Abstract
Objectives
The aim of this pooled analysis of the TOZURA study programme was to evaluate the efficacy and safety of subcutaneous tocilizumab (TCZ-SC) as monotherapy or in combination with conventional synthetic DMARDs (csDMARDs) in patients with moderate to severe RA who had an inadequate response to csDMARD or anti-TNF agent therapy or who were MTX naïve.
Methods
TOZURA is a multinational, open-label, single-arm, common-framework, phase 4 study programme (11 protocols, 22 countries). Patients received TCZ-SC 162 mg each week for ⩾24 weeks, administered at the investigator’s discretion, as monotherapy or in combination with a csDMARD. Efficacy, safety and immunogenicity were evaluated; propensity score–based matching was used for between-group comparisons.
Results
Of 1804 patients, 353 (19.6%) received monotherapy and 1451 (80.4%) received combination therapy. The 28-joint DAS using ESR (DAS28-ESR) in both groups decreased significantly from baseline to week 24 (mean change: monotherapy −3.40, combination therapy −3.46), with no significant difference between groups (P = 0.46). The proportion of patients who achieved DAS28-ESR or Clinical Disease Activity Index remission or ACR 20/50/70/90 responses was similar between groups. Overall, 13.9% of patients withdrew—6.2% for safety reasons and 1.6% for insufficient therapeutic response; 5.8% of patients experienced one or more serious adverse events [14.6/100 patient-years (PY)]; six deaths occurred (0.64/100 PY).
Conclusion
In a common framework of 11 studies in 22 countries, this phase 4 study programme confirmed TCZ-SC’s known efficacy and safety profile with comparable effects as monotherapy and in combination with csDMARDs.
Trial registration
ClinicalTrials.gov (http://www.clinicaltrials.gov) NCT01941940, NCT01941095, NCT01951170, NCT01987479, NCT01988012, NCT01995201, NCT02001987, NCT02011334, NCT02031471, NCT02046603 and NCT02046616.
Keywords: rheumatoid arthritis, biologic therapies, csDMARDs, tocilizumab
Rheumatology key messages
Subcutaneous tocilizumab monotherapy and combination therapy with conventional synthetic DMARDs show comparable efficacy in patients with RA.
The safety of subcutaneous tocilizumab monotherapy and combination therapy in RA is consistent with the known tocilizumab profile.
Subcutaneous tocilizumab’s low immunogenicity confirmed previous findings and has been established for monotherapy in RA.
Introduction
Tocilizumab (TCZ), administered as intravenous (TCZ-IV) or subcutaneous (TCZ-SC) formulations, is the first drug approved worldwide for the treatment of RA that blocks the biologic activity of IL-6. TCZ is a humanized mAb that competitively interferes with the binding of IL-6 to its receptor (IL-6R; also known as IL-6Rα or CD126), thereby disrupting receptor association with glycoprotein 130, a co-signal transducer protein (also known as IL-6Rβ or CD130) necessary for initiating intracellular signalling [1]. The efficacy and safety of TCZ, both as monotherapy and combination therapy with conventional synthetic DMARDs (csDMARDs), have been demonstrated in numerous clinical trials for patients with RA who had an inadequate response to csDMARDs or anti-TNF agents [2–8].
Three phase 3 studies evaluated the efficacy and safety of TCZ-SC as monotherapy or in combination with csDMARDs. SUMMACTA (NCT01194414) demonstrated the non-inferiority of TCZ-SC 162 mg administered once a week (qw) to TCZ-IV 8 mg/kg administered every 4 weeks (q4w) in combination with csDMARDs with regard to a 20% improvement in ACR criteria (ACR20) at 24 weeks [7]. BREVACTA (NCT1232569) demonstrated that TCZ-SC 162 mg every 2 weeks in combination with csDMARDs was superior to placebo in combination with csDMARDs with regard to ACR20 at 24 weeks [8]. MUSASHI reported the non-inferiority of TCZ-SC 162 mg every 2 weeks monotherapy compared with TCZ-IV 8 mg/kg q4w monotherapy with regard to ACR20 at 24 weeks [9]. All of the phase 3 studies had long-term extensions of ⩽2 years that demonstrated the efficacy and safety of TCZ-SC remained comparable to the known profile of TCZ-IV [10–13].
In addition to the rigorous phase 3 randomised clinical trial setting, there is a need to understand the efficacy and safety profile of TCZ-SC both as monotherapy and in combination with csDMARDs in the less stringent phase 4 clinical setting. The objective of the TOZURA common-framework study programme was to evaluate the efficacy, safety and immunogenicity of TCZ-SC 162 mg qw as monotherapy and in combination with csDMARDs over 24 weeks in adult patients with moderate to severe RA in a broad geographic setting.
Methods
Patients
The study population included adult (⩾18 years old) TCZ-naïve patients with active RA, per the revised 1987 ACR criteria or 2010 EULAR/ACR criteria, who had an inadequate response to a csDMARD or an anti-TNF agent or who were MTX naïve. Active RA was defined by local protocol; 10 of 11 protocols (the exception was Israel) defined active RA of at least moderate severity as a 28-joint DAS (DAS28) >3.2 at baseline. Some local protocols also specified one or more of the following as inclusion criteria: minimum CRP levels, minimum ESR, minimum swollen and/or tender joint count and an inadequate response to one or more biologic DMARD (bDMARD) (in Israel, a minimum DAS28 score was not required for inclusion; however, 95 of 100 patients enrolled had DAS28 >3.2). Major exclusion criteria included ongoing rheumatic or inflammatory joint diseases other than RA, functional class IV status, previous treatment with TCZ, treatment with any investigational agent, intra-articular or parenteral glucocorticoids or immunization with a live/attenuated vaccine ⩽4 weeks before screening, treatment with alkylating agents or cell-depleting therapies, any active infections, history of malignancy, positive hepatitis B surface antigen or hepatitis C antibody or serious allergies to biologic agents. bDMARDs were prohibited and were discontinued before initiation of the study.
The final protocols, amendments and informed consent documentation of the studies were approved by the respective local institutional review boards or independent ethics committees of the investigational centres. All patients provided written, informed consent according to the Declaration of Helsinki.
TOZURA phase 4 study programme design
TOZURA is a multinational, open-label, single-arm, common-framework study programme comprising 7 single-country and 4 regional multicountry protocols (22 countries in total), ensuring similar study designs and data collection for the core part of each individual study, which comprises the first 24 weeks.
Patients received TCZ-SC 162 mg qw for 24 weeks, administered at the investigator’s discretion as monotherapy or in combination with a csDMARD. Concomitant csDMARDs (AZA, chloroquine, HCQ, LEF, MTX or SSZ) were permitted if patients maintained a stable dose for ⩾4 weeks before baseline. Concomitant csDMARDs could be used alone or in combination, except for the combination of MTX and LEF. Stable oral NSAIDs and glucocorticoids (⩽10 mg/day prednisone or equivalent) were permitted if initiated ⩾4 weeks before baseline. After the first treatment, TCZ-SC could be administered at home by the patient or caregiver.
Efficacy and safety were evaluated at baseline and weeks 1, 2 and 4 and every 4 weeks thereafter for 24 weeks, with 8 additional weeks for safety follow-up. Clinical efficacy included total tender joint count, total swollen joint count of 28 joints, Patient Global Assessment of disease activity visual analogue scale score, HAQ Disability Index (HAQ-DI), change in DAS28 using ESR (DAS28-ESR), ACR response scores, EULAR response criteria, Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index and glucocorticoid dose reduction and/or discontinuations. Safety assessments included adverse events (AEs) with a focus on AEs of special interest (AESIs), laboratory assessments, physical examinations and vital signs. AESI categories (serious or non-serious AEs) included serious and/or medically significant infections, myocardial infarction/acute coronary syndrome, gastrointestinal perforations, malignancies, anaphylaxis/hypersensitivity reactions, demyelinating disorders, stroke, serious and/or medically significant bleeding events and serious and/or medically significant hepatic events. Immunogenicity assessments were performed at baseline, weeks 12 and 24, study completion or early withdrawal visit and 8 weeks after the last dose of TCZ-SC, as previously described [14].
Statistical methods
Descriptive statistical methods were used to evaluate efficacy and safety. The primary analysis population for efficacy and safety consisted of all patients who received one or more dose of TCZ-SC. The proportion of patients experiencing one or more safety event was estimated with a 95% Clopper–Pearson CI. CIs for event incidence rates by patient-years (PY) with TCZ exposure were analysed based on the Poisson distribution. The Kaplan–Meier method was used for time-to-event data. Propensity score–based 1:1 matching was used for selected between-group comparisons, which employed the Wilcoxon signed-rank test for continuous variables and the McNemar test for categorical variables. For other between-group comparisons, the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables were used.
Results
Patient disposition and baseline characteristics
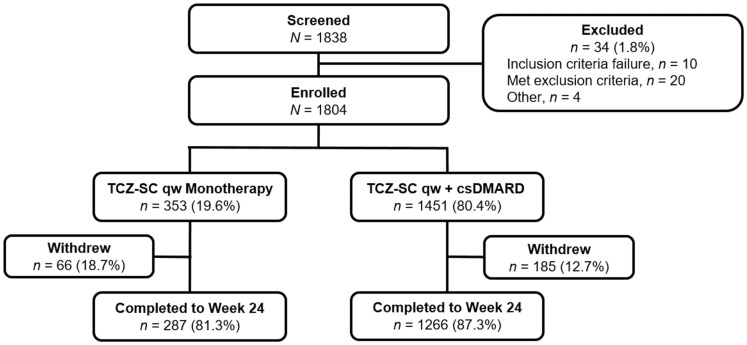
Of the 1804 patients enrolled, 353 patients (19.6%) received TCZ-SC qw monotherapy and 1451 patients (80.4%) received TCZ-SC qw with a concomitant csDMARD (Fig. 1). During the 24-week period, 66 patients (18.7%) discontinued from the TCZ-SC monotherapy group and 185 patients (12.7%) from the combination therapy group. The most common reason for withdrawal during the 24-week study period was AEs. Of the patients who completed 24 weeks, 287 patients (81.3%) were from the monotherapy group and 1266 patients (87.3%) were from the combination therapy group.
Fig. 1.
Patient disposition at baselinecsDMARD: conventional synthetic disease-modifying anti-rheumatic drug; qw: once weekly; TCZ-SC: subcutaneous tocilizumab.
Patient baseline demographics and clinical characteristics were balanced between the TCZ-SC monotherapy and the TCZ-SC combination therapy groups, except for glucocorticoid use, which was less frequent in the TCZ-SC monotherapy group than in the combination group (41.1% vs 51.2%); however, the mean dose was similar between the groups (6.6 vs 6.5 mg/day, respectively) (Table 1). A total of 348 patients (19.3%) had previously received a bDMARD, with etanercept being the most common previous biologic (overall 4.8%, monotherapy 7.1%, combination therapy 4.2%). Patients who received prior bDMARD treatment made up a significantly greater proportion of the monotherapy group than the combination therapy group (monotherapy 31.4%, combination therapy 16.3%; P < 0.001).
Table 1.
Baseline characteristics
| Patient characteristics at baseline (week 1) | Total population (N = 1804) | TCZ-SC monotherapy (n = 353) | TCZ-SC + csDMARD (n = 1451) |
|---|---|---|---|
| Female, n (%) | 1472 (81.6) | 295 (83.6) | 1177 (81.1) |
| Age, mean (s.d.), years | 54.1 (12.3) | 55.0 (12.6) | 53.9 (12.2) |
| Weight, mean (s.d.), kg | 72.8 (16.3) | 72.1 (16.9) | 73.0 (16.1) |
| RA duration, mean (s.d.), years | 7.7 (8.0) | 8.4 (8.2) | 7.6 (7.9) |
| Seropositivity, n (%)a | 1367 (82.7) | 262 (82.1) | 1105 (82.8) |
| RF positive | 1208 (72.6) | 237 (72.5) | 971 (72.7) |
| ACPA positive | 1052 (70.8) | 199 (69.3) | 853 (71.1) |
| Evidence of structural joint damage, n (%) | 723 (46.7) | 172 (56.4) | 551 (44.3) |
| CRP, mean (s.d.), mg/l | 15.0 (21.8) | 17.6 (24.7) | 14.3 (20.9) |
| Disease activity | |||
| DAS28-ESR, mean (s.d.) | 5.77 (1.17) | 5.84 (1.12) | 5.75 (1.18) |
| CDAI, mean (s.d.) | 32.16 (12.81) | 31.75 (12.26) | 32.26 (12.94) |
| HAQ-DI, mean (s.d.) | 1.38 (0.69) | 1.45 (0.67) | 1.36 (0.69) |
| Glucocorticoid use, n (%) | 888 (49.2) | 145 (41.1) | 743 (51.2) |
| Prednisone equivalent daily dose, mean (s.d.), mg | 6.5 (4.0)b | 6.6 (2.9)c | 6.5 (4.2)d |
| Previous bDMARD treatment, n (%) | 348 (19.3) | 111 (31.4) | 237 (16.3) |
Seropositivity, total population positive for RF and/or ACPA.
n = 870.
n = 145.
n = 725. bDMARD: biologic disease-modifying anti-rheumatic drug; CDAI: Clinical Disease Activity Index; csDMARD: conventional synthetic disease-modifying anti-rheumatic drug; DAS28-ESR: Disease Activity Score in 28 joints using erythrocyte sedimentation rate; HAQ-DI: Health Assessment Questionnaire-Disability Index; IR: inadequate response; RA: rheumatoid arthritis; TCZ-SC: subcutaneous tocilizumab.
Efficacy
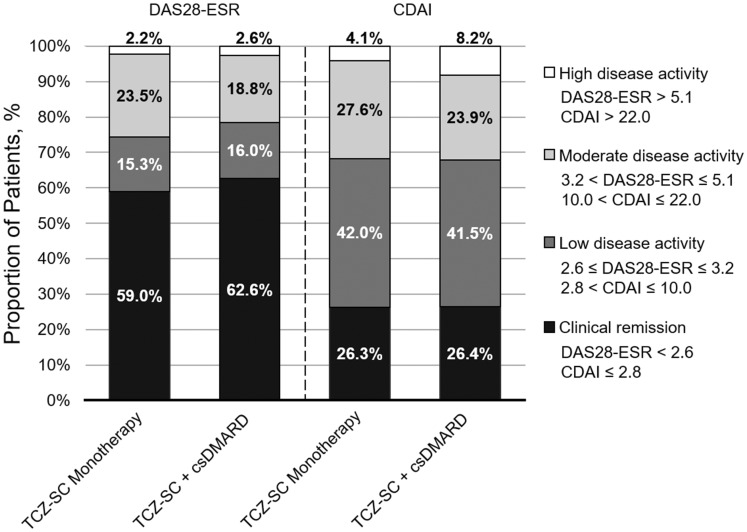
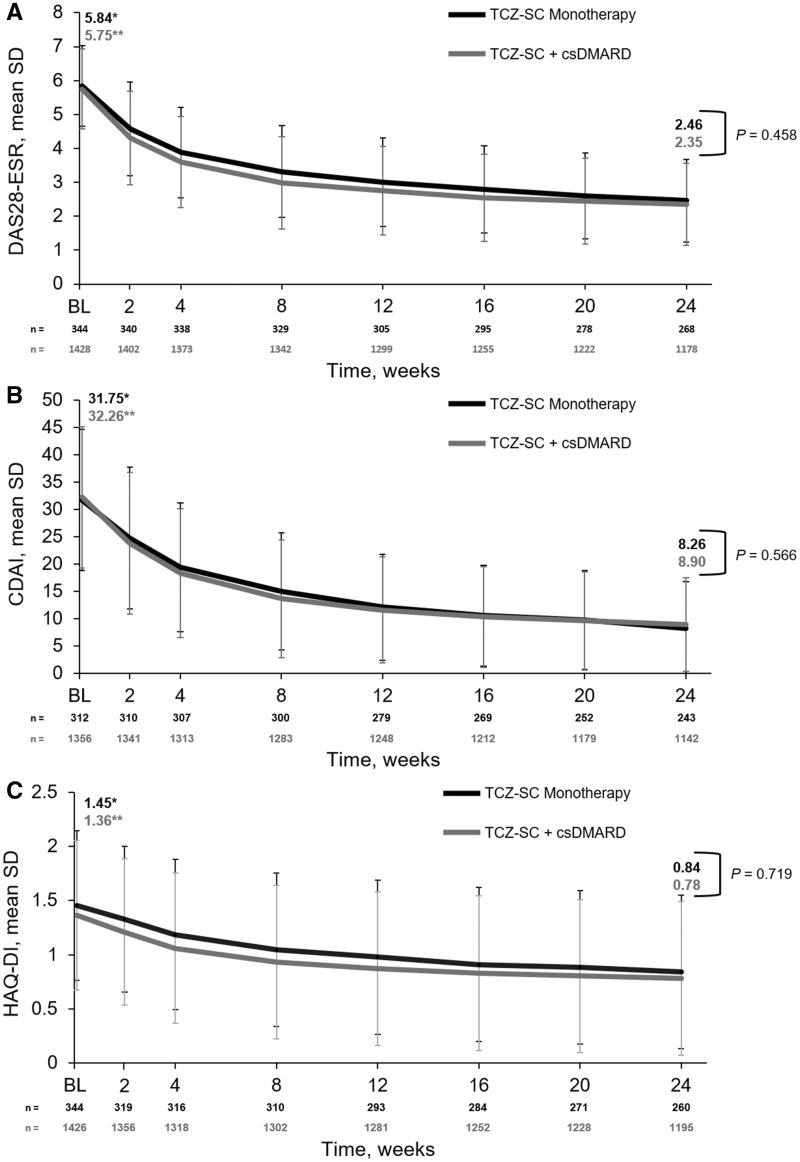
The proportion of patients who achieved DAS28-ESR remission (DAS28-ESR <2.6; TCZ-SC monotherapy 59.0% vs TCZ-SC combination therapy 62.6%), low disease activity (DAS28-ESR 2.6–⩽3.2) or moderate disease activity (DAS28-ESR >3.2–⩽5.1) was similar between groups over 24 weeks (Fig. 2). DAS28-ESR decreased comparably from baseline to week 24 in both the monotherapy and combination therapy groups [mean change: monotherapy −3.40 (s.d. 1.41), combination therapy −3.46 (s.d. 1.43); P < 0.0001 for both], with no significant difference between groups (P = 0.46) (Fig. 3A).
Fig. 2.
DAS28-ESR and CDAI disease activity at week 24
No imputation of data was used. CDAI: Clinical Disease Activity Index; csDMARD: conventional synthetic disease-modifying anti-rheumatic drug; DAS28-ESR: Disease Activity Score in 28 joints using erythrocyte sedimentation rate; TCZ-SC: subcutaneous tocilizumab.
Fig. 3.
Mean (A) DAS28-ESR, (B) CDAI score and (C) HAQ-DI score over 24 weeks
*P < 0.0001 for TCZ-SC monotherapy comparing week 1 with week 24. **P < 0.0001 for TCZ-SC + csDMARD comparing week 1 with week 24. BL: baseline; CDAI: Clinical Disease Activity Index; csDMARD: conventional synthetic disease-modifying anti-rheumatic drug; DAS28-ESR: Disease Activity Score in 28 joints using erythrocyte sedimentation rate; HAQ-DI: Health Assessment Questionnaire-Disability Index; TCZ-SC: subcutaneous tocilizumab.
The proportion of patients who achieved CDAI remission (CDAI ⩽2.8; monotherapy 26.3% vs combination therapy 26.4%), low disease activity (CDAI >2.8–⩽10.0) or moderate disease activity (CDAI >10.0–⩽22.0) was similar between the monotherapy and combination therapy groups (Fig. 2). The CDAI score decreased comparably from baseline to week 24 in both groups [mean change: monotherapy 23.54 (s.d. 13.52), combination therapy −23.83 (s.d. 13.52); P < 0.0001 for both], with no significant difference between groups (P = 0.57) (Fig. 3B).
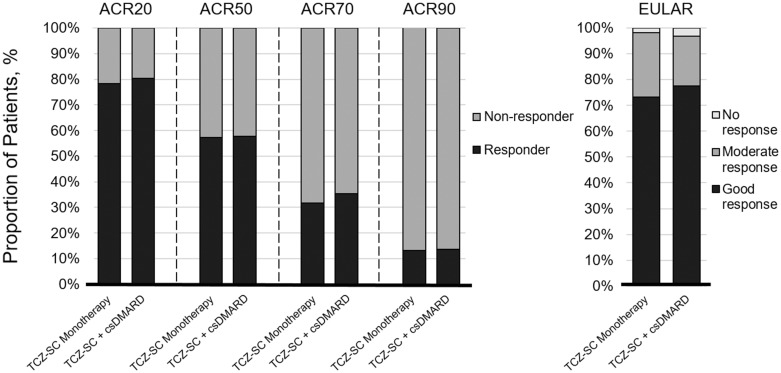
In addition, EULAR and ACR20/50/70/90 response rates were similar between treatment groups at week 24, with 73.3 and 77.5% of patients achieving a EULAR good response and 57.2 and 57.7% achieving an ACR50 response in the monotherapy and combination therapy groups, respectively (Fig. 4). Swollen joint counts and tender joint counts over time were similar between the monotherapy and combination therapy groups (supplementary Fig. S1, available at Rheumatology Online).
Fig. 4.
ACR and EULAR responses at week 24
No imputation of data was used. ACR: American College of Rheumatology; csDMARD: conventional synthetic disease-modifying anti-rheumatic drug; EULAR: European League Against Rheumatism; TCZ-SC: subcutaneous tocilizumab.
The HAQ-DI score decreased comparably from baseline to week 24 in both groups [mean change: monotherapy −0.56 (s.d. 0.63), combination therapy −0.57 (s.d. 0.62); P < 0.0001 for both], with no significant difference between groups (P = 0.72) (Fig. 3C).
Higher body weight was associated with reduced efficacy of TCZ-SC. Compared with patients with high weight (⩾100 kg), the mean decrease in DAS28-ESR from baseline to week 24 was greater for patients with intermediate weight (60–<100 kg; difference, 0.22 DAS28 units) and for patients with low weight (<60 kg; difference, 0.31 DAS28 units). Compared with patients with high weight, the odds ratio for ACR50 for patients with intermediate weight and low weight were 1.37 and 1.84, respectively. The P-value for the weight factor in respective analysis of covariance and in logistic regression models with adjustment for baseline DAS28-ESR and concomitant csDMARDs was 0.085 and 0.021, respectively.
When propensity score–based matched pairs of patients were analysed from the monotherapy and combination therapy groups, no statistical differences were observed for the mean change from baseline in the DAS28-ESR and CDAI score at week 24 (P = 0.86 and P = 0.66, respectively) and in the proportion of patients achieving ACR20 and ACR50 at week 24 (P = 0.71 and P = 0.51, respectively) (supplementary Table S1, available at Rheumatology Online).
Treatment persistence with TCZ-SC through week 24, measured as a binary proportion of patients who continued therapy, was significantly higher in the combination therapy group (supplementary Table S1, available at Rheumatology Online). Kaplan–Meier estimates for the proportion of patients who received TCZ-SC through week 24 were 0.81 (95% CI 0.76, 0.84) for monotherapy and 0.87 (95% CI 0.85, 0.89) for combination therapy (supplementary Fig. S2, available at Rheumatology Online). The difference between treatment groups was statistically significant (P = 0.002, log-rank test; P = 0.029, propensity score–based analysis).
Safety
The total TCZ exposure was 175.7 and 767.6 PY for the monotherapy and combination therapy groups, respectively (Table 2).
Table 2.
Summary of adverse events
| Events | Total population (N = 1804), 943.3 PY | TCZ-SC monotherapy (n = 353), 175.7 PY | TCZ-SC + csDMARD (n = 1451), 767.6 PY |
|---|---|---|---|
| Adverse events | |||
| Patients with ≥1 AE, n (%) | 1508 (83.6) | 282 (79.9) | 1226 (84.5) |
| Rate, per 100 PY | 622.4 | 622.1 | 622.5 |
| Serious adverse events | |||
| Patients with ≥1 SAE, n (%) | 105 (5.8) | 29 (8.2) | 76 (5.2) |
| Rate, per 100 PY | 14.6 | 22.8 | 12.8 |
| Serious infections and infestations | |||
| Patients with ≥1 event, n (%) | 27 (1.5) | 6 (1.7) | 21 (1.4) |
| Rate, per 100 PY | 3.6 | 4.0 | 3.5 |
| Serious GI perforations | |||
| Patients with ≥1 event, n (%) | 3 (0.2) | 1 (0.3) | 2 (0.1) |
| Rate, per 100 PY | 0.32 | 0.57 | 0.26 |
| Serious anaphylactic reactions | |||
| Patients with ≥1 event, n (%) | 2 (0.1) | 0 | 2 (0.1) |
| Rate, per 100 PY | 0.21 | – | 0.26 |
| Serious hypersensitivity reactionsa | |||
| Patients with ≥1 event, n (%) | 9 (0.1) | 2 (0.6) | 7 (0.5) |
| Rate, per 100 PY | 1.3 | 2.8 | 0.91 |
| Adverse events of special interestb | |||
| Patients with ≥1 AESI, n (%) | 123 (6.8) | 25 (7.1) | 98 (6.8) |
| Rate, per 100 PY | 16.1 | 15.4 | 16.3 |
| Withdrawals | |||
| Patients, n (%) | 251 (13.9) | 66 (18.7) | 185 (12.7) |
| Withdrawals due to safety reasonsc | |||
| Patients, n (%) | 111 (6.2) | 31 (8.8) | 80 (5.5) |
| Withdrawals due to insufficient therapeutic response | |||
| Patients, n (%) | 29 (1.6) | 9 (2.5) | 20 (1.4) |
| Deaths | |||
| Patients, n (%) | 6 (0.3) | 1 (0.3)d | 5 (0.3)e |
| Rate, per 100 PY | 0.64 | 0.57 | 0.65 |
SAEs occurring during or within 24 h of the injection/infusion, excluding injection site reactions and not deemed to be unrelated to treatment by the investigator.
AESI categories (serious or non-serious AEs) included serious and/or medically significant infections, myocardial infarctions/acute coronary syndrome, GI perforations, malignancies, anaphylaxis/hypersensitivity reactions, demyelinating disorders, stroke, serious and/or medically significant bleeding events and serious and/or medically significant hepatic events.
Deaths not included.
Coronary artery disease.
Myocardial infarction, pneumonia, pulmonary fibrosis, sepsis and septic shock. AE: adverse event; AESI: AE of special interest; csDMARD: conventional synthetic disease-modifying anti-rheumatic drug; GI: gastrointestinal; SAE: serious adverse event; PY, patient-year; TCZ-SC: subcutaneous tocilizumab.
Overall, the AE rate per 100 PY was 622.4, with similar rates between the monotherapy and combination therapy groups (622.1 vs 622.5) (Table 2). The most common AEs were infections and infestations, occurring in 42.0% of patients (monotherapy 43.1%, combination therapy 41.8%), with nasopharyngitis occurring the most frequently. Overall, 6.2% of patients discontinued the study due to safety reasons (monotherapy 8.8%, combination therapy 5.5%). The most common reasons for withdrawal due to AEs were skin and subcutaneous disorders in the monotherapy group [5 patients (1.4%)] and laboratory findings [16 patients (1.1%)] in the combination therapy group. There were 29 patients (1.6%) who withdrew due to insufficient therapeutic response, slightly more in the monotherapy than in the combination therapy group (Table 2).
AESIs occurred at a rate of 16.1/100 PY in the total population (monotherapy 15.4/100 PY, combination therapy 16.3/100 PY). AESIs that occurred in >0.3% of the total population included pneumonia, herpes zoster infection, transaminase increase, hypersensitivity and injection site erythema. The rate of injection site reactions in the total population was 31.7/100 PY.
Overall, 105 patients (5.8%) reported one or more serious AE (SAE). SAEs were reported in 29 (8.2%) and 76 (5.2%) patients in the monotherapy and combination therapy groups, respectively. The overall SAE rate was 14.6/100 PY (monotherapy 22.8/100 PY, combination therapy 12.8/100 PY). The most common SAEs were infections and infestations, with an overall rate of 3.6/100 PY and similar rates between groups (monotherapy 4.0/100 PY, combination therapy 3.5/100 PY). Three non-fatal gastrointestinal perforations occurred (overall 0.32/100 PY, monotherapy 0.57/100 PY, combination therapy 0.26/100 PY); one diverticular perforation in each group and one duodenal perforation in the monotherapy group also occurred. Three patients had myocardial infarctions classified as SAEs (overall 0.32/100 PY, monotherapy 0.57/100 PY, combination therapy 0.26/100 PY), two in the combination therapy group and one in the monotherapy group, and one patient had a stroke classified as an SAE in the combination therapy group. Overall, two patients had serious anaphylactic reactions, both in the combination therapy group, and nine had serious hypersensitivity reactions (defined as AEs occurring within 24 h of infusion/injection and excluding injection site reactions), two in the monotherapy group and seven in the combination therapy group. One patient had a serious opportunistic infection of disseminated tuberculosis (combination therapy group).
Six deaths occurred (0.64/100 PY), one due to coronary artery disease in the monotherapy group (0.57/100 PY) and five in the combination therapy group (0.65/100 PY) due to myocardial infarction, pneumonia (bacterial), pulmonary fibrosis, sepsis (bacterial) and septic shock (fungal).
Of the patients with a normal neutrophil count at baseline (n = 1395) who developed a low neutrophil count after initiating TCZ-SC, most experienced National Cancer Institute Common Terminology Criteria grade 1 (overall 22.2%, monotherapy 23.2%, combination therapy 22.0%) or grade 2 (18.7%, 15.2% and 19.6%, respectively) neutropenia. No differences were observed between groups for grade 3 or 4 neutropenia [monotherapy 5.4% (grade 3 only), combination therapy 5.9% (5.7% grade 3 and 0.2% grade 4)]. No serious infections occurred following grade 4 neutropenia.
Of the patients with normal alanine aminotransferase (ALT) values at baseline (n = 1361), 46.5% had elevated levels upon initiation of TCZ-SC, with a higher proportion of patients having elevations in the combination therapy group. Most shifts were ⩽3× the upper limit of normal (ULN; overall 41.7%, monotherapy 28.4%, combination therapy 44.9%). Similar results were observed for aspartate aminotransferase (AST) values (n = 1430), with 35.7% of patients having elevated levels after initiating TCZ-SC; most patients had shifts that were ⩽3× ULN (overall 33.6%, monotherapy 20.8%, combination therapy 36.6%). In the monotherapy group, 1.8% and 0.7% had ALT and AST shifts, respectively, from normal at baseline to >3–5× ULN; no patients had shifts from normal at baseline to >5× ULN. In the combination therapy group, 3.7% and 1.5% had ALT and AST shifts, respectively, from normal at baseline to >3–5× ULN; 1.7% and 0.8% had ALT and AST shifts, respectively, from normal at baseline to >5× ULN.
Among 1179 patients screened with the immunogenicity assay, 14 (1.2%) developed treatment-induced anti-TCZ antibodies (monotherapy 1.9%, combination therapy 1.0%) (supplementary Table S2, available at Rheumatology Online). Of these, 13 patients (1.1%) tested positive for neutralizing potential (monotherapy 1.9%, combination therapy 0.9%) and none tested positive for IgE isotype. None of the patients who developed anti-TCZ antibodies experienced anaphylaxis, serious hypersensitivity reactions or loss of efficacy.
Discussion
The TOZURA phase 4 study programme assessed the efficacy, safety and immunogenicity of TCZ-SC qw monotherapy and TCZ-SC qw in combination with a csDMARD in patients with RA who had an inadequate response to csDMARDs or an anti-TNF agent or were MTX naïve. Patients demonstrated significant efficacy improvements over 24 weeks in both groups, with improvements comparable between groups at 24 weeks. TCZ-SC was well tolerated and the safety profile was consistent with the known profile of TCZ. No new safety signals were identified.
Overall, the efficacy data in this study show the benefit of TCZ-SC as monotherapy or combination therapy since patients significantly improved from baseline to week 24 in ACR and EULAR response rates, DAS28-ESR, CDAI and HAQ-DI outcomes. Comparable efficacy responses between TCZ monotherapy and combination therapy have also been observed in clinical trials. The ACT-RAY and FUNCTION clinical trials showed that patients with RA, including early RA, who received TCZ-IV 8 mg/kg monotherapy achieved DAS28-ESR remission at week 24 at similar rates compared with patients who received TCZ-IV 8 mg/kg with concomitant MTX (ACT-RAY 34.8% vs 40.4%, FUNCTION 39% vs 45%) [15, 16]. The ACT-SURE open-label clinical trial of patients with RA who had an inadequate response to prior treatments found that similar proportions of patients who received TCZ-IV as monotherapy and combination therapy achieved DAS28 remission at week 24 (61% vs 62%). Similar to the TOZURA study programme, the ACT-SURE study featured a broad geographic range (25 countries) with less stringent conditions than the phase 3 trials [17].
Treatment persistence with TCZ-SC was lower in the monotherapy group compared with combination therapy. This difference in persistence may be due to unobserved differences in characteristics between the two groups. Patients in the monotherapy group were more likely to have received prior bDMARD treatment and less likely to be receiving glucocorticoids at baseline compared with the combination therapy group. However, the propensity score–based analysis indicated these confounders did not fully explain the difference in persistence.
Safety outcomes at 24 weeks in all patients were consistent with previous studies and were similar between the monotherapy and combination therapy groups. The rate of AEs in this study per 100 PY was consistent with the rate observed in the ACT-SURE study, an open-label clinical trial with a similar patient population (622.4 vs 593), except for the known difference of more frequent injection site reactions occurring with TCZ-SC compared with TCZ-IV. The rate of SAEs for TCZ-SC qw monotherapy in this study per 100 PY was similar to the rate observed for TCZ-SC monotherapy in the 2-year long-term extension of the MUSASHI study, which also studied TCZ-SC qw monotherapy (22.8 vs 16.9) [12]. The rate of SAEs for combination therapy in this study was also similar to the rates in the 97-week long-term extension of the SUMMACTA trial for TCZ-SC with concomitant csDMARDs (12.8 vs 14.61) [10]. Serious infections were the most commonly reported SAE in this study, occurring at rates similar to those reported for TCZ-SC monotherapy in the MUSASHI long-term extension (4.0 vs 5.3) and for combination therapy in the SUMMACTA long-term extension (3.5 vs 3.95) [10, 12].
As also observed in other studies, elevated transaminase levels were more commonly associated with concomitant MTX than with TCZ monotherapy. The proportions of patients who received monotherapy or combination therapy in this study who had shifts in ALT levels from normal to >3–5× ULN and to >5× ULN were similar to those observed in the ACT-RAY study (monotherapy: >3–5× ULN 1.8% vs 0.8% and >5× ULN 0.0% vs 0.4%; combination therapy: >3–5× ULN 3.7% vs 5.7% and >5× ULN 1.7% vs 2.0%); similar trends were observed for shifts in AST levels [15].
Immunogenicity is a concern for mAb biologic therapies because the development of anti-drug antibodies may potentially affect the efficacy of the drug and lead to hypersensitivity reactions [18]. In this study programme, immunogenicity was infrequent in both the monotherapy (not previously addressed in clinical trials) and the combination therapy groups. This result is consistent with the previously reported low immunogenicity for TCZ in patients with RA in clinical trial populations; including interim immunogenicity data from this study [19].
This study programme has several limitations. It did not have a control group; all patients received TCZ-SC. Discontinuation and missing data rates were high and were not balanced between the monotherapy and combination therapy groups. In addition, we did not impute missing values into these analyses. Treatment choice was at investigator discretion, therefore confounders and confounding by indication may have affected the comparison of monotherapy and combination therapy. The propensity score–adjusted results suggest that these potential confounders would not change the overall conclusions.
The TOZURA phase 4 study programme demonstrated that TCZ-SC was efficacious in patients with RA, with combination therapy and monotherapy being comparably effective and with the observed safety profile being consistent with the known TCZ profile.
Supplementary Material
Acknowledgements
Support for third-party writing assistance for this manuscript, furnished by Benjamin L. Ricca, PhD, and Denise Kenski, PhD, of Health Interactions, Inc., was provided by F. Hoffmann-La Roche.
Funding: This work was supported by F. Hoffmann-La Roche.
Disclosure statement: E.C. has received grant research support from Roche, Pfizer and UCB; served as a consultant for Roche, Chugai, Amgen, Eli Lilly, Janssen, Novartis, Regeneron, R-Pharm and Sanofi and has served on speakers bureaus for Roche, Chugai, Bristol-Myers Squibb, Janssen, Pfizer, Regeneron, Sanofi and UCB. R.C. has received honoraria and speaker's fees from AbbVie, Bristol-Meyers Squibb, Eli Lilly, Roche, MSD, Celgene, Sanofi and Pfizer. R.X. has received consultancy and speakers fees from Roche, Pfizer, Eli Lilly, Janssen, UCB and Bristol-Meyers Squibb. B.F. has received consultancy fees from AbbVie, Biogen, Bristol-Meyers Squibb, Celgene, Hospira, Janssen, Eli Lilly, MSD, NORDIC Pharma, Pfizer, Roche, SOBI and UCB. M.B. is an employee of Genentech, a member of the Roche group. C.B. is a contractor of F. Hoffmann-La Roche. A.P.-S. is an employee of F. Hoffmann-La Roche. The other author has declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Eulenfeld R, Dittrich A, Khouri C. et al. Interleukin-6 signalling: more than Jaks and STATs. Eur J Cell Biol 2012;91:486–95. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Beaulieu A, Rubbert-Roth A. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- 3. Genovese MC, McKay JD, Nasonov EL. et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968–80. [DOI] [PubMed] [Google Scholar]
- 4. Emery P, Keystone E, Tony HP. et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones G, Sebba A, Gu J. et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010;69:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kremer JM, Blanco R, Brzosko M. et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609–21. [DOI] [PubMed] [Google Scholar]
- 7. Burmester GR, Rubbert-Roth A, Cantagrel A. et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis 2014;73:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kivitz A, Olech E, Borofsky M. et al. Subcutaneous tocilizumab versus placebo in combination with disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Res 2014;66:1653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogata A, Tanimura K, Sugimoto T. et al. Phase III study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res 2014;66:344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burmester GR, Rubbert-Roth A, Cantagrel A. et al. Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann Rheum Dis 2016;75:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kivitz A, Olech E, Borofsky MA. et al. The efficacy and safety of tocilizumab subcutaneous Q2W and following escalation from Q2W to QW therapy in combination with traditional DMARDs in patients with moderate to severe rheumatoid arthritis at 96 weeks. Arthritis Rheumatol 2014;66:S1076–7. [Google Scholar]
- 12. Ogata A, Amano K, Dobashi H. et al. Longterm safety and efficacy of subcutaneous tocilizumab monotherapy: results from the 2-year open-label extension of the MUSASHI study. J Rheumatol 2015;42:799–809. [DOI] [PubMed] [Google Scholar]
- 13. Kivitz A, Wallace T, Olech E. et al. Long-term safety and efficacy of subcutaneously administered tocilizumab for adult rheumatoid arthritis: a multicenter phase 3b long-term extension study. Rheumatol Ther 2016;3:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stubenrauch K, Wessels U, Birnboeck H. et al. Subset analysis of patients experiencing clinical events of a potentially immunogenic nature in the pivotal clinical trials of tocilizumab for rheumatoid arthritis: evaluation of an antidrug antibody ELISA using clinical adverse event-driven immunogenicity testing. Clin Ther 2010;32:1597–609. [DOI] [PubMed] [Google Scholar]
- 15. Dougados M, Kissel K, Sheeran T. et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 2013;72:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burmester GR, Rigby WF, van Vollenhoven RF. et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis 2016;75:1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bykerk VP, Ostor AJ, Alvaro-Gracia J. et al. Comparison of tocilizumab as monotherapy or with add-on disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and inadequate responses to previous treatments: an open-label study close to clinical practice. Clin Rheumatol 2015;34:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh SK. Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci 2011;100:354–87. [DOI] [PubMed] [Google Scholar]
- 19. Burmester GR, Choy E, Kivitz A. et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:1078–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.