Abstract
BACKGROUND:
Conventional coagulation screening tests such as Prothrombin time, International normalized ratio (INR) and activated partial thromboplastin time are often used to predict bleeding in various clinical situations. We aimed to observe the effect of Fresh-frozen plasma (FFP) on these parameters.
Methods:
Patients' demographics, pre- and post-transfusion coagulation parameters were noted to assess the level of correction. The magnitude of improvement in INR was determined using the formula given by Holland and Brooks. Data was analyzed using IBM SPSS Statistics 20.
RESULTS:
Among 2082 episodes, 4991 units of FFP were transfused at an average of 5 units per patient. Median dose of FFP administered per episode was 10 mL/kg (5.8–13.4). The mean change in INR following transfusion was 8.9% of the pre-transfusion INR and thus considered to be statistically significant.
CONCLUSION:
FFP transfusions as a prophylactic measure especially in patients with mildly deranged conventional coagulation screening tests without any empirical evidence of clinical bleeding needs further scrutiny. Reduction in INR following FFP transfusions was better in cohort having higher pre-transfusion INR value (> 3.0).
Keywords: Blood components, fresh frozen plasma, hemostasis, international normalized ratio, prophylactic transfusion therapy
Introduction
Fresh frozen plasma (FFP) is most often used for transfusion purposes with the abnormality of coagulation screening tests, either therapeutically in the face of bleeding and acute disseminated intravascular coagulation or prophylactically in nonbleeding patients before an invasive procedure. Little evidence exists to inform the best therapeutic transfusion practices, and most studies describe its usage in a prophylactic setting.[1] Conventional coagulation screening parameters, mostly the prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (APTT), and platelet count, are most often considered to be predictive risk factors for bleeding before any invasive procedure or in clinical situations.
Segal and Dzik have suggested that the suboptimal FFP orders occur because of three assumptions, primarily that the elevation of PT or INR will predict bleeding in the setting of an invasive procedure; second, prophylactic usage of FFP will correct the prolonged coagulation screening test results; and finally, fewer bleeding events will occur with its usage.[2] Conventionally, studies have shown that the elevation in coagulation screening tests is generally poor predictor for the risk of bleeding.[3] Thus, the use of FFP prophylactically in such a scenario is not just questionable but often unjustifiable too. Our institution is a 2032-bedded multispecialty tertiary care center. Considering the increasing demand and utilization of FFP, we decided to conduct an analysis on its use with the primary aim to observe the effect of FFP transfusion on the conventional coagulation screening tests as well as adjudicated improvement in the clinical status of patients (based on the clinical judgment of treating physicians).
Subjects and Methods
We conducted a prospective, observational study for the transfusion of FFP requests from December 2012 to September 2013. The patients who were admitted to the hospital and received FFP either with or without the receipt of other blood components were included in the study. The requests for therapeutic plasma exchange were excluded from the study. Likewise for analysis, only that cohort of patients was included who had both pre- and post-transfusion laboratory test results available. The study protocol was approved by the hospital ethics committee before its commencement (IEC 339/2012). The study was conducted in accordance with the Principles of Good Clinical Practices.
Study design
Coagulopathy was defined as a state affecting the coagulability of the blood and specifically causing a tendency to bleed with elevated coagulation screening test results, such as PT (reference range, 12–16 s); INR (>1.5 times above the control were considered elevated), and APTT (reference range, 26.0–36.0 s). Patient's coagulation screening test results within 12 h preceding the requests and posttransfusion screening test results up to 24 h were included for the assessment of the level of corrections. Patients' demographic details such as age, gender, bodyweight, location, and clinical indications for transfusion were noted. Based on indications, they were further classified into groups of “coagulopathy with bleeding” and “coagulopathy without bleeding” (elevated coagulation parameters with no signs of bleed). Data was collected during each transfusion episode (An episode was defined as each time we issued one or more therapeutic units of FFP to the recipients).
Dosage of fresh frozen plasma
During each episode of transfusion, dose of FFP given to the patients based on their bodyweight was observed. For transfusions, a dose of 10–15 ml/kg was considered as a standard dose, in alignment with the British Committee for Standards in Hematology [BCSH] recommendations.[4] Total number of FFP bags transfused and the units per request based on body weight of the patients were noted.
Conventional coagulation screening tests and efficacy
The conventional coagulation screening test results such as PT, INR, and APTT were observed throughout the study population. Based on pretransfusion INR, patients were categorized into three cohorts, namely, Cohort I with no elevation of INR (<1.5), Cohort II with moderate elevation of INR (1.5–3), and Cohort III with severe elevation of INR (>3). The magnitude of correction in INR per unit of FFP against the pretransfusion INR was also calculated. The number of patients who showed a significant correction in their INR was determined using the formula by Holland and Brooks.[5] According to this formula, a decrease of 8.9% or more in a pretransfusion INR per unit of FFP was considered as statistically significant. Any improvement observed in the laboratory parameters such as PT, INR, and APTT were defined as efficacy due to transfusion.
Statistical analysis
Data were analyzed using SPSS software version 20 (IBM Inc., Armonk, NY, USA). Simple descriptive data were expressed as median (interquartile range) and quantitative data were expressed as percentage. Two-tailed t-test for paired data was used to compare coagulation test values before and after transfusion, and Student's t-test was used to calculate the relative confidence intervals. P < 0.05 was considered as statistically significant. For correlation of analysis between two quantitative variables, Pearson's correlation coefficient (P) was used. Values between −0.3 and +0.3 were considered as no correlation.
Results
Patient's demographics
In 2082 episodes, 4991 units of FFP were utilized in 998 patients with an average of five bags per patient. Majority of the recipients were >18 years (82%). Sixty-six percent were males (n = 659/998). The median weight in kg (interquartile range) among the infants, adolescents, and adults were 2.79 (0.5–5.1), 16 (10–29) and 57.0 (51.5–61), respectively. Majority recipients were O blood group (36%). Location-wise FFP was primarily utilized in the operation theaters (33%).
Dosage of plasma administered
Median dose of FFP administered per episode was 10 ml/kg (5.8–13.4). Overall, a median volume of 456.2 ml (17–2800) was administered per episode. Infants received twice the adult dose (15 ml/kg). Ideal dosing of FFP according to the body weight (10–15 ml/kg) was observed in 52% patients (n = 523/998). Those with lower dose required more episodes of transfusion than those who received higher dosage (2.3 vs. 1.9 events). Overdosage was seen in 4% patients (n = 35). With respect to pretransfusion INR, median dosage of FFP given was higher in Cohort III (10.9 ml/kg) against Cohort I (9.9 ml/kg). This difference was statistically significant among both the cohorts (P = 0.008).
Conventional coagulation screening tests and efficacy
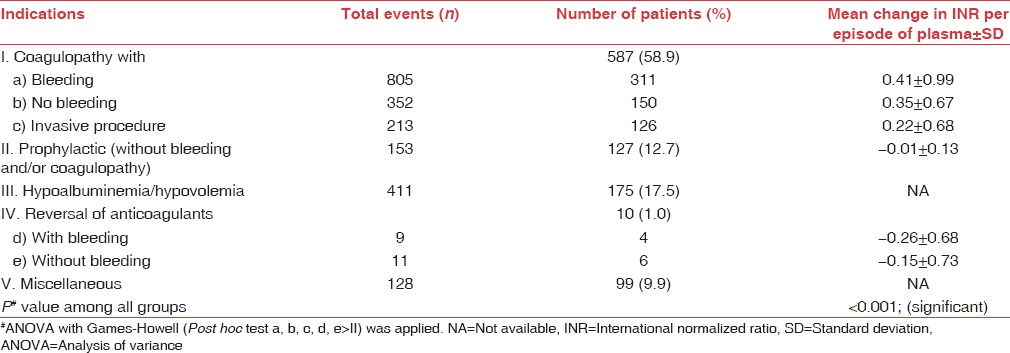
The pretransfusion laboratory values were available in only 73% (n = 724/998) recipients. Overall, the mean change in PT (pre–post) and APTT (pre–post) observed was 36.4–27.8 s and 54.2–45.8 s, respectively. The mean reduction in INR (pre minus post) per episode of FFP transfusion was maximum for bleeding episodes (0.41) against the episodes of coagulopathy without bleeding (0.34). ANOVA with Games-Howell Test was used for comparing mean reduction in INR among various indications. In the regression model, keeping the number of units as a dependent variable, Pearson's positive correlation was found among these seven categories with strongest association found between bleeding and others (P < 0.001) [Table 1].
Table 1.
Mean change in international normalized ratio per episode of fresh frozen plasma transfusion in various indications
A linear relationship was observed between pretransfusion INR and the reduction in INR per 200 ml unit of FFP for every episode. The regression analysis yielded statistically significant difference between the slope and the intercept of the equations. Both mean value change in INR (0.23 ± 0.69) and mean value of pretransfusion INR (2.68 ± 2.09) had a Pearson's correlation (R2 linear = 0.325) [Figure 1]. On applying the Holland and Brooks' formula to our data, the observed change in INR (mean = 0.23) was equal to 8.9% of pretransfusion INR (mean = 2.68). We could thus define this reduction in INR as statistically significant. The mean reduction in INR across Cohort I, II, and III was 0.18 (±0.68), 0.28 (±0.69), and 0.92 (±0.48), respectively. These three cohorts were compared using one-way ANOVA, and the difference was found to be statistically significant (P = 0.000). The laboratory efficacy after FFP transfusions was seen in 73% (n = 527/724) recipients [Figure 2]. Further adjudicated improvement in clinical status of patients was higher among Cohort III (87.5% [n = 371/424]) against Cohort I (31.4% [n = 40/127]) recipients (P = 0.027).
Figure 1.

Effects of plasma on changes in international normalized ratio per unit of plasma transfused
Figure 2.
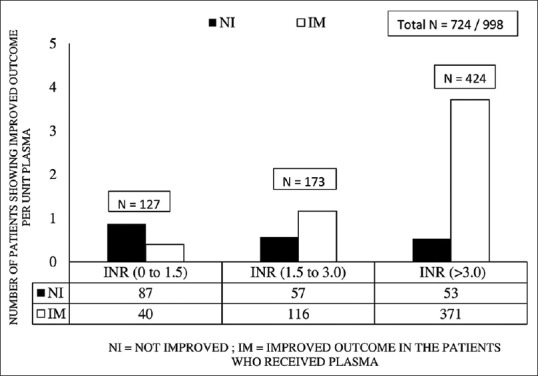
Therapeutic efficacy after plasma transfusion in different INR cohorts
Discussion
FFP is a frequently prescribed blood component and multiple loop holes exist in its ordering as well as optimal utilization. Given the understanding, overall hemostasis depends on the complex interplay among endothelium, platelets, other inflammatory cells, fibrinolysis, and inhibitors as well as pro-coagulant factors; it is not surprising perhaps that an abnormality in one component can derange coagulation screening tests, therefore making them quite an insensitive marker of clinical hemostasis.[1] Many laboratories report conventional coagulation screening tests which helps physicians to base their clinical decisions of transfusing FFP above a certain threshold typically 1.5 times the control. Both PT and APTT results are dependent on reagent and laboratory quality controls and processes and can be prolonged for various reasons not associated with a bleeding risk.[6] For instance, both APTT and PT depend on phospholipids, and the presence of anti-phospholipid antibodies (lupus anti-coagulant) can result in prolonged APTT more than PT. Another example is factor XII deficiency, which is associated with a prolonged APTT but conveys no additional bleeding risk.[7] In our study, the mean reduction in INR per unit of FFP was higher in Cohort III (0.92) against Cohort I (0.18), thereby implying that significant reductions in INR were more when pretransfusion INR was higher. This is in accordance to a Korean study that showed an average decrease in INR was clinically insignificant being 0.03 per unit of FFP transfused for a pretransfusion INR of 1.37.[8] Literature suggests that family history and personal history of clinical bleeding have a direct positive correlation toward the tendency to bleed. Therefore, dependence merely upon conventional tests alone when pretransfusion INR is mildly elevated without any empirical evidence of clinical bleeding is quite unreasonable to decide on prophylactic FFP transfusions. In this study, INR values >1.5 times above the control were considered to be elevated. Based on literature, it is assumed that a reduction in the posttransfusion INR value of >1.5 is representative of an assurance of optimal treatment after FFP transfusions. One more criterion of transfusion efficacy, regardless of the correction in posttransfusion INR values, is the cessation of bleeding; however, clinical assessment of the bleeding could not be performed, thereby adding to the limitation of this study.
According to guidelines, generally, the cited dose of FFP required to reverse a coagulopathy is 10–15 ml/kg. When INR value starts to exceed >1.5 value, factor levels begin to drop below 30%, which for many become a threshold for deranged hemostasis.[8] A small Welsh study extensively evaluated the laboratory parameters of hemostasis including the factor levels, PT and APTT, in ICU patients before and after receiving a median dose of either 12.2 ml/kg (n = 10) or 33.5 ml/kg (n = 22) of plasma. The patients who received the lower dosage failed to achieve the target level of coagulation factor replacement.[9] It should however be noted that, during our study, the mean volume of one unit of FFP was arbitrarily taken as 200 (±10%) ml based on the quality control assessment of blood components conducted in our department on a weekly basis. This could in real instance vary due to biological differences in the hematocrit of various donors and the amount of blood collected 450 ml (±10%) or 350 ml (±10%). In latter instances, amount of FFP present in one unit was generally lower than the former. Further, in a clinical setting, it may not always be possible to weigh a patient before transfusion. Majority of these situations were in an emergency setting. In addition, some requisition forms had documentation of weight in a descriptive manner such as “well built” or “obese.” During data analysis, we included only such cases whose bodyweight was documented (n = 998/1024). FFP is often transfused to nonbleeding patients to correct abnormal coagulation tests in an assumption that it will limit the risk of clinical bleeding. However, a large proportion of the study cohort (n = 175/998) received transfusions for the indication of hypoalbuminemia and/or hypovolemia which were considered inappropriate based upon the BCSH guidelines.[4] The primary reason for usage of FFP in such cases was poor affordability of albumin by the patients. Hence, we selectively excluded this cohort of recipients during the data analysis.
Segal and Dzik further evaluated the patients undergoing invasive procedures with normal and abnormal PT/INR. On conducting 24 observational studies and one clinical trial, they concluded no statistical significance in the association of raised coagulation parameters and risk of bleeding in patients undergoing kidney biopsy, liver biopsy, central vein cannulation, and others.[2] Abdel-Wahab et al. studied plasma recipients from a wide variety of hospital wards. In their retrospective study, 324 FFP units were given to 121 patients who had relatively lower pretransfusion INRs, 1.1–1.85. Very small median reductions in posttransfusion PT and INR were seen; 0.20 s and 0.07, respectively. Those with higher INRs (1.5–1.85) were more likely to correct their coagulation parameters in comparison to those with lower INRs (1.1–1.5).[10] Cheng and Sadek from Canada also found a minimal INR response when mildly coagulopathy patients were transfused with small quantities of FFP.[11] Similar to these studies the Cohort III recipients (INR >3.0) showed significant improvement in laboratory parameters on receiving FFP. However, one of the major limitations in this study was that we could not account for the change in laboratory parameters due to the use of other blood products and/or the dilutional effect of crystalloids used during massive trauma resuscitations.
Conclusion
FFP transfusions, as a prophylactic measure, especially in patients with mildly deranged conventional coagulation screening test results (INR <1.5) without any empirical evidence of clinical bleeding, need further scrutiny. Further, reduction in INR was observed more with higher values of pretransfusion INR (>3.0).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Stanworth SJ. The evidence-based use of FFP and cryoprecipitate for abnormalities of coagulation tests and clinical coagulopathy. Hematology (Am Soc Hematol Educ Program). 2007. 2007;1:179–86. doi: 10.1182/asheducation-2007.1.179. [DOI] [PubMed] [Google Scholar]
- 2.Segal JB, Dzik WH. Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: An evidence-based review. Transfusion. 2005;45:1413–25. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Chee YL, Greaves M. Role of coagulation testing in predicting bleeding risk. Hematol J. 2003;4:373–8. doi: 10.1038/sj.thj.6200306. [DOI] [PubMed] [Google Scholar]
- 4.O'Shaughnessy DF, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, Yates S, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 5.Holland LL, Brooks JP. Toward rational fresh frozen plasma transfusion: The effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006;126:133–9. doi: 10.1309/NQXH-UG7H-ND78-LFFK. [DOI] [PubMed] [Google Scholar]
- 6.Burns ER, Goldberg SN, Wenz B. Paradoxic effect of multiple mild coagulation factor deficiencies on the prothrombin time and activated partial thromboplastin time. Am J Clin Pathol. 1993;100:94–8. doi: 10.1093/ajcp/100.2.94. [DOI] [PubMed] [Google Scholar]
- 7.Desborough MJ, Stanworth SJ. Uses and abuses of fresh-frozen plasma for the prophylaxis of bleeding. Clin Med (Lond) 2013;13:197–9. doi: 10.7861/clinmedicine.13-2-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazer MH. The how's and why's of evidence based plasma therapy. Korean J Hematol. 2010;45:152–7. doi: 10.5045/kjh.2010.45.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdary P, Saayman AG, Paulus U, Findlay GP, Collins PW. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004;125:69–73. doi: 10.1111/j.1365-2141.2004.04868.x. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006;46:1279–85. doi: 10.1111/j.1537-2995.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CK, Sadek I. Fresh-frozen plasma transfusion in patients with mild coagulation abnormalities at a large Canadian transfusion center. Transfusion. 2007;47:748. doi: 10.1111/j.1537-2995.2007.01180.x. [DOI] [PubMed] [Google Scholar]


