Abstract
STUDY QUESTION
Are serum polyunsaturated fatty acids (PUFA) concentrations, including omega-3 (ω3-PUFA) and omega-6 (ω6-PUFA), related to ART outcomes?
SUMMARY ANSWER
Serum levels of long-chain ω3-PUFA were positively associated with probability of live birth among women undergoing ART.
WHAT IS KNOWN ALREADY
Intake of ω3-PUFA improves oocyte and embryo quality in animal and human studies. However, a recent cohort study found no relation between circulating ω3-PUFA levels and pregnancy rates after ART.
STUDY DESIGN SIZE, AND DURATION
This analysis included a random sample of 100 women from a prospective cohort study (EARTH) at the Massachusetts General Hospital Fertility Center who underwent 136 ART cycles within one year of blood collection.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Serum fatty acids (expressed as percentage of total fatty acids) were measured by gas chromatography in samples taken between Days 3 and 9 of a stimulated cycle. Primary outcomes included the probability of implantation, clinical pregnancy and live birth per initiated cycle. Cluster-weighted generalized estimating equation (GEE) models were used to analyze the association of total and specific PUFAs with ART outcomes adjusting for age, body mass index, smoking status, physical activity, use of multivitamins and history of live birth.
MAIN RESULTS AND ROLE OF CHANCE
The median [25th, 75th percentile] serum level of ω3-PUFA was 4.7% [3.8%, 5.8%] of total fatty acids. Higher levels of serum long-chain ω3-PUFA were associated with higher probability of clinical pregnancy and live birth. Specifically, after multivariable adjustment, the probability of clinical pregnancy and live birth increased by 8% (4%, 11%) and 8% (95% CI: 1%, 16%), respectively, for every 1% increase in serum long-chain ω3-PUFA levels. Intake of long-chain ω3-PUFA was also associated with a higher probability of life birth in these women, with RR of 2.37 (95% CI: 1.02, 5.51) when replacing 1% energy of long-chain ω3-PUFA for 1% energy of saturated fatty acids. Serum ω6-PUFA, ratios of ω6 and ω3-PUFA, and total PUFA were not associated with ART outcomes.
LIMITATIONS REASONS FOR CAUTION
The generalizability of the findings to populations not undergoing infertility treatment may be limited. The use of a single measurement of serum fatty acids to characterize exposure may lead to potential misclassification during follow up.
WIDER IMPLICATIONS OF THE FINDINGS
Serum ω3-PUFA are considered biomarkers of dietary intake. The association of higher serum long chain ω3-PUFA levels with improved ART outcomes suggests that increased intake of these fats be may be beneficial for women undergoing infertility treatment with ART.
STUDY FUNDING/COMPETING INTERESTS
NIH grants R01-ES009718 from the National Institute of Environmental Health Sciences, P30-DK046200 and T32-DK007703-16 from the National Institute of Diabetes and Digestive and Kidney Diseases, and L50-HD085359 from the National Institute of Child Health and Human Development, and the Early Life Nutrition Fund from Danone Nutricia US. Dr Rueda is involved in a patent 9,295,662, methods for enhancing, improving, or increasing fertility or reproductive function (http://patents.com/us-9295662.html). This patent, however, does not lead to financial gain for Dr Rueda, or for Massachusetts General Hospital. Dr Rueda does not own any part of the company nor does he have any equity in any fertility related company. As Dr Rueda is not a physician, he does not evaluate patients or prescribe medications. All other coauthors have no conflicts of interest to declare.
Keywords: polyunsaturated fatty acids, omega-3 fatty acids, live birth, clinical pregnancy, ART
Introduction
The utilization of ART for the management of infertility has become increasingly common, with ~150 000 cycles performed per year in the United States alone (Centers for Disease Control and Prevention. American Society for Reproductive Medicine. Society for Assisted Reproductive Technology, 2015). The high prevalence of infertility, around 15% of couples attempting to conceive (Thoma et al., 2013), as well as the relatively low success rates of its treatment (Centers for Disease Control and Prevention. American Society for Reproductive Medicine. Society for Assisted Reproductive Technology, 2015) underscores the importance of identifying potentially modifiable risk factors, such as diet, that impact both natural fecundity and ART outcomes.
Many women facing infertility take dietary supplements such as ω3- polyunsaturated fatty acids (ω3-PUFA) in order to improve ART success despite inconclusive evidence regarding their effects on human fertility. In addition to being incorporated into cell membranes, PUFA are critical precursors for a variety of substrates including inflammatory factors and steroid hormones (Wathes et al., 2007). Animal and human studies suggest that a diet rich in omega-3 polyunsaturated fatty acids (ω3-PUFA) has a positive impact on fertility, possibly through effects on oocyte quality and embryo implantation (Hammiche et al., 2011; Moran et al., 2016; Nehra et al., 2012), reproductive hormones (Mumford et al., 2016) or menstrual cycle function (Deutch, 1995; Harel et al., 1996; Nadjarzadeh et al., 2013). However, other studies have yielded conflicting results (Chavarro et al., 2007a, 2007b; Wakefield et al., 2008; Jungheim et al., 2011). A recent epidemiologic study reported that ω3-PUFA intake (assessed by a diet questionnaire) was associated with higher fecundability in North American pregnancy planners, but no association was found in Denmark, where low intake of ω3-PUFA was rare (Wise et al., 2017). In another epidemiological study, Jungheim et al. did not find an association between serum ω3-PUFA levels and ART outcomes; however, higher embryo implantation, defined as number of gestational sacs on ultrasound/number of embryo transferred, was observed with increasing ratios of serum linoleic acid (LA) to alpha-linolenic acid (Polesel et al. 2006; Jungheim et al., 2013). To further clarify the effects of ω3-PUFAs on infertility treatment outcomes, we investigated the relationship between serum levels of PUFA and ART outcomes.
Materials and Methods
Study population
Women in the study were participating in the Environmental and Reproductive Health (EARTH) Study, an ongoing prospective cohort study conducted at Massachusetts General Hospital Fertility Center, which aims to evaluate the relationships between environmental and nutritional factors with fertility. All participants signed an informed consent after study procedures were explained by a research nurse. To be eligible for this analysis, women had to have completed a food frequency questionnaire (FFQ) (introduced in 2007), provided a blood sample, and subsequently completed at least one ART cycle by May 2013 (n = 232). From this initial pool of eligible women, we used a random number generator to select 100 women for whom stored blood samples taken during their first in-study fresh ART cycle were sent for analysis of fatty acids. Women whose blood samples were analyzed for serum fatty acids did not systematically differ from women who were not included in this analysis (Supplementary Table SI). For the analysis of serum fatty acids, we included the 100 women who underwent 136 cycles within one year of blood sample collection. For the analysis of dietary fatty acids and ART outcomes, we included 168 women who underwent 297 cycles within one year of FFQ completion. Trained research nurses measured height and weight of participants at enrollment, and BMI was calculated as weight (in kilograms) per height (in meters) squared. All participants were given a take-home questionnaire focusing on medical and reproductive history as well as lifestyle factors. The Institutional Review Boards of the Harvard T.H. Chan School of Public Health and the Massachusetts General Hospital approved the study.
Laboratory analyses
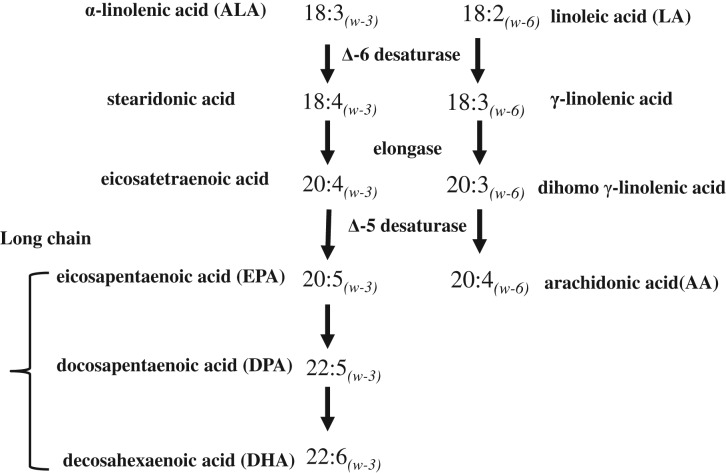
Blood samples were collected from women between Days 3 and 9 of gonadotropin treatment during their first in-study ART cycle. Serum fatty acids were measured at the Biomarker Research Laboratory at the Harvard T.H. Chan School of Public Health. A detailed description of laboratory analyses has been provided elsewhere (Chavarro et al., 2007a, 2007b). Briefly, fatty acids were extracted from whole blood into isopropanol and hexane containing the antioxidant 2.6-di-tert-butyl-p-cresol. Transmethylation was performed with methanol and sulfuric acid. Samples were evaporated and redissolved in iso-octane. Fatty acid levels were quantified by gas–liquid chromatography on a fused silica capillary cis/trans column (SP2560, Supelco) and expressed as percentage of total fatty acids. ChemStation A.08.03 software (Agilent Technologies) was used for analysis. The average between-run coefficients of variance were 1% for alpha-linolenic acid (ALA, 18:3n−3), 3% for eicosapentaenoic acid (EPA, 20:5n−3), 4% for docosapentaenoic acid (DPA, 22:5n−3), 4% for docosahexaenoic acid (DHA, 22:6n−3), 1% for linoleic acid (LA, 18:2n−6) and 6% for arachidonic acid (AA, 20:4n−6). The average within-run coefficients of variance were 1% for ALA, 1% for EPA, 2% for DPA, 1% for DHA, 1% for LA and 1% for AA. Major pathways of PUFA metabolism are shown in Fig. 1.
Figure 1.
Major pathways of polyunsaturated fatty acids.
Dietary assessment
Diet was assessed before ART treatment using a validated FFQ (Rimm et al., 1992). Participants were asked to report how often they consumed specified amounts of 131 food items during the previous year. Multivitamin and supplement users were asked to specify the brand of the multivitamin or supplement, the dose and frequency of use. Nutrient intakes were estimated by summing the nutrient contribution of all food and supplement items. Nutrient contents were obtained from the nutrient database of the US Department of Agriculture with additional information from manufacturers (U.S. Department of Agriculture, 2012).
Clinical outcomes
Clinical data were abstracted from the electronic medical record. Women underwent one of three controlled ovarian stimulation IVF treatment protocols as clinically indicated on the Day 3 of induced menses after taking a cycle of oral contraceptives: (i) luteal phase gonadotropin-releasing hormone (GnRH)-agonist protocol, (ii) follicular phase GnRH-agonist/Flare protocol or (iii) GnRH-antagonist protocol. Patients were monitored during gonadotropin stimulation for serum estradiol, follicle size measurements and counts, and endometrial thickness through 2 days before egg retrieval. hCG was administered approximately 36 hours before the scheduled egg-retrieval procedure to resume oocyte meiosis and to yield mature oocytes at retrieval. Couples underwent ART with conventional IVF or ICSI as clinically indicated. Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II (MII) or degenerated. Embryologists determined the fertilization rate 17–20 h after insemination as the number of oocytes with two pronuclei divided by the number of inseminated MII oocytes. Implantation was defined as a serum β-hCG level >6 mIU/mL measured 15–20 days after egg retrieval, clinical pregnancy as the presence of an intrauterine pregnancy visualized on ultrasound by the presence of a gestational sac, with or without heartbeat, and live birth as the birth of a neonate on or after 24 weeks gestation.
Statistical analyses
Spearman correlation coefficients were used to describe the dependence between serum fatty acid concentrations and dietary intake. Descriptive statistics were calculated for demographic, reproductive, and dietary characteristics according to low (≤33th percentile), medium (34th–67th percentile) and high (≥68th percentile) levels of serum ω3-PUFA. Kruskal–Wallis and Fisher’s exact tests were used to evaluate differences in baseline characteristics according to serum ω3-PUFA concentrations for continuous measures and categorical variables, respectively. Serum fatty acid concentrations were analyzed as continuous linear variables. We also used cubic splines to explore potential non-linearity in the associations of serum fatty acids and ART outcomes. To evaluate the association of total and specific PUFAs with clinical outcomes (implantation, clinical pregnancy, and live birth), cluster-weighted generalized estimating equation (GEE) models with a log link were used to account for within-person correlations between repeated cycles in the presence of non-ignorable cluster size (Williamson et al., 2007; Missmer et al., 2011). Cluster-weighted GEE models with a Poisson distribution and log link function were specified for oocyte counts, and a normal distribution and identity link function were specified for peak estradiol levels. Modified Poisson regression models were used to estimate relative risk for fertilization and clinical outcomes when log binomial models failed to converge (Zou, 2004; Yelland et al., 2011). Robust estimators of the variance were used to compute 95% CIs. Results are presented as population marginal mean adjusted for covariates at their mean for continuous covariates and as a weighted average over levels of categorical variables according to their relative frequency.
Confounding was evaluated using prior knowledge and descriptive statistics from our cohort. Factors that were different across tertiles of PUFA concentrations at P < 0.20 in a univariate analysis were considered as confounders. We decided a priori that age (Malizia et al., 2009), BMI (Luke et al., 2011) and smoking (Soares and Melo, 2008) would be considered as confounders regardless of whether their relation with serum PUFAs was statistically significant since they are well-known predictors of live birth following ART. Covariates included in the final models were age, BMI, smoking status, moderate-to-vigorous physical activity, history of live birth, and intake of multivitamin supplements. Because Day 3 FSH could be either a potential intermediate variable or a confounder, we conducted a sensitivity analysis with additional adjustment for day-3FSH levels as a covariate in the multivariable model. Further, as serum ω3-PUFA are considered as biomarkers of dietary intake (Baylin et al., 2005), we also examined the association of dietary long-chain ω3-PUFA intake and ART outcomes using the multivariable adjusted nutrient density method. Specifically, terms for dietary long-chain ω-3 PUFA intake and other types of fatty acids, protein, and total energy intake were added to the models to represent isocaloric substitution of long-chain ω3 PUFA for other sources of macronutrients. In addition, we adjusted for Prudent and Western dietary pattern scores (to capture overall food choices) in addition to the same set of covariates in the serum fatty acids model. The effect of substituting long-chain ω-3 PUFA for other macronutrients was estimated using linear combinations of the regression coefficients; the 95% CI of a substitution was estimated based on the variance and the covariance of each regression coefficient. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC). Two-sided P values less than 0.05 were considered as statistically significant.
Results
Our analysis of serum fatty acids included 100 women who underwent a total of 136 ART cycles within 1 year of blood sample collection. Of these, 62 cycles (46%) resulted in a clinical pregnancy and 47 cycles (35%) ended in a live birth. Most women were Caucasian (82%), never smokers (71%), nulliparous (88%), with a mean (standard deviation) age of 34.8 (3.8) years and BMI of 24.4 (4.0) kg/m2. The median [25th, 75th percentile] serum ω3- and ω6-PUFA concentrations for the 100 women were 4.7% [3.8%, 5.8%] and 41.8% [39.4%, 45.2%] of total fatty acids, respectively. The Spearman correlations between serum concentrations and dietary intake were 0.49 for long-chain ω3, 0.15 for ALA and 0.18 for LA. Women with higher serum ω3-PUFA concentrations were, on average, leaner, more physically active, more likely to be never smokers, had lower Day 3 FSH levels, had a higher adherence to a Prudent dietary pattern, and consumed higher amounts of ω3-PUFA from diet compared to women with the lowest serum ω3-PUFA concentrations (Table I). Women with higher serum ω6 concentrations were, on average, older and more likely to consume a multivitamin and consume higher amounts of dietary ω6-PUFA compared to women in the lowest tertile of serum ω6-PUFA concentrations.
Table I.
Baseline characteristics of 100 women in Environment and Reproductive Health Study according to tertiles of serum ω-3 polyunsaturated fatty acids.
| Total Cohort | Serum ω-3 PUFAs, % total fatty acids | P value | |||
|---|---|---|---|---|---|
| Group | Low | Median | High | ||
| range of serum ω-3 PUFAs, % | 2.4–4.0 | 4.0–5.4 | 5.4–16.3 | ||
| Number of women | 100 | 33 | 34 | 33 | |
| Mean (SD) or N (%) | |||||
| Personal characteristics | |||||
| Age, years | 34.8 (3.8) | 34.8 (4.0) | 35.4 (4.1) | 34.2 (3.2) | 0.44 |
| BMI, kg/m2 | 24.4 (4.0) | 26.8 (4.9) | 23.6 (2.9) | 22.7 (2.6) | 0.0006 |
| Ever smoker, n (%) | 29 (29.0) | 13 (39.4) | 6 (17.7) | 10 (30.3) | 0.006 |
| White/Caucasian, n (%) | 82 (82.0) | 30 (90.1) | 26 (76.5) | 26 (78.8) | 0.26 |
| Moderate-to-vigorous exercise, hr/week | 4.3 (4.7) | 3.0 (3.7) | 5.2 (5.0) | 4.9 (5.1) | 0.09 |
| Dietary characteristics | |||||
| Total calories, kcal/d | 1815 (566) | 1898 (458) | 1585 (543) | 1973 (638) | 0.004 |
| Carbohydrates, g/d | 227 (82) | 238 (70) | 194 (58) | 252 (104) | 0.97 |
| Protein, g/d | 73.5 (21.4) | 75.1 (20.8) | 66 (15.5) | 79.7 (25.3) | 0.27 |
| Fat, g/d | 67.7 (26.7) | 70.8 (23.2) | 57.9 (21.4) | 74.7 (32.2) | 0.90 |
| SFA, g/d | 21.6 (8.4) | 23.1 (8) | 19.2 (7.5) | 22.7 (9.4) | 0.44 |
| MUFA, g/d | 26.9 (13.1) | 28.7 (12.3) | 22.4 (9.3) | 29.7 (16.1) | 0.61 |
| ω-3 fatty acid, g/d | 1.6 (0.9) | 1.4 (0.7) | 1.4 (0.7) | 2.1 (1.2) | 0.006 |
| ω-6 fatty acids, g/d | 11.6 (5.5) | 11.6 (3.7) | 9.7 (4.4) | 13.6 (7.1) | 0.85 |
| Trans fatty acids, g/d | 1.9 (0.7) | 2 (0.7) | 1.7 (0.7) | 1.9 (0.8) | 0.08 |
| Prudent dietary pattern scores | −0.1 (0.9) | −0.3 (0.8) | -0.3 (0.7) | 0.2 (1.1) | 0.01 |
| Western dietary pattern scores | 0 (0.9) | 0.5 (0.8) | -0.3 (0.8) | -0.1 (0.8) | 0.006 |
| Multivitamin user, n (%) | 91 (91.0) | 28 (84.9) | 32 (94.1) | 31 (93.9) | 0.04 |
| Reproductive characteristics | |||||
| Nulliparous, n (%) | 88 (88.0) | 27 (81.8) | 30 (88.2) | 31 (93.9) | 0.32 |
| Infertility diagnosis, n (%) | 0.67 | ||||
| Female factor | 24 (24.0) | 6 (18.2) | 9 (26.5) | 9 (27.3) | |
| Ovulation disorders | 10 (10.0) | 3 (9.1) | 3 (8.8) | 4 (12.1) | |
| DOR | 3 (3.0) | 1 (3.0) | 2 (5.9) | 0 (0) | |
| Tubal | 7 (7.0) | 2 (6.1) | 0 (0) | 5 (15.2) | |
| Endometriosis | 3 (3.0) | 0 (0) | 3 (8.8) | 0 (0) | |
| Uterine | 1 (1.0) | 0 (0) | 1 (2.9) | 0 (0) | |
| Male factor | 35 (35.0) | 14 (42.4) | 9 (26.5) | 12 (36.4) | |
| Unexplained | 41 (41.0) | 13 (39.4) | 16 (47.1) | 12 (36.4) | |
| Treatment protocol, n (%) | 0.46 | ||||
| Follicular phase GnRH antagonist | 10 (10.0) | 2 (6.1) | 2 (5.9) | 6 (18.2) | |
| Follicular phase GnRH agonist | 9 (9.0) | 4 (12.1) | 3 (8.8) | 2 (6.1) | |
| Luteal phase GnRH agonist | 81 (81.0) | 27 (81.8) | 29 (85.3) | 25 (75.8) | |
| Day 3 FSH, IU/L | 7.0 (2.0) | 7.7 (2.2) | 6.6 (1.7) | 6.7 (2.0) | 0.08 |
| Embryo transfer day, n (%) | 0.69 | ||||
| No embryos transferred | 13 (13.0) | 5 (15.2) | 6 (17.7) | 2 (6.1) | |
| Day 2 | 5 (5.0) | 2 (6.1) | 1 (2.9) | 2 (6.1) | |
| Day 3 | 56 (56.0) | 17 (51.5) | 17 (50.0) | 22 (66.7) | |
| Day 5 | 26 (26.0) | 9 (27.3) | 10 (29.4) | 7 (21.2) | |
| Embryos transferred, n (%) | 0.59 | ||||
| No embryos transferred | 13 (13.0) | 5 (15.2) | 6 (17.7) | 2 (6.1) | |
| 1 embryo | 12 (12.0) | 2 (6.1) | 6 (17.7) | 4 (12.1) | |
| 2 embryos | 59 (59.0) | 21 (63.6) | 17 (50.0) | 21 (63.6) | |
| 3+ embryos | 16 (16.0) | 5 (15.2) | 5 (14.7) | 6 (18.2) | |
DOR, diminished ovarian reserve; PUFAs, polyunsaturated fatty acids.
*P value was calculated based on Kruskal–Wallis test for continuous variable and Fisher exact test for categorical variables.
Serum long-chain ω3-PUFA and ALA were associated with clinical outcomes, but in different directions. Specifically, higher concentrations of serum long-chain ω3-PUFA were associated with higher probability of clinical pregnancy and live birth following ART. In multivariable adjusted models, the probability of clinical pregnancy and live birth increased by 8% (95% CI: 4%, 11%) and 8% (95% CI: 1%, 16%) for every 1% (of total fatty acids) increase in serum long-chain ω3-PUFA concentrations. These positive associations were mainly driven by concentrations of EPA (Table II). Every 1% increase in serum EPA concentrations was associated with a 10% (95% CI: 0%, 20%) and 15% (5%, 26%) increase in the probability of clinical pregnancy and live birth, respectively. On the other hand, serum levels of ALA were associated with lower risks of clinical pregnancy, with a RR of 0.47 (95% CI: 0.24, 0.92) for a 1% increase in ALA levels (Table II). When the analysis was restricted to first cycles only, these estimates were similar albeit with wider confidence intervals, given the loss in statistical power (Supplementary Table SII). Additional adjustment for Day 3 FSH levels had little impact on effect estimates (data not shown). Serum ω6-PUFA, total PUFA, and ratios of ω6: ω3 and LA: ALA were not associated with ART outcomes (Table II). There was no evidence of non-linear relationships between specific or total PUFAs and clinical outcomes.
Table II.
Adjusted risk ratio (95% CI) of clinical outcomes per initiated treatment cycle for every 1% increase in serum fatty acid concentrations in 100 women who underwent 136 ART cycles within 1 year of blood sample collection, the Environment and Reproductive Health (EARTH) Study.
| Serum fatty acids | Implantation | Clinical pregnancy | Live birth |
|---|---|---|---|
| RR1 (95% CI) per 1% increase in serum concentration | |||
| 18:3 ω-3 ALA | 0.57 (0.29, 1.10) | 0.47 (0.24, 0.92) | 0.49 (0.18, 1.31)† |
| 20:5 ω-3 EPA | 1.07 (0.99, 1.16) | 1.10 (1.00, 1.20) | 1.15 (1.05, 1.26)† |
| 22:5 ω-3 DPA | 0.66 (0.22, 2.01) | 0.70 (0.21, 2.30) | 1.46 (0.34, 6.29)† |
| 22:6 ω-3 DHA | 1.09 (0.95, 1.24) | 1.08 (0.94, 1.25) | 1.16 (0.96, 1.40)† |
| Long chain2 | 1.04 (0.98, 1.1) | 1.08 (1.04, 1.11) | 1.08 (1.01, 1.16)† |
| ω-3 PUFA3 | 1.05 (1.01, 1.08) | 1.05 (0.98, 1.12) | 1.07 (1.00, 1.16) |
| 18:2 ω-6 LA | 0.98 (0.95, 1.02) | 0.99 (0.95, 1.03) | 0.97 (0.92, 1.03) |
| 20:4 ω-6 AA | 1.04 (0.97, 1.12) | 1.07 (0.99, 1.16) | 1.05 (0.94, 1.18) |
| ω-6 PUFA | 0.99 (0.96, 1.02) | 1.01 (0.97, 1.04) | 1.00 (0.95, 1.05) |
| Ratio of ω-6 to ω-34 | 1.01 (0.93, 1.10) | 1.02 (0.92, 1.12) | 0.98 (0.84, 1.13)† |
| Ratio of LA to ALA5 | 1.00 (0.97, 1.02) | 1.00 (0.97, 1.02) | 1.00 (0.97, 1.04)† |
| Total PUFA | 1.00 (0.97, 1.03) | 1.01 (0.98, 1.05) | 1.01 (0.97, 1.06) |
AA, arachidonic acid; ALA, alpha linolenic acid; DHA, decosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid, IQR, interquartile range (from 25th to 75th); PUFA, polyunsaturated fatty acids; RR, risk ratio
Bold values represent the following: P = 0.03 for ALA and clinical pregnancy. P = 0.04 for EPA and clinical pregnancy. P < 0.0001 for long chain and clinical pregnancy. P = 0.009 for ω-3 PUFA and implantation.
1Adjusted for age, BMI, smoking status, moderate-to-vigorous physical activity, use of multivitamins and history of live birth.
2Sum of EPA, DPA and DHA.
3Sum of ALA, EPA, DPA and DHA.
4Additional adjusted for ω-6 and ω-3 PUFA concentrations.
5Additional adjusted for LA and ALA concentrations.
†Modified Poisson model was used to estimate RR when GEE log bionomial models did not converge.
Serum specific or total ω3 PUFAs were not associated with peak estradiol, oocyte count, fertilization rates, or embryo quality (Table III). However, serum LA levels were inversely associated with total oocyte and M2 oocyte yield, while the serum ratio of ω6 to ω3-PUFA was inversely associated with peak estradiol levels (Table III). This association was consistent when restricting to the first cycles only (Supplementary Table SIII).
Table III.
Adjusted changes in preclinical outcomes for every 1% increase in serum PUFA concentrations in 100 women who underwent 127 fresh ART cycles1 within 1 year of blood sample collection, the Environment and Reproductive Health (EARTH) Study.
| Peak estradiol (pmol/L) | Total oocytes (count) | MII oocytes (count) | Fertilization (%)† | Probability of ≥1 good embryo quality (%)† | |
|---|---|---|---|---|---|
| Absolute difference per 1% increase in serum concentrations2 | Relative difference per 1% increase in serum concentrations (% (95% CI))2 | ||||
| 18:3 ω-3 ALA | −329 (−978, 320) | 0 (−35.5, 55.0) | 3.5 (−33.8, 61.9) | −14.2 (−32.6, 9.3) | −31.4 (−70.9, 61.6) |
| 20:5 ω-3 EPA | −87 (−233, 59) | −6.5 (−15.2, 3.2) | −4.2 (−13.2, 5.8) | 3.2 (−2.5, 9.2) | 1.0 (−15.2, 20.3) |
| 22:5 ω-3 DPA | −402 (−1492, 689) | −11.6 (−52.0, 62.7) | −8.0 (−49.3, 67.1) | 26.5 (−11.0, 79.9) | −70.2 (−93.9, 45.3) |
| 22:6 ω-3 DHA | 15 (−189, 218) | −3.3 (−12.6, 7.0) | −1.1 (−10.4, 9.1) | 2.1 (−3.4, 7.8) | −3.4 (−21.2, 18.4) |
| Long chain | −23 (−123, 77) | −2.7 (−7.7, 2.6) | −1.5 (−6.6, 4.0) | 1.6 (−1.4, 4.7) | −1.4 (−12.2, 10.7) |
| Total ω-3 PUFA | −28 (−126, 69) | −2.7 (−7.8, 2.7) | −1.4 (−6.7, 4.1) | 1.3 (−1.6, 4.2) | −2.0 (−13.3, 10.6) |
| 18:2 ω-6 LA | −8 (−55, 39) | −2.4 (−4.6, −0.2) | −2.2 (−4.3, 0.0) | −0.8 (−2.8, 1.2) | −1.7 (−6.7, 3.5) |
| 20:4 ω-6 AA | 60 (−23, 143) | 2.1 (−3.4, 7.9) | 2.4 (−3.0, 8.2) | −0.6 (−3.5, 2.4) | 6.4 (−3.1, 16.8) |
| Total ω-6 PUFA | 7 (−29, 43) | −1.2 (−3.4, 1.1) | −1.0 (−3.2, 1.3) | −0.6 (−2.2, 0.9) | −0.3 (−4.3, 3.9) |
| Ratio of ω-6 to ω-33 | −138 (−238, −37) | −5.5 (−12, 1.6) | −4.6 (−10.8, 2.2) | 0.2 (−3.0, 3.6) | 2.2 (−4.6, 9.4) |
| Ratio of LA to ALA4 | 19 (−7, 44) | 0 (−1.2, 1.3) | 0.6 (−0.7, 1.9) | 0.4 (−0.3, 1.2) | 1.2 (−2.0, 4.4) |
| Total PUFA | 2 (−33, 38) | −1.5 (−3.6, 0.8) | −1.1 (−3.3, 1.1) | −50.3 (−51.5, −49.1) | −0.6 (−4.3, 3.3) |
Bold values represent the following: P = 0.03 for total oocyte and LA. P = 0.05 (0.048) for MII oocyte and LA. P = 0.008 for ratio of ω6 to ω3 and peak estradiol.
1Of 136 cycles initiated within 1 year of blood sample collection, nine cryocycles were excluded due to lack of data on fresh oocytes, fertilization, and embryo quality measurement.
2Adjusted for age, BMI, smoking status, moderate-to-vigorous physical activity, and use of multivitamins, and history of live birth.
3Additional adjusted for ω-6 and ω-3 PUFA concentrations.
4Additional adjusted for LA and ALA concentrations.
†Modified Poisson model was used to model fertilization given the weighted GEE log binomial models do not converge.
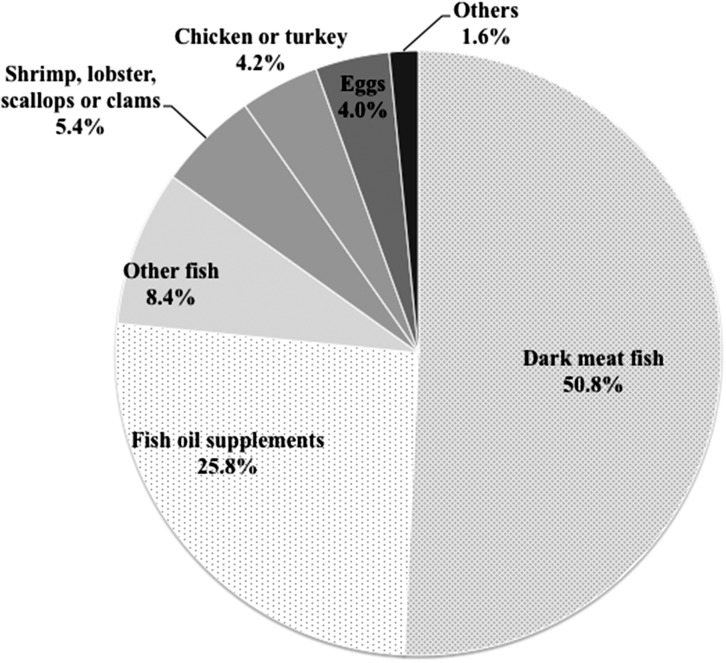
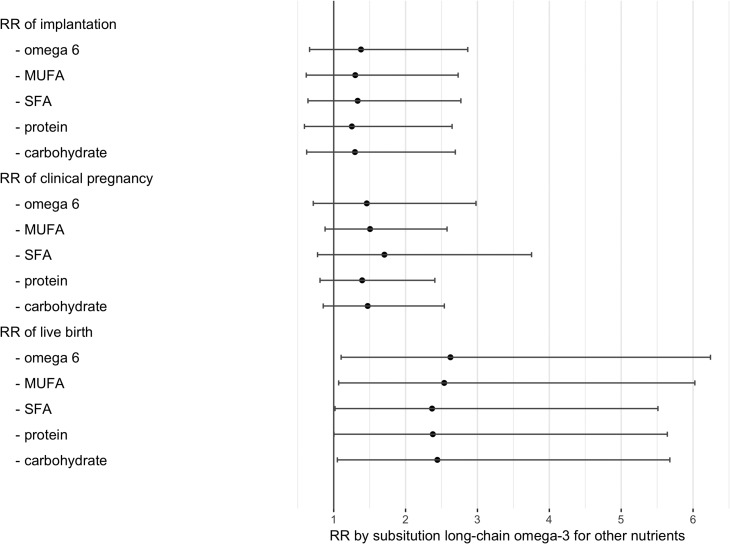
Lastly, we examined the associations of dietary fatty acid intakes and ART outcomes among 168 women who underwent 297 ART cycles after completing their FFQ. The major food sources of long-chain ω3-PUFA were fish (especially dark meat fish) and fish oil supplements (Fig. 2). As shown in Fig. 3 consuming 1% of energy from long-chain ω3-PUFA instead of 1% of energy from saturated fatty acids was associated with 2.37 (95% CI: 1.02, 5.51) times higher risk of live birth. The associations were similar when isocalorically substituting dietary intake of long-chain ω3-PUFA for ω6-PUFA (RR: 2.62, 95% CI: 1.10, 5.24), for monounsaturated fatty acids (2.54, 95% CI: 1.07, 6.03), for protein (RR: 2.38, 95% CI: 1.00, 5.64), or for carbohydrates (RR: 2.44, 95% CI: 1.05, 5.68). Dietary intake of ALA was unrelated to clinical pregnancy or other ART outcomes.
Figure 2.
Major food sources of long-chain ω-3 fatty acids in the EARTH participants. Others included cod liver oil supplement, breaded fish cakes or fish sticks, muffin and cakes. Examples of dark meat fish are tuna, mackerel, salmon, sardines, bluefish, swordfish, etc.
Figure 3.
Risk ratios (RR) of clinical outcomes following ART by substituting long-chain ω-3 fatty acids for different sources of macronutrients among 168 women (297 cycles). Models were adjusted for age, BMI, smoking status, moderate-to-vigorous physical activity, use of multivitamins, history of live birth, total calories, protein intake, dietary patterns and other types of fatty acids. The number in the figure represents the risk ratio (RR) for each of the clinical outcome substituting 1% of calories from long-chain ω-3 fatty acids for 1% of calories from different sources of macronutrients. SFA, saturated fatty acid intake; MUFA, monounsaturated fatty acid intake; PUFA, polyunsaturated fatty acid intake; RR, risk ratio.
Discussion
In this prospective study of women undergoing infertility treatment with ART we found that serum long-chain ω3-PUFA concentrations were associated with a higher probability of clinical pregnancy and live birth. These associations were mainly driven by EPA. Consistent with this finding, intake of long-chain ω3-PUFA was also related to a higher probability of live birth. We also found higher levels of serum ALA were associated with a lower probability of clinical pregnancy although intake of ALA was not related to these same outcomes. On the other hand, serum LA, and ratio of ω6 to ω3-PUFA levels were inversely associated with specific preclinical endpoints such as peak estradiol and oocyte yield (both markers of ovarian response to gonadotropin stimulation), but this did not translate into differences in clinical outcomes. In interpreting these findings it is important to note that ω3-PUFA including ALA and long-chain ω3-PUFA, are biomarkers of usual dietary intake (Baylin et al., 2005), whereas serum levels of AA are under homeostatic regulation and do not change appreciably in response to changes in dietary intake (Baylin et al., 2005; Willett, 2007). Overall, these findings suggest that increased intake of long-chain ω3-PUFAs may be beneficial for women undergoing infertility treatment with ART.
Despite numerous studies that have assessed dietary PUFA intake on ovarian function or infertility treatment outcomes, very few studies have assessed such relationships using serum biomarkers. Consistent with our finding, a prospective cohort of ART patients (n = 105) showed that serum levels of EPA were significantly higher in pregnant women than in non-pregnant patients (Mirabi et al., 2017). Meanwhile, a study of 91 women undergoing assisted reproduction also found that serum ALA concentrations were associated with decreased chance of pregnancy (Jungheim et al., 2011). However, a subsequent publication in the same study cohort using a larger sample size (N = 200 women), found no association between any of the specific serum ω3-PUFAs and ART outcomes, but higher LA to ALA ratio was associated with higher embryo implantation (defined as the number of gestation sacs on ultrasound/number of embryos transferred) and higher probability of clinical pregnancy and live birth (Jungheim et al., 2013). The divergent findings between the latter study and our current analysis could result from the differences in BMI (27.7 versus 24.4 kg/m2) distribution between studies. Further, serum long-chain ω3-PUFAs (mean: 4.28%) among women in our study were considerably higher than plasma long-chain ω3-PUFAs (mean: 1.98%) (Murphy et al., 2015) among a nationally representative sample of US adults. (Of brief note, serum and plasma fatty acid levels are comparable to each other (Moilanen and Nikkari, 1981)). Another possible explanation could be differences in analytical approach, particularly as it relates to the analysis of LA to ALA ratios. Ratio measures are statistical interactions between two variables. Hence, when including them in regression models it is important to also include the main effects of this interaction. Failure to include main effects can result in biased estimates of the effects of the ratio. Clearly, further examination of serum PUFAs and infertility treatment outcomes is warranted.
Although the impact of PUFA on human physiology is complex, investigators have identified several mechanisms by which PUFA composition and concentration may impact female reproduction. PUFAs are the direct precursors of prostaglandins (PGs), compounds which play key roles in reproductive physiology (Wathes et al., 2007; Salleh, 2014). Omega-3 fatty acids promote the generation of the 3-series PGs (such as PGE3 and PGF3α) which are generally categorized as anti-inflammatory, while ω6-PUFA are precursors to the pro-inflammatory PGs (Kohli and Levy, 2009). The precursors for the 3-series PGs compete for the enzymes involved in the ω6-PUFA pathway, creating a system where dietary intake of ω3-PUFAs may inhibit the ω6-PUFA pathway PGs and vice versa (Lands, 1992; Wathes et al., 2007). PG composition within the uterine environment is thought to be critical for successful implantation (Salleh, 2014), although there are conflicting reports regarding the precise roles of each PG. For example, one study suggests that PGE2, a product of the ω6-PUFA pathway (Biondi et al., 2006), inhibits trophoblast invasion, while others demonstrate the opposite (Godbole et al., 2011; Ciepiela et al., 2015).
It has been suggested that PUFAs might exert an effect on pregnancy outcome by influencing oocyte quality and competence. A report by Nehra et al. (2012) demonstrated that a diet rich in ω3-PUFAs resulted in improved fecundity and oocyte quality in aging mice. On the other hand, Wakefield et al. (2008) noted poorer embryo morphology and developmental competence among mice fed a diet rich in ω3-PUFAs. In an epidemiologic study, Hammiche et al. (2011) found improved embryo quality among women with higher ω3-PUFA intake as measured using a questionnaire. In the present study, however, we did not observe any associations between serum ω3-PUFA levels and markers of oocyte or embryo quality despite the positive associations of long-chain ω3-PUFA with clinical pregnancy and live birth. This suggests that even if the positive effect of long-chain ω3-PUFAs on clinical outcome is in fact mediated by oocyte and embryo quality, as other studies have suggested (Wakefield et al., 2008; Hammiche et al., 2011), classifications of quality based on gross morphological criteria may not be sufficiently sensitive to identify these effects. Studies on serum or follicular fluid concentrations of ω6-PUFAs are generally more consistent, suggesting an inverse relationship of LA levels with oocyte yield and quality but not with pregnancy outcomes. For example, a prospective study in humans reported an inverse correlation between follicular fluid levels of LA and number of MII oocytes (Mirabi et al., 2017). Another study in humans evaluated the relationship between follicular fluid ω6-PUFAs and IVF outcomes, showing that elevated concentrations of AA and LA in follicular fluid at the time of oocyte retrieval negatively impacted oocyte competence but did not affect subsequent pregnancy rates (Ciepiela et al., 2015). In the present study, we also observed higher serum LA was associated with lower MII oocyte and total oocyte yield, but these findings did not translate into differences in clinical outcomes. Taken together, this could represent a true association between serum LA and markers of oocyte quality that is mitigated by embryo selection and transfer in the IVF setting. Further examination of the relation between serum fatty acids and fertility outside of the setting of ART is important.
Finally, PUFAs may also impact female reproduction through their influence on steroidogenesis. PUFAs are sources of cholesterol, the precursor molecule for all steroid hormones, and may have direct and indirect effects on steroid synthesis through regulation of enzymes and PGs (Wathes et al., 2007). The results from epidemiologic studies, however, are mixed. Hammiche et al. (2011) reported higher baseline estradiol levels in women who consumed more ALA, a specific type of ω3-PUFA. Another study by Mumford et al. (2016) showed that increased dietary intake of DHA was associated with increased estradiol. Our results, nonetheless, are not consistent with these findings as reflected by a lack of association between serum ω3-PUFAs with peak estradiol levels. However, we found that serum ratio of ω6 to ω3-PUFA was inversely associated with peak estradiol levels, markers of ovarian response to stimulation.
It is unclear why several studies on the relationship between PUFAs and female fertility yield conflicting results. This may be due to the complexity of PUFA metabolism including genetic variation involved in fatty acid metabolism (Simopoulos, 2010; Corella and Ordovas, 2012) and the multitude of endpoints they may impact (i.e. oocyte, steroidogenesis, implantation). Our study adds to the limited data regarding the relationship between PUFAs and human fertility, and suggests that a higher serum ω3-PUFA level may positively impact IVF outcomes. In addition the discrepancy between the relation of serum PUFAs with clinical and preclinical outcomes highlights the need to use ‘hard’ endpoints such as live birth in studies evaluating the clinically relevant effects of potential predictors of ART treatment outcomes.
The strengths of our study include its prospective design and the objective quantification of ω3-PUFA in serum. We were also able to utilize dietary data to complement the results of the serum fatty acids. However, we recognize that this study also has several limitations. First, the use of a single measurement of serum fatty acids to characterize exposure could have led to exposure misclassification during follow-up. However, a previous study showed that the intraclass correlation coefficient for circulating long-chain ω3-PUFAs was 0.75 over the span of 15 months, suggesting that serum PUFAs are good biomarkers of habitual intake (Yuan et al., 2017). Second, our findings may not be generalizable to the general population because participants were recruited through a fertility clinic. Nonetheless, EARTH participants are comparable to fertility patients nationwide (Stephen and Chandra, 2000), suggesting the findings may be generalized to other couples undergoing infertility treatment. Third, as is the case for all observational studies, residual and unmeasured confounding cannot be excluded although we tried to account for many potential confounding factors in our analysis. Moreover, caution must be taken prior to making clinical interventional recommendations. To this end, randomized controlled trials are needed to assess the potential impact of ω3-PUFA supplementation on fertility outcomes. To our knowledge, only one human trial supplementing infertility patients with ω3-PUFA has been performed, suggesting an increase in fertilization rates as presented at a conference but these findings remain unpublished (Kim et al., 2010). Another trial is currently underway (Kermack et al., 2014).
In conclusion, higher serum levels and intake of long-chain ω3-PUFA was positively related to the probability of clinical pregnancy and live birth, among women undergoing ART. Future studies are needed to delineate possible mechanisms as well as understand the potential impact of dietary supplementation with long-chain ω3-PUFAs on fertility.
Supplementary data
Supplementary data are available at Human Reproduction Online.
Authors’ roles
J.E.C. and R.H. designed research; I.S., R.H. and J.E.C. acquired the data; Y.-H.C. analyzed data; Y.-H.C., A.E.K. and J.E.C. wrote the article; M.A. conducted technical reviews; Y.-H.C., A.E.K., A.J.G., M.A., P.L.W., I.S., B.R.R., R.H. and J.E.C. critically revised the article for important intellectual content; Y.-H.C. and J.E.C. had primary responsibility for the final contents.
Conflict of interest
The patent 9,295,662 that Dr Rueda is involved in is ‘Methods for enhancing, improving or increasing fertility or reproductive function’ led by Dr Mark Puder from Children’s Medical Center Corporation. The details of the patent are described in the website (http://patents.com/us-9295662.html). This patent, however, does not lead to any financial gain for Dr Rueda or for Massachusetts General Hospital. Dr Rueda does not own any part of the company nor does he have any equity in any fertility related company. As Dr Rueda is not a physician, he does not evaluate patients or prescribe medications. All other coauthors have declared no conflict of interest.
Funding
NIH grants R01-ES009718 and R01-ES022955 from the National Institute of Environmental Health Sciences (NIEHS), P30-DK046200 from the National Institute of Diabetes and Digestive and Kidney Diseases, and L50-HD085359 from the National Institute of Child Health and Human Development, and the Early Life Nutrition Fund from Danone Nutricia US. K99ES026648 from the NIEHS to A.J.G.
Supplementary Material
Acknowledgments
We acknowledge all members of the EARTH study team, specifically the Harvard T.H. Chan School of Public Health research nurses Jennifer B. Ford and Myra G. Keller, research staff Ramace Dadd, and a special thanks to all the study participants.
References
- Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, Campos H. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol 2005;162:373–381. [DOI] [PubMed] [Google Scholar]
- Biondi C, Ferretti ME, Pavan B, Lunghi L, Gravina B, Nicoloso MS, Vesce F, Baldassarre G. Prostaglandin E2 inhibits proliferation and migration of HTR-8/SVneo cells, a human trophoblast-derived cell line. Placenta 2006;27:592–601. [DOI] [PubMed] [Google Scholar]
- Kim C-H, Yoon J-W, Ahn J-W, Kang H-J, Lee J-W, Kang. BM. The effect of supplementation with omega-3-polyunsaturated fatty acids in intracytoplasmic sperm injection cycles for infertile patients with a history of unexplained total fertilization failure. Fertil Steril 2010;94:S242. , P-518. [Google Scholar]
- Centers for Disease Control and Prevention American Society for Reproductive Medicine. Society for Assisted Reproductive Technology. 2013 Assisted reproductive technology fertility clinic success rates report, 2015. US Department of Health and Human Services.
- Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Dietary fatty acid intakes and the risk of ovulatory infertility. Am J Clin Nutr 2007a;85:231–237. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Stampfer MJ, Li H, Campos H, Kurth T, Ma J. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2007b;16:1364–1370. [DOI] [PubMed] [Google Scholar]
- Ciepiela P, Baczkowski T, Drozd A, Kazienko A, Stachowska E, Kurzawa R. Arachidonic and linoleic acid derivatives impact oocyte ICSI fertilization—a prospective analysis of follicular fluid and a matched oocyte in a ‘one follicle—one retrieved oocyte—one resulting embryo’ investigational setting. PLoS One 2015;10:e0119087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corella D, Ordovas JM. Interactions between dietary n-3 fatty acids and genetic variants and risk of disease. Br J Nutr 2012;107:S271–S283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch B. Menstrual pain in Danish women correlated with low n-3 polyunsaturated fatty acid intake. Eur J Clin Nutr 1995;49:508–516. [PubMed] [Google Scholar]
- Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion. Fertil Steril 2011;95:1278–1283. [DOI] [PubMed] [Google Scholar]
- Hammiche F, Vujkovic M, Wijburg W, de Vries JH, Macklon NS, Laven JS, Steegers-Theunissen RP. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil Steril 2011;95:1820–1823. [DOI] [PubMed] [Google Scholar]
- Harel Z, Biro FM, Kottenhahn RK, Rosenthal SL. Supplementation with omega-3 polyunsaturated fatty acids in the management of dysmenorrhea in adolescents. Am J Obstet Gynecol 1996;174:1335–1338. [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Frolova AI, Jiang H, Riley JK. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J Clin Endocrinol Metab 2013;98:E1364–E1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Elevated serum alpha-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil Steril 2011;96:880–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermack AJ, Calder PC, Houghton FD, Godfrey KM, Macklon NS. A randomised controlled trial of a preconceptional dietary intervention in women undergoing IVF treatment (PREPARE trial). BMC Women’s Health 2014;14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol 2009;158:960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands WE. Biochemistry and physiology of n-3 fatty acids. FASEB J 1992;6:2530–2536. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod 2011;26:245–252. [DOI] [PubMed] [Google Scholar]
- Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med 2009;360:236–243. [DOI] [PubMed] [Google Scholar]
- Mirabi P, Chaichi MJ, Esmaeilzadeh S, Ali Jorsaraei SG, Bijani A, Ehsani M, Hashemi Karooee SF. The role of fatty acids on ICSI outcomes: a prospective cohort study. Lipids Health Dis 2017;16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missmer SA, Pearson KR, Ryan LM, Meeker JD, Cramer DW, Hauser R. Analysis of multiple-cycle data from couples undergoing in vitro fertilization: methodologic issues and statistical approaches. Epidemiology 2011;22:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen T, Nikkari T. The effect of storage on the fatty acid composition of human serum. Clin Chim Acta 1981;114:111–116. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Tsagareli V, Noakes M, Norman R. Altered preconception fatty acid intake is associated with improved pregnancy rates in overweight and obese women undertaking in vitro fertilisation. Nutrients 2016;8, pii: E10. doi: 10.3390/nu8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford SL, Chavarro JE, Zhang C, Perkins NJ, Sjaarda LA, Pollack AZ, Schliep KC, Michels KA, Zarek SM, Plowden TC et al. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am J Clin Nutr 2016;103:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RA, Yu EA, Ciappio ED, Mehta S, McBurney MI. Suboptimal plasma long chain n-3 concentrations are common among adults in the United States, NHANES 2003–2004. Nutrients 2015;7:10282–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjarzadeh A, Dehghani Firouzabadi R, Vaziri N, Daneshbodi H, Lotfi MH, Mozaffari-Khosravi H. The effect of omega-3 supplementation on androgen profile and menstrual status in women with polycystic ovary syndrome: a randomized clinical trial. Iran J Reprod Med 2013;11:665–672. [PMC free article] [PubMed] [Google Scholar]
- Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, Pan AH, Guo L, Rodig SJ, Tilly JL et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 2012;11:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesel J, Talamini R, Montella M, Parpinel M, Dal Maso L, Crispo A, Crovatto M, Spina M, La Vecchia C, Franceschi S. Linoleic acid, vitamin D and other nutrient intakes in the risk of non-Hodgkin lymphoma: an Italian case-control study. Ann Oncol 2006;17:713–718. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126. discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- Salleh N. Diverse roles of prostaglandins in blastocyst implantation. Sci World J 2014;2014:968141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med 2010;235:785–795. [DOI] [PubMed] [Google Scholar]
- Soares SR, Melo MA. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol 2008;20:281–291. [DOI] [PubMed] [Google Scholar]
- Stephen EH, Chandra A. Use of infertility services in the United States: 1995. Fam Plann Perspect 2000;32:132–137. [PubMed] [Google Scholar]
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331. e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture ARS USDA National Nutrient Database for Standard Reference. Release 25 Nutrient Data Laboratory Home Page 2012.
- Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Physiol Endocrinol Metab 2008;294:E425–E434. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod 2007;77:190–201. [DOI] [PubMed] [Google Scholar]
- Willett WC. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J Cardiovasc Med (Hagerstown, MD) 2007;8:S42–S45. [DOI] [PubMed] [Google Scholar]
- Williamson JM, Kim HY, Warner L. Weighting condom use data to account for nonignorable cluster size. Ann Epidemiol 2007;17:603–607. [DOI] [PubMed] [Google Scholar]
- Wise LA, Wesselink AK, Tucker KL, Saklani S, Mikkelsen EM, Cueto H, Riis AH, Trolle E, McKinnon CJ, Hahn KA et al. Dietary fat intake and fecundability in two preconception cohort studies. Am J Epidemiol 2017. Jun 8. doi: 10.1093/aje/kwx204. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol 2011;174:984–992. [DOI] [PubMed] [Google Scholar]
- Yuan CSD, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Rood JC, Harnack LJ, Sampson LK, Willett WC. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol 2017. Oct 4. doi: 10.1093/aje/kwx328. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.