Abstract
Objectives. The aim was to investigate the effects of nicotine on neutrophil extracellular traps (NETs) formation in current and non-smokers and on a murine model of RA.
Methods. We compared spontaneous and phorbol 12-myristate 13-acetate-induced NETosis between current and non-smokers by DNA release binding. Nicotine-induced NETosis from non-smokers was assessed by DNA release binding, NET-specific (myeloperoxidase (MPO)–DNA complex) ELISA and real-time fluorescence microscopy. We also used immunofluorescent staining to detect nicotinic acetylcholine receptors (nAChRs) on neutrophils and performed a functional analysis to assess the role of nAChRs in nicotine-induced NETosis. Finally, we investigated the effects of systemic nicotine exposure on arthritis severity and NETosis in the CIA mouse model.
Results. Neutrophils derived from current smokers displayed elevated levels of spontaneous and phorbol 12-myristate 13-acetate-induced NETosis. Nicotine induced dose-dependent NETosis in ex vivo neutrophils from healthy non-smokers, and co-incubation with ACPA-immune complexes or TNF-α facilitated a synergistic effect on NETosis. Real-time fluorescence microscopy revealed robust formation of NET-like structures in nicotine-exposed neutrophils. Immunofluorescent staining demonstrated the presence of the α7 subunit of the nAChR on neutrophils. Stimulation of neutrophils with an α7-specific nAChR agonist induced NETosis, whereas pretreatment with an nAChR antagonist attenuated nicotine-induced NETosis. Nicotine administration to mice with CIA exacerbated inflammatory arthritis, with higher plasma levels of NET-associated MPO–DNA complex.
Conclusion. We demonstrate that nicotine is a potent inducer of NETosis, which may play an important role in accelerating arthritis in the CIA model. This study generates awareness of and the mechanisms by which nicotine-containing products, including e-cigarettes, may have deleterious effects on patients with RA.
Keywords: neutrophil extracellular traps, rheumatoid arthritis, animal model, nicotine, collagen-induced arthritis
Rheumatology key messages
Current smokers have elevated levels of NETosis, and nicotine induces NETosis in healthy non-smokers.
Nicotine exacerbates arthritis in a CIA model with elevated markers of NETosis.
Use of nicotine-containing e-cigarettes can be harmful in individuals with or at risk for RA.
Introduction
RA is often characterized by the development of highly specific autoantibodies known as ACPAs [1]. Cigarette smoking predisposes the HLA-DRB1-positive population to develop ACPA-positive RA [2] and is also associated with increased RA disease activity in the seropositive form of RA [3–6]. Smoking may predispose to the generation of citrullinated antigens by activating the peptidylarginine deiminase enzyme in the lungs, gums or other sites of inflammation, with subsequent development of ACPA [7]. However, ACPAs may be detected up to a decade before signs or symptoms of RA develop, and thus the mechanism(s) mediating the transition from the asymptomatic ACPA positivity to the development of clinical RA remains incompletely understood [8, 9]. Furthermore, although previous studies have demonstrated increased titres of RA-associated autoantibodies among smokers [10, 11], our recent report showed that disease activity among former smokers is reduced compared with current smokers, whereas ACPA titres in these two groups of individuals are comparable [12]. Thus, both lines of evidence suggest that in addition to the production of ACPAs, there may be other mechanisms by which smoking initiates and drives RA disease activity.
Our recent report suggested that it is not the production of ACPA itself, but rather the generation of local citrullinated antigens (in the presence of circulating ACPA) that initiates inflammatory arthritis [5]. Others have demonstrated that neutrophil extracellular traps (NETs) formation can produce citrullinated proteins that may serve as antigenic targets of the ACPA immune response [13–16]. Indeed, NETosis has been implicated in the pathogenesis of RA, because accelerated NET formation [14] and enhanced signalling elements associated with NETosis have been observed in patients with RA compared with healthy controls [17]. Based on these observations, we hypothesized that nicotine, the most ubiquitous and primary addictive component of cigarette smoke, may contribute to RA by inducing NETosis. The activation of NETosis in the joints results in the generation of local citrullinated antigens and, in the presence of circulating ACPA, may trigger an inflammatory cascade, thus driving the initiation and propagation of RA. In the present study, we investigated the potential role of nicotine as an inducer of NETosis and in accelerating inflammation in an animal model of RA.
Methods
Sample collection and neutrophil isolation
Blood samples were obtained from otherwise healthy current smokers (currently smoking 0.5–2 packs/day, with a history of 8–40 pack-years) and non-smokers. The study, including the protocol, was approved by the Institutional Review Board at Stanford University. A signed consent form was obtained from each patient (both smokers and non-smokers). Neutrophils were isolated from fresh whole blood or buffy coats using the dextran–Ficoll method as previously described [18] or using the EasySep direct human neutrophil isolation kit according to the manufacturer’s instructions (Stemcell Technologies, Vancouver, Canada). Identical isolation methodology was used within each experimental study to assure intra-assay consistency.
Neutrophil activation and measurement of NETosis
Neutrophils isolated from current and non-smokers were immediately resuspended in Roswell Park Memorial Institute medium without phenol red, seeded into black, flat-bottomed, 96-well plates (8 × 104 cells/well), and incubated with PBS or phorbol 12-myristate 13-acetate (PMA) for 4–8 h in the presence 2 μM of SYTOX green (Invitrogen, Carlsbad, CA, USA), a non-cell-permeant DNA-binding dye, to measure spontaneous and PMA-induced NETosis. The plates were analysed in a fluorescence microplate reader at 485 nm (excitation)/520 nm (emission). Separately, neutrophils from healthy non-smokers were incubated in multiple concentrations ranging from 1 to 20 mM of nicotine, and NETosis was measured as described above. In parallel, supernatants were collected from each well, and the levels of MPO–DNA complexes were measured using a novel NET-specific ELISA [19]. Citrullinated H2B immune complexes were generated in flat-bottomed, 96-well plates as previously described [5]. Neutrophils in the presence of SYTOX green were added to wells coated with citrullinated H2B immune complexes or containing recombinant human TNF-α (5 ng/ml; Peprotech, Rocky Hill, NJ, USA) for 30 min before exposure to nicotine. All in vitro cell stimulations were performed in triplicate and in at least two separate experiments. For measurement of MPO–DNA complexes in mouse plasma, a modified capture ELISA was performed. Briefly, NET-associated MPO was captured on coated 96-well plates from the mouse MPO ELISA kit (Abcam, Cambridge, UK), after which the NET-associated DNA backbone was labelled with the detection antibody of the Cell Death Detection ELISAPLUS (Roche, Basel, Switzerland).
Immunofluorescence analysis of NETosis and nicotinic acetylcholine receptors
To visualize the induction of NETosis in response to nicotine stimulation, we coated glass coverslips with human neutrophils and simulated these neutrophils for 2–4 h with PBS, nicotine (2.5 mM) or PMA (100 nM) in the presence of SYTOX green, during which time-lapse images and videos were recorded. For nicotinic acetylcholine receptor (nAChR) staining, neutrophils were placed on chamber slides and fixed with 4% paraformaldehyde in 1 × PBS. Blocking was performed with 1% BSA and 1% normal goat serum. Slides were incubated with α-bungarotoxin conjugated with Alexa Fluor 647® (Thermo Fisher Scientific, Waltham, MA, USA) and a rabbit polyclonal antibody against α7 nAChR (Abcam). All slides were mounted with anti-fade gold containing 4′,6-diamidino-2-phenylindole (Promega, Madison, WI, USA) for visualization of the nuclei. Slides were imaged with a Keyence BZ-X700 digital microscope (Osaka, Japan).
Functional analysis of nAChRs, which mediate nicotine-induced NETosis
Neutrophils were simulated with the α7 nicotinic agonist GTS-21 (0.8 mM; Sigma, St. Louis, MO, USA). In a separate set of experiments, neutrophils were pretreated for 30 min with a non-specific nAChR antagonist, mecamylamine (MCA, 50 μM; Tocris, Bristol, UK), or an α-7 nAChR antagonist, α-bungarotoxin (10 μM; Sigma, St.Louis, MO, USA), before stimulation with nicotine (5 mM).
Mice with CIA
All animal studies were performed in adherence to the Guide for the Care and Use of Laboratory Animals and under protocols approved by the Veterans Affairs Palo Alto Institutional Animal Care and Use Committee. Briefly, male DBA/1J mice (Jackson Laboratory, Bar Harbor, ME, USA) were immunized intradermally in the proximal tail with 100 μg of bovine type II collagen emulsified in Freund’s complete adjuvant. Twenty-one days after immunization, a booster injection was given. Each limb was assigned a score of 0–4, with a maximal possible score of 16 for each mouse. Paw thickness was determined by measuring the thickness of both hindpaws with callipers and calculating the sum of the two measurements. At the termination of the experiment, hindlimbs from mice with autoimmune arthritis were fixed and decalcified for 3 days before being paraffin embedded. Histological assessment of arthritis severity was made by two investigators who were blinded to treatment status, based on a previously described scoring system, as follows: 0 = normal; 1 = mild inflammation, mild hyperplasia of the synovial lining layer and mild cartilage destruction without bone erosion; 2–4 = increasing degrees of inflammatory cell infiltrates, synovial lining hyperplasia and pannus formation, and cartilage and bone destruction [20]. The titre of anti-type II collagen antibodies was measured as previously described [5].
Nicotine pump placement in mice
Under isoflurane anaesthesia and using sterile technique, a small incision was made on the back, between and slightly posterior to the scapulae, and an ALZET mini-osmotic pump model 2006 (DURECT Corporation) was implanted s.c. Animals were monitored daily until the wound clips were removed 10 days post-procedure. Nicotine (15 mg/kg/day) was delivered to mice continuously at 0.15 µl/h from post-immunization day 19 for 42 days. Vehicle control mice received identical pump implantation and PBS infusion at 0.15 μl/h for the same duration. In order to estimate the nicotine exposure accurately in the nicotine-treated mice, the concentration of cotinine, a principal metabolite of nicotine with a substantially longer half-life and the most widely used biomarker of tobacco exposure, was measured at the termination of the experiment using a Mouse/Rat Cotinine ELISA kit (Calbiotech, El Cajon, CA, USA) according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (version 6.04; GraphPad, La Jolla, CA, USA). Statistical comparisons between the groups were analysed using a two-way analysis of variance test followed by Bonferroni’s or Tukey’s post hoc test, or Student’s unpaired t-test. A value of P < 0.05 was considered statistically significant.
Results
Analysis of NETosis in smokers vs non-smokers
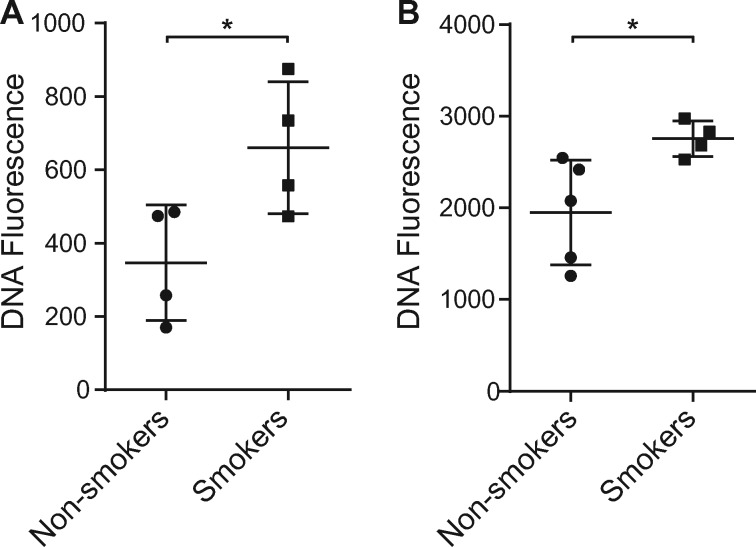
Given the role of smoking in both the initiation and the propagation of RA, we investigated the role of smoking as a contributor to NETosis. To evaluate the effect of smoking on NETosis in humans, we isolated neutrophils from otherwise healthy smokers and non-smokers and measured DNA fluorescence by SYTOX green. As shown in Fig. 1, increased levels of DNA fluorescence were detected in neutrophils derived from current smokers, with or without PMA treatment, indicating an enhanced level of spontaneous NETosis (Fig. 1A) as well as agonist-induced NETosis (Fig. 1B).
Fig. 1.
Effects of cigarette smoking on NETosis
(A) Neutrophils derived from current smokers displayed an enhanced level of spontaneous NETosis compared with those from non-smokers (n = 4 each). (B) Neutrophils derived from current smokers (n = 4) displayed an enhanced level of PMA-induced NETosis compared with those from non-smokers (n = 5, *P < 0.05). Bars represent the mean (s.e.m.). PMA: phorbol 12-myristate 13-acetate.
Nicotine induces dose-dependent NETosis in ex vivo human neutrophils
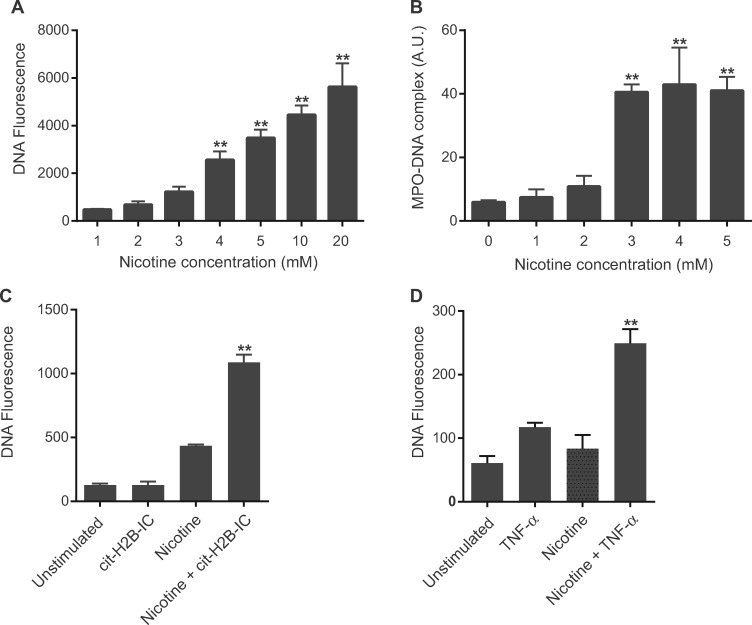
Given that the most ubiquitous and primary addictive component of cigarette smoke is nicotine, we evaluated the effect of nicotine as a stimulant of NETosis. Treatment of neutrophils from healthy non-smokers with nicotine resulted in an increase in SYTOX green staining against DNA in a dose-dependent manner (Fig. 2A). To confirm that the increase in SYTOX green (DNA) staining was attributable to NETosis, we measured levels of NET-associated MPO–DNA complexes using a novel NET-specific ELISA [17, 19]. We observed an increase in MPO–DNA complexes (Fig. 2B) that closely paralleled the increase in SYTOX green florescence, confirming the ongoing NETosis. Moreover, co-incubation of citrullinated H2B immune complexes with nicotine facilitated a synergistic effect on NETosis (Fig. 2C). Likewise, TNF-α exposure exerted a similar synergistic effect on NETosis and significantly lowered the threshold of nicotine concentration for induction of NETosis (Fig. 2D).
Fig. 2.
Nicotine induces NETosis in neutrophils from healthy non-smokers
(A) and (B) Nicotine induced NETosis in neutrophils in a dose-dependent manner as measured by the fluorescent intensity of SYTOX green (A; **P < 0.0001 vs 1 mM nicotine) as well as by NET-specific MPO–DNA complex ELISA (B; **P < 0.0001 vs 0 mM nicotine). (C) Cit-H2B IC and nicotine have a synergistic effect on neutrophil NETosis (**P < 0.001 vs cit-H2B IC or 1 mM nicotine). (D) TNF-α primes neutrophils to undergo NETosis at a lower concentration of nicotine (1 mM; **P < 0.005 vs TNF-α or nicotine). Bars represent the mean (s.e.m.). Cit-H2B IC: citrullinated H2B immune complexes; MPO-DNA: MPO–DNA complexes.
Immunofluorescent visualization and microscopic kinetic analysis of NETosis
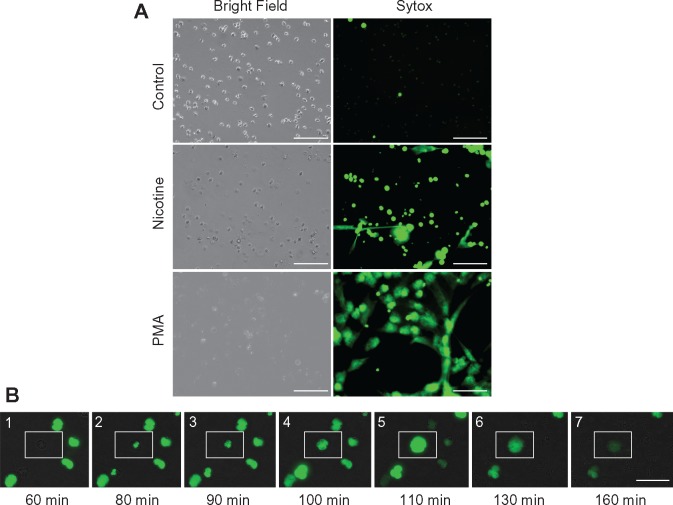
To validate and visualize the induction of NETosis in neutrophils from healthy non-smokers in response to nicotine stimulation, real-time fluorescence video microscopy was recorded during 4 h stimulation with nicotine in the presence of SYTOX green (see supplementary Video, available at Rheumatology Online). We observed robust release of DNA as well as formation of NET-like structures in both the nicotine- and PMA-treated cells (Fig. 3A). Microscopic time-course analysis revealed the characteristic pattern of NET progression, starting at ∼60 min and lasting for a duration of 60–120 min (Fig. 3B), including the following stages: intact neutrophil; loss of nuclear permeability; change in nuclear morphology; chromatin decondensation; nuclear expansion into cytoplasm, mixing nuclear, cytoplasmic and granular components; initial loss of plasma membrane/NET release; and extrusion of DNA and nuclear contents from cells. These findings indicate that the process we observed differs from apoptosis, which is associated with condensed nuclear DNA as opposed to decondensed DNA, that we observed in these neutrophils or necrosis, which is associated with a lack of programmed nuclear decondensation and an early loss of plasma membrane integrity. Notably, induction of necrosis by treatment with 0.01% Triton X-100 resulted in rapid unprogrammed cell death of a pattern characteristically distinct from that observed in response to nicotine or PMA (data not shown).
Fig. 3.
Visualization of nicotine-induced NETosis using SYTOX green DNA staining
(A) Bright field confirms equal cell loading, with induction of NETosis defined as free DNA and characteristic NET-like projections (scale bars: 100 μm). (B) Nicotine induces the classic sequence of NETosis: (1) intact neutrophil; (2) loss of nuclear permeability; (3) change in nuclear morphology; (4) chromatin (nuclear) decondensation; (5) nuclear expansion into cytoplasm, mixing nuclear, cytoplasmic and granular components; (6) initial loss of plasma membrane/NET release; and (7) extrusion of DNA and nuclear contents from cells (scale bar: 50 μm).
Localization of nAChRs and functional analysis
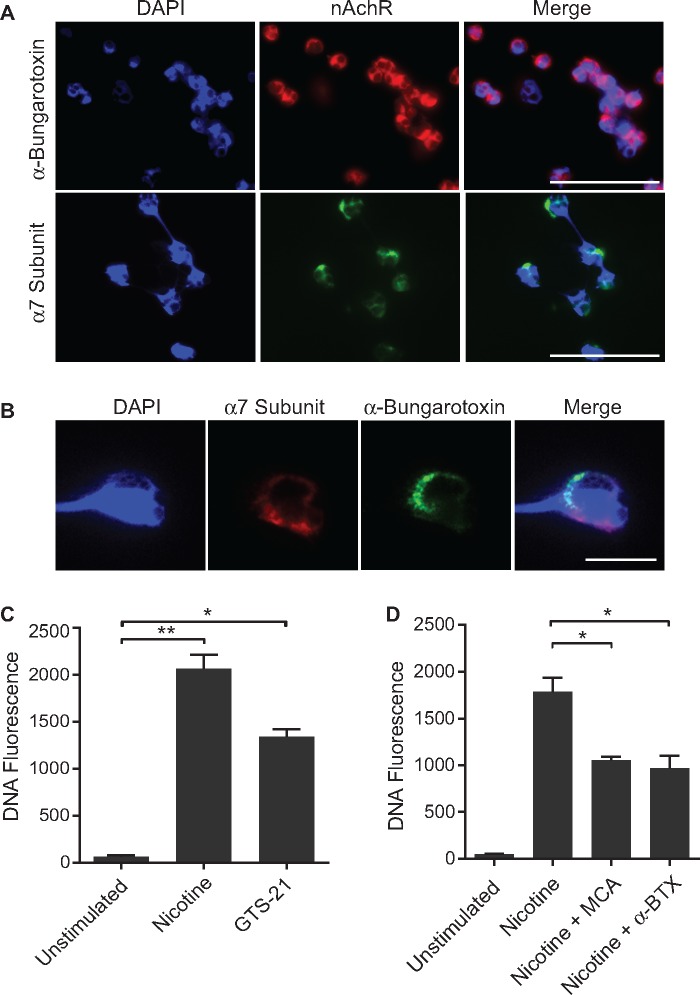
We next examined whether nAChRs are present on neutrophils in healthy non-smokers by immunofluorescent staining using Alexa Fluor-conjugated α-bungarotoxin. Fig. 4A demonstrates a pattern of surface staining on neutrophils, indicating the presence of nAChRs. In addition, we validated the presence of nAChRs by staining neutrophils with an anti-α7-specfic nAChR antibody. Magnified images at higher resolutions confirmed the co-localization of α-bungarotoxin and anti-α7-specfic nAChR antibody staining on the plasma membrane of these neutrophils (Fig. 4B). Notably, we did not detect staining with anti-α3- or α4-nAChR antibodies (data not shown).
Fig. 4.
Identification of nicotinic acetylcholine receptors on neutrophils
(A) Staining of human neutrophils with Alexa Fluor 647-labelled α-bungarotoxin and anti-α7-specfic nAchR antibody, merged with nuclear stain DAPI(4’,6-diamidino-2-phenylindole)), shows a pattern of surface staining (scale bar: 50 μm). (B) Magnified view of neutrophil stained with α-bungarotoxin and an anti-α7-specfic nAchR antibody, demonstrating co-localization on the plasma membrane (scale bar: 10 μm). (C) Stimulation of neutrophils with an α7-nAChR agonist, GTS-21 (0.8 mM), results in nicotine-induced NETosis (*P < 0.05, **P < 0.01). (D) Nicotine-induced NETosis is attenuated by pretreating neutrophils with a non-specific nAChR antagonist (50 μM) or an α7-specfic nAChR antagonist (10 μM) before exposure to nicotine (*P < 0.05). Bars represent the mean (s.e.m.). nAChR: nicotinic acetylcholine receptor.
To confirm that the nAChR pathway is engaged in nicotine-mediated NETosis, we performed functional studies that involved pharmacological modulation of nicotinic acetylcholine receptors. When neutrophils were stimulated GTS-21, an α7-nAChR agonist, nicotine-induced NETosis was observed (Fig. 4C). Alternatively, pretreatment of neutrophils with MCA, a non-specific nAChR antagonist, or α-bungarotoxin, an α-7 nAChR antagonist, resulted in attenuation of nicotine-induced NETosis (Fig. 4D). Interestingly, MCA was not able to attenuate NETosis fully to the level of the unstimulated group, suggesting that other pathways in addition to nAChR may be engaged in the process of nicotine-induced NETosis.
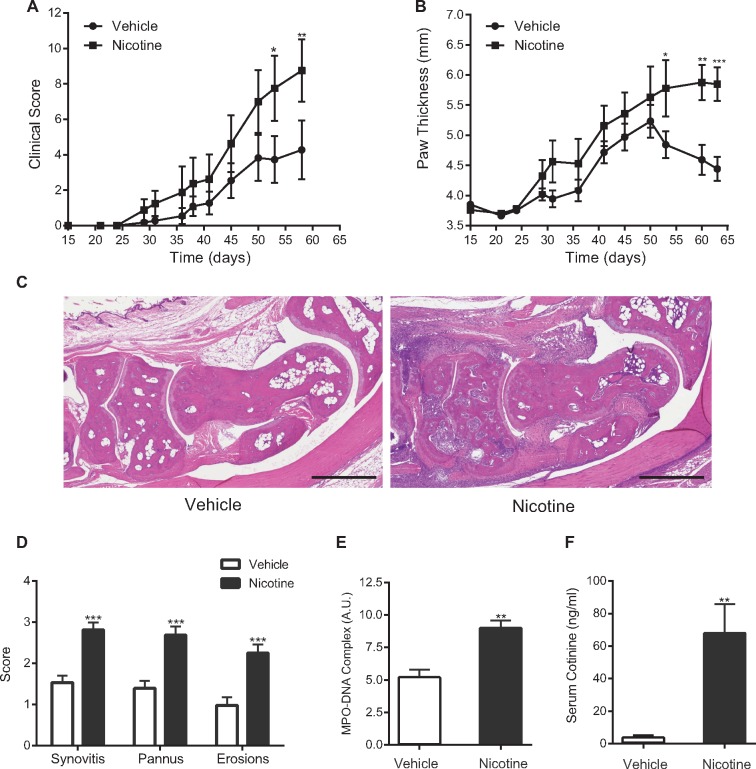
Effect of nicotine on CIA
The observation that nicotine can drive and/or prime NETosis led us to study the systemic effect of nicotine in the CIA mouse model of RA. CIA mice treated with nicotine exhibited more severe arthritis, as measured by clinical score and paw thickness, than mice treated with vehicle (Fig. 5A and B). Histopathological examination revealed more pronounced infiltration of inflammatory cells, cartilage destruction, pannus formation and bony erosions in the nicotine-treated mice (Fig. 5C and D). We observed no significant difference in levels of anti-type II collagen antibodies between treatment groups. However, the levels of NET-associated MPO–DNA complexes were significantly higher in the plasma of nicotine-treated mice compared with vehicle-treated mice (Fig. 5E). Serum cotinine concentrations measured at the end of the experiment were consistent with those observed in the blood of light-to-moderate smokers [67.3 (17.9) and 3.61 (0.8) ng/ml in nicotine- and vehicle-treated groups, respectively; Fig. 5F). The measured concentration of cotinine in the vehicle-treated mice is likely to be lower than the detection limit of the assay. However, we cannot exclude a possibility of low systemic exposure in this group as a result of cohabitation with nicotine-treated mice.
Fig. 5.
Nicotine administration exacerbates arthritis in the CIA mouse model (n = 12 mice per group)
(A) and (B) Significantly increased severity of arthritis is observed in CIA mice treated with nicotine compared with vehicle-treated controls (*P < 0.05, **P < 0.01 and ***P < 0.001). (C) Representative images of haematoxylin- and eosin-stained sections of ankle joints from mice treated with nicotine vs vehicle (scale bar: 500 μm). (D) Histological scores for inflammation, pannus formation and bone or cartilage erosion of ankle joints from mice treated with nicotine vs vehicle (***P < 0.0001). (E) Plasma levels of NET-associated MPO–DNA complexes from nicotine-treated mice are significantly higher than those from vehicle-treated mice (**P < 0.01). (F) Serum concentrations of cotinine from nicotine-treated mice are significantly higher than those from vehicle-treated mice (**P < 0.01). Bars represent the mean (s.e.m.). MPO-DNA: MPO–DNA complexes.
Discussion
In this study, we demonstrate that neutrophils isolated from current smokers display enhanced spontaneous and induced NETosis compared with non-smokers, and that nicotine induces dose-dependent NETosis in isolated human neutrophils. The effect of nicotine on NETosis was primed by ACPA ICs and TNF-α. Moreover, systemic nicotine exposure exacerbated the severity of arthritis in a murine model of RA. These observations, together with findings from our previous studies [5], suggest that autoantigen generation in the setting of circulating autoantibody is crucial to facilitate joint inflammation and that exposure to tobacco, and specifically to nicotine, may facilitate such antigen generation.
Our data provide evidence that nicotine may play a significant role in propagating the production of autoantigens by induction of NETosis and thus generating a robust profile of citrullinated antigens. Indeed, NETosis requires histone citrullination by the peptidylarginine deiminase 4 enzyme [21], and histone citrullination is ubiquitous to sites of neutrophil influx and activation, including the synovial space in RA patients. Consistent with these observations, recent reports have suggested that citrullinated products of NETosis may serve as antigenic targets of the ACPA immune response [13–16]. Furthermore, we have recently demonstrated that immunization with citrullinated H2B immune complexes (a known product of NETosis) can initiate inflammatory arthritis in a murine model of RA, but only in the setting of low-grade joint inflammation [5]. Additionally, it is possible that nicotine-induced NETosis and protein citrullination may provide a nidus not only for disease activity, but also for conversion of preclinical autoimmunity to disease onset. Thus, abrogation of these processes could ameliorate or even prevent the onset of clinical arthritis in the serologically at-risk population [22].
Notably, previous studies reported immunosuppressive effects of nicotine on the immune system. For instance, T cells carry nicotinic and muscarinic acetylcholine receptors, through which nicotine may induce T cell anergy [23]. The nicotinic acetylcholine receptor is thought to be involved in suppressing the inflammatory cytokine response of alveolar macrophages [24], and acetylcholine significantly attenuated the release of inflammatory cytokines, including TNF-α, IL-1 and IL-6, but not anti-inflammatory IL-10 [25]. These immunosuppressive effects of nicotine may be viewed as beneficial or may even be considered as a treatment opportunity for RA patients. However, cautious interpretation is warranted because the present study suggests that nicotine may contribute to neutrophilic inflammation and, through the same immunosuppressive mechanisms described above, may elevate the risk of RA by exposing predisposed populations to an increased risk of chronic infections, including periodontitis and pulmonary inflammation [7]. Furthermore, nicotine has been shown to stimulate IL-8 production by neutrophils via nAChRs by generating peroxynitrite and subsequent NF-κB activation [26].
Cotinine, the major proximate metabolite of nicotine, has been widely used as a biomarker of exposure to tobacco in both active and secondhand tobacco smoke [27]. The serum cotinine concentration of an average US smoker is 211 ng/ml [28]. The serum concentration of cotinine in nicotine-treated mice in our study was less than a third of this value but was enough to potentiate the severity of arthritis, underscoring the importance of abstinence from smoking. Furthermore, a longitudinal study of cotinine in long-term daily users of e-cigarettes showed that cotinine concentrations were similar to those observed in cigarette smokers [29]. Therefore, the results of the present study raise public health safety concerns with regard to the growing use of e-cigarettes, especially among those with or at risk for RA.
Our observation that nicotine exacerbates CIA differs from those reported in previous studies [30–33]. This discrepancy may be explained by a markedly lower dosage of nicotine administered to mice in the previous studies and by the different methods of nicotine administration. Moreover, none of the previous studies measured serum cotinine concentrations and compared these concentrations with those found in average smokers. It is possible that while nicotine may attenuate CIA through stimulating nAChRs at lower concentrations, our data clearly suggest that nicotine exerts deleterious effects on CIA within the concentrations detected in current smokers. In accordance with our results, cigarette smoke condensate, which may have higher nicotine concentrations, has been shown to augment CIA [34, 35].
The present study has several limitations. First, given the fact that ACPA-negative RA patients are likely to have a disease of divergent pathophysiology from those with ACPA-positive RA, the effect of nicotine cannot be applied to the ACPA-negative population. Second, we examined NETosis primarily in healthy neutrophils, rather than in those from patients with RA. However, given previous reports demonstrating exaggerated NETosis among patients with RA [14, 17], use of the RA population would be likely to underestimate the effect of nicotine in the pathogenesis of RA. Additionally, use of neutrophils from established RA patients would not fully mimic a preclinical state, in which nicotine could contribute to disease initiation and perpetuation. Lastly, the nicotine concentration used in ex vivo stimulation of neutrophils may be higher than the physiological nicotine concentration in smokers. Nonetheless, the priming effect on neutrophils by TNF-α and ACPA ICs indicates that much lower concentrations of nicotine may be sufficient to drive NETosis in the pathological synovial microenvironment. Despite these limitations, the present study demonstrates that smoking and nicotine, in addition to being a predisposing factor to asymptomatic autoimmunity, may contribute to RA disease activity and, potentially, the transition of an at-risk population to clinical RA.
In conclusion, our data indicate that nicotine is an inducer of NETosis and may play an important role in RA-associated inflammation. Furthermore, our study provides molecular evidence for public awareness of and potential rationale against the use of nicotine-containing products, including e-cigarettes, in individuals with RA or at risk for the development of RA.
Supplementary Material
Acknowledgements
The authors thank Rong Mao, PhD for her scientific and editorial inputs.
Funding: This work was funded by Department of Veterans Affairs IK2 BX001301, NIH/NHLBI R56HL12277, and a Rheumatology Research Foundation Disease Targeted Research Award.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
Footnotes
Jaejoon Lee and Ayala Luria contributed equally to this study.
References
- 1. Raptopoulou A, Sidiropoulos P, Katsouraki M, Boumpas DT.. Anti-citrulline antibodies in the diagnosis and prognosis of rheumatoid arthritis: evolving concepts. Crit Rev Clin Lab Sci 2007;44:339–63. [DOI] [PubMed] [Google Scholar]
- 2. Padyukov L, Silva C, Stolt P. et al. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 2004;50:3085–92. [DOI] [PubMed] [Google Scholar]
- 3. Manfredsdottir VF, Vikingsdottir T, Jonsson T. et al. The effects of tobacco smoking and rheumatoid factor seropositivity on disease activity and joint damage in early rheumatoid arthritis. Rheumatology 2006;45:734–40. [DOI] [PubMed] [Google Scholar]
- 4. Saag KG, Cerhan JR, Kolluri S. et al. Cigarette smoking and rheumatoid arthritis severity. Ann Rheum Dis 1997;56:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sohn DH, Rhodes C, Onuma K. et al. Local joint inflammation and histone citrullination provides a murine model for the transition from preclinical autoimmunity to inflammatory arthritis. Arthritis Rheumatol 2015;67: 2877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masdottir B, Jónsson T, Manfredsdottir V. et al. Smoking, rheumatoid factor isotypes and severity of rheumatoid arthritis. Rheumatology 2000;39:1202–5. [DOI] [PubMed] [Google Scholar]
- 7. Makrygiannakis D, Hermansson M, Ulfgren AK. et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis 2008;67:1488–92. [DOI] [PubMed] [Google Scholar]
- 8. Rantapää-Dahlqvist S, de Jong BA, Berglin E. et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. [DOI] [PubMed] [Google Scholar]
- 9. Nielen MM, van Schaardenburg D, Reesink HW. et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380–6. [DOI] [PubMed] [Google Scholar]
- 10. Klareskog L, Stolt P, Lundberg K. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 2006;54:38–46. [DOI] [PubMed] [Google Scholar]
- 11. Lee DM, Phillips R, Hagan EM. et al. Quantifying anti-cyclic citrullinated peptide titres: clinical utility and association with tobacco exposure in patients with rheumatoid arthritis. Ann Rheum Dis 2009;68:201–8. [DOI] [PubMed] [Google Scholar]
- 12. Sokolove J, Wagner CA, Lahey LJ. et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: a cross-sectional analysis of US veterans. Rheumatology 2016;55:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romero V, Fert-Bober J, Nigrovic PA. et al. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med 2013;5:209ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013;5:178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dwivedi N, Upadhyay J, Neeli I. et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum 2012;64:982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pratesi F, Dioni I, Tommasi C. et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis 2014;73:1414–22. [DOI] [PubMed] [Google Scholar]
- 17. Sur Chowdhury C, Giaglis S, Walker UA. et al. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther 2014;16:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark RA, Nauseef WM.. Isolation and functional analysis of neutrophils. Curr Protoc Immunol 2001;Chapter 7:Unit 7,23, doi: 10.1002/0471142735.im0723s19. [DOI] [PubMed] [Google Scholar]
- 19. Yoo DG, Floyd M, Winn M, Moskowitz SM, Rada B.. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase–DNA and neutrophil elastase–DNA complexes. Immunol Lett 2014;160:186–94. [DOI] [PubMed] [Google Scholar]
- 20. Deng GM, Zheng L, Chan FK, Lenardo M.. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med 2005;11:1066–72. [DOI] [PubMed] [Google Scholar]
- 21. Li P, Li M, Lindberg MR. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 2010;207:1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerlag DM, Raza K, van Baarsen LG. et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis 2012;71:638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Jonge WJ, Ulloa L.. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 2007;151:915–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsunaga K, Klein TW, Friedman H, Yamamoto Y.. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol 2001;167:6518–24. [DOI] [PubMed] [Google Scholar]
- 25. Borovikova LV, Ivanova S, Zhang M. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458–62. [DOI] [PubMed] [Google Scholar]
- 26. Iho S, Tanaka Y, Takauji R. et al. Nicotine induces human neutrophils to produce IL-8 through the generation of peroxynitrite and subsequent activation of NF-κB. J Leukoc Biol 2003;74:942–51. [DOI] [PubMed] [Google Scholar]
- 27. Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev 1996;18: 188–204. [DOI] [PubMed] [Google Scholar]
- 28. Jain RB. Trends in serum cotinine concentrations among daily cigarette smokers: data from NHANES 1999–2010. Sci Total Environ 2014;472:72–7. [DOI] [PubMed] [Google Scholar]
- 29. Etter JF. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend 2016;160: 218–21. [DOI] [PubMed] [Google Scholar]
- 30. van Maanen MA, Lebre MC, van der Poll T. et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum 2009;60:114–22. [DOI] [PubMed] [Google Scholar]
- 31. Wu S, Luo H, Xiao X. et al. Attenuation of collagen induced arthritis via suppression on Th17 response by activating cholinergic anti-inflammatory pathway with nicotine. Eur J Pharmacol 2014;735:97–104. [DOI] [PubMed] [Google Scholar]
- 32. Yang Y, Yang Y, Yang J. et al. Regulatory effect of nicotine on collagen-induced arthritis and on the induction and function of in vitro-cultured Th17 cells. Mod Rheumatol 2014;24:781–7. [DOI] [PubMed] [Google Scholar]
- 33. Lindblad SS, Mydel P, Jonsson IM. et al. Smoking and nicotine exposure delay development of collagen-induced arthritis in mice. Arthritis Res Ther 2009;11:R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chujo S, Okamoto S, Sunahara R. et al. Cigarette smoke condensate extracts augment collagen-induced arthritis in mice. Int Immunopharmacol 2010;10:1194–9. [DOI] [PubMed] [Google Scholar]
- 35. Okamoto S, Adachi M, Chujo S. et al. Etiological role of cigarette smoking in rheumatoid arthritis: nasal exposure to cigarette smoke condensate extracts augments the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun 2011;404: 1088–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.