Abstract
INTRODUCTION:
With the increased utilization of immunohematology (IH) analyzers in the transfusion medicine, type, and screen policy is the method of choice. Still, the importance of routine crossmatching could not be overruled. Here, we tried to understand the clinical conditions and safety of red cell transfusion and their outcomes.
MATERIALS AND METHODS:
This prospective study was conducted by IH laboratory, Medical College Kolkata, Blood Bank from October 1, 2015 to March 31, 2016. A set of 3cc ethylenediaminetetraacetic acid and clotted blood samples of the patients were received according to sample acceptance criteria. Blood grouping by conventional tube technique followed by crossmatching was performed by column agglutination technology (CAT) in polyspecific (IgG + C3d) gel media. Any positive result was rechecked in duplicate with additional two group-specific donor units. The persistent incompatibility was further evaluated using direct anti-human globulin test, auto control, antibody screening, and antibody identification by CAT.
RESULTS:
On the evaluation of 14,387 sets of patients' sample, only 100 were found to be incompatible (0.69%). Incompatibility rate is higher in females (59%). Eighty-five of these patients were repeatedly transfused. Only 38% of incompatible crossmatch were positive on indirect anti-human globulin test/antibody screening. Antibody could be identified in 16 of them. Seventeen of 100 incompatible samples (17%) presented with panagglutination, were managed with Rh, Kell phenotype/best-matched red cell units. In these 16 patients, 23 alloantibodies were identified; allo anti-E was the most common.
CONCLUSION:
This study showed antibody against the Rh system as the most common cause of incompatibility.
Keywords: Antibody screening, antibody identification, column agglutination test, conventional tube technique, direct anti-human globulin test, immunohematology analyzer, incompatible crossmatch, indirect anti-human globulin test, panagglutination, polyspecific (IgG + C3d) gel media
Introduction
One of the essential goals in crossmatching of red cells is that the transfused blood must be compatible with the patient to provide maximum therapeutic support and minimal red cell destruction. With the increasing utilization of automated immunohematology (IH) analyzers, the routine cross-matching is predominantly replaced by ABO and Rh type and antibody screen or type and screen (T/S) policy. In the Eastern part of India, major cross-matching between the recipient's serum and donor red cells by anti-human globulin is the most common practice in most of the blood banks. These tests are carried out either by the conventional tube techniques (CTT) or by the semi-automated column agglutination technology (CAT). This is due to the constraints related to trained workforce and availability of regular supply of reagents and other logistics.
It appears once the recipient's ABO and Rh blood type is known, a transfusion of compatible blood can be given. However, in practice, donor red blood cells (RBCs) may still be incompatible as it contains other minor antigens against which the recipient is alloimmunized/sensitized. Therefore, a cross-match is done to ensure that the donor RBCs actually do match against the recipient's serum. There are times when even after an exhaustive workup, a unit of compatible red cells becomes unavailable for the patient. The commonly observed clinical conditions and the insights obtained on how safe to transfuse the best unit of blood available was reviewed here along with their outcomes. The clinical and serologic evaluation, which allows for the transfusion of the most compatible (or “least incompatible”) blood, requires a joint effort between the clinician and the transfusion medicine physician.[1]
Materials and Methods
A prospective analysis was conducted in all the incompatible cross-matched blood samples at the IH laboratory of Kolkata Medical College Hospital blood bank since October 1, 2015–March 31, 2016 (6 months). This blood bank is one of the major regional blood transfusion centers in the state of West Bengal (WB), Eastern India, with an average annual blood collection over 30,000 units. The center supplies an overall annual average of 50,000 units of blood components to the patients who were admitted in the hospital itself as well as patients referred from other hospitals located within or outside the city of Kolkata. Red cell concentrates constitute a major volume of the supplied blood components to the extent of 60% approximately. This study had been approved by the Institutional Ethics Committee.
Sample acceptability criteria
Any request for blood component(s) was accompanied by a duly filled and authorized blood requisition form as designed by the WB State Blood Transfusion Council along with properly labeled 3 cc ethylenediaminetetraacetic acid (EDTA) and 3 cc clotted blood samples. The samples should be freshly collected mentioning the name of the patient with patient identity number and phlebotomist's signature. If any nonconformity were observed in the blood samples, they were referred to the blood transfusion officer (BTO) / resident doctor on duty for further decision making. Blood sample(s) showing visible evidence of gross deterioration/hemolysis were excluded from the study. If the patient had a previous history of blood transfusion, the transfusion records related to blood group and any other relevant information were verified.[2]
Preparation of the blood sample and blood grouping
The EDTA and clotted vial were centrifuged at 3000 ×g for 3 min to separate red cells and serum/plasma.[3] ABO and Rh typing was done by CTT using commercially available monoclonal antibodies (Tulip Diagnostics Pvt. Ltd., India). Reverse grouping was performed by CTT using freshly prepared in-house reagent pooled A, B, and O cells.[4] Tests were validated by a negative saline control. Any discrepancy in blood grouping results was resolved according to their type and classification, as per departmental standard operating procedures and recorded. The concerned treating facility was also intimated of their significance.
Cross-matching of patient's sample
Once the blood group of the recipient's sample was determined, a major cross-match using group-specific donor red cell units (1% donor red cell suspension in low ionic strength saline solution) was done by CAT in polyspecific (IgG + C3d) gel media (Matrix gel system, Tulip Diagnostics Pvt. Ltd., India). The tests were performed according to the manufacturer's instructions.
A positive and negative control were run daily in parallel with the tests to validate the test results.[5]
Evaluation of an incompatible cross-matched sample
In case of any incompatible major cross-match results, a repeat cross-match with the same donor unit was performed along with two additional group specific donor units. This repeatation was done to rule out any possibility of technical errors (contamination, direct anti-human globulin test [DAT] positive donor unit, mis-grouping, etc.) as well as clerical/transcriptional errors. If the incompatibility persisted on repetitions in any of these 3 units, a further evaluation of the recipient's sample was done in the departmental IH laboratory. The IH laboratory could not routinely perform T/S policy so such an alternative protocol is chosen.
An initial workup of the recipient's sample was done by DAT, auto-control and antibody screening using commercially available cells or in-house prepared screening cells.[3] Any reaction with a strength of 2+ or above was considered to be strong and below these were weakly reactive. Antibody identification was done in antibody screen positive samples, using commercially available reagent 11 cell panel (Ortho-clinical Diagnostics Inc., USA) by CAT in Ortho BioVue system on polyspecific AHG (IgG + C3d) cassettes. The workflow of evaluation of an incompatible cross-match was shown in Figure 1. However, a detail of clinical history of the patient along with the history of medications and relevant history suggestive of alloimmunization/sensitization is recorded by the transfusion medicine resident doctor, wherever was possible.
Figure 1.
Evaluation of an incompatible cross-match sample
Selection and issue of appropriate donor red cell unit
Wherever any alloantibody(s) were being detected corresponding antigen(s) negative compatible or best-matched unit was issued after consultation with the treating clinician.[1] In situ ations where no specific alloantibody could be pointed, group-specific, extended Rh and Kell phenotype matched (where the patients were transfusion free for more than 3 months) red cell units were provided as emergency lifesaving resort. In situ ations where patients received recent transfusions (within 3 months) best-matched units (less strength than autocontrol) were provided.[6,7]
Each of these transfusions was under the supervision of the treating clinician or the transfusion medicine resident. In the event of any adverse outcome, the transfusion was stopped immediately and the blood bank resident doctor was informed for further proceedings. Every successful transfusion events were monitored with a posttransfusion 24 h increase in hemoglobin (Hb) and clinical improvement of signs and symptoms. A fresh set of blood samples (EDTA and clotted) were required for each and every transfusion requisition, irrespective of the time interval between two consecutive transfusions.
Results
Demographic distribution, clinical history, and history of alloimmunization in the study population
A total of 14,387 sets of patient's samples were accepted at the blood bank during 6 months period. Only 100 (0.69%) of these 14,387 were found to be cross-match incompatible and subjected to evaluation and selection of appropriate donor units [Figure 2]. The cross-match incompatibility was much higher in the females (59%) than the males (41%) [Figure 3]. An overall distribution of incompatibility ranges from 1 to 68 years of age, with a maximum incidence of 39% (n = 39) in the 11–20 years age group. A minimum incidence of 5% (n = 5) was observed in the persons above 50 years of age [Figure 4].
Figure 2.
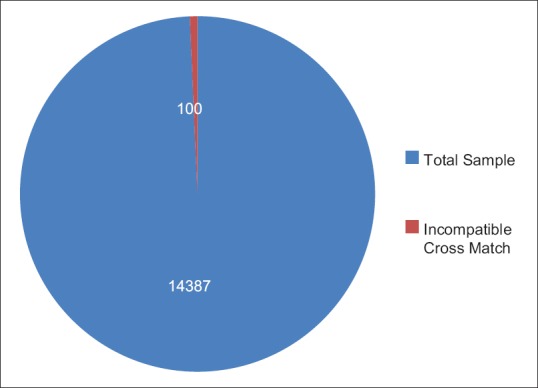
Total sample vs incompatible cross match
Figure 3.
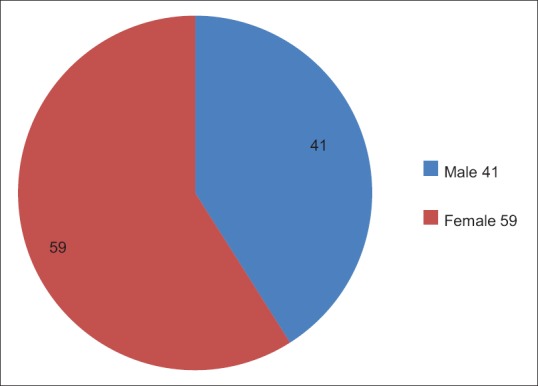
Gender distribution of the incompatible patients
Figure 4.
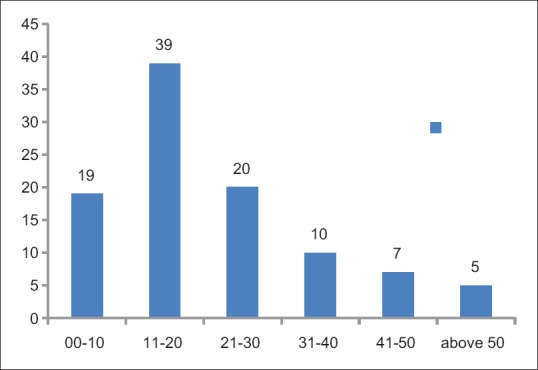
Overall age distribution of 100 incompatible patients
On an overall 14,387 red cell demands, majority were for anemia (n = 8925), surgical procedures (n = 3455), and obstetric cases (n = 1005). The rest of the 1002 belonged to other category which was requested for miscelleneous reasons, namely, acute hemorrhage, trauma, dialysis, etc., [Figure 5]. The majority population of anemic patients were suffering from thalassemia (n = 4115, 46%), hematological malignancies (n = 1865, 21%), autoimmune hemolytic anemia (AIHA) (n = 88, 1%), and other causes of anemia (n = 2857, 32%) [Figure 5a and b].
Figure 5.
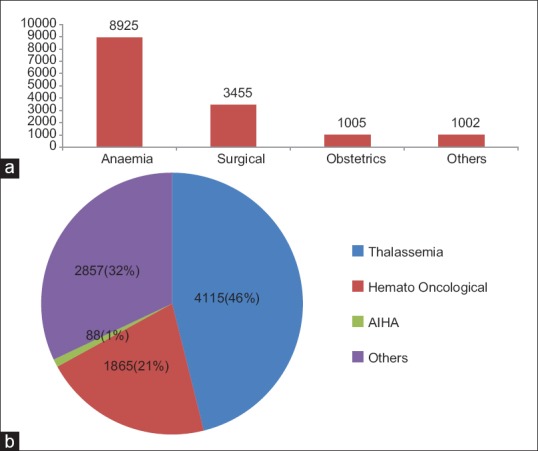
(a) Overall disease distribution in the study population (b) Further distribution of disease under Anemic population
Out of these 100 patients, 85% (n = 85) were repeatedly transfused. Thalassemia, hematological malignancies and autoimmune anemia (primary/secondary) constitute an overall 78% (n = 78) of the total burden of cross-match incompatible samples. Comparative details of the total study population versus the incompatibility results are shown in Figure 6.
Figure 6.
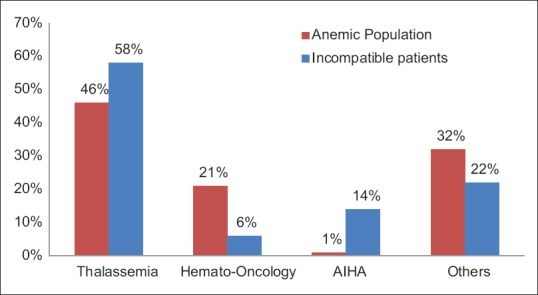
Comparative disease distribution among Anemic population and incompatible patients
Laboratory workup and immunohematology analysis of blood samples
DAT was positive in 53% (n = 53) incompatible samples and 44 of these were strongly positive (more than 2 in strength/ grade) [Figure 7]. Of these 44 strongly positive DAT samples, 21 weakened their strength on auto control.
Figure 7.
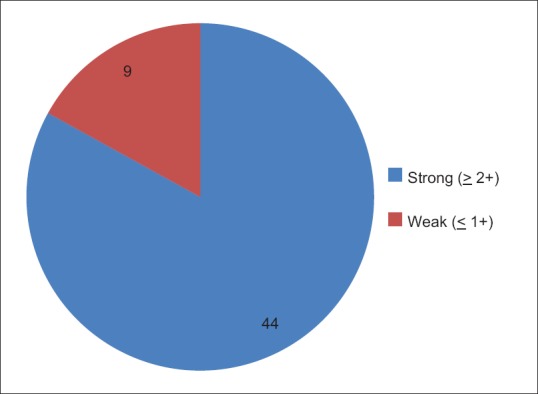
Distribution of DAT +ve samples
A total of 38 (38%) of the incompatible cross-match blood samples were positive on indirect anti-human globulin test (IAT)/antibody screen on CAT. The causative antibody could be identified in 16 of them, with an overall antibody identification rate of 42.10% on IAT/antibody screen positive samples. In the rest 22 of these 38 patients, the specific antibody identification could not be done with the available logistics. On the other hand, 17 of the 100 samples presented with DAT positive and panagglutination, where only blood group specific, best-matched or extended Rh and Kell phenotype-matched red cells were transfused [Figure 8a and b]. The complete analysis of the rest 45 patients could not be done as either they were lost to follow-up or the patient's blood sample was not received again.
Figure 8.
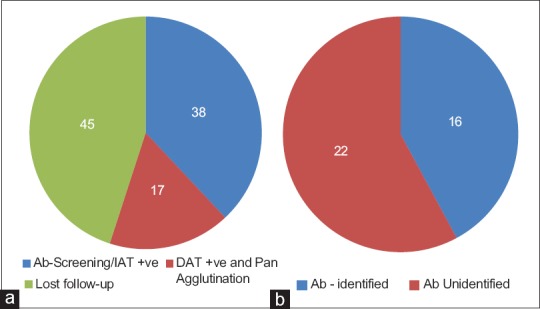
(a) Overall serological status of incompatible samples (n= 100) (b) Results of further evaluation of IAT positive samples (n= 38)
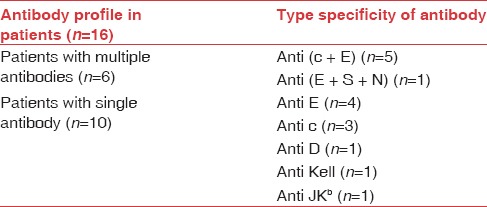
Out of the 16 patients where antibody detection could be done, 6 of them were multiple antibodies and 10 were single. An association of c, E antibodies was observed in 5 out of 6 patients with multiple alloantibodies. The other patient with multiple alloantibody was E, S, and N. The specificity of the alloantibody detected in 16 patients is given in Table 1. A total of 23 alloantibodies were identified in 16 patients. Majority of these antibodies identified were of the Rh system (19/23 [82.60%]) with anti-E being the most common antibody (10/23 [43.47%]).
Table 1.
Antibody profile in incompatible cross-match patients
It was also observed that 6 of these 17 patients (initially showing DAT positivity and panagglutination) who came for further follow-up after receiving best match/phenotype matched red cells transfusion along with steroid/rituximab therapy, recovered uneventfully with an appropriate rise in Hb level and became DAT negative after 3 months. An overall transfusion reaction was observed in 2 of these 17 patients (11.7%). There was no event of death due to adverse outcome.
Discussion
Incompatibility in cross-matching during pretransfusion testing is not uncommon. There is hardly any evidence-based study on frequency of incompatible cross-matched red cells and how to approach these cases for better transfusion practice from the eastern part of India till now.
In our study, we rechecked all the ABO and Rh (D) group specific incompatible cases with the same donor unit (along with two other separate donor units) to exclude clerical error, as clerical error is the most common cause of incompatibility as shown by Stainsby et al. in UK.[8] The incidence of persistent incompatible cases were 0.69%, whereas the study by Bhatt et al. in Western India showed an overall incidence of incompatibility were 0.21%.[9]
In the present study, majority of incompatible crossmatches were found in females (59%) which is comparable to the study conducted by Bhatt et al. in western part of India.[9] Incompatibility was most prevalent in the age group of 11–20 years (39%) and they were mostly thalassemic patient. A total of 58% of incompatible patients were thalassemics. The other important causes of incompatibility were AIHA (14%) and hematological malignancy (6%). This is in contrast to the study conducted by Bhatt et al. where peak incidence seen in AIHA (40%).[9] The present study had shown repeated red cell transfusion was the major factor associated with incompatible cases (85%).
On analysis of these incompatible blood samples, only 38 cases were found to be IAT/antibody screening positive. Among these 38 IAT/antibody screening positive cases alloantibody against red cell antigens was detected in 16 of them (42.1%), panagglutination (agglutination with all reagent cells) with DAT positivity was found in 17 patients (44.73%). A single alloantibody was detected in 10 patients (62.5%), and the rest 6 patients were having multiple alloantibodies (37.5%). A total of seven different types of alloantibodies were observed in these 16 patients [Table 1] having both single and multiple antibodies. Most of the alloantibody detected belonged to the Rh system (82.6%, 19 out of 23), of which anti-E (43.47%) was the most common followed by, Anti-c (34.78%), and anti-D (4.34%). This result is comparable to the study as observed by Goldfinger and Lu.[10]
Conclusion
This study showed the antibodies against Rh system antigens were the most common cause of incompatibility in multi-transfused patients. A significant number of incompatible cross-match were found due to AIHA, presented with positive DAT and panagglutination in antibody screening panel and were managed by best-matched red cells. The treating clinicians were informed about the type of AIHA (warm/cold/mixed) to start the definitive treatment.
A significant number of these AIHA patients were followed up for 3 months, and on follow-up, they showed clinical improvement following steroid/rituximab along with transfusion therapy.
Since the majority of alloantibodies are detected against the Rh system (82.6%), extended Rh phenotyping of the donor red cells and the recipients at the onset of initial transfusion may prevent the development of alloantibodies in the multitransfused patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge the continuous support and patronage from Prof. Krishnendu Mukherjee, Dr. Biswajit Halder, Dr. Sukanya Banerjee and Dr. Krishna Basu Choudhuri.
References
- 1.Petz LD. “Least incompatible” units for transfusion in autoimmune hemolytic anemia: Should we eliminate this meaningless term? A commentary for clinicians and transfusion medicine professionals. Transfusion. 2003;43:1503–7. doi: 10.1046/j.1537-2995.2003.00583.x. [DOI] [PubMed] [Google Scholar]
- 2.Saran RK. Transfusion Medicine Technical Manual. 2nd ed. New Delhi: Directorate General of Health and Family Welfare Government of India; 2003. pp. 117–8. [Google Scholar]
- 3.Datta SS, Mukherjee S, Talukder B, Bhattacharya P, Mukherjee K. Frequency of red cell alloimmunization in thalassemia patient: A report from Eastern India. Adv Hematol. 2015:1–6. doi: 10.1155/2015/610931. Doi:101155/2015/61093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung MK. Technical Manual. 18th ed. Bethesda, Maryland, USA: AABB; 2014. [Methods 2-2] [Google Scholar]
- 5.Fung MK. Technical Manual. 18th ed. Bethesda, Maryland, USA: AABB; 2014. p. 376. [Google Scholar]
- 6.Plapp FV, Beck ML. Transfusion support in the management of immune haemolytic disorders. Clin Haematol. 1984;13:167–83. [PubMed] [Google Scholar]
- 7.Ness PM, Shirey RS, Thoman SK, Buck SA. The differentiation of delayed serologic and delayed hemolytic transfusion reactions: Incidence, long-term serologic findings, and clinical significance. Transfusion. 1990;30:688–93. doi: 10.1046/j.1537-2995.1990.30891020325.x. [DOI] [PubMed] [Google Scholar]
- 8.Stainsby D, Russell J, Cohen H, Lilleyman J. Reducing adverse events in blood transfusion. Br J Haematol. 2005;131:8–12. doi: 10.1111/j.1365-2141.2005.05702.x. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt JK, Patel TR, Gajjar MD, Solanki MV, Bhatnagar NM, Shah SD. Evaluation of incompatible crossmatch at blood bank of a tertiary care teaching hospital in Western India. Pathol Lab Med. 2016;7(1) Available from: www.openventio.org . [Google Scholar]
- 10.Goldfinger D, Lu Q. The incompatible crossmatch. 2013. [Last accessed on 2016 May 30]. Available from: www.uptodate.com .



