Abstract
Thermal manipulation during embryogenesis was previously reported to decrease the occurrence of ascites and to potentially improve cold tolerance of broilers. The objective of our study was to explore the effects of the interaction of cold incubation temperatures and cool ambient temperatures until 21 d of age on performance and body temperature. Ross 308 eggs were incubated either under control conditions I0 (37.6°C) or with cyclic cold stimulations I1 (6 h/d at 36.6°C from d 10 to 18 of incubation) or with 2 cold stimulations I2 (30 min at 15°C) at d 18 and 19 of incubation. These treatments were followed by individual rearing and postnatal exposure to either standard rearing temperature T0 (from 33°C at hatching to 21°C at d 21) or continuously lower temperature T2 (from 28°C at hatching to 21°C at d 21) or exposure to cyclically lower temperature T1 (with circadian temperature oscillations). Treatments I1 and I2 did not significantly alter hatchability compared to control incubation (with 94.8, 95.1, and 92.3%, respectively), or hatching BW and overall chick quality. Hatching body temperature (Tb) was 0.5 and 0.3°C higher in I1 than in I0 and I2 groups, respectively (P = 0.007). A doubled occurrence of health problems was observed with T2 condition, regardless of incubation or sex. At d 3, BW was 2% lower with treatment I1 than with I0 and I2 and was 3% higher in T1 and T2 groups than in T0, but these effects disappeared with age. Group T2 presented a 5% higher feed intake than the control group T0 between 3 and 21 d of age (P = 0.025). Feed conversion ratio (FCR) was affected by experimental conditions (P < 0.001), with low FCR values obtained with I2 incubation in control or cyclically cold postnatal conditions. Maximal FCR values were observed in the continuously cold postnatal conditions, in males submitted to control incubation and in females submitted to I1 incubation, revealing sex-dependent effects of the treatments on performance.
Keywords: incubation, ambient temperature, thermotolerance, cold, broiler chicken
INTRODUCTION
In past decades, a large improvement in poultry production has been made due to genetic selection breeding fast-growing broilers strains (Havenstein et al., 2003; Zuidhof et al., 2014). These advances in chicken growth rate were not associated with a parallel development of the respiratory, metabolic, and locomotor functions. Fast-growing chickens are sensitive to inflammation, disease, and changes in their environment (Cheema et al., 2003; Loyau et al., 2015), which probably results from reduced adaptive capacities. Indeed, broiler chickens have difficulties coping with changes in ambient temperatures. Renwick and Washburn (1982) demonstrated that brooding broiler chicks under cool temperatures (26.7°C vs. 32.2°C) increased mortality and decreased feed efficiency with chicks being particularly sensitive to low temperatures. Under low-temperature conditions, fast-growing chickens have limited capacity to satisfy their oxygen demands, which increases the occurrence of ascites (Olkowski et al., 2005; Druyan et al., 2007).
Mortalities due to ascites syndrome caused severe economic losses up to $1 billion in 1996 (Maxwell and Robertson, 1998). Chicks are not yet homeotherms until the end of the first wk after hatching, and the regulation of body temperature and feedback mechanisms are still immature (Tzschentke, 2007). Mortality rates of chickens were estimated as tripled during the first wk of age compared to the remainder of the grow-out period (Heier et al., 2002; Yassin et al., 2009). Chicks cannot cope with low ambient temperatures without adequate shelter and heating (Collin et al., 2005; Mujahid and Furuse, 2009). Therefore, broiler production uses high amounts of energy for heating in the brooding period and has to compensate for the circadian thermal amplitude in the management of rearing temperature.
It was previously demonstrated that heat acclimation during embryogenesis is a technique that confers to broilers the adaptive capacity to cope with high ambient temperatures (Piestun et al., 2008; Loyau et al., 2015). This was achieved by changes in thermoregulatory and metabolic mechanisms without major alteration of performance (Piestun et al., 2008; Loyau et al., 2013, 2014a). Specific programs of cold acclimation during egg incubation also were investigated in chickens, improving their cold tolerance in the long term. Shinder et al. (2011) provided evidence that early cold conditioning (2 times 30 min at 15°C during d 18 and d 19 of incubation) improved the ability of broilers to maintain body temperature during later cold exposure. This treatment also increased the body weight of chickens reared at both standard and lower ambient temperatures. Furthermore, Yalçın et al. (2012) showed that cyclically cold thermal manipulation during specific phases of embryogenesis (from d 10 to d 18 of incubation) at 36.6°C and 58% relative humidity (RH) enabled broilers to cope with later cool ambient temperatures without altering growth, despite reducing hatchability. By applying the same treatment, Loyau et al. (2014a) also demonstrated that cold incubation induced modifications in antioxidant pathways, as shown by a higher hepatic catalase activity at hatching. With both cold embryo manipulations, experimental results demonstrated fewer incidences of ascites and a reduction of mortality rates during later cold exposure during the growing phase (Shinder et al., 2011; Akşit et al., 2013). These techniques could be interesting in order to increase the robustness of chickens from their first d of life, allowing to decrease ambient temperature in farm buildings at the starting period, especially during the phase when temperature is currently decreased in poultry houses (i.e., until 21 d of age). Thus, the objective of our study was to investigate the interaction of low or standard incubation temperature and postnatal standard or low rearing ambient temperatures, either constant or fluctuating, on performance and body temperatures in fast-growing chickens during the first 3 wk of age.
MATERIALS AND METHODS
All experimental procedures were approved by the Ethics Committee for Animal Experimentation Val de Loire (CEEA Val de Loire, Tours, France, N° 2,014,111,809,444,741 (APAFIS#70).03).
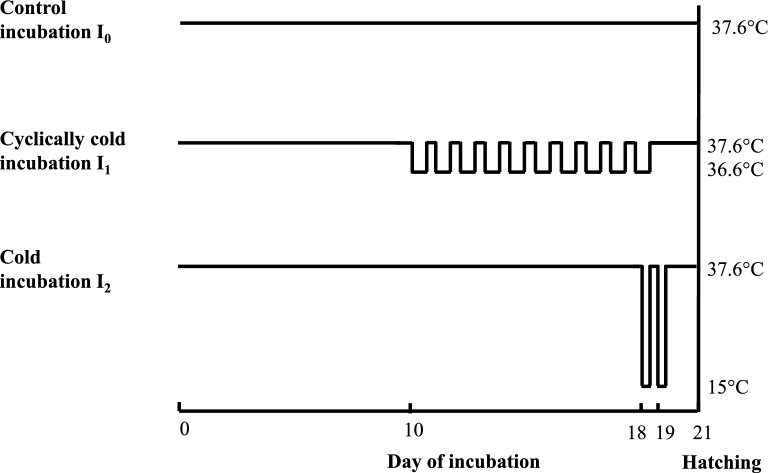
Incubation Process
A batch of 1,188 hatching eggs from Ross 308 broiler breeders at 36 wk of age and 3 d of egg storage was obtained from a commercial hatchery with mean egg weight of 63.5 ± 4.0 g and randomly divided into 3 incubation groups. The first group, including 3 trays of 132 eggs, was placed in standard incubation conditions (I0) at 37.6°C and 56% RH. The second group, including 3 trays of 132 eggs, was incubated in cyclically cold incubation (I1) conditions with reductions by 1°C during 6 h per d from d 10 to d 18 of incubation with RH of 60% (Figure 1), conditions being fixed at 37.6°C and 56% RH for the remainder of incubation. The cold incubation condition (I2) consisted of exposing 3 trays of 132 eggs to 15°C and 81% RH during 30 min on d 18 (transfer from the incubator to a cold room) and d 19 of incubation (transfer from the hatcher to a cold room), eggs being incubated at 37.6°C and 56% RH for the remainder of incubation. At d 18 of incubation, all eggs were moved to a common hatcher set at 37.6°C and 70% RH, with only the 30 min interruption at d 19 for the transfer of group I2 to the cold room.
Figure 1.
Incubation treatments. Incubation I0: Control eggs were incubated until hatch at 37.6°C. Relative humidity was set at 56% until d 18 of embryogenesis. Incubation I1: Eggs were incubated at 37.6°C and 56% RH, except from d 10 to d 18 of incubation when they were exposed to 6 h/d at 36.6°C, 60% RH. Incubation I2: Eggs were incubated at 37.6°C and 56% RH and exposed for 30 min to 15°C at d 18 and 19 of incubation. At d 18, all eggs were transferred to the hatcher where relative humidity was set at 70% until d 21 of incubation.
Hatching
At the end of hatching, all unhatched eggs were opened for macroscopic analysis in order to classify them as “non-fertile” and those with dead embryos. Non-fertile eggs were not included in hatching rate determination. Chicks were wing-tagged, and 50 to 81 chicks were randomly taken in the 3 incubation groups to be macroscopically examined for determining their quality according to the scores proposed by Tona et al. (2003). Briefly, the chick quality was measured on 8 different criteria, such as activity, down and appearance, retracted yolk, eyes, legs, navel area, remaining membrane, and remaining yolk on the navel, that were added to be rated on a total score of 100 points, corresponding to the best chick quality score. Determination of the sex was then realized macroscopically by observation of the cloaca.
Rearing Period
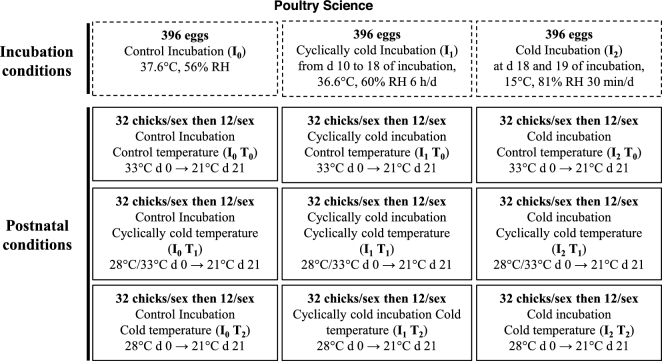
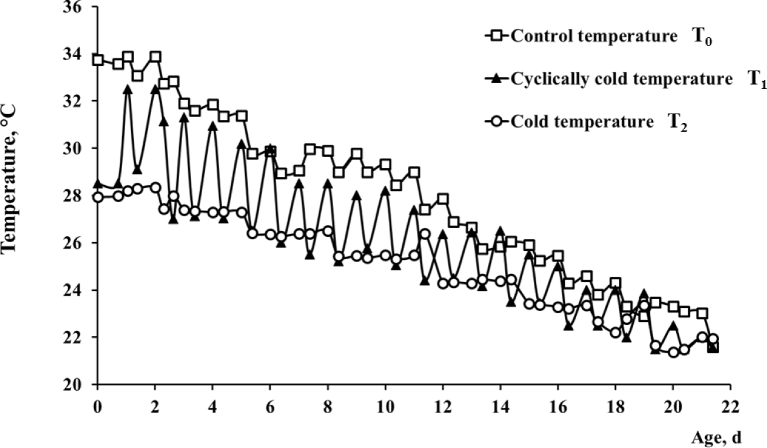
One-hundred-ninety-two chicks from each incubation group were randomly assigned to 3 different rearing rooms. Thirty-two males and 32 females from I0, I1, and I2 (Figure 2) were placed in the control temperature (T0) room where the ambient temperature was gradually decreased until 21 d of age from 33 to 21°C. In a second room, 32 males and 32 females from groups I0, I1, and I2 were exposed to cyclical variations T1 of temperature from 33 to 28°C at d 0 to 21°C at d 21 in a gradual decrease. In a third identical room, 32 males and 32 females from I0, I1, and I2 groups were submitted to cooler ambient temperature T2, decreasing gradually on d 21 from 28 to 21°C. The rearing temperature in each room was monitored by a temperature controller each min during the entire experimental period (Thermo Button, Proges Plus, Willems, France) and data were collected via the software Thermotrack PC for analyses (Figure 3). Chicks were kept under a light:dark regimen of 16:8 after 3 d of age. They were reared in groups of 4 chicks of the same sex per cage (59 cm length × 45 cm width × 59 cm height) from 0 to 3 d of age (at a maximal stocking density of 1,258 g/m2). Thereafter, only 12 chicks per sex and per group were selected to achieve the same group mean BW (±S.E.) to be reared individually in cages up to 21 d of age in each room (at a maximal stocking density of 3,982 g/m2). Standard feed and water were provided ad libitum.
Figure 2.
Experimental design. Eggs were submitted to control incubation I0 (37.6°C and 56% RH), cyclically cold incubation I1, or cold incubation I2, and thereafter exposed from d 1 to d 21 to control temperature T0, cyclically cold temperature T1, and cold temperature T2.
Figure 3.
Rearing temperature recorded in the 3 breeding rooms. Chicks were exposed to control temperature T0 from 33°C at d 0 to 21°C at d 21 or to cyclically cold temperature T1 from 33/28°C at d 0 to 21°C at d 21 or to cold temperature T2 from 28°C at d 0 to 21°C at d 21.
Measurements
The body temperature of chickens was measured individually at d 0 (after opening the hatcher) and d 11 by inserting an electronic thermometer (PX-TH519, Tex, Pelimex, Ingwiller, France) in the cloaca to a depth of 1 cm. Body weight was measured at d 0, d 3, d 11, and d 21 and feed consumption was recorded per cage for the period d 0 to d 3 and thereafter for each chick at d 11 and d 21. Spontaneous mortality and the number of birds removed from the experiment because of health or welfare troubles were recorded daily.
Statistical Analysis
Data were collected and analyzed by statistic software Statview (version 5.0; SAS Institute, Cary, NC) in order to determine the effects of incubation group, postnatal condition, and their interaction on performance traits for each sex. The hatchability and mortality results were analyzed by using a Chi-square test. The responses to treatments and sex and their interactions concerning chick quality, body temperature, and weight were determined using analysis of variance (ANOVA) after having checked the normality of data distribution. The statistical significance of the difference was determined using a Student t test for the comparison of results from each group. Due to a significant heterogeneity of variance evaluated using Levene's test (P < 0.001) on data of FCR, values for this parameter were analyzed by nonparametric tests, including the Kruskal-Wallis test followed by the Mann-Whitney test. Chick quality scores also were analyzed using non-parametric statistic tests (Kruskall-Wallis, followed by Mann-Whitney test). Differences were considered significant when P-values were below 5% (P < 0.05), and values are presented ± standard error in the text or tables.
RESULTS
Performance
Hatchability
Fertile hatchability rates were not different among incubation conditions with 92.3, 94.8, and 95.1% in I0, I1, and I2 groups, respectively.
Chick Quality Scores
No significant overall difference in chick quality was measured among incubation groups I0, I1, or I2 at hatch (Table 1). The score of the navel area of I1 chicks was significantly lower than that of I0 chicks (P = 0.046), while the remaining membrane score was significantly degraded in I2 chicks, as compared to I0 controls (P = 0.037).
Table 1.
Scores of the different parameters of quality in post-hatch chicks after incubation under different conditions.
| Incubation group1 | ||||
|---|---|---|---|---|
| Parameters2 | I0 | I1 | I2 | P-value |
| Activity (/6) | 5.8 ± 0.2 | 5.5 ± 0.2 | 5.8 ± 0.1 | 0.466 |
| Down and appearance (/10) | 10.0 ± 0.0 | 9.8 ± 0.1 | 9.9 ± 0.1 | 0.387 |
| Retracted yolk (/12) | 10.7 ± 0.4 | 11.0 ± 0.3 | 10.7 ± 0.3 | 0.758 |
| Eyes (/16) | 16.0 ± 0.0 | 16.0 ± 0.0 | 16.0 ± 0.0 | 1.000 |
| Legs (/16) | 14.7 ± 0.5 | 15.7 ± 0.2 | 14.9 ± 0.3 | 0.053 |
| Navel area (/12) | 10.7 ± 0.2a | 9.8 ± 0.2b | 10.1 ± 0.2a,b | 0.046 |
| Remaining membrane (/12) | 11.7 ± 0.2a | 11.1 ± 0.3a,b | 10.9 ± 0.2b | 0.037 |
| Remaining yolk (/16) | 15.5 ± 0.2 | 14.6 ± 0.3 | 14.9 ± 0.2 | 0.080 |
| Total quality score (/100) | 95.1 ± 0.8 | 93.6 ± 0.6 | 93.1 ± 0.8 | 0.091 |
1Control incubation I0: eggs were incubated until hatch at 37.6°C and 56% RH (n = 50); I1: eggs were incubated from d 10 to d 18 of incubation, 6 h/d at 36.6°C (n = 77), 60% RH; I2: eggs were exposed for 30 min to 15°C at d 18 and 19 of incubation (n = 81).
2For each parameter, values are given as mean ± standard error.
a,bDifferent letters correspond to significant differences (P < 0.05) between groups.
Chick quality was evaluated according to Tona et al. (2003).
Body Temperature
No significant interaction was observed between sex and incubation condition for body temperature at hatching (Table 2). Higher body temperatures were observed at hatching in females than in males (P < 0.001), and in I1 than in I0 and I2 (P = 0.007). At 11 d of age, only the postnatal conditions had a significant effect on body temperature (P = 0.045) with higher Tb in the cyclically cold rearing temperature T1 group (40.5 ± 0.1°C; n = 71) than in the constantly cold ambient temperature T2 (40.3 ± 0.1°C; n = 68; P = 0.015). The group at standard temperature T0 presented an intermediary Tb value (40.5 ± 0.1°C; n = 70), not significantly different from either other group (P = 0.170 and P = 0.376, respectively).
Table 2.
Body temperature and body weight of males and females at hatch depending on incubation group.
| Incubation group1 | Sex | Incubation × Sex | ||||||
|---|---|---|---|---|---|---|---|---|
| I0 | I1 | I2 | P-value | Male | Female | P-value | P-value | |
| Body temperature (°C)2 | 39.2 ± 0.1b | 39.7 ± 0.1a | 39.4 ± 0.3b | 0.007 | 39.2 ± 0.1b | 39.7 ± 0.1a | 0.001 | 0.053 |
| Body weight (g)3 | 45.4 ± 0.2 | 45.1 ± 0.2 | 45.0 ± 0.2 | 0.477 | 45.4 ± 0.2a | 44.9± 0.2b | 0.043 | 0.911 |
1Control incubation I0: eggs were incubated until hatch at 37.6°C and 56% RH; I1: eggs were incubated from d 10 to d 18 of incubation, 6 h/d at 36.6°C, 60% RH; I2: eggs were exposed for 30 min to 15°C at d 18 and 19 of incubation. For each parameter, values are given as mean ± standard error.
2I0: n = 45; I1: n = 45; I2: n = 46; Males n = 47; Females: n = 62.
3I0: n = 192; I1: n = 192; I2: n = 192; Males n = 272; Females: n = 304.
a,bDifferent letters correspond to significant differences (P < 0.05) between incubation groups or between sexes.
Body Weight
Body weights at hatching were not different among incubation groups in either sex, but males presented significantly higher hatching BW than females (P = 0.043; Table 2). In males as in females, there was no significant effect of the interaction between incubation and postnatal conditions on BW, regardless of the age considered. At 3 d of age, BW was 2% lower in the cyclically cold incubation treatment I1 than in I0 and I2 (P = 0.039; Table 3). It also was affected by postnatal condition with 3% higher BW in T1 and T2 groups than in T0 (P < 0.001), but these effects disappeared with age. An expected, sex effect was significant on BW at 11 (P = 0.007) and 21 d of age (P < 0.001), without significant interaction with incubation or postnatal temperature conditions.
Table 3.
Performance of chickens depending on incubation and rearing temperature conditions. Body weights of males and females were recorded at d 3 and d 21. Feed consumption (FC) was calculated between d 3 and d 21.
| Incubation group (I)1 | Postnatal group (P)2 | Sex (S) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Traits | I0 | I1 | I2 | I effect | T0 | T1 | T2 | P effect | Male | Female | S effect | I × P | I × S | P × S | I × P × S |
| BW at d 3 (g) | 84.4 ± 0.5a | 82.6 ± 0.6b | 84.2 ± 0.6a | 0.039 | 82.1 ± 0.6b | 84.9 ± 0.6a | 84.2 ± 0.5a | <0.001 | 84.1 ± 0.4 | 83.4 ± 0.4 | 0.287 | 0.731 | 0.272 | 0.614 | 0.091 |
| BW at d 11 (g) | 357 ± 4 | 346 ± 4 | 358 ± 4 | 0.066 | 352 ± 4 | 357 ± 4 | 352 ± 4 | 0.523 | 360 ± 3a | 347 ± 3b | 0.007 | 0.880 | 0.597 | 0.153 | 0.839 |
| BW at d 21 (g) | 1069 ± 11 | 1038 ± 15 | 1064 ± 14 | 0.298 | 1053 ± 12 | 1077 ± 12 | 1040 ± 17 | 0.284 | 1108 ± 10a | 1021 ± 8b | <0.001 | 0.750 | 0.937 | 0.968 | 0.065 |
| FC d 3 to d 21 (g) | 1377 ± 14 | 1358 ± 14 | 1372 ± 15 | 0.453 | 1338 ± 13b | 1373 ± 14a,b | 1399 ± 15a | 0.025 | 1414 ± 11a | 1327 ± 10b | <0.001 | 0.760 | 0.970 | 0.872 | 0.443 |
1Control incubation I0: eggs were incubated until hatch at 37.6°C and 56% RH; I1: eggs were incubated from d 10 to d 18 of incubation, 6 h/d at 36.6°C, 60% RH; I2: eggs were exposed for 30 min to 15°C at d 18 and 19 of incubation.
2Control temperature T0 from 33°C at d 0 to 21°C at d 21 or cyclically cold temperature T1 from 33°C/28°C at d 0 to 21°C at d 21 or cold temperature T2 from 28°C at d 0 to 21°C at d 21.
a,bDifferent letters correspond to significant differences (P < 0.05) between groups. For each parameter, values are presented as mean ± standard error. For BW at d 3, n = 189 to 192 chicks per incubation or postnatal group; for BW at d 11, n = 68 to 73 chicks per incubation or postnatal group; for data of FC and BW at 21 d, n = 64 to 67 chicks per incubation or postnatal group.
Feed Consumption From d 3 to d 21
There was no interaction between incubation condition and postnatal ambient temperature on feed consumption in either sex (Table 3). Besides a significant effect of sex (P < 0.001) with 6% higher feed consumption in males than in females, the T2 group submitted to postnatal continuous cold exposure presented a 5% significantly higher feed consumption than the control group T0 (P = 0.025), while T1 group exhibited intermediary values.
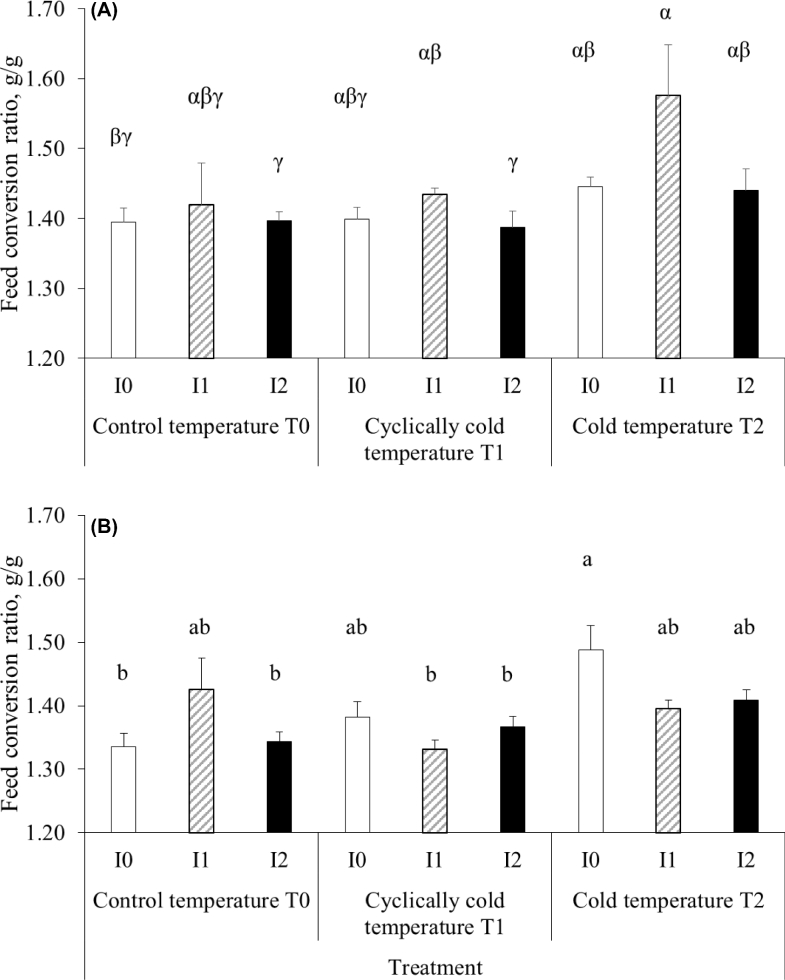
Feed Conversion Ratio
The low incubation temperature or low rearing temperature did not affect FCR from d 0 to d 3 and from d 3 to d 11 (data not shown). Between 3 and 21 d of age, FCR was significantly altered by experimental conditions (P < 0.001; Figure 4). Maximal FCR values were observed in the continuously cold postnatal conditions T2, on the one hand in females submitted to I1 incubation (1.58 g/g, Figure 4A) and on the other hand in males submitted to control incubation I0 (1.49 g/g, Figure 4B). Low FCR values were obtained with I2 incubation in control or cyclically cold postnatal conditions in both sexes: lower FCR values were recorded in the males incubated with I2 or control incubation and reared in the control conditions (I2T0; P = 0.002 or I0T0; P = 0.001, respectively) and in the males incubated with I1 or I2 treatments reared under cyclically cold temperature (I1T1, P = 0.004 or I2T1, P < 0.001, respectively), than in the males with control incubation but constant cold rearing temperature (I0T2). In females, feed efficiency was significantly improved by 4% with I2 incubation as compared to I1 incubation in cyclically cold postnatal conditions (P = 0.011). The other groups were intermediary.
Figure 4.
Feed conversion ratio of chickens between d 3 and d 21. Control eggs were incubated at 37.6°C, 56% RH (I0), I1 eggs were submitted to incubation at 36.6°C and 60% RH for 6 h/d from d 10 to d 18 of incubation, and I2 were submitted to 30 min at 15°C at d 18 and 19 of incubation. Chickens were reared in cages at control temperature T0 from 33°C at d 0 to 21°C at d 21 or at cyclically cold temperature T1 from 33/28°C at d 0 to 21°C at d 21 or at cold temperature T2 from 28°C at d 0 to 21°C at d 21. 4A) Feed conversion ratio calculated in females. Different letters (α, β, γ) correspond to significant differences (P < 0.05) between groups. 4B) Feed conversion ratio calculated in males. Different letters (a, b) correspond to significant differences (P < 0.05) between groups.
Mortality and Morbidity
A total mortality rate of 3.9% was recorded, corresponding to 1.8% of non-eating chicks, 1.2% of cardiac death, and 0.9% of death due to ascites. An occurrence of health and/or welfare troubles at a rate of 11.4% was observed in the present experiment, corresponding to several cases of leg disorders leading to the removal of chickens for ethical reasons. The effect of incubation conditions was not significant within postnatal conditions. Most of the health troubles were observed between 3 and 11 d of age, and were significantly higher in the continuously cool postnatal conditions T2 than in the T0 and T1 conditions (24 chicken vs. 9 and 13, with P = 0.003 and P = 0.021, respectively).
DISCUSSION
In this study investigating the effects of incubation temperature and postnatal temperatures during the first 3 wk of age in broiler chickens, neither of the 2 cold incubation groups affected hatchability in our experimental conditions. By contrast with previous findings of Yalçin et al. (2012) showing a degradation in hatchability by 5 points with I1 treatment as compared to control incubation, hatchability was not different and above 92% of fertile eggs in both I0 and I1 groups. As expected from the study of Shinder et al. (2011), hatchability also was maintained high with the I2 group, including 2 acute stimulations during late embryogenesis.
From our results, high quality scores of chicks over 92/100 evaluated from quality scores of Tona et al. (2003) were observed in all incubation groups, despite cold incubation treatments slightly affecting chick quality due to variations in some criteria. The cyclic cold stimulation I1 degraded the score of navel closure, however, not leading to significantly higher mortality rates post hatch in this group. The quality score related to the remaining membrane was lower in I2 group than with control incubation. Both observations could be due to a greater chick immaturity at hatching in I1 and I2 groups. Moreover, the thermoregulation and development of hatching birds were altered by the incubation treatments. Indeed, body temperature was significantly higher in I1 than in I0 and I2 groups at hatch (P = 0.007); these effects were not observed later on. This observation was not reported previously with the same incubation program I1 (Yalçin et al., 2012; Aksit et al., 2013; Loyau et al., 2014a). However the significantly greater body temperature observed in I1 than in I0 group at hatching is in agreement with the lower body temperature recorded in hatching chicks that were heat-exposed during embryogenesis (Piestun et al., 2008; Loyau et al., 2013), suggesting changes in the thermoregulatory control of metabolism induced by incubation temperature (2014b, 2014b, 2016; Loyau et al., 2015). Consistent with Shinder et al. (2011), cold incubation treatment I2 later during incubation did not affect hatching temperature as compared to control incubation. Females also exhibited higher body temperatures than males (P = 0.001), suggesting differences in thermoregulatory mechanisms between sexes. This result could be explained by differences in times of hatch between sexes, as previously observed by Tzschentke and Halle (2009).
Incubation treatment I1 consisting of repeated cold stimulations at 36.6°C (Yalçin et al., 2012) negatively but transiently affected BW at d 3. The absence of significant effect of incubation treatments on BW at 21 d is consistent with the results of Akşit et al. (2013) showing no effect of lower incubation temperature on body weights in mixed-sex groups of chickens, whereas Shinder et al. (2011) had reported positive effects of I2 incubation on body weights at d 14 and 35 post hatch in males. Both postnatal cold conditions T1 and T2 induced higher BW than control conditions at 3 d of age, inconsistent with previous results showing unchanged to lower BW in chickens exposed to cold ambient temperature at early age (Collin et al., 2003; Shinder et al., 2011). In accordance with the present positive effect on growth, Aksit et al. (2013) showed greater BW of mixed-sex chickens from a young breeder flock exposed to postnatal cold. However, this effect was only transient in our study, whereas they observed a longer effect on growth (until 28 d of age), the difference being likely due to the shorter and lower ambient temperature they applied.
The highest FCR were observed in T2 continuous cold postnatal conditions, in females of I1 group and in males incubated with control incubation conditions, suggesting lower ability of these groups to use nutrients for growth as compared to their respective controls incubated and reared under standard conditions. This effect is likely due to additional heat production to counterbalance the effects of cold in an attempt to maintain a normal body temperature (i.e., thermoregulation), as observed in a previous study dealing with cold rearing temperatures (Collin et al., 2003). It was associated with 5% increased feed consumption in constant cold T2 conditions, as compared to control postnatal temperature (P = 0.025), in an attempt to increase diet-induced thermogenesis for cold acclimation. Such decreases in feed efficiency were not observed under cyclically cold ambient temperatures, suggesting resting phases when temperatures were up again. Interestingly, I2 incubation treatment led to minimal FCR values, however, not significantly differing from the control incubation groups under control or cyclically cooler rearing ambient temperatures for both sexes. The latter result suggests that cold acute stimulation during late embryogenesis, limiting the occurrence of ascites according to Shinder et al. (2011), would not degrade feed efficiency when chickens are submitted to temperature variations in poultry houses from hatching to 21 d of age.
The mortalities and morbidity measured in the present experiment were mainly due to lameness and cardiac death and were observed mostly between 3 and 11 d of age. No significant difference was observed in mortality rates and removal from the experiment resulting from legs problems between incubation groups, somehow inconsistent with previous results from Yalçin et al. (2007), indicating that exposition to low temperature during incubation could induce leg disorders. However, the constantly cool condition T2 applied post hach negatively affected the health of the chicks, probably promoting the survival of the fittest, in accordance with Zhang et al. (2014), pointing out the increased inflammation associated with lipid-metabolism disorders in the livers of chickens exposed to cold stress. Previous data showed that at hatching, chicks have very low storage of minerals (Yair et al., 2015). Furthermore, Angel (2007) reported that the high BW of fast-growing broilers puts lot of pressure on leg bones, which causes a deviation of the corridor and a sprained gastrocnemius. Regarding the number of leg troubles recorded in the present experiment, the postnatal condition T2 was probably too severe for the chickens reared in cages and more susceptible to leg problems than floor-reared chickens (Haye and Simons, 1978) to support growth and optimal muscle-skeleton development. At constantly low temperatures, chicks probably reduced their movements because they allocated available energy to heat production to maintain major functions and homeothermy. To the contrary, the intermittent cyclical cold temperature T1 allowed chicks to recover after the cold period (of around 8 h) of thermoregulatory requirements to bring temperature to equilibrium.
In conclusion, unlike the cyclical exposure to low temperature that barely affected the performance of chicks reared in cages, constant exposure to lower temperature during the first 3 wk post hatch induced some unfavorable sex-specific responses in terms of performance. Regarding the combined effects of incubation and rearing programs observed in the feed efficiency in males and females reared in cages, the underlying physiological and metabolic mechanisms remain to be explored, as well as their effects on floor-reared chicken performance and health status. How these treatments will influence economic (e.g., production cost), social (e.g., animal welfare), and environmental (e.g., non-renewable energy consumption for heating) criteria regarding broiler production is also necessary to investigate before potential applications of alternative incubation programs in poultry industry.
Acknowledgements
D. Nyuiadzi is funded by West Africa Agricultural Productivity Program from Togo (WAAPP-Togo) and Centre d'Excellence Régional en Sciences Aviaires (CERSA)/Lomé University for realizing her PhD. This study was funded by INRA (Department Animal Physiology and Livestock Systems, Eval_Adapt project) and carried out within Technical Mixed Unit (UMT) framework Integrative Biology Research and Development (BIRD) associating ITAVI Poultry Technical Institute and INRA on applied research projects. The authors thank F. Mercerand, J. Delaveau, C. Rat, I. Grimaud-Jottreau, H. Rigoreau, and C. Le Bourhis from Experimental Unit Pôle d'Experimentation Animale de Tours (PEAT), INRA, 37380, Nouzilly, France, for helpful animal care, S. Crochet, N. Couroussé, E. Cailleau-Audouin, P. Chartrin, T. Bordeau, S.-A. David, M. Couty, E. Baéza, M. Bournazel, and A. Jacques from Unité de Recherches Avicoles (URA), INRA, 37380 Nouzilly, France, and C. Souchet from ITAVI, 37380 Nouzilly, France, for their skilled technical assistance. Authors are grateful to I. Bouvarel (Institut Technique de l’Aviculture, F-37380 Nouzilly, France) and C. Leterrier (UMR PRC, INRA, CNRS, IFCE, Université F. Rabelais de Tours, 37380 Nouzilly, France) for helpful discussions.
REFERENCES
- Akşit M., Yalçın S., Siegel P. B., Yenisey Ç., Özdemir D., Özkan S.. 2013. Broilers respond to cooler ambient temperatures after temperature acclimation during incubation and early postnatal age. J. Appl. Poult. Res. 22:298–307. [Google Scholar]
- Angel R. 2007. Metabolic disorders: Limitations to growth of and mineral deposition into the broiler skeleton after hatch and potential implications for leg problems. J. Appl. Poult. Res. 16:138–149. [Google Scholar]
- Cheema M. A., Qureshi M. A., Havenstein G. B.. 2003. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82:1519–1529. [DOI] [PubMed] [Google Scholar]
- Collin A., Buyse J., Van As P., Darras V. M., Malheiros R. D., Moraes V. M. B., Reyns G. E., Taouis M., Decuypere E.. 2003. Cold-induced enhancement of avian uncoupling protein expression, heat production, and triiodothyronine concentrations in broiler chicks. Gen. Com. Endocrinol. 130:70–77. [DOI] [PubMed] [Google Scholar]
- Collin A., Picard M., Yahav S.. 2005. The effect of duration of thermal manipulation during broiler chick embryogenesis on body weight and body temperature of post-hatched chicks. Anim. Res 54:105–111. [Google Scholar]
- Druyan S., Ben-David A., Cahaner A.. 2007. Development of ascites-resistant and ascites-susceptible broiler lines. Poult. Sci. 86:811–822. Erratum in: Poult Sci. 2007 Jun;86(6):1283. [DOI] [PubMed] [Google Scholar]
- Havenstein G., Ferket P., Qureshi M.. 2003. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82:1500–1508. [DOI] [PubMed] [Google Scholar]
- Haye U., Simons P. C.. 1978. Twisted legs in broilers. Br. Poult. Sci. 19:549–557. [DOI] [PubMed] [Google Scholar]
- Heier B. T., Hogasen H. R., Jarp J.. 2002. Factors associated with mortality in Norwegian broiler flocks. Prev. Vet. Med. 53:147–157. [DOI] [PubMed] [Google Scholar]
- Loyau T., Bedrani L., Berri C., Métayer-Coustard S., Praud C., Coustham V., Mignon-Grasteau S., Duclos M. J., Tesseraud S., Rideau N., Hennequet-Antier C., Everaert N., Yahav S., Collin A.. 2015. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: A review. Animal. 9:76–85. [DOI] [PubMed] [Google Scholar]
- Loyau T., Berri C., Bedrani L., Métayer-Coustard S., Praud C., Duclos M. J., Tesseraud S., Rideau N., Everaert N., Yahav S., Mignon-Grasteau S., Collin A.. 2013. Thermal manipulation of the embryo modifies the physiology and body composition of broiler chickens reared in floor pens without affecting breast meat processing quality. J. Anim. Sci. 91:3674–3685. [DOI] [PubMed] [Google Scholar]
- Loyau T., Collin A., Yenisey Ç., Crochet S., Siegel P. B., Akşit M., Yalçin S.. 2014a. Exposure of embryos to cyclically cold incubation temperatures durably affects energy metabolism and antioxidant pathways in broiler chickens. Poult. Sci. 93:1–9. [DOI] [PubMed] [Google Scholar]
- Loyau T., Hennequet-Antier C., Coustham V., Berri C., Leduc M., Crochet S., Sannier M., Duclos M. J., Mignon-Grasteau S., Tesseraud S., Brionne A., Métayer-Coustard S., Moroldo M., Lecardonnel J., Martin P., Lagarrigue S., Yahav S., Collin A.. 2016. Thermal manipulation of the chicken embryo triggers differential gene expression in response to a later heat challenge. BMC Genomics. 17:329 DOI 10.1186/s12864-016-2661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyau T., Métayer-Coustard S., Berri C., Crochet S., Cailleau-Audouin E., Sannier M., Chartrin P., Praud C., Hennequet-Antier C., Rideau N., Couroussé N., Mignon-Grasteau S., Everaert N., Duclos M. J., Yahav S., Tesseraud S., Collin A.. 2014b. Thermal manipulation during embryogenesis has long-term effects on muscle and liver metabolism in fast-growing chickens. PLoS One. http://dx.doi.org/10.1371/journal.pone.0105339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell M. H., Robertson G. W.. 1998. UK survey of broiler ascites and sudden death syndromes in 1993. Br. Poult. Sci. 39:203–215. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Furuse M.. 2009. Oxidative damage in different tissues of neonatal chicks exposed to low environmental temperature. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 152:604–608. [DOI] [PubMed] [Google Scholar]
- Olkowski A. A., Duke T., Wojnarowicz C.. 2005. The aetiology of hypoxaemia in chickens selected for rapid growth. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 141:122–131. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Shinder D., Ruzal M., Halevy O., Brake J., Yahav S.. 2008. Thermal manipulations during broiler embryogenesis: Effect on the acquisition of thermotolerance 1. Poult. Sci. 87:1516–1525. [DOI] [PubMed] [Google Scholar]
- Renwick G. M., Washburn K. W.. 1982. Adaptation of chickens to cool temperature brooding. Poult. Sci. 61:1279–1289. [DOI] [PubMed] [Google Scholar]
- Shinder D., Ruzal M., Giloh M., Druyan S., Piestun Y., Yahav S.. 2011. Improvement of cold resistance and performance of broilers by acute cold exposure during late embryogenesis 1. Poult. Sci. 90:633–641. [DOI] [PubMed] [Google Scholar]
- Tona K., Bamelis F., De Ketelaere B., Bruggeman V., Moraes V. M. B., Buyse J., Onagbesan O., Decuypere E.. 2003. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci. 2003 May;82:736–741. [DOI] [PubMed] [Google Scholar]
- Tzschentke B. 2007. Attainment of thermoregulation as affected by environmental factors. Poult. Sci. 86:1025–1036. [DOI] [PubMed] [Google Scholar]
- Tzschentke B., Halle I.. 2009. Influence of temperature stimulation during the last 4 days of incubation on secondary sex ratio and later performance in male and female broiler chicks. Br. Poult. Sci. 50:634–640. [DOI] [PubMed] [Google Scholar]
- Yair R., Shahar R., Uni Z.. 2015. In ovo feeding with minerals and vitamin D improves bone properties in hatchlings and mature broilers. Poult. Sci. 94:2695–2707. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Molayoğlu H. B., Baka M., Genin O., Pines M.. 2007. Effect of temperature during the incubation period on tibial growth plate chondrocytes differentiation and the incidence of tibial dyschondroplasia. Poult. Sci. 86:1772–1783. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Özkan S., Siegel P., Yenisey Ç., Akşit M.. 2012. Manipulation of incubation temperatures to increase cold resistance of broilers: Influence on embryo development, organ weights, hormones and body composition. J. Poult. Sci. 49:133–139. [Google Scholar]
- Yassin H., Velthuis A. G., Boerjan M., van Riel J.. 2009. Field study on broilers' first-week mortality. Poult. Sci. 88:798–804. [DOI] [PubMed] [Google Scholar]
- Zhang Z. W., Bi M. Y., Yao H. D., Fu J., Li S., Xu S. W.. 2014. Effect of cold stress on expression of AMPKalpha-PPARalpha pathway and inflammation genes. Avian Dis. 58:415–426. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Schneider B. L., Carney V. L., Korver D. R., Robinson F. E.. 2014. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 93:2970–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]