Abstract
The objective of this study was to investigate the effects of supplementation with free myo-inositol (MI) or graded levels of phytase on inositol phosphate (InsP) degradation, concentrations of MI in the digestive tract and blood, bone mineralization, and prececal digestibility of amino acids (AA). Ross 308 broiler hatchlings were allocated to 40 pens with 11 birds each and assigned to one of 5 treatments. The birds were fed a starter diet until d 11 and a grower diet from d 11 to d 22. All diets were based on wheat, soybean meal, and corn. Birds were fed a control diet, calculated to contain adequate levels of all nutrients without (C) or with MI supplementation (C+MI), or one of 3 experimental diets that differed in phytase level (modified E. coli-derived 6-phytase; Phy500, Phy1500, or Phy3000 FTU/kg), with P and Ca levels adapted to the recommendations of the phytase supplier for a phytase level of 500 FTU/kg. The gain:feed ratio (G:F) was increased by MI or phytase in the starter+grower phase by 0.02 g/g. Prececal P and Ca digestibility, P and Ca concentration in blood serum, and tibia ash weight did not differ among treatments (P > 0.05). MI supplementation led to the highest MI concentration in the crop, ileum, and blood plasma across treatments. Phytase supplementation increased MI concentrations in the crop and ileum digesta in a dose-dependent manner and in plasma without any dose effect (P > 0.05). Prececal digestibility of some AA was increased by phytase. These outcomes indicate that MI might have been a relevant cause for the increase in G:F. Therefore, it is likely that the release of MI after complete dephosphorylation of phytate is one of the beneficial effects of phytase, along with the release of P and improvement in digestibility of other nutrients. Simultaneously, MI seems to have no diminishing effects on InsP degradation.
Keywords: myo-inositol, inositol phosphate, phytate, phytase, amino acid
INTRODUCTION
Phosphorus is predominantly bound as phytic acid (myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate); InsP6) and its salt, called phytate, in plant seeds (Eeckhout and de Paepe, 1994; Rodehutscord et al., 2016). In this form, it is only partially available for non-ruminant animals. Stepwise cleavage of the P groups from the phytate molecule by phytases (myo-inositol hexaphosphate phosphohydrolases) or phosphatases is required to make the P available. Along with their P-releasing effect, phytases also increased protein, energy, and trace mineral utilization in poultry by diminishing complexes between phytate and other nutrients (Selle and Ravindran, 2007). However, these effects were not consistent among studies.
After the theoretical complete dephosphorylation of InsP6, 6 phosphate groups and myo-inositol (MI) are potentially available for absorption. Until recently, it was suggested that non-ruminants may not be able to fully degrade InsP6 because 3- and 6-phytases seem to be incapable of splitting off the phosphate group in the axial position from C-atom 2 on the MI molecule (Wyss et al., 1999; Selle and Ravindran, 2007). In recent studies (Beeson et al., 2017; Sommerfeld et al., 2018), however, phytase supplementation to P-deficient and adequate diets led to higher MI concentrations in the digesta of the gizzard and ileum and the excreta of broiler chickens, and similar effects have been shown in pigs (Kühn et al., 2016). These results are a strong indication for the potential for complete InsP6 degradation with subsequent MI release in the digestive tract of broiler chickens. MI is suggested to have several biological functions, e.g., playing a role in cell survival and growth, lipid metabolism, and insulin sensitivity (Huber, 2016). Recent studies assume an effect of MI on growth performance when it is added in its free form to the diet or released as a result of InsP6 breakdown (Zyla et al., 2013; Walk et al., 2014; Cowieson et al., 2015). However, documentation of the effects of free or phytate-released MI in poultry is scarce and often limited to a few measured traits.
Consequently, the first objective was to investigate whether supplementation with free MI results in similar responses to those of MI freed by phytase by complete InsP degradation on concentrations of MI in the digestive tract and blood plasma and performance of broiler chickens.
As P is an end product of InsP6 degradation and is known for its inhibiting effect on phytases (Greiner et al., 1993; Sommerfeld et al., 2018), one might assume the same inhibitory effect of MI on inositol phosphate (InsP) degradation. Thus, the second objective was to investigate whether MI affects the degradation of InsPs. In addition, the possible effects of MI on prececal P, Ca, and amino acid (AA) digestibility and bone mineralization were investigated.
MATERIALS AND METHODS
Birds and Housing
The trial was performed at the Agricultural Experiment Station of the University of Hohenheim and approved by the Regierungspräsidium Tübingen, Germany (Project no. HOH 41/16 TE) in accordance with the German Animal Welfare Legislation. A total of 440 unsexed Ross 308 broiler hatchlings was supplied by a commercial hatchery (Brüterei Süd GmbH & Co. KG, Regenstauf, Germany) and allocated to 40 floor pens (115 × 230 cm ground area, 260 cm height) on deep litter bedding, each holding 11 hatchlings. Eight pens were randomly allocated to each of the 5 treatments in a completely randomized block design. The animals were fed a starter diet until d 11 and a grower diet from d 11 to d 22. Feed and tap water were provided for ad libitum consumption from placement to the end of the trial. From d 14 until the end, the animals were kept on perforated floors. The lighting program was as follows: 24L:0D from hatch to d 3, 22L:2D from d 4 to 7, and 15L:9D from d 8 until the end. The temperature in the barn was set at 34°C on the first d and then gradually decreased every 3 to 5 d to achieve a temperature of 26°C on the last 3 days. The well-being of the animals was checked twice daily, and the occurrence and weight of dead animals as well as the average pen feed consumption up to that day were recorded.
Diets and Treatments
All diets were based on wheat, soybean meal, and corn (Table 1). The control diet (C) was calculated to contain adequate levels of all nutrients according to the Nutrition Specification for ROSS 308 broilers (Aviagen, 2014). All grower diets contained 5 g/kg TiO2 as an indigestible marker. The experimental diets included 3 phytase levels (E.coli-derived, modified 6-phytase; Quantum Blue®, AB Vista, UK; Phy500, Phy1500, and Phy3000 FTU/kg feed) with P, Ca, and Na levels reduced as recommended by the phytase supplier for a level of 500 FTU/kg feed (1.5 g available P, 1.65 g Ca, and 0.3 g Na/kg feed). The fifth diet was the control diet, supplemented with 3.8 (starter) and 3.5 (grower) g/kg MI (C+MI). The C and C+MI diets were produced by first mixing one basal diet that was split and then supplemented with either sand or a sand and MI mixture. A similar procedure was followed for the phytase experimental diets. One basal diet was mixed, split into 3 parts, and then supplemented with individual phytase/sand mixtures to obtain the Phy500, Phy1500, and Phy3000 diets. The pelleting temperature remained below 80°C. Representative samples of each diet were ground through a 0.5 mm sieve or pulverized by a vibrating cup mill (PULVERISETTE 9, Fritsch GmbH, Idar-Oberstein, Germany), based on the analyses described in the Chemical Analysis section. Intended concentrations of Ca, P, and phytase activity were confirmed by analysis of the diets (Table 2).
Table 1.
Ingredients and calculated composition of the experimental diets.
| Starter | Grower | |||||
|---|---|---|---|---|---|---|
| Ingredient, g/kg | C | C+MI | Phy1 | C | C+MI | Phy1 |
| Wheat | 460.0 | 460.0 | 476.8 | 500.5 | 500.5 | 517.4 |
| Extracted soybean meal | 361.7 | 361.7 | 359.3 | 308.1 | 308.1 | 305.8 |
| Corn | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Soybean oil | 29.2 | 29.2 | 23.8 | 40.5 | 40.5 | 35.2 |
| Monocalcium phosphate | 16.1 | 16.1 | 9.1 | 14.4 | 14.4 | 7.5 |
| Limestone | 10.8 | 10.8 | 9.6 | 9.5 | 9.5 | 8.3 |
| Sand | 5.0 | 1.2 | 5.0/4.8/4.5 | 5.0 | 1.5 | 5.0/4.8/4.5 |
| Vitamin+mineral premix2 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Sodium bicarbonate | 2.2 | 2.2 | 1.1 | 2.2 | 2.2 | 1.1 |
| Sodium chloride | 1.9 | 1.9 | 1.9 | 2.0 | 2.0 | 2.0 |
| Choline chloride | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| DL-Methionine | 3.3 | 3.3 | 3.2 | 3.0 | 3.0 | 3.0 |
| L-Lysine | 2.7 | 2.7 | 2.8 | 2.7 | 2.7 | 2.7 |
| L-Threonine | 1.4 | 1.4 | 1.4 | 1.2 | 1.2 | 1.2 |
| L-Isoleucine | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 |
| L-Valine | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Carbohydrase | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Coccidiostat3 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| TiO2 | - | - | - | 5.0 | 5.0 | 5.0 |
| Myo-inositol | - | 3.8 | - | - | 3.5 | - |
| Phytase | - | - | 0.1/0.3/0.6 | - | - | 0.1/0.3/0.6 |
| Calculated composition, g/kg | ||||||
| AMEn, kcal/kg | 3000 | 3000 | 3000 | 3100 | 3100 | 3100 |
| Crude Protein | 235 | 235 | 235 | 214 | 214 | 215 |
| Na | 1.6 | 1.6 | 1.3 | 1.6 | 1.6 | 1.3 |
| Ca | 9.6 | 9.6 | 8.0 | 8.7 | 8.7 | 7.1 |
| Total P | 7.6 | 7.6 | 6.0 | 6.9 | 6.9 | 5.4 |
| InsP6-P | 2.6 | 2.6 | 2.6 | 2.4 | 2.4 | 2.4 |
1Includes treatments Phy500, Phy1500, and Phy3000.
2Vitamin+Mineral Premix Starter (Target Feeds Limited, Shropshire, UK), provided per kg of complete diet: 13,000 IU vitamin A, 5000 IU vitamin D3, 80 IU vitamin E, 3.2 mg vitamin B1, 8.6 mg vitamin B2, 5.4 mg vitamin B6, 0.02 mg vitamin B12, 60 mg nicotinic acid, 15 mg pantothenic acid, 2.2 mg folic acid, 0.3 mg biotin, 250 mg cholinechlorid, 20 mg iron, 120 mg manganese, 9.6 mg copper, 99 mg zinc, 1.25 mg iodine, 0.3 mg selenium, 0.5 mg molybdenum.
2Vitamin+Mineral Premix Grower (Target Feeds Limited, Shropshire, UK), provided per kg of complete diet: 10,000 IU vitamin A, 4500 IU vitamin D3, 65 mg vitamin E, 2.5 mg vitamin B1, 6.5 mg vitamin B2, 3.2 mg vitamin B6, 0.02 mg vitamin B12, 40 mg nicotinic acid, 15 mg pantothenic acid, 1.5 mg folic acid, 0.25 mg biotin, 250 mg cholinechlorid, 20 mg iron, 120 mg manganese, 9.6 mg copper, 99 mg zinc, 1.25 mg iodine, 0.3 mg selenium, 0.5 mg molybdenum.
3Narasin and Nicarbazin 1:1.
Table 2.
Analyzed composition of the experimental grower diets.
| C | C+MI | Phy500 | Phy1500 | Phy3000 | |
|---|---|---|---|---|---|
| Ca, g/kg DM | 9.5 | 10.1 | 7.9 | 7.8 | 7.7 |
| Total P, g/kg DM | 7.5 | 8.0 | 6.1 | 5.9 | 5.9 |
| InsP6-P, g/kg DM | 2.1 | 2.2 | 2.2 | 2.1 | 2.2 |
| MI, μmol/g DM | 1.0 | 14.5 | 1.0 | 1.0 | 2.0 |
| Ins(1,2,3,4,6)P51, μmol/g DM | 0.3 | 0.3 | 0.4 | 0.3 | 0.3 |
| Ins(1,2,3,4,5)P51, μmol/g DM | 0.4 | 0.4 | 0.3 | 0.4 | 0.4 |
| Ins(1,2,4,5,6)P51, μmol/g DM | 0.6 | 0.7 | 0.5 | 0.5 | 0.6 |
| InsP6, μmol/g DM | 11.6 | 11.9 | 11.8 | 11.6 | 12.0 |
| Phytase Activity, FTU/kg | <50 | ∼85 | 361 | 1870 | 3110 |
| Arg, g/kg DM | 14.7 | 15.0 | 15.0 | 14.8 | 14.9 |
| His2, g/kg DM | 6.5 | 6.5 | 6.8 | 6.5 | 6.6 |
| Ile, g/kg DM | 8.9 | 9.1 | 9.2 | 9.1 | 8.7 |
| Leu, g/kg DM | 17.3 | 17.4 | 17.6 | 17.4 | 17.2 |
| Met, g/kg DM | 6.3 | 6.5 | 6.5 | 6.5 | 6.5 |
| Phe2, g/kg DM | 11.3 | 11.5 | 11.4 | 11.4 | 11.3 |
| Thr, g/kg DM | 9.8 | 9.9 | 10.0 | 9.9 | 9.8 |
| Val, g/kg DM | 10.1 | 10.3 | 10.4 | 10.3 | 9.8 |
1No other InsP isomers were detected.
2The concentrations of histidine and phenylalanine may be affected to some extent by the oxidation procedure (Mason et al., 1980).
Sampling and Measurements
Animals and feed were weighed on d 1, d 11, and d 22 in order to determine ADFI and to calculate ADG and the gain:feed ratio (G:F) on a pen basis. In order to avoid time effects, to standardize feed intake, and to ensure the filling of all birds’ crops, the animals were deprived of feed for 1 h, starting 2 h before slaughter on d 22. The feeders were moved back into the pens 1 h before slaughter. One animal per pen was stunned with a gas mixture of 35% CO2, 35% N2, and 30% O2 and killed by decapitation, whereupon the trunk blood was collected in tubes. Tubes containing sodium fluoride and heparin were centrifuged for 10 min at 2,000 × g to separate the plasma. Tubes without chemical supplements were centrifuged for 10 min at 2,000 × g to separate the serum. All other broilers from the same pen were stunned with the gas mixture and euthanized by CO2 asphyxiation. Digesta from the crop and the terminal part of the ileum (last two-thirds of the section between Meckel's diverticulum and 2 cm prior to the ileo-ceco-colonic junction) of all animals from each pen were collected and pooled on a pen basis. The crop was clamped with an arterial clamp to prevent emptying and then upended to remove digesta gently with a spatula without scraping the mucosa. Digesta from the terminal ileum were rinsed with cold double-distilled water. All samples were immediately frozen at −20°C, freeze-dried, and pulverized by a vibrating cup mill. Pulverized samples were stored in airtight containers until further analysis at a temperature below 6°C.
Chemical Analysis
Ground feed samples were analyzed according to the official methods in Germany (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten, 2007) for DM (method no. 3.1).
Pulverized feed and digesta samples were analyzed for P, Ca, and Ti using the modified sulfuric and nitric acid wet digestion method of Boguhn et al. (2009) with subsequent measurement using inductively coupled plasma optical emission spectrometry, described in detail by Zeller et al. (2015a).
The extraction and measurement of InsP3–6 isomers in feed and digesta were carried out using the method of Zeller et al. (2015a) with slight modifications. Samples were extracted twice with a solution of 0.2 M EDTA and 0.1 M sodium fluoride (pH of 8; 4°C) for 30 min under agitation, and were then centrifuged after each extraction at 12,000 × g for 15 minutes. The respective supernatants were combined and a 1-mL sample was centrifuged at 14,000 × g for 15 min and filtered before being centrifuged again at 14,000 × g for 30 minutes. Filtrates were analyzed using high-performance ion chromatography and UV detection at 290 nm after a post-column reaction with Fe(NO3)3 in HClO4 using an ICS-3000 system (Dionex, Idstein, Germany). Some InsP3 isomers could not be identified because the specific standards were unavailable. A clear discrimination between isomers Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3 was not possible because of co-elution; therefore, the term InsP3x will be used for these InsP3 isomers with unknown proportions.
For analysis of MI, samples of feed and digesta were derivatized without sample cleanup. Proteins from plasma samples were precipitated by addition of acetonitrile, and samples were lyophilized prior to derivatization. A two-step derivatization procedure comprising oximation and silanization was carried out. Deuterated MI was used as an internal standard. MI was measured using an Agilent 5977A gas chromatograph/mass spectrometer (Waldbronn, Germany).
AA analysis was performed according to Rodehutscord et al. (2004). In brief, samples were oxidized in an ice bath using a mixture of hydrogen peroxide, phenolic formic acid solution, and phenol. Then, samples were hydrolyzed at 113°C for 24 h in a mixture containing hydrochloric acid and phenol. Norleucine was used as an external standard. AA were separated and detected in an L-8900 AA analyzing system (VWR/Hitachi Ltd, Tokyo, Japan). Methionine and cysteine were determined as methionine sulfone, and cysteic acid, respectively. The concentrations of tyrosine, histidine, and phenylalanine might be affected to some extent by the oxidation procedure (Mason et al., 1980).
Ca and inorganic P (Pi) in blood serum were analyzed at the IDEXX BioResearch Vet Med Labor GmbH (Ludwigsburg, Germany). Ca in blood serum was measured photometrically by the Arsenazo method in a Beckman Olympus AU480. Pi in blood serum was measured photometrically as phosphomolybdate complex in a Beckman Olympus AU480.
The right tibiotarsus (tibia) was removed after slaughter and stored at −20°C. Following defrosting of tibia, adhering soft tissues, cartilage caps, and fibula bones were manually removed. Bones were subsequently rinsed in distilled water and dried at 60°C for 24 h in a convection oven (VL 115, VWR International GmbH, Darmstadt, Germany). The tibia was dried for 48 h at 103°C, weighed, ashed in a muffle furnace (Nabertherm L 40/11/B170, Nabertherm GmbH, Bremen, Germany) at 600°C for 24 h, cooled in a desiccator, and weighed again. Tibia ash was determined for individual bones. We report tibia ash weight rather than ash concentration because ash weight was found to be more sensitive to dietary mineral supply than ash concentration (Shastak et al., 2012).
Feed samples were analyzed for phytase activity by Enzyme Services and Consultancy (Ystrad Mynach, UK) using the analytical method of the supplier (pH 4.5 and 60°C) followed by transfer of the result to commonly used FTU by a validated transfer factor.
Calculations and Statistical Analysis
ADG, ADFI, and G:F were calculated for the experimental periods from d 1 to d 11 (starter), d 11 to d 22 (grower), and d 1 to d 22 (starter+grower) on a pen basis and then divided by the number of remaining animals per pen. Dead animals were taken into account by calculation of their ADG and ADFI per days of life.
The disappearance of InsP6 was calculated based on the analyzed concentration of InsP6 and Ti in feed and digesta. The following generally accepted equation was used:
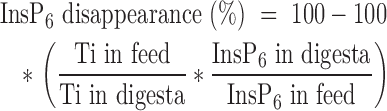 |
Where: Ti and InsP6 are in g/kg DM. The prececal digestibility of P, Ca, CP, and AA was calculated accordingly. A correction for endogenous losses was not applied.
Digested P and Ca (y) was calculated as follows:
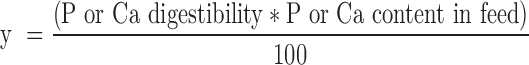 |
Where: y is in g/kg DM; P or Ca digestibility is in %; and P or Ca content in feed is in g/kg DM.
All data were analyzed in a one-factorial analysis of variance using the MIXED procedure of the software package SAS (version 9.3; SAS Institute Inc., Cary, NC). For all traits analyzed in this experiment except the blood and tibia, samples were pooled on a pen basis; thus, the pen was considered as the experimental unit. In the case of the blood and the tibia data that were obtained from individual birds, the bird was considered as the experimental unit. The following model was chosen: Yi = μ + αi + εi, where Yi = response variable, μ = overall mean, αi = effect of dietary treatment, and εi = residual error. Replicates (= blocks) were considered as random effects if significant. Correlations were calculated using the CORR procedure of SAS. Statistical significance was declared at P < 0.05.
RESULTS
Performance Traits
During the starter phase (d 1 to d 11), ADG was slightly increased with dietary supplementation of MI and 500 FTU/kg phytase, but not with 1,500 FTU/kg (Table 3). In treatment Phy3000, it was decreased by 1 g/d. During the grower (d 11 to d 22) and starter+grower phase (d 1 to d 22), no diet effect on ADG was found (P = 0.996 and P = 0.933, respectively). ADFI did not differ among treatments, independent of the growing phase (P = 0.195, P = 0.822, and P = 0.504 for starter, grower, and starter+grower, respectively). However, G:F was increased by MI and 500 FTU/kg phytase during the starter phase (P < 0.001). During the grower phase, G:F was increased by MI and 1,500 or 3,000 FTU/kg phytase (P = 0.037). G:F was increased during the starter+grower phase by MI and all phytase treatments (P = 0.005), but differences among phytase treatments and MI supplemented treatment were not significant.
Table 3.
Effect of phytase and myo-inositol supplementation on performance traits of broiler chickens.1
| Starter (d 1 to d 11) | Grower (d 11 to d 22) | Starter+Grower (d 1 to d 22) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ADG | ADFI | G:F | ADG | ADFI | G:F | ADG | ADFI | G:F | |
| Treatment | g/d | g/g | g/d | g/g | g/d | g/g | |||
| C | 26b | 30 | 0.88c,d | 77 | 96 | 0.80b | 53 | 65 | 0.82b |
| C+MI | 27a,b | 29 | 0.92a | 77 | 95 | 0.81a | 53 | 64 | 0.84a |
| Phy500 | 27a | 30 | 0.91a,b | 77 | 95 | 0.82a,b | 53 | 64 | 0.84a |
| Phy1500 | 26b | 29 | 0.90b,c | 78 | 94 | 0.82a | 53 | 63 | 0.84a |
| Phy3000 | 25c | 29 | 0.87d | 77 | 94 | 0.82a | 52 | 63 | 0.84a |
| Pooled SEM | 0.30 | 0.28 | 0.006 | 1.33 | 1.25 | 0.004 | 0.81 | 0.79 | 0.004 |
| P-value | <0.001 | 0.195 | <0.001 | 0.996 | 0.822 | 0.037 | 0.933 | 0.504 | 0.005 |
1Data are given as treatment means; n = 8 pens per treatment.
a–dMeans within a column not showing a common superscript differ (P < 0.05).
P and Ca in the Digesta of the Terminal Ileum and Blood and Tibia Ash Weight
No effect of MI supplementation on prececal Ca or P digestibility was found (Table 4). Phytase supplementation increased prececal Ca digestibility independent of the phytase level used (P < 0.05). Prececal P digestibility also was increased by all phytase levels, with 1,500 and 3,000 FTU having a greater effect than 500 FTU/kg (P < 0.05). However, these effects on Ca and P digestibility did not translate into differences in prececally digested Ca and P, serum Ca and Pi, or tibia ash weight among dietary treatments.
Table 4.
Effect of phytase and myo-inositol supplementation on prececal Ca and P digestibility up to the terminal ileum1, serum Ca and Pi2, and tibia ash weight3 of broiler chickens on d 22.
| Ca digestibility | P digestibility | Digested Ca | Digested P | Serum Ca | Serum Pi | Tibia ash weight | |
|---|---|---|---|---|---|---|---|
| Treatment | % | g/kg DM | mmol/l | mg | |||
| C | 45b | 60c | 4.3 | 4.6 | 2.98 | 3.48 | 1,201 |
| C+MI | 42b | 58c | 4.3 | 4.6 | 3.06 | 3.73 | 1,187 |
| Phy500 | 51a | 68b | 4.0 | 4.2 | 3.11 | 3.50 | 1,174 |
| Phy1500 | 53a | 75a | 4.2 | 4.5 | 3.04 | 3.75 | 1,171 |
| Phy3000 | 52a | 77a | 3.9 | 4.5 | 2.98 | 3.55 | 1,169 |
| Pooled SEM | 2.5 | 1.7 | 0.2 | 0.1 | 0.09 | 0.17 | 16.6 |
| P-value | <0.001 | <0.001 | 0.319 | 0.064 | 0.599 | 0.663 | 0.555 |
1Data are given as treatment means; n = 8 pens per treatment.
2Data are given as treatment means; n = 8 individuals per treatment.
3Data are given as treatment means; n = 88 individuals per treatment.
a–cMeans within a column not showing a common superscript differ (P < 0.05).
Myo-inositol in Digesta and Blood Plasma
The MI concentration in crop digesta was highest in MI-fed birds (Table 5). Dietary phytase supplementation also increased the MI concentration in the crop, with 3,000 FTU/kg causing a greater increase than 500 and 1,500 FTU/kg, which did not differ from each other. The highest MI concentration in ileum digesta was achieved with MI supplementation. Phytase supplementation increased the MI concentration in ileum digesta in a dose-dependent manner, but even the Phy3000 treatment did not match the C+MI treatment. Plasma MI concentration was highest in the C+MI treatment and also was increased by all phytase doses to a similar level.
Table 5.
Effect of phytase and myo-inositol supplementation on myo-inositol concentrations in crop and ileum digesta1 and in plasma2 of broiler chickens on d 22.
| Crop | Ileum | Plasma | |
|---|---|---|---|
| Treatment | μmol/g DM | mmol/l | |
| C | 1.18d | 3.30e | 0.23c |
| C+MI | 12.16a | 35.90a | 0.52a |
| Phy500 | 1.33c | 10.68d | 0.32b |
| Phy1500 | 1.39c | 18.25c | 0.36b |
| Phy3000 | 2.37b | 23.76b | 0.33b |
| Pooled SEM | 0.14 | 1.11 | 0.03 |
| P-value | <0.001 | <0.001 | <0.001 |
1Data are given as treatment means; n = 8 pens per treatment.
2Data are given as treatment means; n = 8 individuals per treatment.
a–eMeans within a column not showing a common superscript differ (P < 0.05).
InsP6 Disappearance and InsP Isomers in the Crop and Terminal Ileum
Upon feeding with the control diet, 30% of the InsP6 disappeared in the crop (Table 6). InsP6 disappearance was not affected by MI supplementation but increased with increasing phytase supplementation. An asymptote was achieved at Phy1500, resulting in effects similar to those of Phy3000. The concentrations of the InsP5 isomers and Ins(1,2,3,4)P4 were lower in the treatments with phytase addition as compared to treatments C and C+MI.
Table 6.
Effect of phytase and myo-inositol supplementation on InsP6 disappearance and concentration of InsP isomers in the crop digesta of broiler chickens on d 22.1
| InsP3x2 | Ins(1,2,3,4)P4 | Ins(1,2,5,6)P4 | Ins(1,2,3,4,6)P5 | Ins(1,2,3,4,5)P5 | Ins(1,2,4,5,6)P5 | InsP6 | InsP6 disappearance | |
|---|---|---|---|---|---|---|---|---|
| Treatment | μmol/g DM | % | ||||||
| C | 1.1b | 0.6a | 0.8c | 0.3a | 0.7a | 0.4a | 7.9a | 30c |
| C+MI | 1.4a | 0.6a | 1.1a,b | 0.4a | 0.8a | 0.4a | 7.9a | 31c |
| Phy500 | 1.5a | 0.3b | 1.2a | 0.2b | 0.4b | 0.2b | 6.2b | 47b |
| Phy1500 | 0.8c | n.d.3 | 1.0a,b | 0.2b | 0.2c | 0.2b,c | 5.1c | 56a |
| Phy3000 | 0.7c | n.d. | 0.9b,c | <LOQ4 | 0.2c | 0.1c | 5.1c | 58a |
| Pooled SEM | 0.08 | 0.03 | 0.08 | 0.03 | 0.03 | 0.03 | 0.54 | 4.5 |
| P-value | <0.001 | <0.001 | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
1Data are given as treatment means; n = 8 pens.
2At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
3n.d., not detectable in the majority of samples.
4<LOQ, not quantifiable in the majority of samples.
a–cMeans within a column not showing a common superscript differ (P < 0.05).
InsP6 disappearance up to the terminal ileum was 31 and 28% in treatments C and C+MI, respectively, and did not differ between these treatments (Table 7). InsP6 disappearance was significantly increased by supplementation with 500 FTU/kg, and even more so by the 1,500 and 3,000 FTU/kg feed treatments, which did not differ from one another. The concentrations of Ins(1,2,4,5,6)P5 and Ins(1,2,3,4,6)P5 did not differ between treatments C and C+MI and were decreased or not detectable in the phytase-supplemented treatments. Ins(1,2,3,4,5)P5 was significantly decreased in treatments Phy1500 and Phy3000. The concentrations of Ins(1,2,5,6)P4 (except for Phy3000) and InsP3x were significantly higher and the concentration of Ins(1,2,3,4)P4 significantly lower in the phytase treatments as compared to treatments C and C+MI.
Table 7.
Effect phytase and myo-inositol supplementation on concentration of InsP isomers in and InsP6 disappearance up to the terminal ileum of broiler chickens on d 22.1
| InsP3x2 | Ins(1,5,6)P3 | Ins(1,2,3,4)P4 | Ins(1,2,5,6)P4 | Ins(1,2,3,4,6)P5 | Ins(1,2,3,4,5)P5 | Ins(1,2,4,5,6)P5 | InsP6 | InsP6 disappearance | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment | μmol/g DM | % | |||||||
| C | 1.1b | n.d.3 | 1.0a,b | 2.2d | 0.7a | 3.3b | 1.4a | 30.0a | 31c |
| C+MI | 1.4b | n.d. | 1.2a | 2.8c,d | 0.7a | 3.9a | 1.6a | 30.1a | 28c |
| Phy500 | 2.3a | <LOQ4 | 0.9b | 5.2a | 0.2b | 3.7a,b | 1.1b | 13.4b | 70b |
| Phy1500 | 2.7a | n.d. | 0.2c | 4.2a,b | n.d. | 0.8c | 0.3c | 3.3c | 93a |
| Phy3000 | 2.5a | n.d. | n.d. | 3.8b,c | n.d. | 0.6c | 0.2c | 2.8c | 94a |
| Pooled SEM | 0.28 | - | 0.07 | 0.41 | 0.04 | 0.22 | 0.09 | 1.29 | 2.7 |
| P-value | <0.001 | - | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
1Data are given as treatment means; n = 8 pens.
2At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
3n.d., not detectable in the majority of samples.
4<LOQ, not quantifiable in the majority of samples.
a–dMeans within a column not showing a common superscript differ (P < 0.05).
Prececal Amino Acid Digestibility
The prececal digestibility of all AA was affected by dietary treatment (P < 0.05; Table 8 and Table 9). Dietary supplementation of MI either had no effect (His, Lys, Met, Thr, Val, Ala, Cys, Gly, Ser) or decreased prececal digestibility by 1 to 2 percentage points (Arg, Ile, Leu, Phe, Asp, Glu, Pro, Tyr). Dietary supplementation with 500 FTU/kg phytase had no effect on AA digestibility except in the case of Ile and Lys, which were increased by 1 and 2 percentage points, respectively. In treatment Phy1500, prececal digestibility of most AA was increased by 1 to 3 percentage points, with the exception of His, Thr, Cys, Gly, and Pro. Supplementation with 3,000 FTU/kg phytase increased the prececal digestibility of most AA by 1 to 3 percentage points, with the exception of His, Thr, Val, Cys, and Gly.
Table 8.
Effect of phytase and myo-inositol supplementation on prececal essential AA digestibility (%) in broiler chickens on d 22.1
| Treatment | Arg | His2 | Ile | Leu | Lys | Met | Phe2 | Thr | Val |
|---|---|---|---|---|---|---|---|---|---|
| C | 88b | 84a,b | 85c | 86c | 87b | 92b | 86b | 82a,b | 84b,c |
| C+MI | 87c | 83b | 84d | 84d | 87b | 92b | 85c | 81b | 83c |
| Phy500 | 89b | 85a | 86b | 86b,c | 89a | 93a,b | 87b | 82a | 85b |
| Phy1500 | 90a | 85a | 88a | 88a | 90a | 93a | 88a | 83a | 86a |
| Phy3000 | 90a | 85a | 87b | 87a,b | 90a | 93a | 88a | 83a | 85b |
| Pooled SEM | 0.31 | 0.44 | 0.32 | 0.35 | 0.30 | 0.20 | 0.35 | 0.43 | 0.35 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 |
1Data are given as treatment means; n = 8 pens.
2The concentrations of histidine and phenylalanine may be affected to some extent by the oxidation procedure (Mason et al., 1980).
a–dMeans within a column not showing a common superscript differ (P < 0.05).
Table 9.
Effect of phytase and myo-inositol supplementation on prececal non-essential AA digestibility (%) in broiler chickens on d 22.1
| Treatment | Ala | Asp | Cys | Glu | Gly | Pro | Ser | Tyr2 |
|---|---|---|---|---|---|---|---|---|
| C | 82b,c | 82b | 78a,b | 90c | 81a,b | 88b | 83b,c | 85c |
| C+MI | 81c | 81c | 77b | 89d | 80b | 87c | 82c | 84d |
| Phy500 | 83a,b | 83b | 78a | 91b,c | 81a | 88a,b | 84a,b | 86b,c |
| Phy1500 | 84a | 84a | 79a | 92a | 82a | 89a,b | 85a | 87a |
| Phy3000 | 84a | 84a | 79a | 91a,b | 82a | 89a | 85a | 86a,b |
| Pooled SEM | 0.46 | 0.47 | 0.54 | 0.30 | 0.47 | 0.35 | 0.46 | 0.41 |
| P-value | 0.001 | <0.001 | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
1Data are given as treatment means; n = 8 pens.
2The concentrations of tyrosine may be affected to some extent by the oxidation procedure (Mason et al., 1980).
a–dMeans within a column not showing a common superscript differ (P < 0.05).
The digestibility of all AA was negatively correlated with the concentration of InsP6 (P < 0.03, −0.707 < r < −0.347) and Ins(1,2,4,5,6)P5 in the ileum (P < 0.05, −0.682 < r < −0.318). The digestibility of most AA (except Cys and His) was negatively correlated with Ins(1,2,3,4,5)P5 (P < 0.03, −0.615 < r < −0.352). The digestibility of Glu, Val, Ile, Leu, Phe, Lys, and Arg was negatively correlated with the concentration of Ins(1,2,3,4,6)P5 (P < 0.03, −0.640 < r < −0.447). The digestibility of most AA (except Cys, His, and Pro) was negatively correlated with Ins(1,2,3,4)P4 (P < 0.03, −0.685 < r < −0.385). Positive correlations were observed between the digestibility of Met, Ala, Val, Ile, Leu, His, and Lys and the concentration of Ins(1,2,5,6)P4 (P < 0.03, 0.345 < r < 0.443) and between the digestibility of most AA (except Cys, Asp, Pro, and Gly) and InsP3x (P < 0.05, 0.317 < r < 0.516).
DISCUSSION
Effects of Myo-inositol Supplementation
The aim of this study was to investigate the effects of MI supplementation on performance traits, InsP degradation, MI concentrations in the crop, ileum, and blood, and the prececal P, Ca, and AA digestibility of broiler chickens. Previous studies on MI supplementation of broiler or layer diets have focused on effects on performance traits or blood metabolites, but not blood MI (Żyła et al., 2004; Pirgozliev et al., 2007; Żyła et al., 2012; Cowieson et al., 2013).
As might be expected, MI supplementation increased MI concentrations in the crop and ileum digesta and blood plasma. However, no effects of MI supplementation on any other measured trait were observed. This means that MI had no impact on the degradation of InsP6. This is in contrast to P, which also is released during phytate degradation and is known for its end-product inhibition effects on phytate degradation (Greiner et al., 1993; Angel et al., 2002; Olukosi and Fru-Nji, 2014; Shastak et al., 2014; Zeller et al., 2015b). As reviewed by Lee and Bedford (2016), MI is thought to be involved in bone formation and bone mineral density in mice. This was not supported by the tibia ash data obtained in the present study. However, P and Ca levels were not limiting in this study, which suggests that the benefits of MI may be more apparent under more limiting diets. It also cannot be ruled out that other criteria of bone mineralization, such as breaking strength or mineral concentration, could have shown different results.
Supplementation with MI increased G:F in the starter+grower phase as a result of its numerically lower ADFI, while maintaining the same ADG as in treatment C. As other measured traits in treatment C+MI did not differ from those in treatment C, the assumption is that MI had a direct effect on feed efficiency. Possible causes of enhanced feed efficiency as a result of MI supplementation have been described in the literature (Żyła et al., 2004; Cowieson et al., 2013; Walk et al., 2014). However, no effects of MI supplementation on broiler performance were found in a study using a different broiler strain than that used in the present study (Linares et al., 2017). MI is involved in several signaling pathways that play roles in cell survival and growth, lipid metabolism, and insulin sensitivity (Huber, 2016; Lee and Bedford, 2016). Although the numerically low increase in G:F owing to the supplementation of MI was highly significant, further studies should be conducted to confirm this outcome and to investigate the metabolic mode of action of MI in the animal.
Effects of Supplementation of Graded Levels of Phytase
Phytase supplementation led to an increased G:F, but there was no difference in phytase levels among treatments. Because the amount of prececally digested P and total tibia ash did not differ among treatments, the improvement in G:F could not have been the result of P release by phytase. Prececal AA digestibility was not increased for most AA when 500 FTU/kg were added to the diets, but increased for several AA by 1 to 3 percentage points when 1,500 or 3,000 FTU/kg were added. However, the concentration of AA in the diets was calculated to meet the birds’ requirements, and the digestibility of all AA was already very high. Clearly, no G:F benefit of the additional AA digestibility was achieved with the higher phytase doses, suggesting that the birds’ requirements were met, and the additional AA absorbed were likely surplus to their needs. Indeed, at over 1,200 g and a G:F of 0.84 g/g, the birds were performing almost 20% ahead of breeder target weights and 10% ahead of G:F targets. Such growth rates leave little room for improvement. It is therefore unlikely that the increase of 1 to 3 percentage points in AA digestibility resulting from the high phytase levels caused the higher G:F.
It is likely that the MI released by phytase addition, as shown by higher MI concentrations in the crop, ileum, and blood plasma, led to better feed efficiency, as suggested by the direct MI supplementation data. Phytase supplementation was previously shown to result in increased MI concentrations in the gizzard by Beeson et al. (2017) and Walk et al. (2014), in the ileum and excreta by Beeson et al. (2017), and in blood plasma of broilers by Cowieson et al. (2013).
Based on the outcomes of this study, it seems that MI released from phytate and free MI added to the diets lead to comparable effects. The accompanying metabolomics study of Huber et al. (2017) revealed increased serotonin and dopamine concentrations in blood plasma after the addition of MI to diets. Further, the authors found a positive relationship between the MI concentration in plasma and the serotonin and dopamine levels in plasma across all treatments, including those with phytase supplementation (A. Kenéz, University of Hohenheim, Germany, personal communication). This leads to the conclusion that MI, regardless of origin, is equally available for metabolic processes. The relationship between MI and serotonin and dopamine opens new avenues for study regarding MI effects, e.g., bird behavior and thus welfare.
In this study, the supplementation of 500 FTU/kg phytase to a diet reduced in P and Ca according to the assumed P equivalency of the phytase led to similar levels of prececally digested P and Ca and tibia ash weights but a higher G:F than treatment C. Thus, the assumed P equivalency of 500 FTU/kg phytase was achieved. Higher phytase levels did not further increase growth rate or efficiency.
Phytase supplementation also increased MI concentration in the crop and ileum in a dose-dependent manner, which is in agreement with Beeson et al. (2017). Supplementation with phytase increased MI concentration in the blood plasma, but there was no difference among the three phytase levels. This confirms the results of Cowieson et al. (2015), who also found higher MI concentrations in the blood plasma of broilers with phytase supplementation. In their first trial, they found a significant difference only between treatments with 1,000 and 3,000 FTU/kg phytase; no difference between treatments supplemented with 1,000 and 2000 FTU/kg or between 2,000 and 3,000 FTU/kg phytase was found. In their second trial, no significant differences were found among treatments supplemented with 1000, 2000, or 3000 FTU/kg phytase. Based on our outcomes, we conclude that there must be an effective MI transport from the intestine into the blood. Lerner and Smagula (1979), working with incubated intestinal segments of a broiler cross, suggested that MI is transported at high substrate concentrations by diffusion. However, the ability to absorb MI varies substantially among individual birds. There may be several reasons for the similarity in blood MI levels and difference in ileum MI levels. As the plasma MI concentration of treatment C+MI was much higher than that of Phy3000, it might have been absorbed most effectively in the duodenum, and perhaps the conversion of InsP1 to MI took place further down the digestive tract, whereas MI would start to be absorbed as soon as it enters the small intestine. Further, a metabolism step in the epithelial cells could have had an effect on the blood level, or perhaps the MI was transported into tissues at different speeds. However, this cannot be distinguished at this time. Further studies investigating the transport of MI in broilers and MI concentrations in different tissues would help to clarify these processes.
Relationship Between Amino Acid Digestibility and InsP Isomers
In the present study, negative correlations between the digestibility of most AA and concentrations of InsP6, Ins(1,2,4,5,6)P5, Ins(1,2,3,4,5)P5, and Ins(1,2,3,4)P4 in the ileum were found. This is in accordance to Bedford and Walk (2016), who also reported negative relationships among ileal concentrations of InsP6, InsP5, InsP4, and InsP3, without differentiation among the respective isomers, and with higher significance for InsP4 and InsP3. Their conclusion was that InsP4 and InsP3 might have a direct diminishing effect on the digestibility of several nutrients. In the present study, when no phytase was added, concentrations of InsP6 and InsP5 isomers were high because of limitations of endogenous microbial and epithelial phytases in degrading phytate. With phytase addition, the concentrations of InsP6 and InsP5 isomers were decreased and the digestibility of AA partially increased, possibly owing to diminished phytate–protein complexes or reduced endogenous losses (Selle et al., 2012). Ins(1,2,3,4)P4 also was decreased, and the main InsP4 isomer generated by the phytase, Ins(1,2,5,6)P4, was increased. All this led to a subsequent increase in InsP3x; thus, the positive relationship between Ins(1,2,5,6)P4 and InsP3x and the negative relationships between InsP6, Ins(1,2,4,5,6)P5, Ins(1,2,3,4,5)P5, and Ins(1,2,3,4)P4 and AA digestibility are a logical consequence of the differences in the phytate degradation pattern. Thus, it is likely that the relationship between AA digestibility and the concentration of the respective InsP isomers is not causal but rather an effect of phytase provoking a shift in the InsP isomer pattern while simultaneously releasing AA. However, the question of whether further degradation of lower InsPs would have led to even higher AA digestibility needs to be investigated.
CONCLUSION
In the present study, a higher G:F was achieved in treatments with either phytase or MI addition. The results of all other measured traits indicate that MI might have been the main reason for this increase. Therefore, it is likely that the release of MI after complete dephosphorylation of InsP6 is one of the benefits of phytase addition, independent of its effects on the release of P or the improvement in digestibility of other nutrients. MI seems to have had no effect on InsP degradation or the prececal digestibility of P, Ca, and AA. Further studies should be conducted to confirm these results.
ACKNOWLEDGEMENTS
The authors express their gratitude to Aviagen, Midlothian, for providing the experimental diets and Dr. Leonardo Linares for discussions on the subject. This study was supported by the Studienstiftung des deutschen Volkes through a doctoral scholarship for Vera Sommerfeld.
REFERENCES
- Angel R., Tamim N. M., Applegate T. J., Dhandu A. S., Ellestad L. E.. 2002. Phytic acid chemistry: Influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poult. Res. 11:471–480. [Google Scholar]
- Aviagen 2014. Ross 308 Broiler Nutrition Specifications. [Google Scholar]
- Bedford M. R., Walk C. L.. 2016. Reduction of phytate to tetrakisphosphate (IP4) to trisphosphate (IP3), or perhaps even lower, does not remove its antinutritive properties. Pages 45–51 in Phytate destruction. Walk C. L., Kühn I., Stein H. H., Kidd M. T., Rodehutscord M., eds., Wageningen Academic Publishers, Wageningen. [Google Scholar]
- Beeson L. A., Walk C. L., Bedford M. R., Olukosi O. A.. 2017. Hydrolysis of phytate to its lower esters can influence the growth performance and nutrient utilization of broilers with regular or super doses of phytase. Poult. Sci. 96:2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguhn J., Baumgärtel T., Dieckmann A., Rodehutscord M.. 2009. Determination of titanium dioxide supplements in different matrices using two methods involving photometer and inductively coupled plasma optical emission spectrometer measurements. Arch. Anim. Nutr. 63:337–342. [DOI] [PubMed] [Google Scholar]
- Cowieson A. J., Aureli R., Guggenbuhl P., Fru-Nji F.. 2015. Possible involvement of myo-inositol in the physiological response of broilers to high doses of microbial phytase. Anim. Prod. Sci. 55:710–719. [Google Scholar]
- Cowieson A. J., Ptak A., Mackowiak P., Sassek M., Pruszynska-Oszmalek E., Żyła K., Swiatkiewicz S., Kaczmarek S., Józefiak D.. 2013. The effect of microbial phytase and myo-inositol on performance and blood biochemistry of broiler chickens fed wheat/corn-based diets. Poult. Sci. 92:2124–2134. [DOI] [PubMed] [Google Scholar]
- Eeckhout W., de Paepe M.. 1994. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed Sci. Technol. 47:19–29. [Google Scholar]
- Greiner R., Konietzny U., Jany K.-D.. 1993. Purification and characterization of two phytases from Escherichia coli. Arch. Biochem. Biophys. 303:107–113. [DOI] [PubMed] [Google Scholar]
- Huber K. 2016. Cellular myo-inositol metabolism. Pages 53–60 in Phytate Destruction. Walk C. L., Kühn I., Stein H. H., Kidd M. T., Rodehutscord M., eds., Wageningen Academic Publishers, Wageningen. [Google Scholar]
- Huber K., Kenéz A., Rodehutscord M.. 2017. Dietary myo-inositol enhances serotonin and dopamine concentrations in plasma of 21-day-old broilers. Proc. Soc. Nutr. Physiol. 26:100. (Abstr.). [Google Scholar]
- Kühn I., Schollenberger M., Männer K.. 2016. Effect of dietary phytase level on intestinal phytate degradation and bone mineralization in growing pigs. J. Anim. Sci. 94:264–267. [Google Scholar]
- Lee S. A., Bedford M. R.. 2016. Inositol - An effective growth promotor? World's Poult. Sci. J. 72:743–760. [Google Scholar]
- Lerner J., Smagula R. M.. 1979. Myo-inositol transport in the small intestine of the domestic fowl. Comp. Biochem. Physiol. 62:939–945. [Google Scholar]
- Linares L., Sacranie A., Willemsen H.. 2017. Supplementation of exogenous phytase and inositol on performance of male broilers from 0 to 46 days. Proc. Eur. Symp. Poult. Nutr. 21:249. (Abstr.). [Google Scholar]
- Mason V. C., Rudemo M., Bech-Andersen S.. 1980. Hydrolysate preparation for amino acid determinations in feed constituents 6. The influence of phenol and formic acid on the recovery of amino acids from oxidized feed protein. Z. Tierphysiol. Tierernahr. Futtermittelkd. 43:35–48 [PubMed] [Google Scholar]
- Olukosi O. A., Fru-Nji F.. 2014. The interplay of dietary nutrient level and varying calcium to phosphorus ratios on efficacy of a bacterial phytase: 2. Ileal and total tract nutrient utilization. Poult. Sci. 93:3044–3052. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V., Allymehr M., Sarwar S., Acamovic T., Bedford M. R.. 2007. Effect of dietary inositol on performance and mucin excretion, when fed to chickens. Br. Poult. Abstr. 3:4–5. [Google Scholar]
- Rodehutscord M., Kapocius M., Timmler R., Dieckmann A.. 2004. Linear regression approach to study amino acid digestibility in broiler chickens. Br. Poult. Sci. 45:85–92. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Rückert C., Maurer H. P., Schenkel H., Schipprack W., Bach Knudsen K. E., Schollenberger M., Laux M., Eklund M., Siegert W., Mosenthin R.. 2016. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch. Anim. Nutr. 70:87–107. [DOI] [PubMed] [Google Scholar]
- Selle P. H., Cowieson A. J., Cowieson N. P., Ravindran V.. 2012. Protein-phytate interactions in pig and poultry nutrition: A reappraisal. Nutr. Res. Rev. 25:1–17. [DOI] [PubMed] [Google Scholar]
- Selle P. H., Ravindran V.. 2007. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 135:1–41. [Google Scholar]
- Shastak Y., Witzig M., Hartung K., Bessei W., Rodehutscord M.. 2012. Comparison and evaluation of bone measurements for the assessment of mineral phosphorus sources in broilers. Poult. Sci. 91: 2210–2220. [DOI] [PubMed] [Google Scholar]
- Shastak Y., Zeller E., Witzig M., Schollenberger M., Rodehutscord M.. 2014. Effects of the composition of the basal diet on the evaluation of mineral phosphorus sources and interactions with phytate hydrolysis in broilers. Poult. Sci. 93:2548–2559. [DOI] [PubMed] [Google Scholar]
- Sommerfeld V., Schollenberger M., Kühn I., Rodehutscord M.. 2018. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. in press, doi: 10.3382/ps/pex404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA) 2007. Handbuch der landwirtschaftlichen Versuchs und Untersuchungsmethodik (VDLUFA–Methodenbuch), Bd. III: Die chemische Untersuchung von Futtermitteln. Darmstadt, Germany: VDLUFA-Verlag. [Google Scholar]
- Walk C. L., Santos T. T., Bedford M. R.. 2014. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult. Sci. 93:1172–1177. [DOI] [PubMed] [Google Scholar]
- Wyss M., Brugger R., Kronenberger A., Rémy R., Fimbel R., Oesterhelt G., Lehmann M., van Loon A. P.. 1999. Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): Catalytic properties. Appl. Environ. Microbiol. 65:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Kühn I., Rodehutscord M.. 2015a. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 4:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Witzig M., Shastak Y., Kühn I., Hoelzle L. E., Rodehutscord M.. 2015b. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 94:1018–1029. [DOI] [PubMed] [Google Scholar]
- Żyła K., Duliński R., Pierzchalska M., Grabacka M., Józefiak D., Swiatkiewicz S.. 2013. Phytases and myo-inositol modulate performance, bone mineralization and alter lipid fractions in the serum of broilers. J. Anim. Feed Sci. 22:56–62. [Google Scholar]
- Żyła K., Mika M., Duliński R., Swiatkiewicz S., Koreleski J., Pustkowiak H., Piironen J.. 2012. Effects of inositol, inositol-generating phytase B applied alone, and in combination with 6-phytase A to phosphorus-deficient diets on laying performance, eggshell quality, yolk cholesterol, and fatty acid deposition in laying hens. Poult. Sci. 91:1915–1927. [DOI] [PubMed] [Google Scholar]
- Żyła K., Mika M., Stodolak B., Wikiera A., Koreleski J., Swiatkiewicz S.. 2004. Towards complete dephosphorylation and total conversion of phytates in poultry feeds. Poult. Sci. 83:1175–1186. [DOI] [PubMed] [Google Scholar]


