Abstract
Several putative deep brain photoreceptors (DBPs) have been identified, such as melanopsin, opsin 5, and vertebrate ancient opsin. The aim of this study was to elucidate the role of DBPs in gonadal regulation in the Pekin drake. As previously reported, we observed opsin-like immunoreactivity (-ir) in the lateral septum (LS), melanopsin-ir in the premammillary nucleus (PMM), and opsin 5-ir in the periventricular organ. To determine the sensitivity of the DBPs to specific wavelengths of light, drakes were given an acute exposure to red, blue, or white light. Blue light stimulated an increase (P < 0.01) in the immediate early gene fra-2-ir co-expression in melanopsin-ir neurons in the PMM, and red light increased (P < 0.05) fra-2-ir co-expression in opsin-ir neurons, suggesting these neurons are blue- and red-receptive, respectively. To further investigate this photoperiodic response, we exposed drakes to chronic red, long-day white, short-day white, or blue light. Blue light elicited gonadal regression, as testes weight (P < 0.001) and plasma luteinizing hormone (LH) levels (P < 0.001) were lower compared to drakes housed under long-day white light. Photo-regressed drakes experienced complete gonadal recrudescence when housed under long-day red and blue light. qRT-PCR analyses showed that gonadally regressed drakes showed reduced levels (P < 0.01) of gonadotropin releasing hormone (GnRH) mRNA but not photoreceptor or GnIH mRNAs compared to gonadally functional drakes. Our data suggest DBP in the LS may be rhodosin and multiple DBPs are required to fully maintain gonadal function in Pekin drakes.
Keywords: rhodopsin, melanopsin, opsin 5, vertebrate ancient opsin, gonadal recrudescence, luteinizing hormone
INTRODUCTION
Pekin ducks were domesticated from the wild mallard (Anas platyrhynchos) between 4,000 to 10,000 years ago in southern China. Despite domestication, the Pekin duck has maintained many of the growth and reproductive characteristics of its wild predecessor (Cherry and Morris, 2008). A prominent physiological characteristic is seasonal reproduction and their photosensitivity to increasing day length that triggers a rapid gonadal recrudescence as a component of seasonal breeding. During the non-breeding season, the drake's testes regress to less than 10% of their reproductively active size, and to a near pre-pubertal state of seminiferous tubule differentiation (Cherry and Morris, 2005). However, during breeding season, the activation of the drake's hypothalamic-pituitary-gonadal axis (HPG) ultimately stimulates testicular hypertrophy, spermatogenesis, and androgen secretion. Despite decades of research, the precise neural mechanisms within the hypothalamus that underlie photoactivation of gonadotropin releasing hormone (GnRH) remains unclear.
In mammals, photoreceptivity includes the activity of rods and cones within the retina, as well as non-image forming photosensitive retinal ganglion cells. Non-image-forming retinal cells regulate environmental light responses, including circadian entrainment (Panda et al., 2002; Panda et al., 2003a,b) and the pupillary light reflex (Heaton, 1971; Hattar et al., 2002; Lin et al., 2008). These cells are also known to activate diencephalic nuclei, such as the suprachiasmatic nucleus, and in turn affect melatonin secretion from the pineal gland (for review, see Wagner et al., 2008). In birds, the removal of these retinal cells does not affect the seasonal changes in the HPG (Oliver and Bayle, 1982). However, if light is prevented from penetrating the skull, a loss in photoresponsiveness leads to gonadal regression (Menaker et al., 1970; Underwood and Menaker, 1970a,b,c). Hence, in birds there is compelling evidence that photoresponsiveness is mediated—at least in part—by non-retinal neurons that express photosensitive chemicals. These neurons have been referred to as deep brain photoreceptors (DBPs; reviewed in (Li and Kuenzel, 2008)).
The identities of the DBPs have been somewhat elusive. Over the past 2 decades, some success in identifying putative DBPs has come from using anti-Ret-P1 antibodies. Photo-responsive receptors are transmembrane G-protein coupled receptors that transduce light energy into a neuronal signal (Hattar et al., 2002). Success in identifying putative DBPs has come by staining for 4 different, albeit related, photoreceptors: opsin (Ret-P1; Silver et al., 1988; Saldanha et al., 2001), opsin 5 (neuropsin or OPN5; Nakane et al., 2010), vertebrate ancient opsin (VA; Halford et al., 2009), and melanopsin (ONPN4; Hattar et al., 2002; Panda et al., 2002; Nayak et al., 2007). The VA opsin has been found in the mediobasal preoptic area and paraventricular nucleus with fiber terminals shown in the medial basal hypothalamus, particularly the median eminence (Halford et al., 2009). OPN5 has been found in the paraventricular organ in the medial basal hypothalamus.
OPN4 is a member of a well-conserved family of photoreceptive proteins and its gene has been identified in numerous species including humans, birds, amphibians and fish (for review see (Bellingham et al., 2006)). In birds, OPN4 immunoreactivity (-ir) has been localized to the premammillary nucleus (PMM) and is co-localized with tyrosine hydroxylase-ir neurons—presumably dopaminergic (El Halawani et al., 2009; Kang et al., 2010). Several studies have suggested that melanopsin receptors are responsive to blue-specific light wavelengths in several species (Iyilikci et al., 2009; Bailes and Lucas, 2013; Tsunematsu et al., 2013; Ramos et al., 2014; Takeuchi et al., 2014; Walmsley et al., 2015) and that melanopsin plays a role in photoperiodic responsiveness (El Halawani et al., 2009; Kang et al., 2010). Male quail appear to respond equally in testicular growth to either blue or full-spectrum white light (Nakane et al., 2010). Although in some species, blue light is sufficient and even desirable for increased growth, reproduction, or other favorable behaviors (Levenick and Leighton, 1988; Prayitno et al., 1997; Rozenboim et al., 2004; Iyilikci et al., 2009; Zhang et al., 2014), this condition does not appear to be the case for ducks (Campbell et al., 2015). It has been suggested that for optimal growth (Campbell et al., 2015) and reproductive development to occur in drakes, both red and blue wavelengths are required (Benoit, 1961; Benoit, 1964).
Thus, we first set out to determine if Ret-P1, OPN4, and OPN5 can be observed within the diencephalon of the drake. Second, we set out to determine the effects of short (blue) or long (red) wavelengths of light on the DBPs and reproduction in the Pekin drake. We found that blue light was not able to maintain gonadal activity in drakes following either an acute or long-term exposure. Further, our data suggests that the Ret-P1-ir in lateral septum (LS)may be indicative of rhodopsin (OPN2).
METHODS
Animals and Housing
All ducks were sexually active Pekin drakes (∼45 wk of age) obtained from Maple Leaf Farms, Inc. (Leesburg, IN) and housed in the Hope College aviary at a density of about ≥0.3 m2 per duck. Lights were set on a 18:6 h light:dark cycle. Birds arrived at Hope College in early May; they were housed in floor pens and allowed ad libitum access to water via pin-metered water lines. Ducks were fed standard Pekin duck breeder chow and given access to the feed for 7 h/d, following typical standards of care for breeder ducks. Ducks were raised on pine litter with fresh litter spread over the entire pen twice daily. Birds were housed for 1 wk prior to the start of the experiment. All housing and experimental procedures were approved by Hope College Animal Care and Use Committee.
Experiment 1: Immunocytochemistry for Localization of Ret-P1, OPN4, and OPN5
Antibodies
Several primary antibodies were utilized for this experiment. The first is a commercially available mouse anti-Ret-P1 (Ret-P1, Abcam, Inc., Cambridge, MA) monoclonal antibody raised against the extracellular fragment of the Ret-P1 (opsin) peptide. This antibody has been successfully utilized in other non-mammalian species (Silver et al., 1988). The second primary antibody was a rabbit anti-OPN4 polyclonal that was generously provided by Dr. Mohamed El Halawani (Department of Animal Sciences, University of Minnesota). The third primary antibody was also a mouse monoclonal against avian OPN5 and it was generously provided by Dr. Yoshimura (Avian Bioscience Research Center, Graduate School of Bioagricultural Sciences, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Japan). For double-staining studies described below, we also utilized a rabbit anti-fra2 polyclonal antibody (Santa Cruz Biotech, Dallas, TX) combined with a mouse monoclonal anti-tyrosine hydroxylase (TH; Immunostar, Inc., Hudson, WI). Fra-2 is an immediate early gene and is a fos-related transcriptional factor known to stimulate the AP-1 promoter binding domain and that is induced by photostimulation in birds (Nishina et al., 1990; Suzuki et al., 1991; Peczely and Kovacs, 2000).
Tissue Preparation
Immunocytochemical procedures were carried out as described previously (Fraley and Kuenzel, 1993; Fraley and Ulibarri, 2002; Fraley, 2006; Johnson and Fraley, 2008; Constant et al., 2012). Briefly, brains (n = 5 gonadally active drakes) were collected 4 hrs after lights-on and static-fixed in 4% paraformaldehyde for 72 hrs, then cryoprotected in a 30% sucrose solution (PB; 0.1 M phosphate buffer, pH = 7.4) for 96 h then frozen on dry ice. Brains were stored at −80°C until sectioned. Four parallel series of 40 μm coronal sections of brain tissue were cut on a sliding microtome (American Optical Company, Buffalo, NY) from the diagonal band of Broca (DBB) through the mammillary bodies, and stored at −20°C in cryopreservative (0.9% NaCl, 30% sucrose, 1% polyvinylpyrolidine mw 40,000, 30% ethylene glycol in 0.05 M PB) solution until processed. Free-floating immunocytochemistry for each of the 3 primary DBP antibodies (Ret-P1, melanopsin or OPN5) was performed on one set of hypothalamic sections by a standard ABC (avidin/biotin complex) reaction, as previously described (Saldanha et al., 2010). Briefly, sections were washed in PB, incubated in 10 mM sodium citrate (pH = 4.3, 80°C for 30 min) followed by 0.3% H2O2 in PB (30 min at room temp.). After washing in 0.1 M PB, sections were then transferred to solution containing PB with 0.4% triton-X-100 (anti-Ret-P1, 1:2000; anti-OPN4, 1:1000; anti-OPN5, 1:500) and incubated for 48 h at 4°C with agitation. After 3 PB washes, sections were incubated for 3 h at room temperature in blocking solution with secondary antibody (1:500, biotinylated-anti-rabbit or biotinylated-anti-mouse as appropriate, Vector Laboratories, Burlingame, CA). Primary antibody immunoreactivity was visualized with the standard ABC reaction with Ni-3,3΄,5,5΄ diaminobenzidine (DAB) as the chromagen to produce a blue-black reaction product (DAB Chromagen Kit No. PK6100, Vector Laboratories, Burlingame, CA). Sections were mounted on Superfrost Plus slides (VWR Scientific, West Chester, PA), air-dried, dehydrated with graded ethanol series, cleared with Citrosolv, after which glass coverslips were applied.
Co-localization of fra-2-ir with other primary antibodies was peformed as described above for the single-label studies using the standard ABC reaction, but with the addition of Ni+ to the DAB solution to produce a blue-black reaction product within the cellular nuclei of fra-2 positive-ir. The second primary antibody (anti-TH or anti-Ret-P1) was visualized using DAB alone, producing a brown reaction product in the cytoplasm of positive-ir neurons.
Immunocytochemical Controls
For both the single- and double-label staining, elimination of either the primary or secondary antibody prevented cell body or fiber staining for that peptide. Preabsorption of tissue sections with 10-fold higher molar concentration of immunogen (Table 1) eliminated specific respective DBP staining (Figure 1, panels F - H).
Table 1.
Antibody characteristics used in single- and double-label immunocytochemistry.
| Target antigen | Catalog Number | Manufacturer; species & type | Antibody class | Immunogen | Dilution | Citation/Reference |
|---|---|---|---|---|---|---|
| Fra-2 | Sc-604 | Santa Cruz; rabbit polyclonal | IgG | N-terminus of Fra-2 | 1:1000 | (Nishina et al., 1990; Suzuki et al., 1991; Peczely and Kovacs, 2000) |
| Tyrosine hydroxylase | 22941 | Immunostar, Mouse monoclonal | IgG1 | TH from Rat PC-12 cells | 1:2000 | (Goodson et al., 2012; Kang et al., 2010; Riters et al., 2000; Riters et al., 2007) |
| Opsin clone Ret-P1 | O 4886 | Sigma-Aldrich; mouse monoclonal | IgG1 | Amino acid residues 4–10 at N-terminus | (Silver et al., 1988; Saldanha et al., 2001) | |
| Opsin-5 | N/A | Gift from Takashi (Nakane et al., 2010), PhD; rabbit polyclonal | IgG | C-terminal region of OPN-5 | 1:1000 | (Nakane et al., 2010) |
| melanopsin | N/A | Gift from Muhamad El Halawani; rabbit polyclonal | IgG | 1:500 | (Kang et al., 2010) |
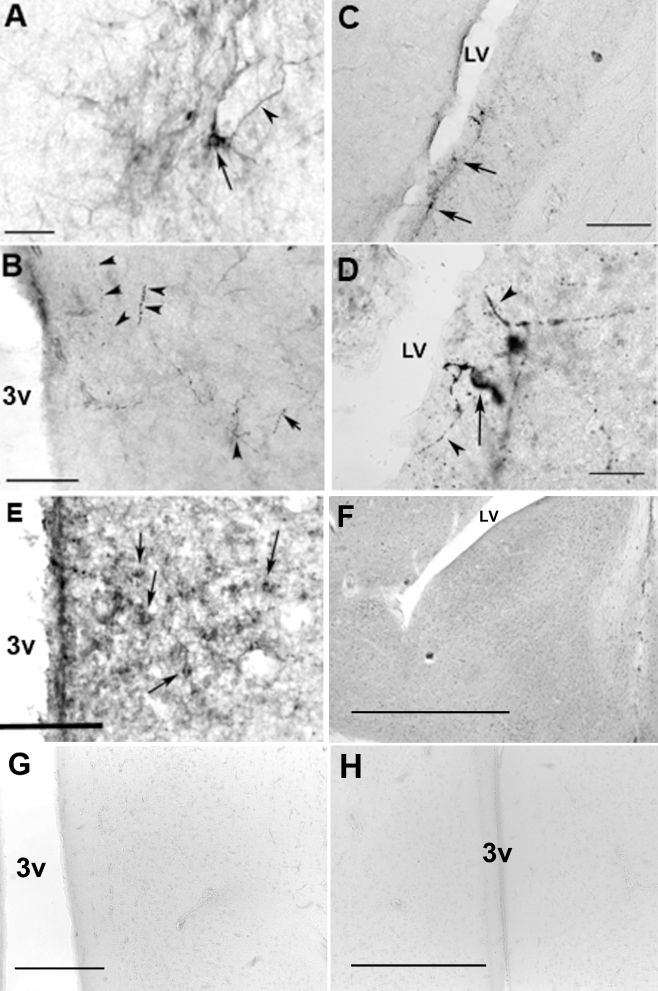
Figure 1.
Photomicrographs of DBPs in the drake brain. (A) OPN4-ir in the PMM. Arrow = OPN-5-ir cell bodies, bar = 25 μm. (B) OPN4-ir fibers (arrow heads) in the PMM. 3v = third ventricle, bar = 50 μm. (C & D) Ret-P1-ir neurons (arrows) and fibers (arrow heads) in the LS. LV = lateral ventricle, bar = 50 & 25 μm respectively. (E). OPN-5-ir in the PVO, arrows = cell bodies, bar = 50 μm. Photomicrographs of immunocytochemistry following preabsorption of sections with appropriate immunogen (Table 1) in LS (F), PVO (G) and PMM (H). Bars = 150, 50, or 100 μm, respectively.
Analyses of Immunocytochemistry
All sections that contained immunoreactive neurons were analyzed under bright-field illumination (Leica Microsystems DM5100, Wetzlar, Germany). All immunoreactive neurons were counted bilaterally at 20× magnification. Single-labeled DBP neurons were counted if brown neurons were observed with clear nuclei lacking dark staining. Double-labeled neurons were counted bilaterally if dark blue-black nuclei were observed within neurons containing brown-colored cytoplasm. All slides were coded so that the identity of the treatment was unknown during analysis.
Experiment 2: Effects of an Acute Exposure to Colored vs. White Light
The purpose of this experiment was to determine if an acute exposure of drakes to single-colored light would alter luteinizing hormone (LH) secretion compared to drakes under white light. Three light-proofed pens (n = 12 drakes per pen) contained different colored lights; red light (peak excitation @ 625 nm), blue light (peak excitation @ 425 nm) or white light. The experiment was repeated on a new set of birds for each treatment until a final N = 6 pens per light treatment was achieved. For each repeated experiment, we rotated the light among the different pens to minimize any pen-location affects. All lights were from fluorescent bulbs and the photonic energy was normalized to 1.6 × 103 μM photons/m2/sec at the level of the ducks’ heads in each pen, as we have described previously (Campbell et al., 2015). Blood samples were collected from all drakes during the scotophase 1 h prior to light exposure. Thirty minutes prior to lights-on, 2 drakes from each pen (n = 6) were euthanized for blood and brain analyses as dark controls. The remaining drakes were exposed to red, blue, or white light for 90 min, and then euthanized (IP FatalPlus, 400 mg/kg) 90 min after lights-on. Blood samples were collected from theses drakes as well. Brain sections were analyzed for the immediate-early gene product, fra-2, to determine cell stimulation by the different colors of light. In an attempt to identify the phenotype of stimulated neurons, we also co-localized fra-2 with anti-Ret-P1, anti-OPN5 (OPN5), or anti-tyrosine hydroxylase (TH) to identify OPN4 neurons.
Experiment 3: Effects of a Chronic Exposure to Colored vs. White Light
The purpose of the third experiment was to determine the effects of a long-term exposure to red or blue light compared to white light on reproduction in the drake. In order to accomplish this, we set up floor pens with adult, sexually active Pekin drakes in the same light-proof pens as described in Experiment 2. However, Experiment 3 drakes were exposed to light sources and subsequently euthanized after 4 wk. As for Experiment 2, we repeated the experiment until a final N = 6 pens per treatment was achieved. We also included 2 additional light-treatment groups. A group of drakes were housed on a white-light, short day (SD 6:18, n = 12) that is well established to cause gonadal regression. After 4 wk, one-half of the short-day group was euthanized and the remaining drakes (n = 6) placed back on long day exposure (18:6) by replacing the lights (during scotophase) to a combination of the red and blue lights, again normalized to the quantal energy described for Experiment 2. The red + blue light ducks were then euthanized 4 wk later. Blood was collected prior to the start of the experiment and then weekly throughout the experiment starting with wk 2. Drakes were euthanized using an overdose of pentobarbital (400 mg/kg intraperitoneal) between 3–4 hrs after lights-on. After the drakes reached a deep surgical plane of anesthesia, the brain was removed within 3 min and immediately frozen on dry ice. Phalluses were then measured (length × width at proximal end), testes were collected and weighed, and plasma obtained. Plasma was stored at −20°C until processed for LH levels via radioimmunoassay (RIA). Brains were stored at −80°C until processed for qRT-PCR analyses.
Plasma Hormone Analyses
Plasma was collected and stored at −20C until analyzed for LH levels. A radioimmunoassay was used to measure LH in a single assay by the Sharp Laboratory (University of Edinburgh, UK) as described previously (Sharp et al., 1987; Saldanha et al., 2010); the antibody was generated in rabbit against chicken LH and the intra-assay coefficient of variation was 8%.
qRT-PCR Analyses of Diencephalic mRNA
Tissue Preparation
The tissue samples were removed from the −80°C freezer and allowed to thaw enough to cut the brain without fractures. The diencephalic tissue was removed rostrally from the septomesencephalic tract. The tissue was then cut caudally at the third cranial nerve and dorsally at the Lobus parafactorius (LPO). The tissue was then cut ventrally from the base of the brain with both the optic chiasm and tract trimmed off. Manufacturers instructions for the QIAGEN RNeasy Midikit protocol were followed for RNA extraction from the tissues collected.
qRT-PCR
A Superscript VILO Invitrogen (Carlsbad, CA) cDNA synthesis kit was used to complete qRT-PCR analysis. Single-stranded cDNA was synthesized from 2 μg total cellular RNA using oligo(dT)16 primer and superscript II Reverse Transcriptase (Gibco BRL, Invitrogen Corp., Carlsbad, CA,), as recommended by the manufacturer. The RNA from dissected tissue was extracted by RNAqueous®-4PCR system (Ambion). Average yield was 80–100 ng/100 μl/turkey total RNA. Each sample was concentrated into 10 μl, and then amplified by MessageAmpTM II aRNA Amplification Kit (Ambion) to yield an average of 10–15 μg aRNA (antisense amplified RNA). Five micrograms of aRNA was used to perform a reverse transcription reaction using second round primers, and then second strand was synthesized using oligo(dT) primer. The 3΄ end specific oligonucleotide primers were designed within 300 bp from the 3΄ end of the transcript and used in qRT-PCR for each of the DPBs or neuropeptides (see Table 2). The amplification profile of β-actin, DBPs and the neuropeptides consisted of 36 cycles each for 1 min at 95°C, 30 s at 54°C, and 1 min at 72°C, respectively. The cycles were previously determined to be within the linear range. Final qRT-PCR was performed using the iTaq SYBR Green Supermix (BioRad Inc.; Hercules, CA) following manufacturers recommendation using a CFX96 Tougch REalt Time System (BioRad Inc.; Hercules, CA). Fold changes were determined following by first averaging the ct values for all samples. The delta ct was determined by taking the gene of interest average minus the β-actin average. The delta-delta ct was determined by subtracting the treatment delta ct value from the control delta ct value; fold-change was calculated by taking the delta-delta ct value (x) and calculating 2^-x.
Table 2.
Primer sequences utilized for qRT-PCR.
| Name | Forward | Reverse |
|---|---|---|
| Beta actin | CAC AAT GTA CCC GGG CAT CG | ACA TCT GCT GGA AGG TGG AC |
| GnRH-I | ATC GCA AAC GAA ATG GAA AG | CTG GCT TCT CCT TCG ATC AG |
| GnIH | TAA CAC CGC ATG GTA TGT GC | CTC CTC TGC TCT TCC TCC AA |
| OPN4 (melanopsin) | AAG GTT TCG CTG TCA TCC AGC | CTG CTG CTG TTC AAA CCA AC |
| VA opsin | TAG CCA CTG CAT ACC CTT CC | TGGGTGAGTGTTGCTCTCTC |
| OPN5 | TTT CTC ACC GCT GGA TCT TT | CAG GCA GAT AAA GGC ATG GTG T |
| OPN2 (rhodopsin) | TAC GCT GGG CGG TGA AAT C | ATG ATC CAG GAG AAC GCG AC |
Statistical Analyses
Data were analyzed ad hoc by analysis of variance (ANOVA) using Mac JMP (JMP 9; SAS Institute, Raleigh, NC) followed by a Fisher's PLSD post hoc to determine differences between pairs of treatment groups. A P-value < 0.05 was considered significant.
RESULTS
Experiment 1: Immunocytochemical Localization of Ret-P1, OPN4, and OPN5
Many Ret-P1-ir neurons were observed in the LS with dense fibers found throughout the diagonal band of Broca (DBB) and the external zone of the median eminence (ME) as previously reported (Saldanha et al., 2001). A few OPN4-ir neuronal cell bodies were observed in the PMM with fibers found throughout the hypothalamus, but densely within the external zone of the ME; similar to the distribution described by others (El Halawani et al., 2009). Lastly, a very few OPN5-ir cell bodies were observed in the periventricular organ (PVO) also similar to that described by others (Nakane et al., 2010), however very few OPN5-ir fibers were observed. Figure 1 illustrates these results.
Experiment 2: Effects of an Acute Exposure to Colored vs. White Light
LH Secretion
No differences were observed in circulating LH levels among the drakes when sampled during the scotophase regardless of ultimate light treatment. However, after the lights were turned back on, drakes under white light maintained similar levels of circulating LH compared to dark conditions. Drakes under red and blue light showed slight, but reduced (P < 0.05), circulating LH levels compared to both of their own pre-lights-on samples, and to drakes maintained under white light (Figure 2).
Figure 2.
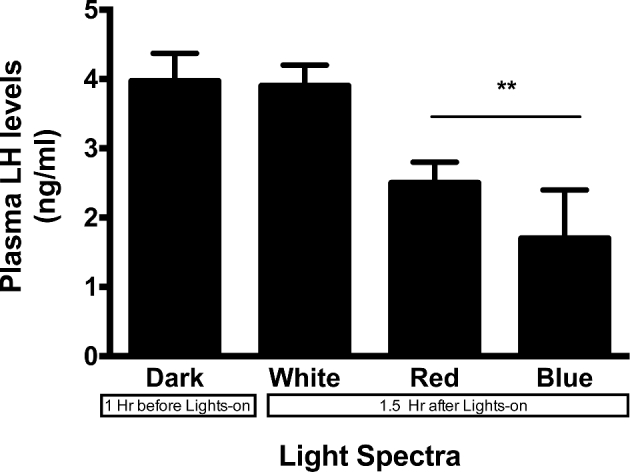
Plasma LH levels following acute exposure to red, blue or white light. Red and blue light could not maintain circulating LH levels to that of drakes under white light 1.5 h after lights-on. **P < 0.01.
Light Effects on Fra-2 Expression in DBP Neurons
Observations from Experiment 1 showed that the anti-OPN5 staining resulted in very few observable neurons within the PVO, thus making double-staining results with fra-2 difficult to interpret. Similarly, results with the anti-OPN4 antibody also showed limited results; however, it is known that all OPN4-containing neurons within the PMM contain TH (El Halawani et al., 2009; Kang et al., 2010). Thus this portion of the experiment focused on Fra-2-ir co-localized with either Ret-P1-ir or TH-ir.
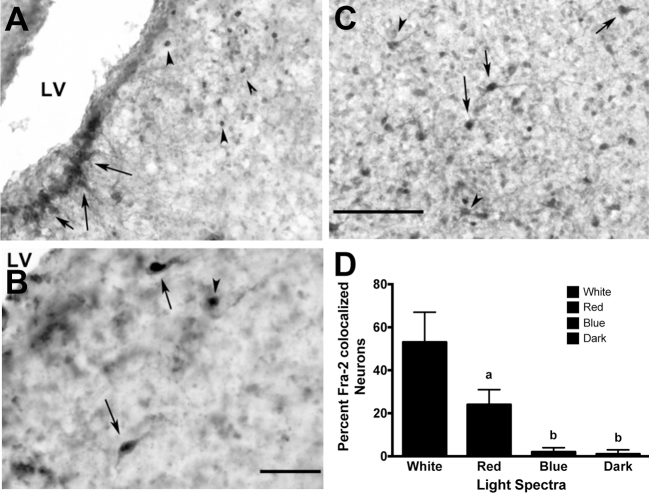
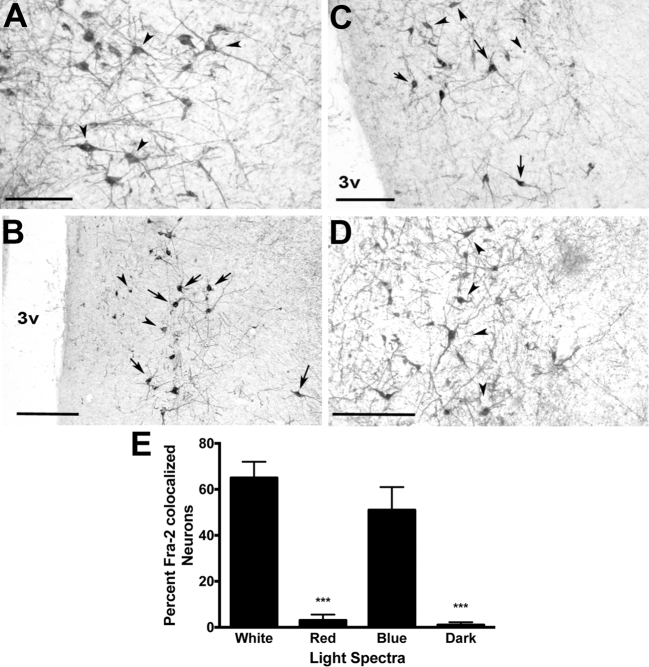
Drakes exposed to 90 min of white light showed increased (P < 0.01) percentage of Fra-2-ir nuclei co-localized with Ret-P1-ir in the LS and with TH-ir within the PMM compared to drakes euthanized in the dark. A greater (P < 0.05) number of fra-2-ir nuclei were co-localized with Ret-P1-ir in the LS in drakes housed under red lights compared to dark conditions, but not under blue lights. Drakes housed under red light showed no fra-2 co-localization with TH-ir in the PMM. However, drakes housed under blue light did show considerably increased (P < 0.01) percentage of fra-2/TH-ir co-localization in the PMM compared to dark conditions, though not to the level of white light. Figures 3 and 4 illustrate these results.
Figure 3.
Fra-2- and Ret-P1-ir co-localization in the LS under (A) White light (arrow = fra-2/Ret-P1 co-localized neurons, arrow heads = fra-2-ir), (B) red light (arrow = fra-2/Ret-P1 co-localized neurons), and (C) white light (arrow = fra-2/Ret-P1 co-localized neurons, arrow heads = Ret-P1-ir neurons) exposure. (D) Only white and red light conditions elicited fra-2 expression within Ret-P1-ir neurons, though red light elicited co-expression to a lesser degree than white light. Blue and dark conditions showed significantly fewer fra-2/TH-ir co-localized neurons. LV = lateral ventricle, letters = P < 0.01, Bar (A & B) = 100 μm, Bar (B) = 50 μm.
Figure 4.
Fra-2- and TH-ir co-localization in the PMM. (A) Dark (arrowheads indicate single-labeled TH-ir), (B) blue (arrows indicate co-localized neurons, arrowheads fra-2-ir), (C) white (arrows indicate co-localized neurons, arrowheads fra-2-ir), (D) dark conditions (arrowheads indicate single labeled TH-ir). (E) Only white and blue light conditions elicited fra-2 expression within TH-ir neurons. Red and dark conditions showed significantly fewer fra-2/TH-ir co-localized neurons. 3v = third ventricle, ***P < 0.001, Bar (A) = 50 μm, Bar (B,C,D) = 100 μm.
Experiment 3: Effects of a Chronic Exposure to Colored vs. White Light
LH Analyses
At the start of the experiment, there were no differences in plasma LH levels between the treatment groups. Beginning 2 wk after the change in lighting, a reduction (P < 0.01) in LH levels in drakes housed under short-day white light was observed compared to drakes kept on long-day white light. At wk 2, drakes raised under red or blue lights also showed a reduction (P < 0.01) in circulating LH levels, similar to that under short-day white light. Interestingly, by wk 3 the drakes housed under red light recovered LH levels to return to similar levels of drakes under long-day white light and these levels were maintained through wk 4. However, drakes under blue light and short-day white light continued to show reduced (P < 0.001) levels of plasma LH compared to the other treatment groups. After the gonadally regressed drakes from the short-day white light group were placed on long-day cycle with combined red and blue lights, they showed increased plasma LH levels within 2 wk. Plasma LH levels further increased until the end of the experiment to levels that were comparable to those of the drakes under long-day white light (8 wk into experiment; Figure 5).
Figure 5.
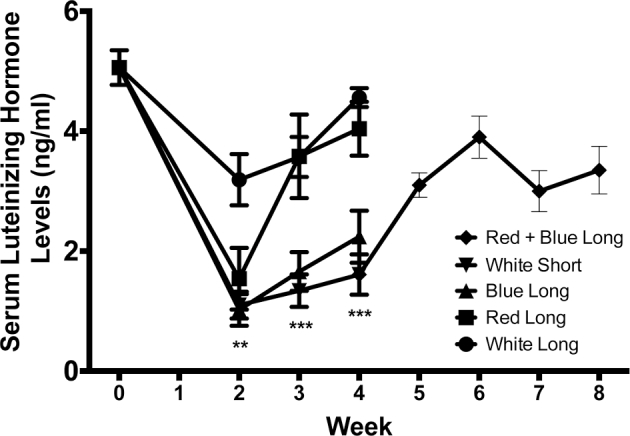
Plasma LH levels following long-term exposure to white, red, or blue light. Drakes raised under red, blue or short day white lights showed significant reduction in plasma LH levels compared to ducks on long day, white. Ducks who were switched from short-day white light, to long-day, red + blue light showed gonadal recrudescence and increased LH levels. **P < 0.01, ***P < 0.001.
Relative Testes Weights
As expected, drakes under short-day white light showed complete gonadal regression with relative testes weights that were considerably smaller (P < 0.001) compared to drakes under long-day white light. Neither red nor blue light were able to maintain relative gonadal size compared to drakes under long-day white light. However, drakes under red light showed testes that were not fully regressed and were larger (P < 0.05) than ducks under short-day white light. Gonadally regressed drakes who were returned to long-day, red + blue light, showed gonadal recrudescence after 4 wk to a size comparable to that of drakes housed under long-day white light (Figure 6).
Figure 6.
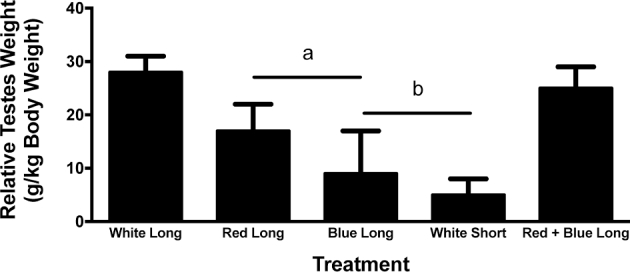
Relative testes size following long-term exposure to white, red, or blue lights. Blue or red light could not maintain relative teste size compared to white light on long day lengths. Ducks that were switched from short-day white light to long-day, red + blue light showed gonadal recrudescence. Letters indicate treatment groups that illustrated statistical differences, P < 0.01.
qRT-PCR mRNA Levels
There were no significant differences observed in relative mRNA levels of OPN4, OPN-2, OPN5, or GnIH mRNAs between any of the treatment groups. As expected, there was a significant (P < 0.01) reduction in the relative amount of GnRH mRNA in drakes housed under short-day, white light and blue light compared to long-day white, red, and long-day red + blue drakes (Figure 7).
Figure 7.
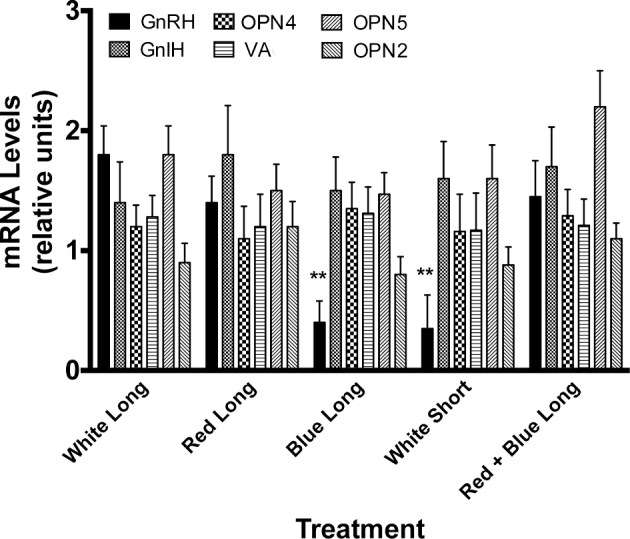
mRNA expression following long-term exposure to white, red, or blue light. Blue and short-day white-light exposure elicited a reduction in GnRH mRNA compared to other treatment groups. No differences among treatments were observed in DBP or GnIH mRNAs. **P < 0.01.
DISCUSSION
The specific mechanisms that underlie HPG photoactivation in any species still remain ambiguous. Evidence suggests that the activation of and the maintenance of the HPG axis is governed by multiple deep brain photoreceptors (DBPs), at least in birds (Kang and Kuenzel, 2015). These DBPs include melanopsin (OPN4), vertebrate ancient opsin (VA), OPN5, and in the drake rhodopsin (OPN2). OPN4 immunoreactive neurons, located in the PMM, are responsive to blue specific light wavelengths, whereas rhodopsin is responsive to red light. In this study, we wanted to determine the location of rhodopsin, melanopsin, and OPN5 within the diencephalon of the drake. We also set out to determine the effects of short (blue) or long (red) wavelengths of light on the DBPs and reproduction in the Pekin drake. We found that drakes were unable to fully maintain reproductive status when exposed to 18-h day lengths of long (red) or short (blue) wavelength light. However, drakes continued to maintain active reproductive status under 18 h of a combination of red and blue light, similar to that of drakes housed under long-day white light.
Past studies have reported that both blind and sighted Japanese quail (Coturnix japonica), were comparable in their response to differential light energies, suggesting that even low intensity energy reaches photoreceptors in birds regardless of their ability to see (Oishi and Lauber, 1973a,b). Our study suggests that blue light alone could not maintain HPG function during either acute or chronic exposure but red light could nearly maintain HPG function. Drakes housed under red light, after 3 wk, had similar LH levels to those housed under long-day white light. Interestingly, the acute exposure to red light did show a partial, but significant, decrease in plasma LH levels. A similar response was observed under the chronic red light conditions in that a reduction in plasma LH levels was initially observed, but quickly recovered. It is possible that initial change from white to red light at the onset of the experiment resulted in an environmental stress that elicited a transient reduction in circulating LH levels. Once drakes habituated to the environment, red light was able to maintain gonadal function. In contrast, drakes housed under blue light continued to have decreased LH levels. The only efficient low intensity photostimulator was red wavelength, which is able to penetrate tissue and reach photoreceptors to a greater extent than shorter wavelengths (Oishi and Lauber, 1973a,b). In chickens, red light was more effective in stimulating sexual maturation due to its ability to reach deeper brain tissue as well (Baxter et al., 2014). Our data suggest that partial or complete inactivation of the HPG axis can be attributed to, at least acutely, by red light alone. Quails and chickens, similar to drakes, sustain reproductive functions when exposed to low-energy red light (Baxter et al., 2014). However, the higher energy, blue light, has equivocal effects to maintain reproduction among galliforms (Glass and Lauber, 1981; Levenick and Leighton, 1988; Rozenboim et al., 2004). However, some studies suggest that male quail appear to respond equally in testes growth when exposed to either blue or full-spectrum white light (Nakane et al., 2010). Despite these observations in commercial poultry settings, a DBP was recently described that is primarily sensitive to blue light and appears to be a critical component of photoactivation in avian species (Hattar et al., 2002; Panda et al., 2002; Bailey and Cassone, 2005; Chaurasia et al., 2005).
One DBP phenotype that has been found in mammalian and non-mammalian species is OPN4 (Hattar et al., 2002). In both mammals and birds, short wavelengths appear to have an activational effect on OPN4 molecules (Hattar et al., 2002; Chaurasia et al., 2005; Holthues et al., 2005; Iyilikci et al., 2009). OPN4-containing retinal cells in mammals are specialized for measuring ambient illumination, regulating pupil size, modulating sleep cycles, suppressing pineal melatonin secretions and contributing to visual discrimination along with the synchronization of circadian clocks to light:dark cycles (Bailes and Lucas, 2010; Bailes and Lucas, 2013). OPN4 expression has been observed in the mouse, rat, cat, zebrafish, frog, fly, and human (Nayak et al., 2007). Most notably, the expression of this photoreceptor has recently been reported in birds within the lateral septal region of the thalamus, the pineal gland, and the retina (Hattar et al., 2002; Panda et al., 2002; Bailey and Cassone, 2005; Chaurasia et al., 2005). In the retina, OPN4 mRNA showed expression throughout all retinal layers (Bailey and Cassone, 2005). In the pineal gland, OPN4 has been described throughout the parenchyma of the pineal gland (Bailey and Cassone, 2005). Previous studies have shown that photoresponsive cells of the retina and the pineal gland are not responsible for the seasonal photo-activation of gonads in birds (Menaker et al., 1970; Underwood and Menaker, 1970a,b,c), but rather the avian photoresponsiveness is due to DBPs.
In the bird, the OPN4-expressing neurons appear to be localized in the PMM, where they are co-localized with tyrosine hydroxylase (presumably dopaminergic), tryptophan hydroxylase, and melatonin (El Halawani et al., 2009; Kang et al., 2010). Other studies in the chick have shown that c-fos gene expression in the PMM is increased 30 min after photostimulation (Thayananuphat et al., 2007a). Fra-2 is a transcription factor related to fos, and protein expression was increased in the PMM in the drake 90 min following blue-light stimulation, similar to these previous in the chicken (Thayananuphat et al., 2007a). Further, previous studies in the chick have shown maximal tyrosine hydroxylase expression in the PMM from 2 to 8 h after lights-on (Thayananuphat et al., 2007b) in photostimulated birds. In the drake, we measured DBP mRNAs within that time frame but yet, we did not obtain significant differences possibly due species differences in temporal expression of synthetic enzyme or DBP mRNAs. Future studies will examine more closely the temporal expression of these elements in relation to photostimulation. However, we did note that the combination of both red and blue light had a superior effect to photoactivate or maintain reproduction in the drake compared to either color alone. Our results in the drake are similar to those recently reported in which at least 3 DBPs are required to prime the diencephalic structures associated with reproduction (Kang and Kuenzel, 2015).
Others have identified opsin-like cells in the lateral septum (Silver et al., 1988), and there has been some discussion as to the actual phenotype of these photoreceptive neurons. Some have identified these neurons as opsin (Silver et al., 1988; Vigh and Vigh-Teichmann, 1998; Saldanha et al., 2001), while others have identified them as OPN4 (Chaurasia et al., 2005). However, none of these studies attempted to determine which light wavelengths stimulate these neurons in the LS. OPN2 is activated by longer wavelengths of light (Wada et al., 2000; Kasahara et al., 2002; Bailey and Cassone, 2004). In the Pekin drake, red light does appear to activate neurons in the LS due to the fra-2 activation and co-localization within Ret-P1-stained neurons. Furthermore, we were able to measure OPN2 specific mRNA in the brain of the drake. Others have postulated that OPN2 exists in the avian brain and is involved in the photoactivation of the gonads (Foster et al., 1985). Regardless of the specific phenotype, these neurons have been shown to interact with GnRH-neurons which may help to regulate photoperiodic changes (Silver et al., 1988). It was determined in the Japanese quail that 492 nm (red) wavelengths are needed to activate OPN2 (Foster et al., 1994). However, in our study, fra-2 activation following red light exposure was not at the level of that seen following white light exposure. Another transcription factor could have been involved in activation of rhodopsin neurons in the duck. Alternatively, a combination of DBP activation could play a critical role in photostimulation of the drake as described recently in the chicken (Kang and Kuenzel, 2015).
In years past, data have been collected regarding VA and OPN5 in the avian brain. OPN5 has been found in the paraventricular organ in the medial basal hypothalamus. VA was originally isolated in the eyes of salmon by Soni and Foster in 1997 (Minamoto and Shimizu, 2002; Grone et al., 2007; Davies et al., 2010). Its discovery led to further studies looking at VA in avian species and their ability to produce photosensitive receptors. It has been found in the mediobasal preoptic area and paraventricular nucleus with fiber terminals shown in the medial basal hypothalamus, particularly the median eminence (Halford et al., 2009). Non-mammalian OPN5 was first found in the quail brain in the paraventricular organ (Nakane et al., 2010). In our study, we could not observe sufficient numbers of OPN5 or VA neurons using available antibodies, and thus could not ascertain if any of the light-treatment groups were able to activate these neurons. Nonetheless, we were able to measure mRNA for OPN5 and VA but none of the light treatments elicited any change in these photoreceptor mRNAs. Our results do not suggest that these photosensitive receptors are not involved in the regulation of the drake HPG axis, simply that we were not able to resolve their role given our experimental design and brain-sampling milieu.
Research to date from many groups shows that deep brain photoreceptors in birds include OPN2, OPN4, OPN5, and VA and each DBP responds to different wavelengths of light triggering the stimulation of reproductive activity, and the drake is no exception. Our study suggests that PMM OPN4 is responsive to blue light as it is in other species (Iyilikci et al., 2009; Bailes and Lucas, 2013; Tsunematsu et al., 2013; Ramos et al., 2014; Takeuchi et al., 2014; Walmsley et al., 2015), but blue light alone is not able to maintain gonadal function in drakes, although these observations do not suggest that OPN4 activity is not necessary for gonadal recrudescence. Further research should include further analyses of plasma for reproductive and thyroid hormones, qRT-PCR and in situ hybridization to analyze brains for photoneuroendocrine peptides and GnRH mRNAs, histology of testes over a long-term period, and immunolesion technology to specifically eliminate DBP neurons in the diencephalon.
Acknowledgments
The authors wish to thank Maple Leaf Farms, Inc. for their support for this research project. This work was funded in part by the National Science Foundation REU award 0754293 to GSF. We also wish to thank the Department of Biology at Hope College for their continued support of our research. SLM acknowledges Roslin Institute strategic grant funding from the Biotechnology and Biological Sciences Research Council (BB/J004316/1 and BB/J004332/1). We also thank Dr. S.M. Fraley and Dr. Wayne J. Kuenzel for their critical comments on this manuscript.
REFERENCES
- Bailes H. J., Lucas R. J.. 2010. Melanopsin and inner retinal photoreception. Cell Mol. Life Sci. 67:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes H. J., Lucas R. J.. 2013. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc. Biol. Sci. 280:20122987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M. J., Cassone V. M.. 2004. Opsin photoisomerases in the chick retina and pineal gland: characterization, localization, and circadian regulation. Invest OphthalMol. Vis. Sci. 45:769–775. [DOI] [PubMed] [Google Scholar]
- Bailey M. J., Cassone V. M.. 2005. Melanopsin expression in the chick retina and pineal gland. Brain Res. Mol. Brain Res. 134:345–348. [DOI] [PubMed] [Google Scholar]
- Baxter M., Joseph N., Osborne V. R., Bedecarrats G. Y.. 2014. Red light is necessary to activate the reproductive axis in chickens independently of the retina of the eye. Poult. Sci. 93:1289–1297. [DOI] [PubMed] [Google Scholar]
- Bellingham J., Chaurasia S. S., Melyan Z., Liu C., Cameron M. A., Tarttelin E. E., Iuvone P. M., Hankins M. W., Tosini G., Lucas R. J.. 2006. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 4:e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit J. 1961. Opto-sexual reflex in the duck: physiological and histological aspects. Yale J. Biol. Med. 34:97–116. [PMC free article] [PubMed] [Google Scholar]
- Benoit J. 1964. The Role of the Eye and of the Hypothalamus in the Photostimulation of Gonads in the Duck. Ann. N.Y. Acad. Sci. 117:204–216. [DOI] [PubMed] [Google Scholar]
- Campbell C. L., Colton S., Haas R., Rice M., Porter A., Schenk A., Meelker A., Fraley S. M., Fraley G. S.. 2015. Effects of different wavelengths of light on the biology, behavior, and production of grow-out Pekin ducks. Poult. Sci. 94:1751–1757. [DOI] [PubMed] [Google Scholar]
- Chaurasia S. S., Rollag M. D., Jiang G., Hayes W. P., Haque R., Natesan A., Zatz M., Tosini G., Liu C., Korf H. W., Iuvone P. M., Provencio I.. 2005. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J. Neurochem. 92:158–170. [DOI] [PubMed] [Google Scholar]
- Cherry P., Morris T. R.. 2005. The maintenance requirement of domestic drakes. Br. Poult. Sci. 46:725–727. [DOI] [PubMed] [Google Scholar]
- Cherry P., Morris T. R.. 2008. Domestic Duck Production: Science and Practice. CABI, Wallingford, Oxfordshire, UK, Cambridge, MA. [Google Scholar]
- Constant J. P., Fraley G. S., Forbes E., Hallas B. H., Leheste J. R., Torres G.. 2012. Resveratrol protects neurons from cannulae implantation injury: implications for deep brain stimulation. Neuroscience. 222:333–342. [DOI] [PubMed] [Google Scholar]
- Davies W. L., Hankins M. W., Foster R. G.. 2010. Vertebrate ancient opsin and melanopsin: divergent irradiance detectors. PhotoChem. PhotoBiol. Sci. 9:1444–1457. [DOI] [PubMed] [Google Scholar]
- El Halawani M. E., Kang S. W., Leclerc B., Kosonsiriluk S., Chaiseha Y.. 2009. Dopamine-melatonin neurons in the avian hypothalamus and their role as photoperiodic clocks. Gen. Comp. Endocrinol. 163:123–127. [DOI] [PubMed] [Google Scholar]
- Foster R. G., Follett B. K., Lythgoe J. N.. 1985. Rhodopsin-like sensitivity of extra-retinal photoreceptors mediating the photoperiodic response in quail. Nature. 313:50–52. [DOI] [PubMed] [Google Scholar]
- Foster R. G., Grace M. S., Provencio I., Degrip W. J., Garcia-Fernandez J. M.. 1994. Identification of vertebrate deep brain photoreceptors. NeuroSci. Biobehav. Rev. 18:541–546. [DOI] [PubMed] [Google Scholar]
- Fraley G. 2006. Immunolesions of glucoresponsive projections to the arcuate nucleus alter glucoprivic-induced alterations in food intake, luteinizing hormone secretion, and GALP mRNA but not sex behavior in adult male rats. Neuroendocrinology. 83:97–105. [DOI] [PubMed] [Google Scholar]
- Fraley G. S., Kuenzel W. J.. 1993. Immunocytochemical and histochemical analyses of gonadotrophin releasing hormone, tyrosine hydroxylase, and cytochrome oxidase reactivity within the hypothalamus of chicks showing early sexual maturation. Histochemistry. 99:221–229. [DOI] [PubMed] [Google Scholar]
- Fraley G. S., Ulibarri C.. 2002. Development of androgen receptor and p75(NTR) mRNAs and peptides in the lumbar spinal cord of the gerbil. Brain Res. Dev. Brain Res. 137:101–114. [DOI] [PubMed] [Google Scholar]
- Glass J. D., Lauber J. K.. 1981. Sites and action spectra for encephalic photoreception in the Japanese quail. Am. J. Physiol. 240:R220–R228. [DOI] [PubMed] [Google Scholar]
- Grone B. P., Sheng Z., Chen C. C., Fernald R. D.. 2007. Localization and diurnal expression of melanopsin, vertebrate ancient opsin, and pituitary adenylate cyclase-activating peptide mRNA in a teleost retina. J. Biol. Rhythms. 22:558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S., Pires S. S., Turton M., Zheng L., Gonzalez-Menendez I., Davies W. L., Peirson S. N., Garcia-Fernandez J. M., Hankins M. W., Foster R. G.. 2009. VA opsin-based photoreceptors in the hypothalamus of birds. Curr. Biol. 19:1396–1402. [DOI] [PubMed] [Google Scholar]
- Hattar S., Liao H. W., Takao M., Berson D. M., Yau K. W.. 2002. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 295:1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton M. B. 1971. Ontogeny of vision in the Peking duck (Anas platyrhynchos): the pupillary light reflex as a means for investigating visual onset and development in avian embryos. Dev. Psycho. Biol. 4:313–332. [DOI] [PubMed] [Google Scholar]
- Holthues H., Engel L., Spessert R., Vollrath L.. 2005. Circadian gene expression patterns of melanopsin and pinopsin in the chick pineal gland. BioChem. Biophys. Res. Commun. 326:160–165. [DOI] [PubMed] [Google Scholar]
- Iyilikci O., Aydin E., Canbeyli R.. 2009. Blue but not red light stimulation in the dark has antidepressant effect in behavioral despair. Behav. Brain Res. 203:65–68. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Fraley G. S.. 2008. Rat RFRP-3 alters hypothalamic GHRH expression and growth hormone secretion but does not affect KiSS-1 gene expression or the onset of puberty in male rats. Neuroendocrinology. 88:305–315. [DOI] [PubMed] [Google Scholar]
- Kang S. W., Kuenzel W. J.. 2015. Deep-brain photoreceptors (DBPs) involved in the photoperiodic gonadal response in an avian species, Gallus gallus. Gen. Comp. Endocrinol. 211:106–113. [DOI] [PubMed] [Google Scholar]
- Kang S. W., Leclerc B., Kosonsiriluk S., Mauro L. J., Iwasawa A., El Halawani M. E.. 2010. Melanopsin expression in dopamine-melatonin neurons of the premammillary nucleus of the hypothalamus and seasonal reproduction in birds. Neuroscience. 170:200–213. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Okano T., Haga T., Fukada Y.. 2002. Opsin-G11-mediated signaling pathway for photic entrainment of the chicken pineal circadian clock. J. NeuroSci.. 22:7321–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenick C. K., Leighton A. T. Jr.. 1988. Effects of photoperiod and filtered light on growth, reproduction, and mating behavior of turkeys. 1. Growth performance of two lines of males and females. Poult. Sci. 67:1505–1513. [DOI] [PubMed] [Google Scholar]
- Li H., Kuenzel W. J.. 2008. A possible neural cascade involving the photoneuroendocrine system (PNES) responsible for regulating gonadal development in an avian species, Gallus gallus. Brain Res. Bull. 76:586–596. [DOI] [PubMed] [Google Scholar]
- Lin B., Koizumi A., Tanaka N., Panda S., Masland R. H.. 2008. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc. Natl. Acad. Sci. USA. 105:16009–16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaker M., Roberts R., Elliott J., Underwood H.. 1970. Extraretinal light perception in the sparrow. 3. The eyes do not participate in photoperiodic photoreception. Proc. Natl. Acad. Sci. USA. 67:320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamoto T., Shimizu I.. 2002. A novel isoform of vertebrate ancient opsin in a smelt fish, Plecoglossus altivelis. BioChem. Biophys. Res. Commun. 290:280–286. [DOI] [PubMed] [Google Scholar]
- Nakane Y., Ikegami K., Ono H., Yamamoto N., Yoshida S., Hirunagi K., Ebihara S., Kubo Y., Yoshimura T.. 2010. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. USA. 107:15264–15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S. K., Jegla T., Panda S.. 2007. Role of a novel photopigment, melanopsin, in behavioral adaptation to light. Cell Mol. Life Sci. 64:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina H., Sato H., Suzuki T., Sato M., Iba H.. 1990. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc. Natl. Acad. Sci. USA. 87:3619–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi T., Lauber J. K.. 1973a. Photoreception in the photosexual response of quail. I. Site of photoreceptor. Am. J. Physiol. 225:155–158. [DOI] [PubMed] [Google Scholar]
- Oishi T., Lauber J. K.. 1973b. Photoreception in the photosexual response of quail. II. Effects of intensity and wavelength. Am. J. Physiol. 225:880–886. [DOI] [PubMed] [Google Scholar]
- Oliver J., Bayle J. D.. 1982. Brain photoreceptors for the photo-induced testicular response in birds. Experientia. 38:1021–1029. [DOI] [PubMed] [Google Scholar]
- Panda S., Hogenesch J. B., Kay S. A.. 2003a. Circadian light input in plants, flies and mammals. Novartis Found. Symp. 253:73–82; discussion 82-78, 102–109, 281-104. [PubMed] [Google Scholar]
- Panda S., Provencio I., Tu D. C., Pires S. S., Rollag M. D., Castrucci A. M., Pletcher M. T., Sato T. K., Wiltshire T., Andahazy M., Kay S. A., Van Gelder R. N., Hogenesch J. B.. 2003b. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 301:525–527. [DOI] [PubMed] [Google Scholar]
- Panda S., Sato T. K., Castrucci A. M., Rollag M. D., DeGrip W. J., Hogenesch J. B., Provencio I., Kay S. A.. 2002. Melanopsin (OPN4) requirement for normal light-induced circadian phase shifting. Science. 298:2213–2216. [DOI] [PubMed] [Google Scholar]
- Peczely P., Kovacs K. J.. 2000. Photostimulation affects gonadotropin-releasing hormone immunoreactivity and activates a distinct neuron population in the hypothalamus of the mallard. NeuroSci. Lett. 290:205–208. [DOI] [PubMed] [Google Scholar]
- Prayitno D. S., Phillips C. J., Omed H.. 1997. The effects of color of lighting on the behavior and production of meat chickens. Poult. Sci. 76:452–457. [DOI] [PubMed] [Google Scholar]
- Ramos B. C., Moraes M. N., Poletini M. O., Lima L. H., Castrucci A. M.. 2014. From blue light to clock genes in zebrafish ZEM-2S cells. PLoS One. 9:e106252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters L. V., Eens M., Pinxten R., Duffy D. L., Balthazart J., Ball G. F.. 2000. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris). Horm. Behav. 38:250–261. [DOI] [PubMed] [Google Scholar]
- Riters L. V., Olesen K. M., Auger C. J.. 2007. Evidence that female endocrine state influences catecholamine responses to male courtship song in European starlings. Gen. Comp. Endocrinol. 154:137–149. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Biran I., Chaiseha Y., Yahav S., Rosenstrauch A., Sklan D., Halevy O.. 2004. The effect of a green and blue monochromatic light combination on broiler growth and development. Poult. Sci. 83:842–845. [DOI] [PubMed] [Google Scholar]
- Saldanha C. J., Silverman A. J., Silver R.. 2001. Direct innervation of GnRH neurons by encephalic photoreceptors in birds. J. Biol. Rhythms. 16:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha C. J., Walters B. J., Fraley G. S.. 2010. Neurons that co-localize aromatase- and kisspeptin-like immunoreactivity may regulate the HPG axis of the Mallard drake (Anas platyrhynchos). Gen. Comp. Endocrinol. 166:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. J., Dunn I. C., Talbot R. T.. 1987. Sex differences in the LH responses to chicken LHRH-I and -II in the domestic fowl. J. Endocrinol. 115:323–331. [DOI] [PubMed] [Google Scholar]
- Silver R., Witkovsky P., Horvath P., Alones V., Barnstable C. J., Lehman M. N.. 1988. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res. 253:189–198. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Okuno H., Yoshida T., Endo T., Nishina H., Iba H.. 1991. Difference in transcriptional regulatory function between c-Fos and Fra-2. Nucleic Acids Res. 19:5537–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Imamura S., Sawada Y., Hur S. P., Takemura A.. 2014. Effects of different colors of light on melatonin suppression and expression analysis of Aanat1 and melanopsin in the eye of a tropical damselfish. Gen. Comp. Endocrinol. 204:158–165. [DOI] [PubMed] [Google Scholar]
- Thayananuphat A., Kang S. W., Bakken T., Millam J. R., El Halawani M. E.. 2007a. Rhythm-dependent light induction of the c-fos gene in the turkey hypothalamus. J. Neuroendocrinol. 19:407–417. [DOI] [PubMed] [Google Scholar]
- Thayananuphat A., Kang S. W., Bakken T., Millam J. R., El Halawani M. E.. 2007b. Rhythmic dependent light induction of gonadotrophin-releasing hormone-I expression and activation of dopaminergic neurons within the premammillary nucleus of the turkey hypothalamus. J. Neuroendocrinology. 19:399–406. [DOI] [PubMed] [Google Scholar]
- Tsunematsu T., Tanaka K. F., Yamanaka A., Koizumi A.. 2013. Ectopic expression of melanopsin in orexin/hypocretin neurons enables control of wakefulness of mice in vivo by blue light. NeuroSci. Res. 75:23–28. [DOI] [PubMed] [Google Scholar]
- Underwood H., Menaker M.. 1970a. Extraretinal light perception: entrainment of the biological clock controlling lizard locomotor activity. Science. 170:190–193. [DOI] [PubMed] [Google Scholar]
- Underwood H., Menaker M.. 1970b. Photoperiodically significant photoreception in sparrows: is the retina involved? Science. 167:298–301. [DOI] [PubMed] [Google Scholar]
- Underwood H., Menaker M.. 1970c. Photoreception in sparrows: response to photoperiodic stimuli. Science. 169:893. [DOI] [PubMed] [Google Scholar]
- Vigh B., Vigh-Teichmann I.. 1998. Actual problems of the cerebrospinal fluid-contacting neurons. Microsc. Res. Tech. 41:57–83. [DOI] [PubMed] [Google Scholar]
- Wada Y., Okano T., Fukada Y.. 2000. Phototransduction molecules in the pigeon deep brain. J. Comp. Neurol. 428:138–144. [DOI] [PubMed] [Google Scholar]
- Wagner G. C., Johnston J. D., Clarke I. J., Lincoln G. A., Hazlerigg D. G.. 2008. Redefining the limits of day length responsiveness in a seasonal mammal. Endocrinology. 149:32–39. [DOI] [PubMed] [Google Scholar]
- Walmsley L., Hanna L., Mouland J., Martial F., West A., Smedley A. R., Bechtold D. A., Webb A. R., Lucas R. J., Brown T. M.. 2015. Colour as a signal for entraining the mammalian circadian clock. PLoS Biol. 13:e1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Cao J., Wang Z., Dong Y., Chen Y.. 2014. Effect of a combination of green and blue monochromatic light on broiler immune response. J. Photochem. Photobiol. B Biol. 138:118–123. [DOI] [PubMed] [Google Scholar]