Abstract
STUDY QUESTION
Is testicular transplantation of in vitro propagated spermatogonial stem cells associated with increased cancer incidence and decreased survival rates in recipient mice?
SUMMARY ANSWER
Cancer incidence was not increased and long-term survival rate was not altered after transplantation of in vitro propagated murine spermatogonial stem cells (SSCs) in busulfan-treated recipients as compared to non-transplanted busulfan-treated controls.
WHAT IS KNOWN ALREADY
Spermatogonial stem cell autotransplantation (SSCT) is a promising experimental reproductive technique currently under development to restore fertility in male childhood cancer survivors. Most preclinical studies have focused on the proof-of-principle of the functionality and efficiency of this technique. The long-term health of recipients of SSCT has not been studied systematically.
STUDY DESIGN, SIZE, DURATION
This study was designed as a murine equivalent of a clinical prospective study design. Long-term follow-up was performed for mice who received a busulfan treatment followed by either an intratesticular transplantation of in vitro propagated enhanced green fluorescent protein (eGFP) positive SSCs (cases, n = 34) or no transplantation (control, n = 37). Using a power calculation, we estimated that 36 animals per group would be sufficient to provide an 80% power and with a 5% level of significance to demonstrate a 25% increase in cancer incidence in the transplanted group. The survival rate and cancer incidence was investigated until the age of 18 months.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Neonatal male B6D2F1 actin-eGFP transgenic mouse testis were used to initiate eGFP positive germline stem (GS) cell culture, which harbor SSCs. Six-week old male C57BL/6 J mice received a single dose busulfan treatment to deplete the testis from endogenous spermatogenesis. Half of these mice received a testicular transplantation of cultured eGFP positive GS cells, while the remainder of mice served as a control group. Mice were followed up until the age of 18 months (497–517 days post-busulfan) or sacrificed earlier due to severe discomfort or illness. Survival data were collected. To evaluate cancer incidence a necropsy was performed and tissues were collected. eGFP signal in transplanted testis and in benign and malignant lesions was assessed by standard PCR.
MAIN RESULTS AND THE ROLE OF CHANCE
We found 9% (95% CI: 2–25%) malignancies in the transplanted busulfan-treated animals compared to 26% (95% CI: 14–45%) in the busulfan-treated control group, indicating no statistically significant difference in incidence of malignant lesions in transplanted and control mice (OR: 0.3, 95% CI: 0.1–1.1). Furthermore, none of the malignancies that arose in the transplanted animals contained eGFP signal, suggesting that they are not derived from the in vitro propagated transplanted SSCs. Mean survival time after busulfan treatment was found to be equal, with a mean survival time for transplanted animals of 478 days and 437 days for control animals (P = 0.076).
LARGE SCALE DATA
NA.
LIMITATIONS, REASONS FOR CAUTION
Although we attempted to mimic the future clinical application of SSCT in humans as close as possible, the mouse model that we used might not reflect all aspects of the future clinical setting.
WIDER IMPLICATIONS OF THE FINDINGS
The absence of an increase in cancer incidence and a decrease in survival of mice that received a testicular transplantation of in vitro propagated SSCs is reassuring in light of the future clinical application of SSCT in humans.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by KiKa (Kika86) and ZonMw (TAS 116003002). The authors report no financial or other conflict of interest relevant to the subject of this article.
Keywords: spermatogonial stem cell transplantation, cancer incidence, long-term follow-up, pre-clinical animal study, spermatogonial stem cells, childhood cancer survivors, male infertility
Introduction
Childhood cancer regimens do not merely cause damage in cancer cells, but germ cells are affected as well (van der Meer et al., 1992; Meistrich, 2013; Poganitsch-Korhonen et al., 2017). Due to this collateral damage, male childhood cancer survivors often have to face subfertility (Howell and Shalet, 2005). For adult male cancer patients, fertility can be preserved by cryopreservation of semen. Since spermatogenesis has not commenced in prepubertal boys, cryopreservation of ejaculated or surgically retrieved spermatozoa is not feasible. Therefore there is a need for a clinical application to preserve and restore fertility in these boys.
It was shown for the first time in 1994 that germ cell transplantation can restore fertility in an infertile mouse model (Brinster and Avarbock, 1994; Brinster and Zimmermann, 1994). Since then the field of reproductive biology has advanced in translating this experimental technique into a clinical application for childhood cancer survivors (Ginsberg et al., 2010; Picton et al., 2015). Spermatogonial stem cell autotransplantation (SSCT) is an experimental technique still in a preclinical phase (Struijk et al., 2013; Mulder et al., 2016). The treatment involves a testicular biopsy taken prior to cancer therapy to preserve the unharmed SSCs for testicular transplantation after successful cancer treatment and proven sterility. In theory, these transplanted SSCs would resume spermatogenesis and result in a restoration of fertility, thereby enabling these men to father a child by natural conception. Currently, in many centers across the globe, cryopreservation of testicular biopsies is offered to children with cancer (Picton et al., 2015)
The functionality of this germ cell transplantation has been shown to restore spermatogenesis in recipients in a wide range of animal models, including rodent (Kanatsu-Shinohara et al., 2003; Ryu et al., 2007; Kubota et al., 2009; Yuan et al., 2009; Goossens et al., 2009; Wu et al., 2012), large mammals (Honaramooz et al., 2002; Izadyar et al., 2003), zebrafish (Nobrega et al., 2010; Kawasaki et al., 2012) and non-human primates (Schlatt et al., 1999; Hermann et al., 2012). Moreover, animal experiments in goat and mouse have shown that donor-derived offspring could be produced by natural conception (Honaramooz et al., 2003; Kanatsu-Shinohara et al., 2005c; Yuan et al., 2009; Goossens et al., 2009). These results strengthen the notion that SSCT is a plausible therapy for humans as well.
The transplantation efficiency depends largely on the number of transplanted SSCs (Dobrinski et al., 1999; Nagano, 2003). Due to the low number of SSCs present in a prepubertal testicular biopsy, in vitro propagation of these stem cells is thought to be an indispensable step in SSCT. The first efficient long-term murine SSC propagation system was established in 2003, proving that primary cultures of undifferentiated spermatogonia, designated as germline stem (GS) cells, kept stem cell capacity to colonize a recipient testis and initiate spermatogenesis after transplantation (Kanatsu-Shinohara et al., 2003, 2005a). Two milestones towards clinical applications for SSCT were achieved in 2009 and 2011, when propagation of human adult (Sadri-Ardekani et al., 2009) and human prepubertal (Sadri-Ardekani et al., 2011) spermatogonial stem cells could be demonstrated by xenotransplantation of cultured human testicular cells, proving the capacity of these cells to migrate to the basal membrane of the seminiferous tubules where the stem cell niche is located. Subsequently, several other culture systems to propagate human SSCs in vitro have been established as well (Lim et al., 2010; Akhondi et al., 2013; Kossack et al., 2013; Guo et al., 2015). However, as no spermatogenic differentiation of human in vitro propagated SSCs can be achieved after xenotransplantation to mice, these results are merely indicative of the presence of SSCs in culture. Ultimate proof will only be acquired with clinical application of SSCT in humans.
These culture systems are designed to stimulate cell division, while in vivo a delicate balance between self-renewal and differentiation is in place. In most mouse and human SSC culture systems self-renewal is stimulated by the addition of growth factors, including FGF2, GDNF, EGF and LIF (Kanatsu-Shinohara et al., 2003, 2005c; Sadri-Ardekani et al., 2009, 2011; Lim et al., 2010; Akhondi et al., 2013; Guo et al., 2015). Growth factors such as FGF2 and EGF have been implicated in malignant cell transformation and stimulate progression in a variety of cells and cancers (Jaye et al., 1988; Sasada et al., 1988; Zhang et al., 2010; Nayak et al., 2015). To date, information on the possible tumourigenic potential of transplanted long-term in vitro propagated SSCs has been limited. Studies have focused on the proof-of-concept that SSCT is able to restore fertility and generate offspring (Kanatsu-Shinohara et al., 2003, 2005b; Yuan et al., 2009), and have included analysis of the genetic and epigenetic profile of generated spermatozoa and selected tissues from offspring (Lee et al., 2009; Goossens et al., 2009, 2010; Wu et al., 2012). Also the elimination of lingering cancer cells originating from the primary tumor before transplantation has been studied (Fujita et al., 2005; Geens et al., 2007; Hermann et al., 2011; Sadri-Ardekani et al., 2014). However, the long-term health effects and potential increased tumor incidence of cultured SSC transplanted recipients has largely been neglected.
With the present preclinical animal study, we aimed to gain more insight in the long-term cancer incidence and survival rates of SSC transplantation recipients. To achieve this, we designed a mouse study resembling a clinical prospective study design in which we performed testicular transplantations of in vitro propagated SSCs and followed these mice until the age of 18 months.
Materials and Methods
Ethical approval
All animal experiments were approved according to the European legislation of animal experimentation and were evaluated and approved by the Animal Ethical Committee (DEC) of the Academic Medical Center, Amsterdam.
Murine SSC culture
Donor animals were generated by crossing male DBA/2 J (Charles River, France) to female C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ (Jackson Laboratory, United States of America, stock No: 006567, designated ‘Green’), designated C57BL/6-eGFP, hence creating B6D2F1 actin-eGFP offspring expressing eGFP in all cells except hair and erythrocytes (Okabe et al., 1997). Correct phenotypes (eGFP+) were identified using a hand-held Long-Wave UV lamp (Bio-Rad, United Kingdom). Donor mice were sedated using 4% isoflurane total body anesthesia prior to euthanization by decapitation followed by inactivation of the brain. Testes from 4- to 8-day-old mice were collected, the tunica albuginea was removed and tissue was cryopreserved in supplemented MEM (Gibco™, ThermoFisher Scientific, USA) with 20% FCS and 8% DMSO in a Coolcell® freezing container and stored in −196 °C for later spermatogonial cell isolation. Spermatogonia were isolated and cultures were initiated and maintained as described previously (Kanatsu-Shinohara et al., 2003), hereby always using testes of a mixed population of mice with an age ranging from 4 to 8 d.p.p. Briefly, from Passage 3, germline stem cells (GS) were maintained on a feeder layer of primary mitomycin C inactivated MEFs originated from 13 d.p.c. 129/SV (Charles River, France) embryos. If formed, embryonic stem cell like (ES-like) colonies were removed mechanically. At Passage 5 or 6, GS cells, harboring SSCs, were harvested. Residual feeder cells were removed by plating on 0.1% gelatin coated plates for 1 h prior to transplantation or cryopreservation.
Testicular SSC transplantation
Animals (C57BL/6 J, Charles River, France) received a single administration of 38 mg/kg busulfan (Sigma-Aldrich, USA) at the age of 6 weeks to deplete endogenous spermatogenesis (Zohni et al., 2012; Qin et al., 2016). Within 4–8 weeks post-busulfan treatment, half of the animals were transplanted with cultured GS cells via the efferent duct as described previously (Kanatsu-Shinohara et al., 2003) under 2–3% isoflurane total body anesthesia, while the other half of animals did not undergo spermatogonial stem cell transplantation and served as a control group. Mice were selected at random for transplantation, but no computerized randomization program was used. In general, animals were transplanted unilaterally with 121.300 ± 53.550 (mean ± SD) GS cells in a total volume of 12.5 μl. Appropriate analgesics (0.05 mg/kg temgesic, Reckitt Benckiser Healthcare, UK) were giving prior and for 1–2 day after transplantation. Immunosuppression was achieved by intraperitoneal injection of 0.50 μg anti-CD4 (eBioscience, Austria, Clone GK1.5) on Days 0, 2 and 4 (Benjamin and Waldmann, 1986).
Long-term follow-up and post-mortem examination
Animals were housed socially in individually ventilated cages with food (CRM (P), Technilab, the Netherlands) and water ad libitum. Control and transplanted animals were mixed if possible; the location of the cages was altered at variable intervals. Animals were followed until the age of 18 months. To reduce any perception bias from the researcher, discomfort was scored by an independent animal caretaker using an arbitrary scoring system weekly. The animal caretakers were blinded for the differences between the experimental and control group, nevertheless it is mandatory that all handling of each animal is reported in an experimental logbook that is kept inside the animal room. Animals were weighed at regular intervals. If needed, advice from an independent veterinarian or animal welfare officer was sought. If animals became ill or sudden death occurred, a necropsy was performed as soon as possible. In a few cases a necropsy could not be performed. At the age of 18 months (497–517 days post-busulfan), the surviving animals were sacrificed and examined. Animals were euthanized under total anesthesia induced by a mixture of C02 and O2 followed by C02 asphyxiation and finally cervical dislocation.
After death, the body was palpated to identify abnormalities. General health and cancer incidence were studied by evaluating the organs of the animals macroscopically. In general, the following organs were collected and fixated in 4% PFA: testis, epididymis, urine bladder, spleen, intestine, stomach, liver, kidney, lungs, heart, thymus and brain. To allow DNA isolation of selected organs, a piece was snap frozen in liquid nitrogen. These included testis, urine bladder, spleen, small intestine, liver (right lateral lobe), kidney, lung (caudal), thymus and brain cortex. If abnormalities were observed, a portion was fixated and a biopsy was snap frozen. In cases with abnormalities, animals were photographed. Findings were reported in a necropsy report. In case an animal was found deceased, an estimation of time of death was made. The majority of necropsies were performed by a single person.
Histological examination
Relevant tissues (i.e. testis and abnormal organs) were embedded in paraffin. Sections (5 μM) were stained with a standard HE staining and analyzed using an Olympus BX41 microscope. Diagnosis of benign lesions and malignancies was confirmed by a blinded pathologist. In order to identify eGFP expression in neonatal donor spermatogonia, co-localization of Lin 28 (Abcam, United Kingdom, ab46020), a marker for spermatogonia, and eGFP (Clontech, USA, 632375), a marker for donor transplanted cells was performed by immunofluorescent staining. In short, antigen retrieval was performed using 0.01 M tri-sodium citrate dihydrate after which slides were blocked using 1% BSA/PBS. Primary antibodies were applied in blocking solution at a concentration of 1:1000 (LIN28) and 1:4000 (eGFP). IgG was used as a negative isotype control. Secondary antibodies were applied in blocking solution at a concentration of 1:1000, utilizing goat α-Mouse Alexa-488 and Donkey α-Rabbit Alexa-555 (both Life technologies, USA), respectively. DAPI was used as a nuclear counterstain. Sections were mounted in Prolong-Gold (Cell Signaling Technology, USA) and analyzed using a Leica DM5000B microscope. In order to identify eGFP positive tubules in transplanted testis, we performed an immunohistochemical staining using anti-GFP antibody (Dilution: 1:1000. Abcam, United Kingdom, ab6556), after antigen retrieval using 0.01 M tri-sodium citrate dihydrate. Isotype IgG was used as a negative control. The signal was visualized by incubation with goat-anti Mouse/Rabbit poly HRP secondary antibody (Polyvision, Immunologic, the Netherlands) followed by incubation with Bright DAB (Immunologic, the Netherlands). Hematoxylin was used as counterstaining and slides were evaluated on an Olympus BX41 microscope. The researcher who performed the evaluation was blinded for the origin of the testicular tissue (i.e. transplanted or contralateral control).
PCR for eGFP
To assess whether benign and malignant lesions originated from eGFP transplanted GS cells and to verify the presence of an eGFP specific sequence in testes, we performed a standard PCR for eGFP on genomic DNA from these tissues. Genomic DNA was isolated using a QIAGEN DNA mini isolation kit. Organs from adult B6D2F1 actin-eGFP mice were used as positive control. The following primersets were used: FW primer 5′-GGACGACGGCAACTACAAGA-3′ RW primer 5′-AAGTCGATGCCCTTCAGCTC-3′ (product size 89 bp) based on GenBank: EU056363.1 ‘Mus musculus transgenic enhanced green fluorescent protein (Egfp) mRNA, complete cds’ and: FW primer 5′-CCACCATGTACCCAGGCATT-3′ RW primer 5′-AGGGTGTAAAACGCAGCTCA-3′ (product size 253 bp) based on GenBank: AK147787.1 ‘Mus musculus melanocyte cDNA, RIKEN full-length enriched library, clone:G270037P22 product: actin, beta, cytoplasmic, full insert sequence’. Using Taq DNA polymerase and PCR buffer (Roche, Switzerland), PCR amplification was performed as follows: 3 min at 95°C, followed by 35 cycles of 30 s at 95°C, 30 s at 65°C and 30 s at 72°C, followed by a final extension step of 8 min at 72°C. PCR products were stored cool at 4°C until visualization using agarose gel electrophoresis.
Statistical analysis
Our primary outcome was development of cancer in the transplanted and control groups at the age of 18 months. Our secondary outcome was the incidence of benign lesions and the survival rate in the two groups until the age of 18 months. We hypothesized that transplanted animals were more likely to develop cancer than control animals. A brief literature study revealed that 10–15% of wild-type mice have developed cancer at the age of 18 months (Bashford and Murray, 1909; Myers et al., 1970; Weindruch and Walford, 1982; Pugh et al., 1999; Ranger et al., 2003; Van Remmen et al., 2003; Yang et al., 2008). Based on these estimations, we calculated that this study would require 36 animals per group to provide an 80% power with a 5% level of significance to demonstrate a 25% increase in cancer incidence in the transplanted group. We expressed development of cancer as number and proportions with a 95% confidence interval calculated with a correction for continuity and compared differences using Fisher’s exact one-sided analyses. Cumulative survival curves were compared using a log-rank test. Risk estimates are given as odds ratios. Statistical analyses were done using IBM SPSS Statistics 23. VassarStats was used specifically for the computation of proportions and their respective confidence intervals (Newcomb, 1998).
Results
In vitro propagation and transplantation of murine spermatogonial stem cells
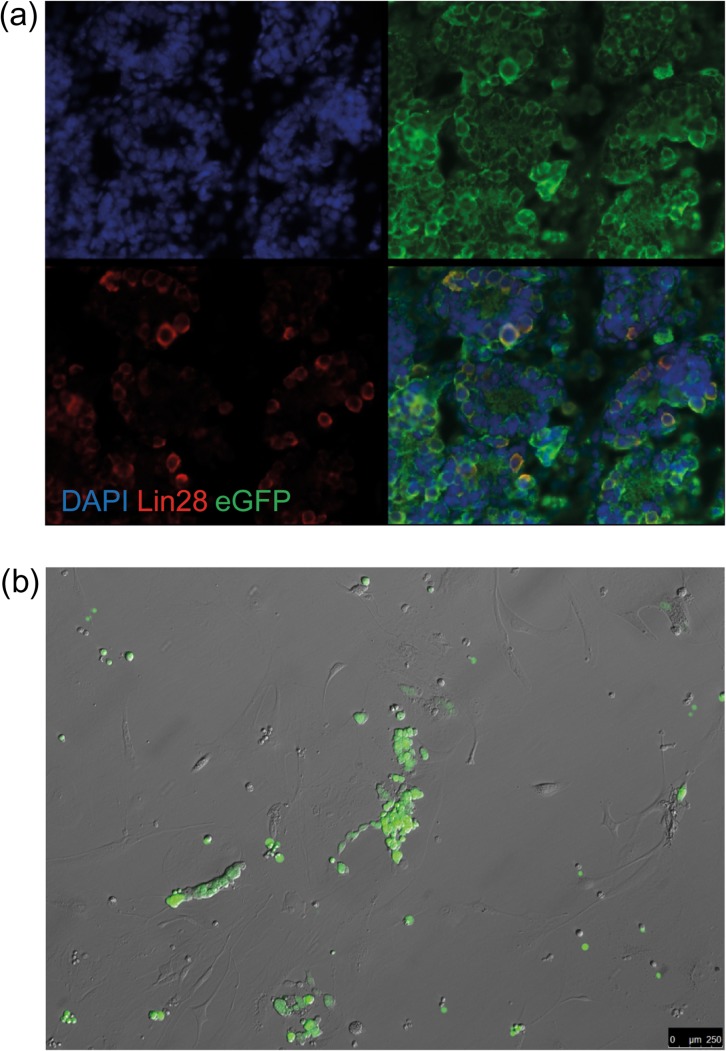
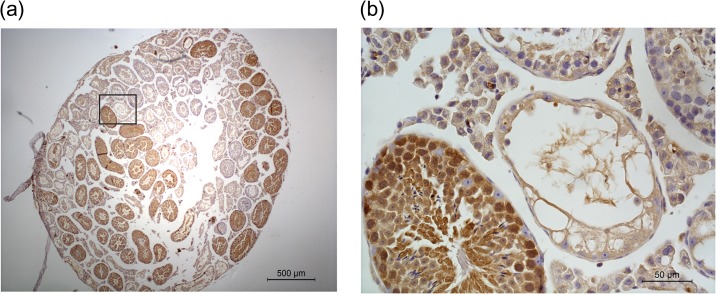
Primary testicular cultures (n = 4) from cryopreserved 4–8 d.p.p. B6D2F1 actin-eGFP hybrid donor testis were initiated and maintained up to Passage 5 or 6 (2–3 months of culture). By that time, the cultures exhibited the typical GS cell culture phenotype. Immunohistological staining of donor testis confirmed the presence of eGFP+/LIN28+ spermatogonia (Fig. 1a). Expression of eGFP was stable throughout culture and detectable 1.5 years after transplantation in a busulfan-treated sterile male by PCR and immunohistochemistry (Figs 1b, 2 and 3). We could find eGFP signal in the testis in 87% (27/31) of transplanted mice (8 months after transplantation (n = 1/1), 16 months after transplantation (n = 26/30)). Three transplanted mice could not be evaluated for eGFP signal because of loss of the sample (e.g. no autopsy or severe autolysis upon autopsy). On the immunohistochemical level, 18 out of 31 transplanted mice showed tubules with positive spermatogenesis by immunohistochemistry for eGFP (Fig. 3 shows a representative section). No eGFP DNA signal or tubules with eGFP positive spermatogenesis by immunohistochemistry were found in any of the contralateral untransplanted control testes. The discrepancy between immunohistochemistry and PCR is attributed to the fact that the testis was divided in two halves (one for immunohistochemistry and one for PCR), and due to the uneven distribution of GS cells during transplantation and the fact that the number of colonies decreases over time (Klein et al., 2010; Klein and Simons, 2011). Out of all testes examined on the histological level, no eGFP nor endogenous spermatogenesis could be found in 10 cases (5 transplanted, 5 control testes). In all other testes, differentiating germ cells could be identified. In transplanted testes with eGFP positive colonies, occurrence of both transplantation derived and endogenous spermatogenesis was common (occurring in 72% of these cases).
Figure 1.
B6D2F1 actin-eGFP neonatal spermatogonia express eGFP in vivo and after long-term culture. (a) An immunofluorescent staining of neonatal B6D2F1 actin-eGFP testicular sections for the spermatogonial marker LIN28 and eGFP. LIN28 and eGFP co-localize in the cytoplasm of the cell, indicating that spermatogonia of these mice indeed express eGFP. (b) eGFP positive GS cell aggregates of B6D2F1 actin-eGFP (Passage 5) under green excitation light have a typical GS cell aggregate structure and display intercellular bridges. Note the eGFP negative wild-type feeder layer.
Figure 2.
eGFP is expressed in transplanted testis, but not in benign and malignant lesions outside of the testis. Gel electrophoresis of a standard PCR for eGFP performed on genomic DNA from adult testis and lesions found in transplanted animals. Genomic DNA of adult B6D2F1 actin-eGFP testis serves as a positive control. Mouse 541570 serves as an example for all transplanted mice, and was transplanted unilaterally with eGFP positive GS cells, which can be confirmed by PCR 14 months after transplantation with a positive eGFP signal in the transplanted testis and no signal in the contralateral untransplanted testis. Mouse 541555 developed a hematoma in the transplanted testis, explaining a positive read-out in this case. eGFP could not be detected in all other benign and malignant lesions. An amplicon for β-actin is present in all samples. LN, lymph node; LCL, diffuse large cell lymphoma.
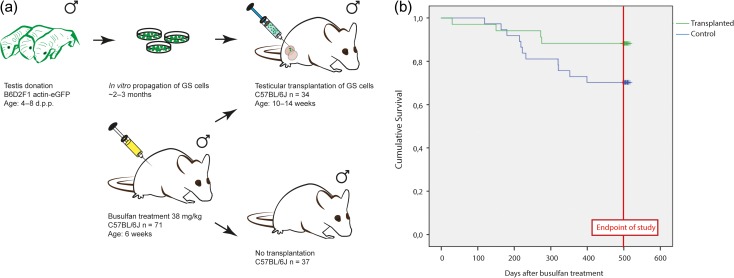
Figure 4.
Testicular transplantation of in vitro propagated GS cells has no effect on survival. (a) Experimental overview of the SSCT mouse model. Donor testis were collected from B6D2F1 actin-eGFP neonatal mice (4–8 d.p.p.), expressing eGFP in all cells except for erythrocytes and hair. Testis were decapsulated and GS cells were propagated for 2–3 months in vitro. Recipient and control C57BL/6 J mice received a single dose busulfan treatment (38 mg/kg) at the age of 6 weeks. Approximately half of these animals received a testicular transplantation of in vitro propagated eGFP + GS cells from B6D2F1 actin-eGFP neonatal mice (4–8 d.p.p.) 4–8 weeks after busulfan treatment. The remainder of mice serve as a control group. (b) In this Kaplan–Meier survival curve, survival is depicted in number of days survival after busulfan treatment. The green line represents the transplanted population, while the blue line represents the control population (busulfan only). Mice that survived up to the age of 18 months were euthanized between 497 and 517 days after busulfan treatment.
Disease and cancer incidence in transplanted animals
To gain more insight in cancer incidence in the recipients of SSC transplantation, a long time follow-up until the age of 18 months was performed. A total of 71 mice were included in this study, all of which received a busulfan injection. Of these mice, 34 underwent testicular transplantation of cultured eGFP positive GS cells and 37 mice served as a control group (Fig. 4a). Shortly after death, a comprehensive post-mortem examination was performed, except for three animals (n = 1 for transplanted, n = 2 for control) which were lost to follow-up.
Figure 3.
Donor-derived spermatogenesis is shown by eGFP expression in the germ cells of the seminiferous tubules of transplanted C57BL/6 J mice transplanted with eGFP positive GS cells. (a) In this representative image, both endogenous and transplantation eGFP-derived (brown) spermatogenesis can be recognized. (b) Spermatogenic cells are positive for eGFP, while endogenous Sertoli cells are negative. Bar represents 500 μM (a) and 50 μM (b).
Malignancies were found in three transplanted animals (9%, 95% CI: 2–25%) and in nine control animals (26%, 95% CI: 14–45%). Despite the high cancer frequency in the control group, a statistical significant difference could not be attributed (OR: 0.3, 95% CI: 0.1–1.1). When considering the malignant lesions (Table I), three types of cancer could be diagnosed. Remarkably, lymphoblastic lymphoma, localized in the thymus and occasionally in the kidney or lung, was solely detected in control animals who died prematurely (d.o.d. 119–320). Another enlarged thymus was detected in a control mouse (d.o.d. 275), however could not be diagnosed as a lymphoblastic lymphoma due to severe autolysis of the cadaver and was therefore excluded from statistical analysis for malignancies. Diffuse large cell lymphoma was found in both groups at the end of the experiment at 18 months of age located primarily in the abdomen and in one transplanted case also in the spleen with no signs of discomfort. A liposarcoma of the liver was apparent in one control animal at term age.
Table I.
Malignant and benign lesions found upon necropsy.
| Malignant lesions | Transplanted animals (n = 34) | Control animals (n = 37) | ||
|---|---|---|---|---|
| Premature death (n = 15) | Total | 4 | Total | 11 |
| No necropsy | 1a | No necropsy | 2a | |
| Enlarged thymus | 1b | |||
| Lymphoblastic lymphoma | 6 | |||
| No malignant lesions | 3 | No malignant lesions | 2 | |
| Death at term (n = 56) | Total | 30 | Total | 26 |
| Diffuse large cell lymphoma | 3 | Diffuse large cell lymphoma | 2 | |
| Liposarcoma | 1 | |||
| No malignant lesions | 27 | No malignant lesions | 23 | |
| Benign lesions | Transplanted animals (n = 34) | Control animals (n = 37) | ||
|---|---|---|---|---|
| Premature death (n = 15) | Total | 4 | Total | 11 |
| No necropsy | 1a | No necropsy | 2a | |
| Benign lymph node hyperplasia | 1 | |||
| No benign lesions | 3 | No benign lesions | 8 | |
| Death at term (n = 56) | Total | 30 | Total | 26 |
| Adhesion of epidydimal fat pad | 1 | Benign adrenal gland hyperplasia | 1 | |
| Hematoma of the testis | 1 | |||
| Benign lymph node hyperplasia | 3 | |||
| Hemangioma of the liver | 1 | |||
| No benign lesions | 24 | No benign lesions | 25 | |
aNo necropsy could be performed, therefore these animals are excluded from this analysis.
bCould not be diagnosed as a lymphoma due to severe autolysis of the tissue, therefore this animal is excluded from analysis.
Benign lesions were found in both transplanted and control animals, however discrepancies exist in type of lesions and age at death (Table I, and Supplementary Figs S1 and S2). During the necropsy, benign lesions were found in six transplanted animals (18%, 95% CI: 8–36) and in two control animals (6%, 95% CI: 1–20). There was no statistical difference in the frequencies of benign lesions between the transplanted and non-transplanted group (OR: 2.9, 95% CI:0.5–16.4). Benign hyperplasia was found in both groups, and mainly appeared in the mesenteric lymph nodes. One case of adrenal gland hyperplasia was found in a control animal, and one transplanted animal that presented with a small hemangioma of the liver.
In the transplanted group, a few reproductive abnormalities were found. A single mouse had to be euthanized 3 days after transplantation because of a post-surgical abdominal infection. At the end of the experiment at the age of 18 months, reproductive abnormalities were found in two mice upon necropsy (see Table I and Supplementary Fig. S1). In one case severe adhesions of the epididymal fat pad to the abdominal viscera had resulted in a cryptorch testis. Another case had developed a post-surgical hematoma of the testis. Both animals did not experience perceivable discomfort. A third case was discovered during histological analysis of the testis. This mouse had a testicular obstruction, resulting in an accumulation of spermatids in the rete testis (Supplementary Fig. S2). All transplanted testes were analyzed histologically, revealing no testicular cancer or teratoma formation.
Malignant lesions are not associated with the transplanted germ cells
It is of key importance to ascertain that malignant lesions found in transplanted animals were not associated with the testicular transplantation. Since we utilized transplantation of GS cells derived from B6D2F1-eGFP donor mice, we could investigate whether the malignancies were derived from these transplanted eGFP GS cells. We were able to detect eGFP in the transplanted, but not in the untransplanted, testis using PCR in 87% of transplanted mice, including a positive read-out for the case of post-surgical hematoma of the transplanted testis. The other benign and malignant lesions were devoid of eGFP signal, indicating that tumors did not originate from GS cells (Fig. 2).
Survival of transplanted animals
Kaplan–Meier analysis revealed no statistical significant difference in overall survival between animals that received a testicular transplantation and controls (Fig. 4b). Premature death occurred in 4/34 (12%, 95% CI: 4–28) transplanted animals and in 11/37 (30%, 95% CI: 16–47) control animals (OR: 0.8, 95% CI: 0.6–1.0). A few of those animals were found deceased in their cage (n = 2 for transplanted, n = 4 for control), the other animals had to be sacrificed due to severe discomfort. The surviving animals were sacrificed at the age of 18 months (497–517 days post-busulfan), n = 30 for transplanted (88%, 95% CI: 72–96) animals and n = 26 for control (70%, 95% CI: 53–84) animals (P = 0.058). Mean survival time after busulfan treatment was found to be equal in both groups, with a mean survival time for transplanted animals of 478 days (95% CI: 439–516 days) and 437 days (95% CI: 395–479 days) for control animals (P = 0.076).
Discussion
To our knowledge, this is the first preclinical animal study to show that SSCT with cultured GS cells does not generate long-term adverse effects in the recipient. In this follow-up study where mice were followed to the age of 18 months, malignant and benign and lesions were present in both groups at an equal rate. eGFP could specifically be detected in transplanted testes, while malignant and benign lesions were devoid of eGFP. Survival rates were similar in busulfan-treated animals transplanted with eGFP positive GS cells to that of animals who received only busulfan. This strongly suggests that the lesions found are not related to the transplantation.
Pre-clinical studies assessing the safety of experimental reproductive techniques are of extreme importance, even though historically not standardly performed in the field of reproductive medicine. The existing preclinical animal data on the risks of SSCT for the recipient are limited. Reports mainly focus on the proof-of-principle that SSCT is a functional technique, and information on the health of the recipient mice is limited to fertility recovery. Reassuringly, no tumor formation is reported in key publications on transplantation of both uncultured SSCs 48–300 days after transplantation (Brinster and Avarbock, 1994; Brinster and Zimmermann, 1994) and cultured mouse SSCs 8 weeks after transplantation (Kanatsu-Shinohara et al., 2003, 2005a) although none of the studies have examined it systematically. Another study specifically mentions no tumor formation 8 weeks after transplantation of sorted uncultured germ cells out of a suspension containing both leukemic cells and germ cells in six mice (Fujita et al., 2005). Given the fact that the lifespan of mice is approximately 1.5 years, and that future human recipients have to live safely with their transplants through their entire life, there was a need for a large scale study where mice were followed up during their full lifespan after transplantation of cultured SSCs. With the present study, to our knowledge, we are the first to confirm and strengthen the previously published data in a systematic way showing no increased tumor incidence after SSCT of in vitro propagated GS cells in mice.
One of the greatest strengths of this study is that it is similar to a human prospective study design. We attempted to mimic the future clinical therapy to restore fertility of infertile male childhood cancer survivors by transplantation of cultured SSCs from a small testicular biopsy cryopreserved before cancer treatment. Therefore, we opted for a long-term follow-up study for busulfan-treated recipient mice through their entire lifespan and transplantation of cultured SSCs from neonatal donor mouse testis. We used a well described culture method (Kanatsu-Shinohara et al., 2003), to culture the GS cells up to 2–3 months on average. It has been demonstrated that, in a similar murine primary testicular culture system, a single SSC can produce 5000 clones within a time-span of 72 days of culture resulting from a cell cycle time of 6 days (Kubota et al., 2004). In a clinical context, a 1300-fold increase of human SSCs is required to repopulate an adult testis when taking into account the biopsy size of 0.2 ml, adult testis size of 13 ml and colonization efficiency of 5% (Sadri-Ardekani et al., 2009). Based on the 18 450-fold increase in SSCs observed in 64 days in our human SSC culture system, the cell cycle time for human SSCs would be 4.5 days and therefore 47 days of culture would be necessary to achieve this 1300-fold increase. For mouse SSCs with a cell cycle time of 6 days, hence a 1300-fold increase would be achieved in 62 days. Therefore, a 2–3 month culture period would be more than sufficient to mimic the human future clinical application. It has been shown that mouse GS cells remain genetically and epigenetically stable in the culture period of 3–24 months (Kanatsu-Shinohara et al., 2005b). Human spermatogonia also remain genetically stable during a culture period of 50 days (Nickkholgh et al., 2014). Of course, it is of paramount importance to study (epi)genetic stability in more detail prior to clinical implementation to assure the safety of both the patient and his offspring (Struijk et al., 2013).
Despite our efforts to resemble clinical SSCT, some factors had to be altered. Regardless of the immense value of animal models in science, mice are not equal to men, and therefore the mouse model that we used might not reflect all aspects of the future clinical setting. A genuine difference in murine and future human germ cell transplantation is the route of administration. In both cases SSCs are injected into the rete testis. In this case, we performed transplantation via the efferent duct which, because of practical reasons, can only be reached effectively by abdominal surgery, while the future human transplantation will likely occur by a direct injection into the rete testis through the scrotum under ultrasound monitoring (Hermann et al., 2012; Ning et al., 2012; Faes et al., 2013). In this study, four transplanted mice experienced post-surgical complications. Post-surgical infection and abdominal adhesions could directly be linked to the abdominal surgery and will therefore likely not occur in the future human application. Likewise, we found one case of an obstruction which we expect was a consequence of accidental damage of the efferent duct during transplantation. Bleeding and subsequent hematoma of the testis is a risk of testicular surgery and in patients it would be recognized shortly after transplantation in standard post-surgery follow-up. Moreover, a discrepancy between the chosen mouse model and a clinical application is that in this study an allogeneic transplantation rather than a more clinically relevant autotransplantation was opted for. C57BL6/J was deemed most applicable as a recipient strain, since it is the most used mouse strain in animal research (Johnson, 2012), and more importantly it was shown that mice of a B6-background are suitable for busulfan treatment to remove endogenous spermatogenesis to prepare for SSC transplantation (Kanatsu-Shinohara et al., 2010; Zohni et al., 2012). We chose B6D2F1-eGFP hybrid as donor animals due to the necessity of a fluorescent marker and the difficulty of propagating GS cells from C57BL6/J in vitro (Kanatsu-Shinohara et al., 2003; Aoshima et al., 2013). These choices harbor some limitations, such as the need to use immunosuppressive agents in transplanted animals (Benjamin and Waldmann, 1986; Kanatsu-Shinohara et al., 2010). The use of immunosuppressive agents in general is associated with a decreased cancer immunosurveillance, and therefore malignancies are prevalent in organ transplanted patients who require immunosuppressive therapy (Chapman et al., 2013). Since in the current study no increased cancer incidence was found in the transplanted group compared to the non-transplanted control, we estimate that the using anti-CD4 as an immunosuppressant did not affect the chance of de-novo tumorigenesis in these animals.
In spite of the similar overall cancer incidence in transplanted and control animals, a difference in the type and onset of disease could be observed. The discrepancy in disease progression could be indicative of an insufficient power. At the time of power calculation, cancer incidence of busulfan-treated mice was unknown, and we had to resort to literature on wild-type animals (Bashford and Murray, 1909; Myers et al., 1970; Weindruch and Walford, 1982; Pugh et al., 1999; Ranger et al., 2003; Van Remmen et al., 2003; Yang et al., 2008). Retrospectively, we found that the overall cancer incidence to be 18% (12 out of 67) in our entire busulfan-treated cohort (transplanted and control), which is slightly higher compared to the percentage described previously for wild-type untreated animals (10–15%). Remarkably, we found a high frequency of premature loss of animals due to lymphoblastic lymphoma in the control group. Lymphoma is quite common in C57BL6 mice (Brayton, 2007), and busulfan is known to cause lymphoma in mice (Bhoopalam et al., 1986). Therefore, the reason we only found lymphoblastic lymphoma in control animals is most likely due to chance. Including an untreated control group of C57BL6/J animals would have enabled us to account for the spontaneous cancer rate in this mouse strain in our animal facility. However, since SSCT is a proposed therapy to preserve subfertility primarily in childhood cancer patients who all have to experience a gonadotoxic therapy prior to the transplantation, we opted to include only a busulfan-treated control group in this study.
Taken together, we have shown in a preclinical animal study that SSCT with cultured SSCs is not associated with an increased cancer incidence or a decreased survival rate. These reassuring data on safety of SSCT has brought us one step closer to restoring fertility in childhood cancer survivors.
Supplementary data
Supplementary data are available at Human Reproduction Online.
Authors' roles
Experiments were designed by A.M.M.P., S.R. and C.L.M. Preparation of in vitro propagated murine SSCs was performed by C.L.M., Y.Z. and L.A.E.C. Transplantations were performed by A.M.M.P, C.L.M, L.A.E.C with assistance of C.M.W.K. Long-term follow-up including macro- and histopathological analysis was primarily done by C.L.M. Diagnosis of benign and malignant lesions was performed by S.P. C.M.W.K, L.A.E.C. and S.K.M.D. assisted with the histological analysis of testes. Statistical analyses were performed by C.L.M and M.W. C.L.M drafted the original manuscript. All authors critically reviewed and revised the manuscript and approved the final version.
Funding
This study was funded by KiKa (KiKa86) and ZonMw (TAS 116003002).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We would like to thank Julia Chikhovskaya for the isolation of primary mouse embryonic fibroblasts and Susanne Borgman for her assistance on DNA isolations from testis biopsies and benign and malignant lesions.
References
- Akhondi MM, Mohazzab A, Jeddi-Tehran i M, Sadeghi MR, Eidi A, Khodadadi A, Piravar Z. Propagation of human germ stem cells in long-term culture. Iran J Reprod Med 2013;11:551–558. [PMC free article] [PubMed] [Google Scholar]
- Aoshima K, Baba A, Makino Y, Okada Y. Establishment of alternative culture method for spermatogonial stem cells using knockout serum replacement. PLoS One 2013;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford E, Murray J. The incidence of cancer in mice of known age. Proc R Soc London Ser B Contain Pap a Biol Character 1909;81:310–313. [Google Scholar]
- Benjamin R, Waldmann H. Induction of tolerance by monoclonal antibody therapy. Nature 1986;320:449–551. [DOI] [PubMed] [Google Scholar]
- Bhoopalam N, Price K, Norgello H, Barone-Varelas J, Fried W. Busulfan and chloramphenicol induced T cell lymphoma: cell surface characteristics and functional properties. Clin Exp Immunol 1986;64:646–655. [PMC free article] [PubMed] [Google Scholar]
- Brayton C. Spontaneous diseases in commonly used mouse strains. Mouse Biomed Res 2007;2:623–717. [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A [Internet] 1994;91:11303–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A 1994;91:11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Webster AC, Wong G. Cancer in the transplant recipient. Cold Spring Harb Perspect Med 2013;3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod 1999;61:1331–1339. [Internet]. [DOI] [PubMed] [Google Scholar]
- Faes K, Tournaye H, Goethals L, Lahoutte T, Hoorens A, Goossens E. Testicular cell transplantation into the human testes. Fertil Steril 2013;100:981–988. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Fujita K, Ohta H, Tsujimura A, Takao T, Miyagawa Y, Takada S, Matsumiya K, Wakayama T, Okuyama A. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest 2005;115:1855–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geens M, Van de Velde H, De Block G, Goossens E, Van Steirteghem A, Tournaye H. The efficiency of magnetic-activated cell sorting and fluorescence-activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Hum Reprod [Internet] 2007;22:733–742. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, Carlson CA, Lin K, Hobbie WL, Wigo E, Wu X, Brinster RL, Kolon TF. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum Reprod [Internet] 2010;25:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens E, De Rycke M, Haentjens P, Tournaye H. DNA methylation patterns of spermatozoa and two generations of offspring obtained after murine spermatogonial stem cell transplantation. Hum Reprod 2009;24:2255–2263. [DOI] [PubMed] [Google Scholar]
- Goossens E, de Vos P, Tournaye H. Array comparative genomic hybridization analysis does not show genetic alterations in spermatozoa and offspring generated after spermatogonial stem cell transplantation in the mouse. Hum Reprod 2010;25:1836–1842. [DOI] [PubMed] [Google Scholar]
- Guo Y, Liu L, Sun M, Hai Y, Li Z, He Z. Expansion and long-term culture of human spermatogonial stem cells via the activation of SMAD3 and AKT pathways. Exp Biol Med [Internet] 2015;240:1112–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Salati J, Sheng Y, Chu T, Orwig KE. Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Hum Reprod 2011;26:3222–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G et al. . Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 2012;11:715–726. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod 2003;69:1260–1264. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod [Internet] 2002;66:21–28. [DOI] [PubMed] [Google Scholar]
- Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr [Internet] 2005;34:12–17. [DOI] [PubMed] [Google Scholar]
- Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD et al. . Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction [Internet] 2003;126:765–774. [PubMed] [Google Scholar]
- Jaye M, Lyall RM, Mudd R, Schlessinger J, Sarver N. Expression of acidic fibroblast growth factor cDNA confers growth advantage and tumorigenesis to Swiss 3T3 cells. EMBO J [Internet] 1988;7:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. Laboratory mice and rats. Mater Methods 2012;2:1–13. [Google Scholar]
- Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod 2005. a;72:985–991. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 2003;69:612–616. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y et al. . Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development [Internet] 2005. b;132:4155–4163. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Miki H, Inoue K, Morimoto H, Takashima S, Ogura A, Shinohara T. Genetic influences in mouse spermatogonial stem cell self-renewal. J Reprod Dev [Internet] 2010;56:145–153. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Toyokuni S, Shinohara T. Genetic selection of mouse male germline stem cells in vitro: offspring from single stem cells. Biol Reprod 2005. c;72:236–240. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Saito K, Sakai C, Shinya M, Sakai N. Production of zebrafish offspring from cultured spermatogonial stem cells. Genes Cells 2012;17:316–325. [DOI] [PubMed] [Google Scholar]
- Klein AM, Nakagawa T, Ichikawa R, Yoshida S, Simons BD. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 2010;7:214–224. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Klein AM, Simons BD. Universal patterns of stem cell fate in cycling adult tissues. Development 2011;138:3103–3111. [DOI] [PubMed] [Google Scholar]
- Kossack N, Terwort N, Wistuba J, Ehmcke J, Schlatt S, Schöler H, Kliesch S, Gromoll J, Scholer H, Kliesch S et al. . A combined approach facilitates the reliable detection of human spermatogonia in vitro. Hum Reprod 2013;28:3012–3025. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A [Internet] 2004;101:16489–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Schmidt JA, Brinster RL. Spermatogonial stem cells derived from infertile Wv/Wv mice self-renew in vitro and generate progeny following transplantation. Biol Reprod [Internet] 2009;81:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Ogonuki N, Miki H, Inoue K, Morimoto T, Morimoto H, Ogura A, Shinohara T. Heritable imprinting defect caused by epigenetic abnormalities in mouse spermatogonial stem cells. Biol Reprod 2009;80:518–527. [DOI] [PubMed] [Google Scholar]
- Lim JJ, Sung SY, Kim HJ, Song SH, Hong JY, Yoon TK, Kim JK, Kim KS, Lee DR. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif 2010;43:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer Y, Huiskamp R, Davids JA, van der Tweel I, de Rooij DG. The sensitivity of quiescent and proliferating mouse spermatogonial stem cells to X irradiation. Radiat Res [Internet] 1992;130:289–295. [PubMed] [Google Scholar]
- Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril 2013;100:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder CL, Zheng Y, Jan SZ, Struijk RB, Repping S, Hamer G, van Pelt AMM. Spermatogonial stem cell autotransplantation and germline genomic editing: a future cure for spermatogenic failure and prevention of transmission of genomic diseases. Hum Reprod Update 2016;22:561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers DD, Meier H, Hueber RJ. Prevalence of murine C-type RNA virus group specific antigen in inbred strains of mice. Life Sci II 1970;9:1071–1080. [DOI] [PubMed] [Google Scholar]
- Nagano M. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod [Internet] 2003;69:701–707. [DOI] [PubMed] [Google Scholar]
- Nayak S, Goel MM, Makker A, Bhatia V, Chandra S, Kumar S, Agarwal SP. Fibroblast growth factor (FGF-2) and its receptors FGFR-2 and FGFR-3 may be putative biomarkers of malignant transformation of potentially malignant oral lesions into oral squamous cell carcinoma. PLoS One 2015;10:e0138801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;30:2635–2650. [DOI] [PubMed] [Google Scholar]
- Nickkholgh B, Mizrak SC, van Daalen SK, Korver CM, Sadri-Ardekani H, Repping S, van Pelt AM. Genetic and epigenetic stability of human spermatogonial stem cells during long-term culture. Fertil Steril [Internet] 2014;102:1700–1707.e1. [DOI] [PubMed] [Google Scholar]
- Ning L, Meng J, Goossens E, Lahoutte T, Marichal M, Tournaye H. In search of an efficient injection technique for future clinical application of spermatogonial stem cell transplantation: infusion of contrast dyes in isolated cadaveric human testes. Fertil Steril [Internet] 2012;98:1443–1448. [DOI] [PubMed] [Google Scholar]
- Nobrega RH, Greebe CD, van de Kant H, Bogerd J, de Franca LR, Schulz RW. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS One 2010;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett 1997;407:313–319. [DOI] [PubMed] [Google Scholar]
- Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, Mitchell RT, Pennings G, Rives N, Tournaye H et al. . A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod 2015;30:2463–2475. [DOI] [PubMed] [Google Scholar]
- Poganitsch-Korhonen M, Masliukaite I, Nurmio M, Lähteenmäki P, van Wely M, van Pelt AMM, Jahnukainen K, Stukenborg J-B. Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia 2017;31:1460–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh TD, Oberley TD, Weindruch R. Dietary intervention at middle age: Caloric restriction but not dehydroepiandrosterone sulfate increases lifespan and lifetime cancer incidence in mice. Cancer Res 1999;59:1642–1648. [PubMed] [Google Scholar]
- Qin Y, Liu L, He Y, Wang C, Liang M, Chen X, Hao H, Qin T, Zhao X, Wang D. Testicular busulfan injection in mice to prepare recipients for spermatogonial stem cell transplantation is safe and non-toxic. PLoS One 2016;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A 2003;100:9324–9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang T-T et al. . Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics 2003;16:29–37. [DOI] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Lin CC, Chang LJ, Avarbock MR, Brinster RL. Efficient generation of transgenic rats through the male germline using lentiviral transduction and transplantation of spermatogonial stem cells. J Androl 2007;28:353–360. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA 2011;305:2416–2418. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Homburg CH, van Capel TM, van den Berg H, van der Veen F, van der Schoot CE, van Pelt AM, Repping S. Eliminating acute lymphoblastic leukemia cells from human testicular cell cultures: a pilot study. Fertil Steril 2014;101:1072–1078.e1. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJ, van der Veen F et al. . Propagation of human spermatogonial stem cells in vitro. JAMA 2009;302:2127–2134. [DOI] [PubMed] [Google Scholar]
- Sasada R, Kurokawa T, Iwane M, Igarashi K. Transformation of mouse BALB/c 3T3 cells with human basic fibroblast growth factor cDNA. Mol Cell Biol 1988;8:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatt S, Rosiepen G, Weinbauer GF, Rolf C, Brook PF, Nieschlag E. Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod 1999;14:144–150. [DOI] [PubMed] [Google Scholar]
- Struijk RB, Mulder CL, Van Der Veen F, van Pelt AMM, Repping S. Restoring fertility in sterile childhood cancer survivors by autotransplanting spermatogonial stem cells: are we there yet? Biomed Res Int 2013; 2013:1–12, http://dx.doi.org/10.1155/2013/903142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 1982;215:1415–1418. [DOI] [PubMed] [Google Scholar]
- Wu X, Goodyear SM, Abramowitz LK, Bartolomei MS, Tobias JW, Avarbock MR, Brinster RL. Fertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 years. Hum Reprod [Internet] 2012;27:1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W et al. . Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res 2008;68:7803–7810. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Hou R, Wu J. Generation of mice by transplantation of an adult spermatogonial cell line after cryopreservation. Cell Prolif 2009;42:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Nie D, Chakrabarty S. Growth factors in tumor microenvironment. Front Biosci (Landmark Ed [Internet] 2010;15:151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohni K, Zhang X, Tan SL, Chan P, Nagano MC. The efficiency of male fertility restoration is dependent on the recovery kinetics of spermatogonial stem cells after cytotoxic treatment with busulfan in mice. Hum Reprod 2012;27:44–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.