Abstract
Objectives
Our aim was to describe the burden of early dcSSc in terms of disability, fatigue and pain in the European Scleroderma Observational Study cohort, and to explore associated clinical features.
Methods
Patients completed questionnaires at study entry, 12 and 24 months, including the HAQ disability index (HAQ-DI), the Cochin Hand Function Scale (CHFS), the Functional Assessment of Chronic Illness Therapy-fatigue and the Short Form 36 (SF36). Associates examined included the modified Rodnan skin score (mRSS), current digital ulcers and internal organ involvement. Correlations between 12-month changes were also examined.
Results
The 326 patients recruited (median disease duration 11.9 months) displayed high levels of disability [mean (s.d.) HAQ-DI 1.1 (0.83)], with ‘grip’ and ‘activity’ being most affected. Of the 18 activities assessed in the CHFS, those involving fine finger movements were most affected. High HAQ-DI and CHFS scores were both associated with high mRSS (ρ = 0.34, P < 0.0001 and ρ = 0.35, P < 0.0001, respectively). HAQ-DI was higher in patients with digital ulcers (P = 0.004), pulmonary fibrosis (P = 0.005), cardiac (P = 0.005) and muscle involvement (P = 0.002). As anticipated, HAQ-DI, CHFS, the Functional Assessment of Chronic Illness Therapy and SF36 scores were all highly correlated, in particular the HAQ-DI with the CHFS (ρ = 0.84, P < 0.0001). Worsening HAQ-DI over 12 months was strongly associated with increasing mRSS (ρ = 0.40, P < 0.0001), decreasing hand function (ρ = 0.57, P < 0.0001) and increasing fatigue (ρ = −0.53, P < 0.0001).
Conclusion
The European Scleroderma Observational Study highlights the burden of disability in early dcSSc, with high levels of disability and fatigue, associating with the degree of skin thickening (mRSS). Impaired hand function is a major contributor to overall disability.
Keywords: early diffuse cutaneous systemic sclerosis, disability, hand function, fatigue, pain
Rheumatology key messages
Early dcSSc is associated with a high burden of disability, fatigue and pain.
In early dcSSc, impaired hand function is a major contributor to overall disability.
Patients with early dcSSc should be referred to a skilled multidisciplinary team.
Introduction
Patients with the diffuse cutaneous subtype of SSc have a high mortality due to early internal organ involvement of their disease. It is therefore understandable that in patients with this subtype of SSc, clinicians have tended to focus on early recognition and treatment of lung, heart and kidney involvement. Perhaps less well recognized is the substantial burden of disability, fatigue and pain of early dcSSc, caused in large part by progressive skin tightening and musculoskeletal manifestations: levels of disability have been found to be higher in patients with high than with low skin scores [1, 2], and in those with joint pain, tendon friction rubs and contractures [1]. Thus, early dcSSc can have a major impact on quality of life.
A Canadian study published in 2011 specifically addressed the impact of SSc in terms of ability to carry out everyday activities [3], and highlighted the importance of fatigue, pain and limitation of hand function. However, only 59 of the 464 patients studied definitely had the diffuse cutaneous subtype of SSc (and of unspecified duration). Functional impact, fatigue and pain specifically relating to early diffuse disease have been little studied. The European Scleroderma Observational Study (ESOS) [4] was a prospective observational study of treatment outcome in 326 patients with early dcSSc: data collected included a number of self-administered questionnaires relating to functional ability and quality of life. ESOS therefore afforded a unique opportunity to perform a detailed evaluation of disease impact in a large multinational cohort with very early disease (median disease duration from onset of skin thickening 11.9 months). Our aim was to describe, in the ESOS cohort, the burden of early dcSSc in terms of disability, fatigue and pain and to explore disease features that associate with this burden both at the baseline visit and over the subsequent 12–24 months. Although some summary data have been previously reported in the ESOS paper describing treatment efficacy [4], here we focus (and expand on) the different measures of disability, fatigue and pain.
Methods
ESOS study design
The study design is described fully elsewhere [4]. In summary, patients with early dcSSc were recruited into a prospective, observational cohort study (ClinicalTrials.gov Identifier: NCT02339441), the overall aim of which was to compare the effectiveness of four different treatment protocols (MTX, MMF, CYC or no immunosuppressant), selected on the basis of clinician and patient preference. A secondary objective of ESOS was to benchmark the severity of disability in patients with early dcSSc, examine the associates of disability and describe its patterns of change over the 2-year study period. The main inclusion criteria for ESOS were early dcSSc (skin involvement extending to proximal to elbow or knee and/or involving the trunk [5] and within 3 years of the onset of skin thickening, to ensure that patients were comparable to those being included into randomized controlled trials of early disease) and age >18 years. Patients attended 3-monthly for 12–24 months. The primary outcome measure of ESOS was modified Rodnan skin score (mRSS) [6] and secondary outcome measures included questionnaire-based measures of disability and fatigue as described below. The Ethics Committee of each participating centre approved the ESOS study, which this paper was part of, and each patient gave written informed consent.
Patients
Patient recruitment took place between July 2010 and September 2014. Demographic and clinical characteristics including age, gender, smoking habit, ethnicity, antibody status [anti-topoisomerase-1 (anti-Scl-70), anti-RNA polymerase III, anticentromere] and presence of visceral organ involvement were recorded for all patients. Three hundred and twenty-six patients from 50 centres (in 19 countries) were recruited: 65 started on MTX, 118 on MMF, 87 on CYC and 56 had no immunosuppressant [4]. The baseline characteristics of the patients are described in supplementary Table S1, available at Rheumatology Online.
Outcome measures relating to functional ability, fatigue and pain
At the baseline, 12- and 24-month visits, patients were asked to complete a set of patient questionnaires to assess functional ability, fatigue and pain. The patient questionnaires were the Scleroderma Specific HAQ [sHAQ, including the HAQ disability index (HAQ-DI)], the Cochin Hand Function Scale (CHFS), the Functional Assessment of Chronic Illness Therapy (FACIT) fatigue score and the Short Form 36 Health Survey (SF36), with brief descriptions and justification for inclusion as follows.
Scleroderma HAQ
Global disability in patients with SSc is usually measured by the HAQ, a self-report questionnaire consisting of 20 items divided into eight categories (dressing and grooming, arising, eating, walking, hygiene, reach, grip and activities), which are averaged into the final HAQ-DI score [7]. Items are rated from 0 (no difficulty) to 3 (unable to do). The sHAQ questionnaire [8] includes the generic HAQ-DI index and six additional disability measures, graded on a visual analogue scale (VAS) from 0 (least disability) to 100 (most disability). Using these VAS scales, patients self-assess the extent to which (in addition to pain), gastrointestinal symptoms, breathing problems, RP and digital ulcers interfere with their daily activities, with an additional scale for perceived overall disease severity from SSc. The HAQ-DI is a validated outcome measure for use in clinical studies in patients with SSc as per OMERACT criteria [9]. The HAQ-DI and sHAQ have both been widely applied in studies of SSc [10].
Cochin (Duruöz) Hand Function Scale
The CHFS (Duruöz) [11] index corresponds to the sum of 18 questions relating to the difficulty of daily manual activities at the time of assessment. Each individual question is ranked on a Likert scale from 0 (without difficulty) to 5 (impossible to do). The total score is obtained by adding the scores of all items (range 0–90). The reliability and validity of the CHFS have been demonstrated in patients with SSc [12, 13]. Because of translational issues, patients from certain centres did not complete the CHFS.
FACIT fatigue scale
The FACIT fatigue index is derived from a 13-item questionnaire, measuring the extent of a patient’s fatigue over the past week [14]. The final FACIT fatigue score is graded from 0 (higher fatigue) to 52 (lower fatigue).
SF36
The Medical Outcome Study 36-item SF36 questionnaire is a generic measure of health-related quality of life in relation to the previous 4 weeks. This self-administered questionnaire covers eight areas: physical function, physical role, bodily pain, general health, vitality, social function, emotional role and mental health. For each area, the score ranges from 0 (poorer health status) to 100 (better health status). Scores can also be summarized in two global scores: the physical (PCS) and mental (MCS) component summaries [15].
Statistical analysis
In analysing baseline and follow-up data, the immunosuppressant treatment assignment was not accounted for, given that the goal of this paper was not to establish a causal effect between any of these treatments and the progression of disability.
Baseline associations of disability
In order to assess baseline associations between patient characteristics and levels of disability, the distribution of each disability indicator was compared between levels of categorical variables (e.g. gender or presence of organ involvement) using the Kruskal–Wallis test. For continuous variables, Spearman’s correlation (ρ) was used to examine associations with the disability indicators. Each pair of continuous variables uses the subset of observations available to compute each ρ. Unadjusted P-values are reported, with an additional significance level supplied in Table 1 corresponding to the Šidák correction for multiple testing.
Table 1.
Baseline associates of disability indicators
| HAQ-DI(0–3), n = 307[3 most disabled] | CHFS(0–90), n = 230[90 most disabled] | FACIT fatigue(0–52), n = 310[0 most disabled] | SF36 physical index (0–100), n = 311[0 most disabled] | SF36 mental index(0–100), n = 311[0 most disabled] | sHAQ Pain VAS(0–100), n = 309[100 most disabled] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall indicator median (IQR) | 1.0 (0.4–1.8) | 11.0 (3.0–29.0) | 31.0 (20.0–41.0) | 37.4 (29.9–45.0) | 38.3 (34.3–44) | 29.0 (8.7–52.7) | ||||||
| Binary variables | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No |
| Female | 1.0 (0.4–1.9) | 0.8 (0.4–1.4) | 12.0 (3.0–29.0) | 9.0 (3.0–19.0) | 30.7 (19.0–41.0) | 32.5 (24.5–41.5) | 37.1 (29.3–44.7) | 37.7 (31.7– 45.4) | 37.3 (33.2–44.0)* | 39.8 (35.9–44.4)* | 30.0 (9.3–53.3) | 27.7 (4.7–49.0) |
| Current or previous steroid use | 1.3 (0.5–2.0)*** | 0.9 (0.4–1.5)*** | 16.0 (5.0– 34.0)*** | 8.0 (2.0–22.0)*** | 27.5 (17.2–38.5)*** | 34.0 (24.0–42.0)*** | 35.6 (28.2–41.8)*** | 38.6 (32.0– 45.8)*** | 39.1 (34.4–44.4) | 37.8 (34.3–43.5) | 35.0 (10.7–59.0)** | 24.7 (6.0–46.7)** |
| Previous use of immunosuppressants | 0.9 (0.5–2.0) | 1.0 (0.4–1.6) | 10.0 (3.0–22.0) | 11.0 (3.0–29.0) | 35.0 (24.0–38.0) | 31.0 (20.0–41.0) | 37.9 (29.2–43.0) | 37.3 (30.4–45.0) | 36.6 (34.3–42.5) | 38.5 (34.3–44.0) | 24.3 (5.7–48.0) | 29.2 (8.8–54.0) |
| Current digital ulcers | 1.5 (0.6–2.3)*** | 0.9 (0.4–1.6)*** | 18.5 (3.5–43.5)** | 10.0 (3.0–26.0)** | 27.0 (20.0–37.5)* | 32.3 (20.0–42.0)* | 36.0 (28.5–41.8) | 37.5 (30.8–45.1) | 37.6 (32.7–43.2) | 38.5 (34.4–44.2) | 37.0 (6.3–63.7) | 27.3 (9.0–50.3) |
| Pulmonary fibrosis | 1.5 (0.8–2.1)*** | 0.9 (0.4–1.6)*** | 23.5 (11.0–40.0)** | 10.0 (3.0–26.0)** | 21.5 (14.0–30.5)**** | 33.0 (22.0–42.0)**** | 31.8 (24.9–38.9)*** | 37.8 (31.0–45.1)*** | 37.9 (33.9–42.3) | 38.3 (34.3–44.1) | 44.0 (21.0–67.3)** | 26.7 (6.7–50.7)** |
| Pulmonary hypertension | 1.4 (0.7–2.2)* | 0.9 (0.4–1.6)* | 13.0 (1.0–21.0) | 11.0 (3.0–29.0) | 22.5 (15.5–31.0)*** | 32.5 (21.0–42.0)*** | 31.4 (24.2–42.4)* | 37.5 (30.7–45.0)* | 38.8 (33.6–43.9) | 38.2 (34.3–44.0) | 30.0 (4.5–69.0) | 29.0 (8.8–51.0) |
| Renal involvement | 1.2 (0.6–2.1) | 1.0 (0.4–1.6) | 18.0 (4.0–33.0) | 11.0 (3.0–26.0) | 23.5 (17.0–34.0)** | 32.0 (21.0–42.0)** | 35.9 (24.5–45.7) | 37.4 (30.7–44.7) | 35.7 (32.0–40.6) | 38.5 (34.4–44.1) | 30.0 (5.0–59.0) | 29.0 (9.0–52) |
| Cardiac involvement | 1.3 (0.9–2.3)*** | 0.9 (0.4–1.7)*** | 11.0 (5.0–39.0) | 11.0 (3.0–27.0) | 23.5 (12.0–34.0)**** | 32.0 (21.0–42.0)**** | 32.0 (24.1–40.2)*** | 37.6 (30.8–45.1)*** | 36.1 (32.7–41.9)* | 38.5 (34.4–44.2)* | 45.2 (16.3–59.3)* | 27.7 (7.3–51.7)* |
| Muscle involvement | 1.9 (1.0–2.3)*** | 0.9 (0.4–1.6)*** | 16.0 (5.0–55.0) | 10.0 (3.0–26.0) | 25.5 (16.0–38.0)* | 32.0 (21.0–42.0)* | 31.5 (25.5–39.2)*** | 37.6 (30.8–45.1)*** | 38.6 (32.4–45.0) | 38.3 (34.4–43.9) | 40.7 (5.7–64.7) | 28.3 (8.7–51.0) |
| Anti-topoisomerase, anti-ScL70 | 0.9 (0.4–1.7) | 1.0 (0.5–1.8) | 11.0 (3.0–26.0) | 10.0 (3.0–29.0) | 30.3 (19.0–41.0) | 32.0 (22.0–42.0) | 37.4 (29.7–44.5) | 37.4 (31.0–45.1) | 38.5 (34.3–43.7) | 38.1 (34.4–44.0) | 31.0 (10.7–58.7)* | 25.3 (3.7–49.0)* |
| Anti-RNA polymerase III | 1.0 (0.6–1.6) | 0.9 (0.4–1.7) | 10.5 (6.0–29.0) | 11.0 (2.0–26.0) | 29.6 (18.0–41.0) | 30.9 (20.5–41.5) | 35.6 (29.6–42.7) | 37.6 (30.6–45.0) | 38.9 (33.5–45.6) | 37.9 (33.6–43.7) | 27.5 (10.5–51.2) | 30.2 (8.7–54.7) |
| Anticentromere | 0.6 (0–1.5)* | 1.0 (0.4–1.8)* | 8.5 (0.0–26.0) | 11.0 (3.0–27.0) | 34.5 (22.0–44.0) | 31.0 (20.0–41.0) | 38.5 (33.0–46) | 37.3 (29.9–44.7) | 37.6 (31.9–45.3) | 38.2 (34.4–44.0) | 36.0 (15.3–47.0) | 27.3 (7.0–53.7) |
| Continuous variables | Spearman's ρ | Spearman's ρ | Spearman's ρ | Spearman's ρ | Spearman's ρ | Spearman's ρ | ||||||
| Age | −0.05 | −0.04 | 0.04 | 0.08 | 0.10* | −0.11* | ||||||
| Months since onset of skin thickening | 0.01 | −0.01 | −0.01 | 0.01 | 0.01 | 0.02 | ||||||
| mRSS, 0–51 | 0.34**** | 0.35**** | −0.20**** | −0.27**** | 0.03 | 0.17*** | ||||||
| mRSS in fingers and hand dorsa, 0–12 | 0.23**** | 0.32**** | −0.13** | −0.20**** | 0.02 | 0.13** | ||||||
| Haemoglobin (g/l) | −0.29**** | −0.18*** | 0.24**** | 0.26**** | 0.03 | −0.14** | ||||||
| White blood count, ×109/l | 0.08 | 0.06 | −0.08 | −0.14** | 0.04 | 0.01 | ||||||
| Platelets, ×109/l | 0.21**** | 0.20*** | −0.08 | −0.21**** | 0 | 0.10* | ||||||
| ESR, mm/h | 0.23**** | 0.22*** | −0.23**** | −0.27**** | 0.07 | 0.16** | ||||||
| CRP, mg/l | 0.34**** | 0.27**** | −0.22**** | −0.34**** | 0.03 | 0.21*** | ||||||
| Plasma creatinine, μmol/l | −0.09 | −0.09 | 0.01 | 0.03 | −0.01 | −0.03 | ||||||
| eGFR, ml/min | 0.01 | 0.04 | 0.02 | −0.02 | −0.04 | 0.06 | ||||||
| FVC, % predicted | −0.20**** | −0.16** | 0.24**** | 0.22**** | −0.06 | −0.09 | ||||||
| DLCO, % predicted | −0.21**** | −0.19*** | 0.22**** | 0.25**** | −0.01 | −0.06 | ||||||
| HAQ-DI, 0–3 | 1 | 0.84**** | −0.67**** | −0.72**** | −0.18**** | 0.57**** | ||||||
| CHFS, 0–90 | 0.84**** | 1 | −0.63**** | −0.61**** | −0.19*** | 0.59**** | ||||||
| FACIT fatigue score, 0–52 | −0.67**** | −0.63**** | 1 | 0.67**** | 0.29**** | −0.52**** | ||||||
| SF36 physical component, 0–100 | −0.72**** | −0.61**** | 0.67**** | 1 | −0.02 | −0.61**** | ||||||
| SF36 mental component, 0–100 | −0.18**** | −0.19*** | 0.29**** | −0.02 | 1 | −0.12** | ||||||
| sHAQ overall, 0–100 | 0.59**** | 0.55**** | −0.59**** | −0.58**** | −0.17*** | 0.62**** | ||||||
| sHAQ pain, 0–100 | 0.57**** | 0.59**** | −0.52**** | −0.61**** | −0.12** | 1 | ||||||
| sHAQ Raynaud's phenomenon, 0–100 | 0.37**** | 0.43**** | −0.41**** | −0.36**** | −0.16*** | 0.54**** | ||||||
| sHAQ finger ulcers, 0–100 | 0.26**** | 0.29**** | −0.23**** | −0.30**** | −0.04 | 0.39**** | ||||||
| sHAQ intestinal problems, 0–100 | 0.36**** | 0.37**** | −0.45**** | −0.40**** | −0.18*** | 0.49**** | ||||||
| sHAQ breathing problems, 0–100 | 0.36**** | 0.33**** | −0.43**** | −0.45**** | −0.06 | 0.43**** | ||||||
Index medians (IQR) across levels of binary variables and correlations with continuous variables. For binary variables, P-values from the Kruskal–Wallis test, comparing distribution of disability indicator between levels of each binary variable. For continuous variables, P-values for the correlation (ρ) significance. *P < 0.10, **P < 0.05, ***P < 0.01, ****P < 1.385 × 10−3 (Šidák significance threshold, adjusted for multiple testing for α = 0.05 and 37 tests). Each pair of variables in the table above used the subset of patients available, according to data availability. The number of patients having data for each disability index was equal to or higher than 93.9% except for the pairs involving the following variables: anti-RNA polymerase III, ESR, CRP, plasma creatinine, eGFR and DLCO. For those variables, the coverage rates did not go below 71.8%. DLCO: carbon monoxide diffusing capacity; eGFR: estimated glomerular filtration rate; FVC: forced vital capacity; HAQ-DI: Health Assessment Questionnaire disability index; mRSS: modified Rodnan skin score (17 sites); sHAQ: Scleroderma Health Assessment Questionnaire; CHFS: Cochin Hand Function Scale; SF36: Short Form 36 Health Survey; FACIT: Functional Assessment of Chronic Illness Therapy.
Changes in disability
Patterns of change were examined by computing the shares of improvers and regressors (of any magnitude) for each indicator during the first 12 months.
Associates of changes in disability
Spearman’s correlation (ρ) was used to examine associations between the 12-month evolution of each disability indicator with the evolution in corresponding continuous variables. For instance, we evaluated the association between the increase in skin fibrosis (mRSS) and the change in disability. The numbers of observations for each pair of variables differ due to data availability and loss to follow-up. Simple linear regression was used to translate these correlations into marginal effects due to changes.
Results
Baseline values
Baseline distribution of HAQ-DI
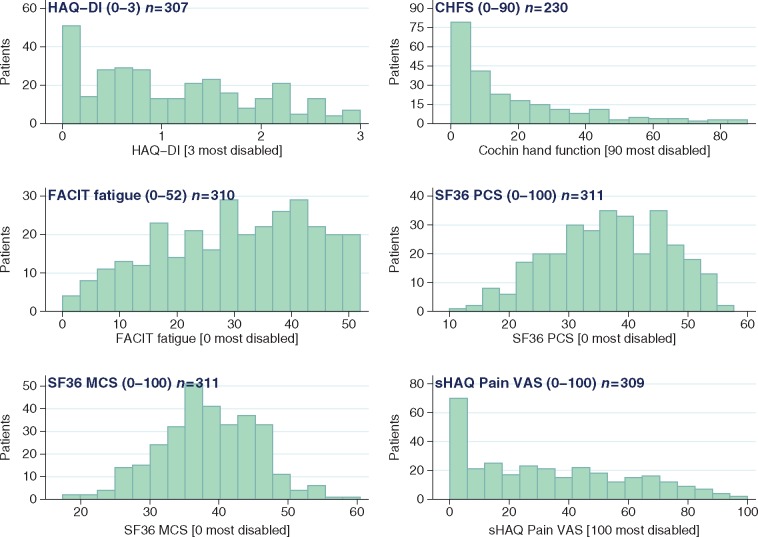
The mean (s.d.) and median (interquartile range, IQR) HAQ-DI scores were 1.1 (0.83) and 1.0 (0.4–1.8), respectively (Fig. 1 and Table 1). Fifty (16.3%) patients reported a HAQ-DI lower than 0.15, including 36 null scores, indicating no or very low disability for these patients. On the other hand, 10 (3.3%) patients reported a score of 2.75 or higher, indicating severe disability.
Fig. 1.
Baseline distribution of disability indicators
CHFS: Cochin Hand Function Scale; FACIT: Functional Assessment of Chronic Illness Therapy; HAQ-DI: HAQ disability index; MCS: mental component summary; PCS: physical component summary; SF36: Short Form 36 Health Survey; sHAQ: Scleroderma HAQ.
By subdividing the HAQ-DI index into its eight components, grip and activity contributed the most, with 49.0% and 39.0% of patients being ‘unable’ or reporting much difficulty in the corresponding questions (Fig. 2). Rising and walking were the items that contributed the least to disability with minimal impact on the total HAQ-DI score (78.0 and 75.8% were able to perform these tasks without any or only some difficulty).
Fig. 2.
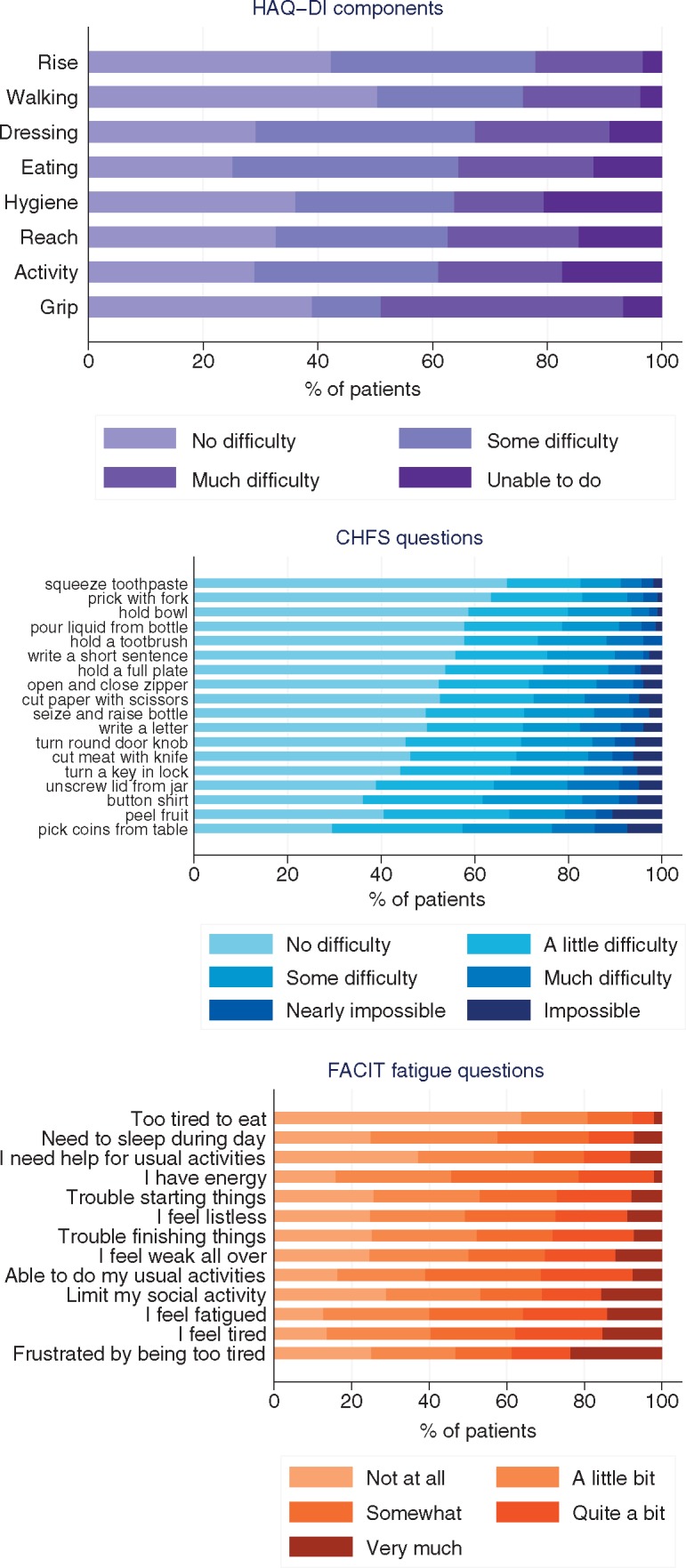
Composition of disability indicators at baseline
For each questionnaire item, the distribution of answers is displayed based on the number of respondents for each individual question. CHFS: Cochin Hand Function Scale; FACIT: Functional Assessment of Chronic Illness Therapy; HAQ-DI: HAQ disability index.
Baseline distribution of CHFS
Out of 230 patients with a CHFS score at baseline, the mean (s.d.) and median (IQR) CHFS were 18.7 (20.7) and 11 (3.0–29.0), respectively (Fig. 1 and Table 1), while 28 (12.2%) patients reported no impairment in hand function according to the CHFS.
Out of 18 activities assessed by the hand function questionnaire, picking up coins, peeling fruit and buttoning a shirt (i.e. activities involving fine finger movements) had the highest mean difficulty (Fig. 2). On the other hand, squeezing a new tube of toothpaste, pricking things with a fork and holding a bowl (activities requiring less dexterity) had the lowest mean difficulty.
Baseline distribution of FACIT fatigue
The mean (s.d.) and median (IQR) scores were 30.2 (13.0) and 31.0 (20.0–41.0), respectively (Fig. 1 and Table 1). Among the questionnaire items, 110 patients (35.8%) reported feeling quite a bit or very much fatigued. By the same approach, 120 (38.7%) patients reported frustration from being too tired to do the things they want to do and 23 (7.4%) reported being too tired to eat (Fig. 2).
Other measures
The mean (s.d.) and median (IQR) scores for the SF36 physical component (PCS) were 36.9 (9.7) and 37.4 (29.9–45.0), respectively, and were 38.5 (7.1) and 38.3 (34.3–44.0) for the mental component (MCS). For the sHAQ pain scale, the mean (s.d.) and median (IQR) scores were 32.9/100 (26.9) and 29.0/100 (8.7–52.7) (Fig. 1 and Table 1).
Baseline associations
HAQ-DI [0–3 (3 most disabled)]
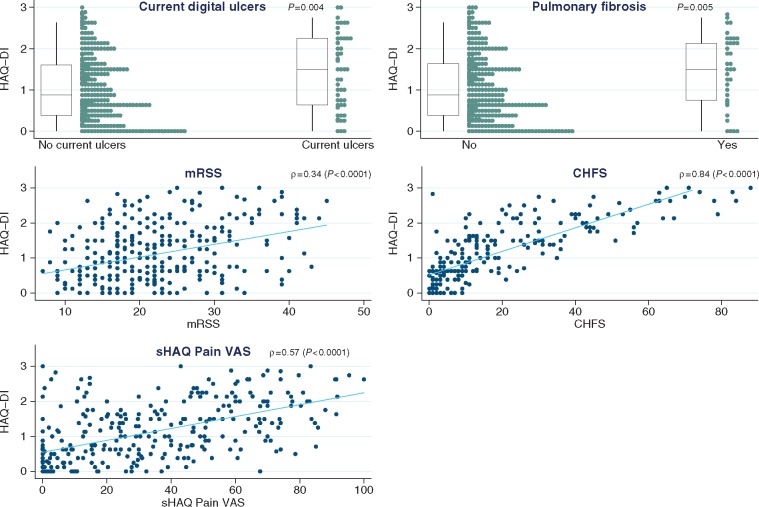
Table 1 and Fig. 3 indicate that many clinical and laboratory features of dcSSc were associated with increased disability: current or previous steroid use (P = 0.002), current digital ulcers (P = 0.004, with a difference in medians of 0.6 unit between those with and without ulcers), pulmonary fibrosis [P = 0.005, and with reduced forced vital capacity (FVC; P = 0.001) and carbon monoxide diffusing capacity (DLCO; P = 0.001)], cardiac involvement (P = 0.005) and muscle involvement (P = 0.002). Disability was not correlated with the duration of skin thickening, although there was a correlation with total skin thickening (ρ = 0.34, P < 0.0001) as well as with skin thickening as measured in the fingers and dorsum of hand (ρ = 0.23, P < 0.0001). Lower levels of haemoglobin and higher levels of platelets, ESR and CRP associated with higher HAQ-DI scores (P < 0.0005 for all four). As expected, the HAQ-DI score was strongly associated with the other disability indexes and in particular the CHFS (ρ = 0.84, P < 0.0001) (Fig. 3).
Fig. 3.
Associates of HAQ disability index
For the relation between HAQ-DI and binary variables (here current digital ulcers and pulmonary fibrosis), box plots (with median and interquartile ranges) summarize the distribution of the index within each level of the binary variable. In addition, a strip plot shown next to each box plot gives more detail on the dispersion of all individual points. For the relation between HAQ-DI and continuous variables (here mRSS, CHFS and sHAQ Pain VAS), a scatter plot is shown for each pair and superimposed with a linear regression line to describe the correlation direction. CHFS: Cochin Hand Function Scale; mRSS: modified Rodnan skin score; sHAQ: Scleroderma HAQ.
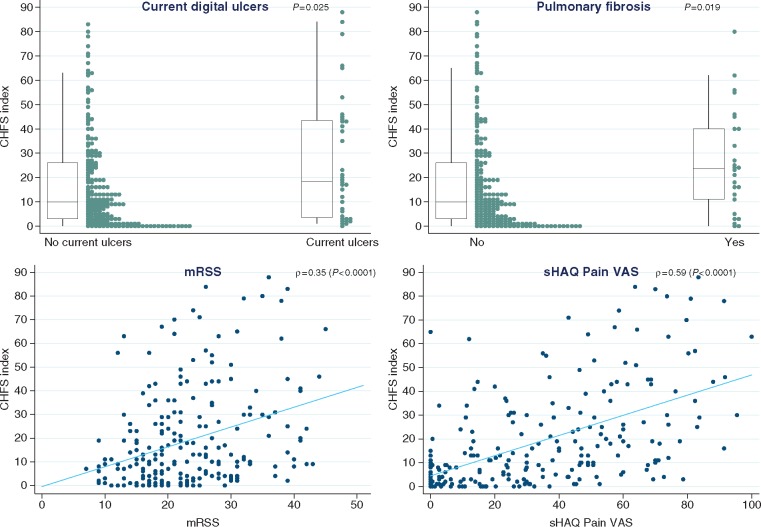
CHFS hand function [0–90 (90 most disabled)]
The following were associated with reduced hand function: current or previous corticosteroid use (P = 0.009), current digital ulcers (P = 0.025 and 8.5 difference in median CHFS scores) and pulmonary fibrosis [P = 0.019, and with reduced FVC (P = 0.016) and DLCO (P = 0.008)] (Table 1 and Fig. 4). Skin thickening was also correlated with impaired hand function (ρ = 0.35, P < 0.0001) although its duration was not (P = 0.901). Among other disability indicators, pain and the severity of RP (from the sHAQ questionnaire) were both strongly associated with impaired hand function (ρ = 0.59 for pain and ρ = 0.43 for Raynaud’s, P < 0.0001 for both).
Fig. 4.
Associates of Cochin hand function scale
For the relation between CHFS and binary variables (here current digital ulcers and pulmonary fibrosis), box plots (with median and interquartile ranges) summarize the distribution of the index within each level of the binary variable. In addition, a strip plot shown next to each box plot gives more detail on the dispersion of all individual points. For the relation between CHFS and continuous variables (here mRSS and sHAQ Pain VAS), a scatter plot is shown for each pair and superimposed with a linear regression line to describe the correlation direction. CHFS: Cochin Hand Function Scale; mRSS: modified Rodnan skin score; sHAQ: Scleroderma HAQ.
FACIT fatigue [0–52 (0 most disabled)]
Associated with increased fatigue were current or previous use of corticosteroids (P = 0.004) and several indicators of organ involvement: pulmonary fibrosis [P < 0.0005, and with reduced FVC (P < 0.0001) and DLCO (P < 0.0005)] and pulmonary hypertension (P = 0.006), and renal and cardiac involvement (P = 0.013 and P = 0.001, respectively) (Table 1). Skin thickening was also associated with more fatigue (ρ = −0.20, P = 0.0005). Lower levels of haemoglobin (P < 0.0001) and higher levels of CRP (P < 0.001) and ESR (P < 0.0005) were associated with more fatigue.
SF36 physical and mental components [0−100 (0 most disabled)]
As shown in Table 1, associations of lower SF36 physical component (PCS) scores (representing increased physical disability) included current or previous use of corticosteroids (P = 0.004), pulmonary fibrosis [P = 0.004, and with reduced FVC (P < 0.0005) and DLCO (P < 0.0001)], cardiac (P = 0.007) and muscle involvement (P = 0.009), and increased skin thickening (P < 0.0001). The mental component (SF36 MCS) was not significantly associated with organ involvement measures and was not significantly correlated with the extent of skin thickening. The strongest association of the SF36 MCS score was with fatigue (ρ = 0.29, P < 0.0001).
sHAQ Pain VAS scale [0−100 (100 most disabled)]
Patients with pulmonary fibrosis (P = 0.033) and cardiac involvement (P = 0.088) reported more pain compared with those without (Table 1). Older patients tended to report slightly lower levels of pain (ρ = −0.11, P = 0.055). Skin thickening was associated with more pain (ρ = 0.17, P = 0.002), as were higher levels of ESR and CRP (P = 0.015 and P = 0.002, respectively). Pain correlated strongly with other disability measures, including HAQ-DI, hand function and fatigue (P < 0.0001 for all three).
Changes in disability
Trajectories of disability
The 12-month changes in all indicators followed an approximately normal distribution centred around zero and underline the heterogeneity in the evolution of disability (supplementary Fig. S1, available at Rheumatology Online). For the HAQ-DI, changes ranged from −1.95 to 1.67 in the first 12 months. There was a tendency, for each measure of disability, for half of the cohort to improve while the other became more disabled.
Supplementary Fig. S2 available at Rheumatology Online plots the baseline values of the HAQ-DI, CHFS, FACIT fatigue, SF36 (physical and mental components) and sHAQ Pain VAS against their change in the 12 months. In general, those with more disability at baseline tended to improve while those with the least disability tended to become worse (suggesting regression to the mean). Supplementary Fig. S3, available at Rheumatology Online, describes the evolution of these indicators from baseline to 24 months, further underlining the variability in individual trajectories.
Associates of changing disability
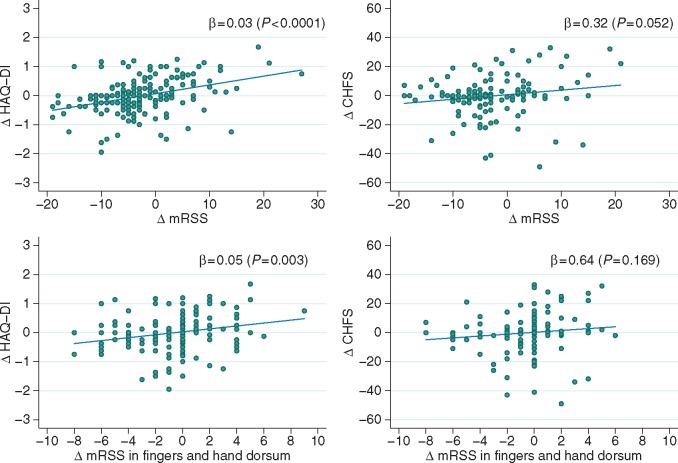
The data on 12-month changes are shown in supplementary Table S2, available at Rheumatology Online, with changes in several of the measures of disability correlating with each other. Worsening disability according to HAQ-DI was strongly associated with increasing overall skin thickening (ρ = 0.40, P < 0.0001), decreasing hand function (ρ = 0.57, P < 0.0001) and increasing fatigue (ρ = −0.53, P < 0.0001). In addition, worsening hand function was also associated with increasing fatigue (ρ = −0.50, P < 0.0001). In a regression setting, a 5 unit increase in the skin score resulted in a 0.15 unit increase in the HAQ-DI (0–3) and a 1.58 increase in the CHFS index (0–90) (Fig. 5).
Fig. 5.
Co-movements of skin score and disability indicators in first 12 months
These four scatter plots describe the 12 month changes in mRSS (overall and in fingers and hand dorsum) with respect to the change in HAQ-DI and CHFS. In each axis, the symbol Δ denotes change. A regression line shows in each case the marginal effect of a 1 unit increase in the skin score, equal to the regression coefficient (β), shown here alongside its significance P-value. CHFS: Cochin Hand Function Scale; HAQ-DI: HAQ disability index; mRSS: modified Rodnan skin score.
Discussion
ESOS has benchmarked the high burden of disability, fatigue and pain in patients with early dcSSc. Although several recent studies (as discussed below) have reported the functional disability, fatigue and pain associated with SSc, ESOS is the first to make such a detailed analysis specifically in patients with the early dcSSc subtype, and has provided new insights into the associates of disability and fatigue in this patient population. Disability, fatigue and pain were all associated with mRSS (the greater the degree of skin thickening, the greater the disability, fatigue and pain), with hand function, and with internal organ involvement. In addition, HAQ-DI and hand function were reduced in patients with current digital ulcers, reinforcing previous reports of the functional impact of SSc-related digital ulcers [16–20].
Patients with the early diffuse cutaneous subtype of SSc have a particular set of problems, with rapidly progressive skin thickening (often with early contractures) and internal organ involvement. We have shown that this skin thickening is likely to be a key driver of disability, in large part through its effect on hand function: not only were HAQ-DI and CHFS associated with mRSS (reflecting the extent of skin thickening) at baseline, but changes in HAQ-DI over 12 months correlated with changes in mRSS (including specifically with changes in skin score relating to the hands). This suggests the changes are not generated simply by regression to the mean, and makes clinical sense: stiff, painful, hands (due to skin thickening) impact significantly on an individual’s ability to perform activities of everyday living, and this is further exacerbated if there is concomitant finger ulceration.
The association between both disability and fatigue with internal organ involvement is also of interest. Here inter-relationships are likely to be more complex. In part, internal organ involvement will be an index of disease severity (associating with extent of skin thickening [4, 21]), but some internal organ involvements, for example pulmonary fibrosis and muscle involvement, are in themselves fatiguing.
Steen and Medsger [8] reported a mean HAQ-DI of 1.22 in 222 patients with early dcSSc (<3 years’ duration), and 0.72 in patients with (any duration of) lcSSc. This value of 1.22 is comparable to that in the ESOS cohort (1.1), and indicates a high level of disability. Steen and Medsger also reported that among the 163 patients with contemporaneous HAQ-DI and skin score data, increasing and decreasing skin scores were associated (respectively) with an increase and decrease in HAQ-DI, with changes in skin score correlating with changes in HAQ-DI (as we have also found). Although it is unclear how many of these 163 patients had diffuse cutaneous disease, 55% of the whole cohort had dcSSc [8]. The HAQ-DI score of 1.1 also closely matches scores in a meta-analysis of clinical trials in dcSSc including 629 patients [22]. High HAQ-DI scores have, unsurprisingly, been associated with work disability in patients with SSc [23], just one reflection of how functional disability impacts on patients’ lives in many different ways.
Previous studies have reported that hand function is more compromised in patients with dcSSc than lcSSc [12, 13], but these were not studies conducted specifically in patients with early disease. In the ESOS cohort, the degree of hand impairment correlated with skin score, and activities involving fine finger movement were especially affected. Although Brower and Poole [12] did not report a significant correlation between CHFS and skin score, most patients in this study had established limited cutaneous disease (mean duration 11 years) and therefore it is possible that other factors often associated with disease duration, for example severity of digital vasculopathy and degree of contracture, could have been more influential than skin tightening in impairing hand function (as was found by Mouthon et al. [16]). Our finding suggests that in early dcSSc, skin thickening in itself is a major contributor to hand disability, and that impaired hand function is a major contributor to overall disability, confirming the results obtained by Rannou et al. [13] who identified that hand disability explained 75% of the variance of global disability in a cohort of patients with either lcSSc or dcSSc. In that study [13], a significant difference was observed between patients with lcSSc and dSSc for mean CHFS score [11.07 ± 11.04 vs 23.48 ± 19.45, respectively (P = 0.01)]. In the ESOS cohort, the median CHFS was 11, which is lower than in other studies, a finding possibly explained by the early disease: Brower and Poole [12] reported a mean score of 21.1 in their cohort of 40 patients with a mean disease duration of 11 years [12], whereas Mouthon et al. [16] reported a mean score of 20.15 in a cohort of 213 patients with a mean disease duration of 10.4 years.
Fatigue is now recognized as a very major symptom in most patients with SSc with a number of recent studies describing fatigue and its associates [3, 24–28], previously reported associates of fatigue including pain and poor physical function [25], symptoms suggestive of internal organ involvement (breathing problems and gastrointestinal symptoms [26]) and ability to work [27]. Again, the contribution of ESOS has been to quantify fatigue and describe the associates of fatigue specifically in early dcSSc: these associates include extent of skin thickening and internal organ involvement. SF36 MCS scores were lower than in some other studies of SSc [13, 29], indicating more disability, perhaps due to all patients in ESOS having early diffuse cutaneous disease.
Pain, as measured by VAS, was also associated with degree of skin thickening. We found that pain was also associated with all measures of disability, confirming results from a previous study of 89 patients (67 had dcSSc) in whom pain was correlated with both physical and mental components of the SF36 [30], and from a very recent study from the Canadian Scleroderma Research Group [31]. However, the majority of patients in the latter study had limited cutaneous disease [31]. The pain associated with the skin thickening of early dcSSc has in the past been insufficiently recognized.
In conclusion, the message of our analysis is straightforward—early dcSSc is disabling, fatiguing and associated with severe compromise in hand function and with pain. As options for treating the life-threatening organ-based complications improve, the non-lethal burden is likely to require increasing attention for therapy. Pending the development of effective treatments to prevent or reverse progression of this devastating disease, clinicians need to be aware of this huge burden of disability, and recognize each patient’s need for multidisciplinary input including physiotherapy [32], occupational therapy and pain management, to minimize the impact on the individual. Ideally all patients with early dcSSc should be referred at the earliest opportunity to a skilled multidisciplinary team.
Supplementary Material
Acknowledgements
We are grateful to Dr Holly Ennis for study set-up and to her and Dr Graham Dinsdale for project co-ordination during the earlier phases of the study. Also to members of the independent oversight board: Stephen Cole, Dinesh Khanna and Frank Wollheim.
Funding: This study was a part of ESOS, which was funded by a grant from the EULAR (European League Against Rheumatism) Orphan Disease Programme. Additional funding from Scleroderma and Raynaud’s UK allowed a one-year extension of the study.
Disclosure statement: H.G. has done consultancy work and received honoraria from Actelion, UK. C.P.D. has done consultancy for GlaxoSmithKline (GSK), Actelion, Bayer, Inventiva and Merck-Serono, received research grant funding from GSK, Actelion, CSL Behring and Inventiva, received speaker’s fees from Bayer and given trial advice to Merck-Serono. A.R. receives funding from AstraZeneca. A.L.H. has done consultancy work for Actelion, served on a Data Safety Monitoring Board for Apricus, received research funding and speaker’s fees from Actelion, and speaker’s fees from GSK. S.P. has received research grants from Actelion Pharmaceuticals Australia, Bayer, CSL Biotherapies, GlaxoSmithKline Australia and Pfizer and speaker fees from Actelion. L.C. has done advisory board work for Gilead and Actelion and served on Data Safety Monitoring Boards for Cytori and Reata. O.D. had consultancy relationships and/or has received research funding from 4D Science, AbbVie, Actelion, Active Biotec, Bayer, BiogenIdec, Bristol-Myers Squibb (BMS), Boehringer Ingelheim, ChemomAb, EpiPharm, espeRare foundation, Genentech/Roche, GSK, Inventiva, iQone Healthcare, Lilly, medac, Mepha, MedImmune, Mitsubishi Tanabe Pharma, Pharmacyclics, Pfizer, Sanofi, Serodapharm and Sinoxa in the area of potential treatments of scleroderma and its complications and has a patent mir-29 for the treatment of systemic sclerosis licensed. U.M.-L. was funded in part by EUSTAR, EULAR and the European Community (Desscipher FP 7 Program). J.M.v.L. has received honoraria from Arthrogen, BMS, Eli Lilly, MSD, Pfizer, Roche, and research grants from AstraZeneca, Genentech and MSD. T.P.S. has received support from UCB, Roche, Pfizer and Novartis. W.G. has received teaching fees from Pfizer. M.E.A. has undertaken advisory board work and received honoraria from Actelion and received speaker’s fees from BMS. F.H. had her attendance at the Systemic Sclerois World Congress, Lisbon 18–20 February 2016 sponsored by Actelion Pharmaceutical and Actelion Pharmaceutical part-funded Specialist Nurse 2017–19 (£50 848). N.D. has done consultancy work for Abbvie, Pfizer, Roche and MSD, received speaker’s fees from AbbVie, Boehringer-Ingelheim, Pfizer, Richter Gedeon, Roche and MSD. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Poole JL, Steen VD.. The use of the Health Assessment Questionnaire (HAQ) to determine physical disability in systemic sclerosis. Arthritis Care Res 1991;4:27–31. [DOI] [PubMed] [Google Scholar]
- 2. Hudson M, Thombs BD, Steele R.. Clinical correlates of quality of life in systemic sclerosis measured with the World Health Organisation Disability Assessment Schedule II. Arthritis Care Res 2008;59:279–84. [DOI] [PubMed] [Google Scholar]
- 3. Bassel M, Hudson M, Taillefer SS. et al. Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian National Survey. Rheumatology 2011;50:762–7. [DOI] [PubMed] [Google Scholar]
- 4. Herrick AL, Pan X, Peytrignet S. et al. An observational study of treatment outcome in early diffuse cutaneous systemic sclerosis: the European Scleroderma Observational Study (ESOS). Ann Rheum Dis 2017;76:1207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. LeRoy EC, Black C, Fleischmajer R. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 6. Clements P, Lachenbruch P, Siebold J. et al. Inter- and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995;22:1281–5. [PubMed] [Google Scholar]
- 7. Merkel PA, Herlyn K, Martin RW. et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum 2002;46:2410–20. [DOI] [PubMed] [Google Scholar]
- 8. Steen VD, Medsger TA.. The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum 1997;40:1984–91. [DOI] [PubMed] [Google Scholar]
- 9. Furst DE. Outcome measures in rheumatologic clinical trials and systemic sclerosis. Rheumatology 2008;47:v29–30. [DOI] [PubMed] [Google Scholar]
- 10. Johnson SR, Hawker GA, Davis AM.. The health assessment questionnaire disability index and scleroderma health assessment questionnaire in scleroderma trials: An evaluation of their measurement properties. Arthritis Care Res 2005;53:256–62. [DOI] [PubMed] [Google Scholar]
- 11. Duruoz MT, Poiraudeau S, Fermanian J. et al. Development and validation of a rheumatoid hand functional disability scale that assesses functional handicap. J Rheumatol 1996;23:1167–72. [PubMed] [Google Scholar]
- 12. Brower LM, Poole JL.. Reliability and validity of the Duruöz Hand Index in persons with systemic sclerosis (scleroderma). Arthritis Care Res 2004;51:805–9. [DOI] [PubMed] [Google Scholar]
- 13. Rannou F, Poiraudeau S, Berezne A. et al. Assessing disability and quality of life in systemic sclerosis: Construct validities of the Cochin Hand Function Scale, Health Assessment Questionnaire (HAQ), Systemic Sclerosis HAQ, and Medical Outcomes Study 36-Item Short Form Health Survey. Arthritis Care Res 2007;57:94–102. [DOI] [PubMed] [Google Scholar]
- 14. Webster K, Cella D, Yost K.. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ware JE, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). 1. Conceptual framework and item selection. Med Care 1992;2:473–83. [PubMed] [Google Scholar]
- 16. Mouthon L, Mestre-Stanislas C, Bérezné A. et al. Impact of digital ulcers on disability and health-related quality of life in systemic sclerosis. Ann Rheum Dis 2010;69:214–7. [DOI] [PubMed] [Google Scholar]
- 17. Khimdas S, Harding S, Bonner A. et al. Associations with digital ulcers in a large cohort of systemic sclerosis: Results from the Canadian Scleroderma Research Group Registry. Arthritis Care Res 2011;63:142–9. [DOI] [PubMed] [Google Scholar]
- 18. Bérezné A, Seror R, Morell-Dubois S. et al. Impact of systemic sclerosis on occupational and professional activity with attention to patients with digital ulcers. Arthritis Care Res 2011;63:277–85. [DOI] [PubMed] [Google Scholar]
- 19. Matucci-Cerinic M, Kreig T, Guillevin L. et al. Eludicating the burden of recurrent and chronic digital ulcers in systemic sclerosis: long-term results from the DUO registry. Ann Rheum Dis 2016;75:1770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brand M, Hollaender R, Rosenberg D. et al. An observational cohort study of patients with newly diagnosed digital ulcer disease secondary to systemic sclerosis registered in the EUSTAR database. Clin Exp Rheumatol 2015;33(Suppl. 91):S47–54. [PubMed] [Google Scholar]
- 21. Clements PJ, Hurwitz EL, Wong WK. et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis. Arthritis Rheum 2000;43:2445–54. [DOI] [PubMed] [Google Scholar]
- 22. Merkel PA, Silliman NP, Clements PJ. et al. Patterns and predictors of change in outcome measures in clinical trials in scleroderma: An individual patient meta-analysis of 629 subjects with diffuse cutaneous systemic sclerosis. Arthritis Rheum 2012;64:3420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ouimet JM, Pope JE, Gutmanis I, Koval J.. Work disability in scleroderma is greater than in rheumatoid arthritis and is predicted by high HAQ scores. Open Rheum J 2008;2:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Lankveld WG, Vonk MC, Teunissen H, van den Hoogen FH.. Appearance self-esteem in systemic sclerosis—subjective experience of skin deformity and its relationship with physician-assessed skin involvement, disease status and psychological variables. Rheumatology 2007;46:872–6. [DOI] [PubMed] [Google Scholar]
- 25. Sandusky SB, McGuire L, Smith MT, Wigley FM, Haythornthwaite JA.. Fatigue: an overlooked determinant of physical function in scleroderma. Rheumatology 2009;48:165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thombs BD, Hudson M, Bassel M, Taillefer SS, Baron M.. Canadian Scleroderma Research Group. Sociodemographic, disease, and symptom correlates of fatigue in systemic sclerosis: evidence from a sample of 659 Canadian Research Group Registry patients. Arthritis Care Res 2009;61:966–73. [DOI] [PubMed] [Google Scholar]
- 27. Sandqvist G, Scheja A, Hesselstrand R.. Pain, fatigue and hand function closely correlated to work ability and employment status in systemic sclerosis. Rheumatology 2010;49:1739–46. [DOI] [PubMed] [Google Scholar]
- 28. Strickland G, Pauling J, Cavill C, McHugh N.. Predictors of health-related quality of life and fatigue in systemic sclerosis: evaluation of the EuroQol-5D and FACIT-F assessment tools. Clin Rheumatol 2012;31:1215–22. [DOI] [PubMed] [Google Scholar]
- 29. Khanna D, Ahmed M, Furst DE. et al. Health values of patients with systemic sclerosis. Arthritis Care Res 2007;57:86–93. [DOI] [PubMed] [Google Scholar]
- 30. Georges C, Chassany O, Toledano C. et al. Impact of pain in health related quality of life of patients with systemic sclerosis. Rheumatology 2006;45:1298–302. [DOI] [PubMed] [Google Scholar]
- 31. Racine M, Hudson M, Baron M, Canadian Scleroderma Research Group. The impact of pain and itch on functioning and health-related quality of life in systemic sclerosis: an exploratory study. J Pain Symptom Manage 2016;52:43–53. [DOI] [PubMed] [Google Scholar]
- 32. Mouthon L, Poole JL.. Physical and occupational therapy In: Varga J, Denton CP, Wigley FM, Allanore Y, Kuwana M, eds. Scleroderma. New York: Springer, 2016, 603–13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.