patient, age, and gender
Type of blood component transfused
First time or repeat transfusion
Causalty assessment.
General observations
A total of 3903 transfusion reactions were reported to the HvPI, National Co-ordinating Centre, NIB, Noida. These 3903 reactions had occurred in 3807 patients, thus 96 patients had more than one transfusion reaction. Age-wise distribution of the patients is shown in Table 1.
Table 1.
Age-wise distribution of the patients

Out of 337 pediatric patients, 8 were neonates. The distribution among males and females was 1955 and 1852 patients, respectively. Recovery from the reaction was recorded in 3542 patients, recovery was associated with sequelae in 20 patients, and the outcome was not known in 228 patients. Mortality was reported in 17 patients; however, only in five patients, it was related to the transfusion reaction, and in the remaining 12 patients, the mortality occurred due to underlying clinical condition and the transfusion episode was coincidental.
Although 3903 reactions occurred in 3807 patients, for the purposes of analysis, each reaction is described separately.
Analysis as per the nature of adverse transfusion reactions
Immunological hemolysis due to ABO incompatibility
Twenty-two hemolytic transfusion reactions due to ABO incompatibility were reported, 6 in male patients and 16 in females. The age range was 12–70 years. One reaction occurred in a pediatric patient, and the rest were reported in adults. Sixteen patients had received packed red blood cell (PRBC) transfusion, 5 whole blood, and in one patient, apheresis unit had been transfused. In five patients, a bedside sampling error – “wrong blood in tube” – led to the mismatched transfusion; in two patients, there was a blood grouping error; in one case, a blood bag labeling error; and in one patient, bedside administration error was reported. In 13 patients, the cause of ABO-mismatched transfusion was not recorded.
Immunological hemolysis due to other alloantibodies
Fifty-eight such reactions were reported, the age range of the patients was 2–92 years; 8 reactions were in children, 31 reactions occurred in male patients, and 27 in female patients. Fifteen patients had thalassemia major and nine patients had chronic hemolytic anemia (not specified further). Out of these patients, 19 were on regular transfusion therapy. Prior history of transfusion was noted in 18 other patients, and in eight patients, the reaction occurred after the first transfusion episode. Eleven out of the 27 female patients also had a history of past pregnancy. Alloantibodies (anti-Jka, anti-E, and anti-Jkb) were reported in only three patients.
Nonimmunological hemolysis
Eighty-four reactions were reported, age range of the patients was neonate to 79 years. Ten reactions occurred in the pediatric and 74 in the adult patients. Male and female patients had equal number of reactions (42 in each gender group). Handling and storage errors were recorded in nine patients as follows:
Freezing and thawing of PRBC – 1
Prolonged storage in the ward – 4
Warming of blood in hot water – 2
Storage of blood in chiller tray in ward refrigerator – 2
Hemolysis during leukofiltration – 1
During massive transfusion – 1.
Transfusion-transmitted bacterial infection
This adverse reaction was reported in 18 patients, 7 females and 11 males. The age range was neonate to 68 years. The implicating blood component was PRBCs (14 patients), apheresis platelets (1), both PRBC and fresh frozen plasma (FFP) (one patient), while three patients who underwent therapeutic plasma exchange received FFP. In nine cases, the bacterial culture from the blood bag was positive, and the bacteria grown were coagulase-negative staphylococci (1), Escherichia coli (1), Klebsiella (1), Staphylococcus epidermidis (1), and micrococci (2). Two of the patients were reported to be on antibiotics.
Anaphylaxis/hypersensitivity
A total of 495 reactions were reported in this category, the age range was infancy to 93 years; 38 reactions occurred in pediatric patients. The male–female distribution was 276 versus 219 cases. The implicated blood components in decreasing order of frequency were PRBC (215 cases), FFP (157), whole blood (58), random donor platelets (47), apheresis platelets (15), cryoprecipitate (3), and solvent detergent-treated plasma (2).
First-time transfusions were recorded in 355 patients (71.4%).
Transfusion-related acute lung injury
Transfusion-related acute lung injury (TRALI) was reported in ten patients, all adults, in the age range of 23–66 years, five males, and five females. The blood components implicated were PRBCs (7 cases), FFP (2 cases), and whole blood (1 case). The clinical diagnoses as recorded in the TRRF were as follows: postbone marrow transplant, prostate carcinoma, liver laceration, lung carcinoma with pulmonary thrombus, pancytopenia, stomach carcinoma, colon carcinoma with metastasis and sepsis, severe anemia, postoperative patient, and an antenatal patient.
Febrile nonhemolytic transfusion reactions
Febrile nonhemolytic transfusion reactions (FNHTRs) constituted 1594 adverse transfusion reaction, age range was neonate to 96 years, and 166 reactions occurred in the pediatric age group. The male–female distribution was 784 versus 810. In order of decreasing frequency, the implicated blood components were PRBCs (1260 cases), whole blood (224 cases), FFP (67 cases), random donor platelets (39 cases), and apheresis platelets (8 cases). The clinical conditions were varied and included both medical and surgical conditions. FNHTRs occurred in 1140 patients after the first transfusion episode. In 68 patients, FNHTR occurred alongwith another transfusion reaction.
Transfusion-associated dyspnea
Transfusion-associated dyspnea (TAD) was reported in 93 patients, age range was 1–90 years, 7 were pediatric patients, 43 were males, and 50 were female patients. In order of decreasing frequency, the implicated blood components were PRBCs (69 cases), whole blood (7 cases), random donor platelets (7), and apheresis platelets (3).
Transfusion-associated circulatory overload
Transfusion-associated circulatory overload (TACO) was reported in 26 patients, age range was 7–72 years only, and one patient was in the pediatric age group. There were 11 male and 15 female patients. In order of decreasing frequency, the implicated blood components were PRBCs (24 cases), whole blood (one case), and FFP (one case). All the patients had moderate-to-severe anemia and in one patient rapid transfusion was recorded.
Transfusion-associated-graft versus host disease
Transfusion-associated-graft versus host disease (TA-GvHD) was reported in one patient with the underlying clinical condition of septicemia. There were no supportive diagnostic or clinical criteria mentioned in the TRRF.
Transfusion-associated parasitic infection
One case of transfusion-transmitted (TT) malarial infection was reported. However, on investigation by the reporting center, the patient's pretransfusion sample was positive for malarial parasite and the donor unit was negative.
Posttransfusion purpura
Posttransfusion purpura (PTP) was reported in 25 patients, age range was 1–55 years, and three patients belonged to the pediatric age group. The male–female distribution was 5 males and 20 females. Whole blood was transfused to 18 patients and PRBCs to 7.
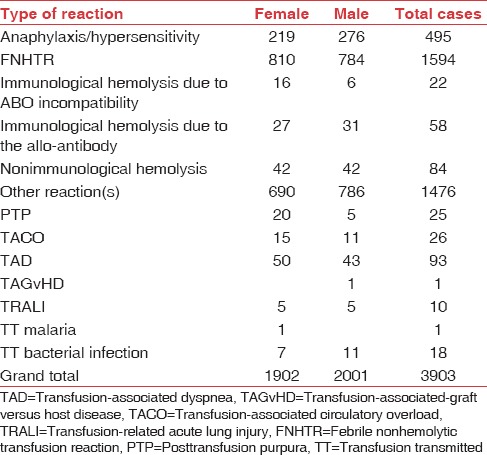
Other Reactions
All reactions which could not be defined into the specific categories were reported as “others.” There were a total of 1476 such reactions reported on the TRRF; 786 in males and 690 in females. These included mild allergic reactions (1064), mild fever and/or chills (196), hypotension (48), dyspnea/mild breathlessness (30), pain at infusion site (3), abdomen (2), flanks (4), and calf muscle (3), chest pain or discomfort (11), and headache (3). Tingling and numbness were reported in three patients, hypertension in five, anxiety in five, early detection of ABO mismatch in three cases, and Rh D-incompatible transfusion in one patient. In 95 patients, the reaction was not specified.
Summary of reports
FNHTRs constituted the most frequently reported transfusion reaction (40.84%). Mild allergic reactions which were reported in “other reaction” category comprised 27.26% of the reactions. Anaphylactic/hypersensitivity reactions were 12.68% and hemolytic transfusion reactions were 4.31% (164 out of 3903). Out of these 164 hemolytic transfusion reactions, 22 (0.56%) were due to ABO mismatch, 58 (1.49%) were due to non-ABO alloantibodies, and 84 (2.15%) were due to nonimmune causes. There was incomplete information on cause/error which led to the ABO mismatch. In 9 out of 22 cases where information was available, 6 cases had a bedside sampling/administration error. Alloantibodies were identified and reported in three patients only. In the rest, immune-hematology workup was not available for review in the TRRF. The nonimmune hemolytic transfusion reactions were mainly due to ward/bedside storage and handling errors as per the available information. The remaining categories of transfusion reactions reported were TAD (2.38%), TACO (0.67%), PTP (0.64%), TTBI (0.46%), TRALI (0.26%), TT malaria (0.03%), and TAGvHD (0.03%). In the category of “other reactions,” majority were mild allergic reactions (27.26%) and mild FNHTRs (5.02%), and the rest were either not specific or symptoms not possible to classify into a specific reaction [Table 2 and Figure 2]. The distribution of these reactions with respect to age and blood group is shown in Figures 2–4 and Tables 2–4.
Table 2.
Distribution of transfusion reactions out of 3903 reported reactions
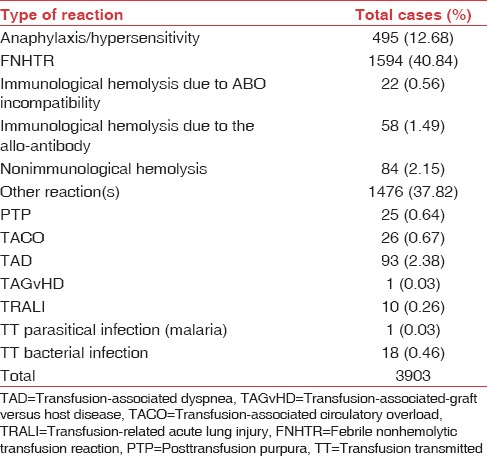
Figure 2.
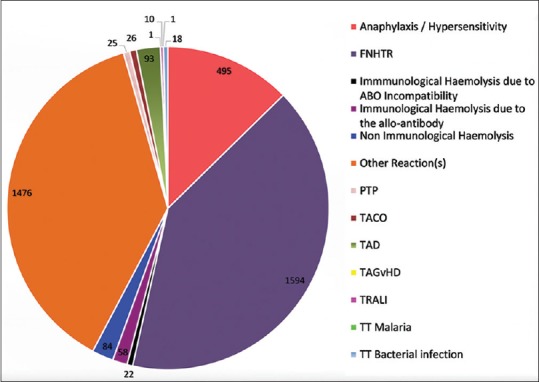
Distribution of transfusion reactions out of 3903 reported reactions
Figure 4.
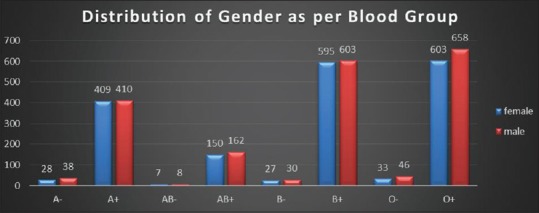
Distribution of gender as per blood group reported in 3807 patients
Table 4.
Distribution of gender as per blood group reported in 3807 patients
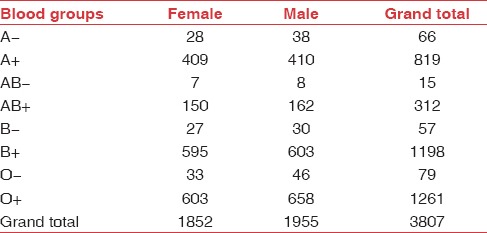
Figure 3.
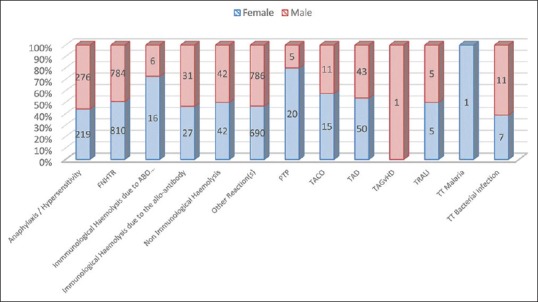
Distribution of transfusion reactions out of 3903 reported reactions (gender wise)
Table 3.
Distribution of transfusion reactions out of 3903 reported reactions (gender wise)
Mortality was reported in 17 patients; in ten patients, it was clearly due to the underlying critical condition of the patient and transfusion was not causally related. Two deaths were associated with hemolytic transfusion reactions, one each with TT bacterial infection, TACO, TRALI, TAD, and severe FNHTR. However, the patient with TACO was a postpartum female with severe anemia, the patient with TRALI was a case of carcinoma colon with metastasis and sepsis, the patient with TAD was a trauma patient with poor general condition, and the patient with severe FNHTR was a severely anemic female. It is possible that the underlying clinical condition might have played a significant contributory role in mortality. Hence, only three deaths might have a probable causal relationship. The causalty assessment has not been analyzed in detail as most of the centers needed further clarification on this analytic parameter, and from the one-page TRRF, it was not possible for the expert reviewers to reassess the causality.
Recommendations
The recommendations for the role and responsibility of various stakeholders in ensuring success of the haemovigilance programme in blood safety are as follows:
Central Drugs Standard Control Organization – the regulatory authority for blood banks
The most significant positive outcome of the programme has been the steady increase in the number of centers reporting to the HvPI since the launch of the programme. The report is based on 3903 reactions reported from 104 centers out of the 368 enrolled centers. This reflects the increasing confidence in the programme on the reporting of sensitive data and has resulted from the persistent and dedicated efforts of the core committee of HvPI and its outreach to the transfusion medicine community and clinicians through CMEs, workshops, and newsletters.
However, there are 2760 licensed blood banks across the country and the aim of the programme is to get all the blood banks enrolled with HvPI. An office memorandum has been issued from the office of Drug Controller General, India, dated December 4, 2015, “instructing all licensed blood banks to obtain their user id and password from NIB to uplink their adverse transfusion reaction data to Haemo-Vigil software.” There is a need to implement these instructions by all the licensed blood banks. The blood banks maintain records of adverse transfusion events as per the licensing rules; however, a suitable amendment to the rules directing blood banks to uplink these records to the HvPI is essential.
Blood centers/departments of transfusion medicine
Twenty-two hemolytic transfusion reactions due to ABO incompatibility were reported; in two patients, there was a blood grouping error; and in one case, there was a blood bag labeling error. Both these types of errors are preventable. There is a need to improve and implement standard technology for blood grouping and should have quality-tested blood grouping reagents and trained staff
In cases of 58 HTRs due to other alloantibodies, only three centers have reported the “other” alloantibody identified. The technologies for alloantibody screening and identification need to be introduced in blood banks for proper investigation and diagnosis of immune HTRs and also to issue compatible blood in complicated cases. Fifteen patients had thalassemia major and nine patients had chronic hemolytic anemia (not specified further). Out of these patients, 19 were on regular transfusion therapy. Hence, blood banks/departments of transfusion medicine supporting transfusion therapy in thalassemic patients need to implement these facilities on priority
Some of the reactions reported were rare and their diagnosis is not supported by investigations. Further awareness is required on how to improve the accuracy of diagnosis and imputability of the reaction to the transfusion
FNHTRs constituted the most frequently reported transfusion reaction (40.84%). These reactions are mediated by leukocytes in red cell and platelet products. They can be minimized by usage of leukofiltered red cells and platelets. Blood banks should be encouraged to prepare leukofiltered products and setting up plateletpheresis technology for good quality platelets.
Clinicians
The clinicians are the prescribers of blood and blood components. The clinical bedside staff is also responsible for bedside administration and monitoring of transfusion reactions, including early detection of reaction and reporting to the blood bank for further investigations.
There is a need to create awareness about the different types of blood components, their appropriate clinical use, and detection and reporting of adverse events. Training modules need to be prepared and CMEs must be organized for the awareness of clinicians. In fact, national guidelines on appropriate clinical use for various categories of patients are lacking in India and this programme could put expert groups in place to formulate guidelines
Nonimmune HTRs due to blood handling and storage errors were recorded in nine patients. These reactions can be prevented by proper guidelines on bedside transfusion practices. All hospitals should have hospital transfusion committees which formulate good transfusion practices and ensure that all bedside staff including house physicians, interns, residents, and nursing staff are periodically trained. Good bedside transfusion practices should include raising appropriate blood requisition, obtaining informed consent of patient/next of kin, obtaining blood sampling, bedside recognition, identity check of blood bag and patient, documentation, and monitoring. At least one senior nursing staff may be designated as haemovigilance nurse to track transfusion events and liase with medical officer of the blood bank. Alternately, a transfusion safety officer may be nominated.
Expert committees of Haemovigilance Programme of India
One-page TRRF was initially introduced to simplify the reporting mechanism and motivate centers to start reporting. However, there is no information as to how the reporting center classified the transfusion reaction or performed the causality assessment.
Hence, switch over to the new detailed TRRF is recommended at the earliest to improve the quality of data captured for analysis
Statistical tools for data analysis are required to sort out the data into various groups to identify critical causative factors
Training of reporting centers – both new enrollment and problem sorting for already enrolled centers – should be an ongoing process
Development of training modules for clinicians and organizing CMEs for clinicians.
Ministry of Health and Family Welfare
To issue circulars to all state health departments and central government medical institutions to direct medical superintendent/civil surgeons of all hospitals to constitute and make functional hospital transfusion committees
To issue circulars to state health departments and central government medical institutions to designate haemovigilance nurse/transfusion safety officers
To issue instructions to the Medical Council of India to ensure that clinical staff is appropriately trained for prescribing and transfusing blood and blood components
To allocate resources for immunohematology technology upgrading in blood transfusion services
To allocate resources for preparation of leukofiltered blood components.
Conclusion
The most significant positive outcome of the programme has been the steady increase in the number of centers reporting to the HvPI since the launch of the programme. The report is based on 3903 reactions reported from 104 centers out of the 368 enrolled centers. This reflects the increasing confidence in the programme on the reporting of sensitive data and has resulted from the persistent and dedicated efforts of the core committee of HvPI and its outreach to the transfusion medicine community and clinicians through CMEs, workshops, and newsletters.
The categorization of transfusion reactions finalized as per the current TRRF is based on the reporting center. One-page TRRF was to simplify the reporting mechanism and motivate centers to start reporting. However, there is no information as to how the reporting center classified the transfusion reaction or performed the causality assessment. In cases of HTRs due to other alloantibodies, only three centers have reported the “other” alloantibody identified. No investigations were reported to authenticate the diagnosis of transfusion reactions. For nonimmune HTRs which are largely preventable, only 6/84 reactions were substantiated by the labeling handling and storage error. Here, a very important message has been communicated by those few centers and that is the need for sensitization of clinicians for good bedside transfusion practices.
The categorization of reactions reported as PTP and TA-GVHD needs to be reviewed. Wherever recovery with sequelae has been reported, the residual effect has not been mentioned in the TRRF. The review process is restrained by inadequate information and hence switch over to the new detailed TRRF is recommended at the earliest to improve the quality of data captured for analysis. Statistical tools for data analysis are required to sort out the data into various groups to identify critical causative factors. Further awareness is required on how to improve the accuracy of diagnosis and imputability of the reaction to the transfusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
HvPI is thankful to all blood centers which have reported their data of various transfusion reactions to the central data base. Assistance of Ms. Ruchi Rao, Technical Consultant, HvPI, is acknowledged.
References
- 1.Haemovigilance Programme of India. [Last accessed on 2018 Jan 15]. Available from: http://www.nib.gov.in/haemovigilance1.html .
- 2.Bisht A, Singh S, Marwaha N. Hemovigilance program-India. Asian J Transfus Sci. 2013;7:73–4. doi: 10.4103/0973-6247.106744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Society of Blood Transfusion Working Party on Haemovigilance. Proposed Standard Definitions for Surveillance of Non Infectious Adverse Transfusion Reactions; July, 2011. [Last accessed on 2018 Jan 15]. Available from: http://www.isbtweb.org/Haemovigilance/ISBT_definitions_final_2011 .


