Abstract
Colour duplex sonography (CDS) of temporal arteries and large vessels is an emerging diagnostic tool for GCA. CDS can detect wall oedema, known as a halo, throughout the length of the vessel and shows higher sensitivity compared with biopsy. Specificity reaches 100% in case of bilateral halos. A positive compression sign has been demonstrated to be a robust marker with excellent inter-observer agreement. The assessment of other large vessels, particularly the axillary arteries, is recognized to further increase the sensitivity and to reliably represent extra-cranial involvement in other areas. Nevertheless, CDS use is still not widespread in routine clinical practice and requires skilled sonographers. Moreover, its role in the follow-up of patients still needs to be defined. The aim of this review is to provide the current evidence and technical parameters to support the rheumatologist in the CDS evaluation of patients with suspected GCA.
Keywords: giant cell arteritis, large vessel vasculitis, colour duplex sonography, ultrasound
Rheumatology key messages
Colour duplex sonography for the diagnosis and follow-up of GCA is supported by increasing robust evidence.
Colour duplex sonography of both temporal arteries and axillary arteries represents the core set assessment for suspected GCA.
We described the main parameters and pitfalls to support the colour duplex sonography evaluation of GCA.
Introduction
GCA is the most common form of large vessel vasculitis (LVV), typically affecting adults older than 50 years [1]. It is characterized by inflammatory involvement of large and medium-sized vessels, typically the cranial branches of the carotid artery but also the aorta and its major branches [2, 3]. It is associated with significant morbidity due to ischaemic manifestations leading to permanent visual loss or stroke, and the increased risk of extracranial involvement, potentially complicated by aortic aneurysm formation and dissection [3]. Patients are treated with high dose glucocorticoids (GCs), often for long periods of time, with frequent and relevant side effects [4].
The gold standard for diagnosis has been temporal artery biopsy (TAB), but, although highly specific, it lacks adequate sensitivity and can yield false-negative results in up to 60% of cases, due to delay in performing the test following initiation of GCs, sampling errors or the segmented nature of the abnormalities [5]. Furthermore, TAB is not free from potential complications such as haematoma, scalp necrosis, infections and neurological damage [6].
Efforts have been directed to finding a more accessible, rapid and less invasive diagnostic tool. Colour duplex sonography (CDS) of temporal arteries (TAs) and large vessels is an emerging diagnostic tool for GCA [7]. CDS can provide information about the presence of vessel wall oedema, known as a halo, throughout the length of the vessel, potentially overcoming the problem of skip lesions often affecting the results of histological examination [8]. Nevertheless, CDS use is still not widespread in routine clinical practice and requires skilled sonographers, being highly operator dependent.
The aim of this review is to provide the current evidence and technical parameters to support the rheumatologist in the CDS evaluation of patients with suspected GCA.
Indications for the US of TAs
Role of CDS in the diagnosis of GCA
Prompt diagnosis and treatment of GCA is important in order to prevent serious vascular complications, particularly permanent visual loss, which occurs in 15–20% of patients [9, 10].
In 1997, Schmidt et al. [11] proposed the use of CDS in the diagnosis of temporal arteritis with sufficient confidence to avoid the need for TAB in selected patients.
Compared with TAB, US is a safe, well tolerated, rapid and less costly procedure. Moreover, CDS can be repeated, offers the advantage of allowing examination of the full-length superficial TA, and of extending the evaluation to other accessible cranial or extra-cranial vessels [12].
Three meta-analyses have confirmed the validity of CDS in diagnosing GCA [6, 12, 13]. The first, of studies available through April 2004 [6], concluded that the presence of halo (overall sensitivity 69%, specificity 82% compared with TAB), stenosis or occlusion (sensitivity 68%, specificity 77%) improved the diagnostic yield for GCA, with a pooled sensitivity of 87% and a pooled specificity of 96% compared with the 1990 ACR criteria for GCA, despite significant heterogeneity between studies. In 2010, Ball and colleagues [13] analysed the results of 17 studies (n = 998 participants) and concluded that CDS was relatively accurate for the diagnosis of GCA, recommending it for first-line investigation, with biopsy reserved for patients with negative scans. A third meta-analysis from Arida and colleagues [12] reported that the presence of unilateral halo sign was associated with an overall sensitivity of 68% and specificity of 91%, rising to a specificity of 100% in the presence of bilateral halos. Pooled odds ratio for a diagnosis of GCA in patients with a halo compared with those with a negative CDS was 34 (95% CI: 8.21, 138.23) and increased to 65 (95% CI: 17.86, 236.82) if the halo was bilateral. Another study from the same group demonstrated that, unlike halo, blood flow alterations (stenosis and/or occlusions) detected on TAs were neither specific nor sensitive for GCA, being equally common among elderly individuals due to atherosclerotic changes [12, 14]. These findings were recently confirmed by the OMERACT task force [15].
Buttgereit et al. [9] undertook a systematic literature review, reporting the sensitivity of CDS across 10 studies (n = 696 patients) ranging from 55% to 100% and specificity from 78% to 100%. The presence of a positive compression sign, defined as the persistence of a visible vessel wall on compression of the lumen with the US probe in the presence of inflammation-induced wall thickening, had a sensitivity of 75–79% and a specificity of 100% for a diagnosis of GCA in a study of 140 suspected cases [16, 17]. The compression sign is a simple and robust sonographic marker with excellent inter-observer agreement [17]. Patients with typical clinical features and characteristic imaging findings may not require a biopsy to confirm the diagnosis of GCA [9].
Fast-track US clinics for early diagnosis of GCA are emerging and have significantly reduced the rate of permanent visual impairment (up to 88% lower compared with the conventional standard of care), and led to significant savings due to reduction of inpatient care [18, 19].
Careful clinical evaluation and assessment of the pre-test probability of diagnosis of GCA are still required, because the halo sign can rarely be found in other forms of vasculitis (granulomatosis with polyangitiis or polyarteritis nodosa) and infections with secondary vasculitis [14, 20]. Anecdotal reports suggested a possible association of a positive CDS with malignancies, particularly lymphoproliferative disorders involving the TA [21, 22].
Role of CDS in predicting a positive TAB
Some authors have suggested that CDS has a good positive predictive value up to 80% in predicting TAB positivity [23] and that CDS-guided selection of the arterial segment to be biopsied can increase TAB sensitivity [14]. However, other groups have reported that the use of CDS only modestly increased the probability of biopsy-proven GCA and did not improve the diagnostic accuracy of a careful physical examination; nevertheless, these results were affected by poor quality of the US equipment and an unrealistically high cut-off value of 1.0 mm for the intima–media thickness of TA [24].
A recent randomized study from Germanò et al. [25] reported that CDS-guided TAB did not improve the sensitivity of biopsy to diagnose GCA compared with standard TAB. Muratore and colleagues [26] compared CDS findings with histological patterns and showed a poor correlation between CDS and specific histological patterns in GCA. An abnormal CDS was significantly less common in patients with histological evidence limited to periadventitial small vessel vasculitis (SVV) and/or vasa vasorum vasculitis (VVV), compared with classic transmural inflammatory infiltrate. The sensitivity of the halo sign was 20% and with an 80.6% specificity for the diagnosis of SVV and/or VVV, compared with a sensitivity of 82.5% and comparable specificity for classic biopsy-proven GCA. Moreover, bilateral halos (known to be highly specific for GCA) were detected in 16.7% of patients with SVV or VVV compared with 69.5% of the patients with classic GCA.
Role of CDS in the follow-up of patients with GCA and influence of treatment
The role of CDS in the follow-up of patients with GCA, particularly in the detection of disease flares and remission still needs to be defined. Moreover, the timing for resolution of CDS findings in response to GC treatment is unclear, with previous reports ranging from 2 days to several weeks [26]. Hauenstein and colleagues [27] evaluated the impact of the duration of GC treatment on CDS findings in a cohort of 59 patients, demonstrating that the sensitivity of CDS rapidly decreases with treatment (ranging from 85% showing resolution within the first day of GC treatment, to 50% in patients scanned within 2–4 days or >4 days from GC initiation). In one study patients were scanned twice weekly until halo resolution. The halo sign disappeared within a mean of 16 days (range, 7–56 days) [11]. In a study of 32 consecutive patients, CDS was performed at weeks 2, 4, 8 and 12 from the first detection of an abnormal result. The halo was reported to disappear after a mean of 21 days following initiation of treatment. De Miguel et al. [28] explored the sensitivity to change of CDS in monitoring changes in GCA in 30 consecutive patients. They reported a mean time until halo disappearance of 11 weeks, with 50% of cases showing halo resolution within the first 8 weeks of observation. Patients with a smaller number of affected branches experienced halo resolution more quickly. Among the 13 relapsing patients, the halo characteristics seemed to differ in terms of fewer branches affected, lower ESR values and a shorter time to achieve a negative halo, particularly in patients experiencing their first flare.
In the Temporal Artery Biopsy vs Ultrasound in Diagnosis of Giant Cell Arteritis (TABUL) study, a cross-sectional analysis of 312 patients with GCA and positive CDS was performed; the size of the halo was significantly smaller in patients who had received >4 days of GC treatment, compared with those receiving up to 4 days of treatment, as well as correlating with the presence of ischaemic symptoms, supporting the early use of US as a potential prognostic marker and monitoring tool [11, 29, 30].
In extracranial arteries, like the axillary arteries (AXs), the wall thickening can persist for months or years although vessel wall diameter decreases and echogenicity increases with treatment [31].
Which vessels to assess
When examining the TAs in patients with suspected GCA, the complete length of the common superficial TA with its frontal and parietal branches in transverse and longitudinal views should always be examined bilaterally.
Involvement of extra-cranial arteries is common in GCA [16, 32, 33] and has been described in up to 83% of cases [34]. The thoracic aorta (45–65%) and the subclavian/AXs (30–75%) have been reported to be the most affected sites. PET and MRI studies have shown that subclavian/AX inflammation is virtually always accompanied by thoracic aortitis [32]. Up to 50% of patients may present with extra-cranial GCA without concomitant involvement of TAs [35, 36]. Ultrasonographic studies have demonstrated that the AXs are the most frequently involved extra-cranial vessels accessible by CDS, and that inflammation is often bilateral [32, 37, 38].
Therefore, the evaluation of AXs in addition to TAs can increase the diagnostic yield of CDS in GCA [36]. Patients with concomitant involvement of large vessels are typically females, of younger age, with a lower incidence of overt symptoms of cranial GCA such as headache, jaw claudication or anterior ischaemic optic neuropathy and whose disease course correlates less well with inflammatory markers [16, 20, 32, 36].
Among a series of 46 patients with GCA, 17 (37%) had involvement of large vessels assessed by CDS [20]. Two patients (12%) with large vessel involvement (carotid or AXs) had a negative CDS of the TAs. Of the patients with a halo of the TAs, 12 (70%) had concomitant involvement of the AXs, 1 (5.8%) of the carotid artery and 2 (12%) had both carotid and AX involved. Having a positive US of the TAs, common carotid and AXs raised sensitivity to 100%, with a specificity of 96%. Czihal et al. [32] reported that up to 61% of a cohort of 43 patients with GCA showed signs of extra-cranial vessel involvement, 39% of them showing exclusive extra-cranial CDS signs of vasculitis. All patients with extra-cranial vasculitis had bilateral involvement of subclavian or AX arteries. Carotid artery involvement was found in 12 (28%) cases, with only 3 (7%) patients showing concomitant involvement of all branches (temporal, axillary, subclavian and carotid arteries). Interestingly, patients with concomitant TA and subclavian/axillary involvement experienced a significantly higher number of flares and more frequently required steroid-sparing agents.
These studies highlight the importance of evaluating large vessels in all patients with a suspicion of GCA.
An extensive US evaluation of 11 peripheral large vessel sites (common carotid, internal and external carotid, vertebral, subclavian and axillary, deep and superficial femoral and popliteal arteries) showed that involvement of the carotid artery was invariably associated with at least one other site, usually the subclavian or AX, and in one case by the TA [38]. Schmidt et al. [39] explored the value of ultrasonographic evaluation of peripheral arteries (occipital, facial, vertebral, carotid, subclavian, axillary, brachial, ulnar, radial, femoral, popliteal, posterior tibial, dorsal pedal arteries and the abdominal aorta) in 33 consecutive patients with temporal arteritis. Upper arm proximal arteries were the most commonly involved, together with the external carotid and/or facial arteries. In all of these cases, either the TA or the AX showed a halo in addition to the findings in the other vascular areas.
We therefore recommend that the minimum acceptable CDS assessment of patients with suspected GCA should routinely include both the TAs and the AXs. This core assessment is recognized to increase the sensitivity of the technique and to reliably represent the presence of extra-cranial involvement in other areas.
Equipment and general settings
Probe type and frequency
CDS assessment of arteries in patients with suspected GCA requires the use of a linear probe with a grey-scale (GS) frequency of 10 MHz or greater and a colour Doppler (CD) frequency of at least 6 MHz, using a vascular pre-set and applying CD mode.
For TA branches a linear high GS frequency, preferably of 15 MHz or greater, linear or hockey stick transducer is recommended.
For axillary, vertebral, subclavian, carotid and femoral arteries a linear transducer with a GS frequency can be lower. When scanning subclavian arteries in obese individuals, a linear or curved transducer with a lower frequency (<10 MHz) is preferable.
GS general settings
We recommend a vascular pre-set on the US machine. Table 1 shows the typical GS settings required for assessing arteries for GCA.
Table 1.
Definitions and details of typical grey-scale and colour Doppler settings required for assessing arteries in GCA
| Settings | Description | Example of recommended valuea |
|---|---|---|
| Grey-scale settings | ||
| Frequency | Regulates the length of the US wave and therefore the penetration of the beam | 18 MHz |
| Focus | Represents the level (or levels if multiple focuses are selected) of depth at which the US beam is focused. Depth can then be adjusted to detect AX or other branches according to the patient’s characteristics | 5 mm for TA, 2–3 cm for AX |
| Depth | Determines the penetration depth of the US beam. (The penetration of the US beam, however, is determined by the frequency) | 1–2 cm for TA, 3–4 cm for AX |
| B-mode gain | This function can be adapted to adjust brightness; it should be kept within ranges ideal to avoid false reading of halo because of excessive or inadequate brightness. Gain can also be adjusted through the time gain compensation controls, which allows selecting gain in specific areas of the image (near-, mid- and far-field) | 35–45 dB |
| Line density | This adjusts the spatial resolution of the image by regulating the number of scan lines. Increasing the line density increases image quality and detail but decreases the frame rate | 3 |
| Frame rate | Indicates the number of frames per second that can be acquired. It can be considered as temporal resolution. A high frame rate is crucial in small, fast moving tissues | >15 images per second |
| Dynamic range | Regulates the intensity between the shades of grey displayed | 40–66 dB |
| Colour Doppler settings | ||
| Frequency | Regulates the length of the US wave and therefore the penetration of the beam. | About 10 MHz |
| Pulse repetition frequency (PRF) | Represents the Doppler sampling frequency (how many pulses of sounds are emitted per second into the tissue). High PRF should be selected when analysing high blood velocities to filter underlying noise signals; however, these filters also remove slow flows, and therefore the PRF should be adjusted during scanning and adapted to the vessels’ flow velocity. PRF should also be adjusted (increased) to avoid systolic aliasing, an artefact leading to the visualization of pixels with an opposite direction from the surrounding flow, arising when the Doppler shift of the moving blood is higher than half of the PRF. When the PRF is too high, diastolic flow gaps might be enhanced. | 2–3 kHz for TA, >3 kHz for AX (dependent on machine and flow velocity) |
| Wall filter | Wall filters are added to remove noise (e.g. pulsation from moving vessel walls). The lowest possible wall filter (strictly connected with low PRFs) should be selected to identify low velocity flow | Low values, may have to increase to assess AX |
| Colour box | This requires an angle steer correction to obtain an angle between the scan lines and the direction of blood flow ≤60° to avoid poor CD signals and inaccurate readings | ≤60° |
| Colour flow gain | This setting needs to be constantly adjusted to ensure precise filling of the vessel lumen with colour, while avoiding under- or over-filling, and therefore creating a potential misinterpretation for halo or a blooming/bleeding artefact | 2–18 |
| Flow direction | It is useful to confirm the direction of flow, particularly in vertebral arteries in order to exclude subclavian steal syndrome. Conventionally, the flow is red if it is directed towards the transducer, typically applying to arteries, and blue if the flow is directed away, usually for veins. The direction of flow is influenced by the probe position (it will change if the transducer is rotated 180°) or if the box is steered to the opposite direction. When this happens the colour interpretation should be inverted to avoid confusion | Invert function off |
CD settings
CD should be used to aid visualization of the vessel’s lumen. Power Doppler can be applied in the case of very low blood flow or occlusion, or in deep or tortuous vessels.
We recommend a high CD frequency for TAs (>6 MHz). The frequency can be lowered when examining larger vessels. Table 1 shows CD settings for assessment of arteries for GCA. Supplementary Table S1, available at Rheumatology Online, shows further GS and CD settings that can be adjusted to optimize CDS assessment of GCA.
Scanning technique
When performing the CDS of the TAs, patients should lie in a recumbent or semi-recumbent position on their side or on their back. The first part of the common superficial TA can be detected at the level of the tragus. The probe should be applied in the transverse and subsequently the longitudinal plane or vice versa. After completing a sweep of the artery in one plane, the probe is rotated by 90° and a further sweep performed in the opposite plane. The level of the bifurcation between frontal and parietal branches of TA serves as the marker point to define the start of the frontal and parietal branches, respectively.
The AX is examined by placing the US probe over the mid axillary line, and sweeping along the expected course of the artery. The transducer should be applied in either the longitudinal or the transverse plane and swept along until the posterior humeral circumflex artery is identified. The area directly distal to the humeral circumflex artery that represents the proximal brachial artery should also be examined. The sweep will be repeated with the probe rotated at 90°, so that both longitudinal and transverse scans are performed.
Abnormal US findings in GCA
Blood vessels are normally visualized as anechoic longitudinal tubular structures in the longitudinal plane and as round structures in the transverse plane that may be compressed. A list of the definitions of the main ultrasonographic findings in GCA is presented in Table 2.
Table 2.
Definition of ultrasonographic findings in large vessel vasculitis and parameters to be assessed
| US Finding | Definition |
|---|---|
| Normal |
|
| Halo | A halo appears to be present. This observation should be made before applying the compression test (for the temporal artery and its branches). The halo is defined as homogeneous, hypoechoic wall swelling, well delineated towards the luminal side, visible both in longitudinal and transverse planes; it is most commonly concentric in transverse scans |
| Positive compression sign | This applies to the temporal artery and its branches. Any apparent halo seen should be confirmed using this technique. The thickened arterial wall remains visible upon compression. The hypoechogenic vasculitic vessel wall thickening contrasts with the mid- to hyperechogenic surrounding tissue |
| Halo extends for the whole length of anatomical site? | The halo is visible along the entire length of the segment of artery being scanned |
| Concentric or eccentric halo at the site of measurement of maximum halo size | The halo (viewed in transverse plane) is present uniformly surrounding the vessel (or if eccentric, the halo is predominantly present over one area of the artery in the transverse plane) |
| Maximum halo size (measured in mm in longitudinal view) | The longitudinal plane of view should be used to document the size of the halo (halo size is most commonly between 0.4 and 1.0 mm on temporal arteries, and above 1.0 mm on axillary arteries) |
| Significant vessel tortuosity present? | This applies to the temporal artery and its branches. Is the artery difficult to visualize in one plane along its course, potentially making it difficult to interpret the presence or absence of halo or arteriosclerosis. Most temporal arteries and their branches are a little tortuous, but this should be recorded if the tortuosity makes it difficult to interpret the scan findings |
| Arteriosclerosis present? | Arteriosclerosis is defined as heterogeneous and in part hyperechoic, irregularly delineated, eccentric vessel wall alteration. This may be present in arteries, independently of the presence or absence of halo |
| Stenosis | Localized increase of blood flow velocity, usually defined as a velocity that is more than twice the rate recorded in the area before or behind the stenosis, possibly accompanied by flow turbulence and reduced velocity behind the area of stenosis. With older equipment detection of stenosis helped to increase sensitivity. Modern equipment usually shows a halo at the level of the stenosis. For extracranial arteries it is important to calculate the degree of stenosis |
| Occlusion | Absence of flow and hypoechoic material in former vessel lumen. A halo is often present proximal to the occlusion |
A halo is defined as an hypoechoic (most commonly concentric) rim of wall swelling around the artery lumen that is visible in two planes and does not disappear upon compression [11, 16].
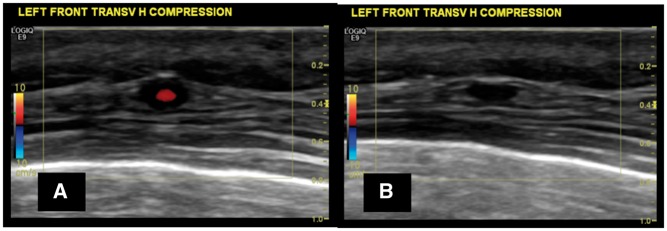
The compression sign should always be performed in the presence of a suspected halo (Fig. 1).
Fig. 1.
US image showing a halo around the temporal artery and a positive compression sign
(A) Halo sign at the level of the frontal branch of the right temporal artery, transverse view, before applying compression, in a patient with active GCA. (B) Evidence of a positive compression sign with the halo persisting despite firm compression applied with the transducer.
If a halo is detected, the sonographer should document the maximum thickness of halo observed in the longitudinal plane in millimetres. The measurement should start as close to the limit of the vessel wall as possible, and include the halo thickness up to the limit of the colour flow signal. Cut-off measurements to identify a thickness that is regarded as consistent with active inflammation have been described. The limit for a pathological halo in the TA has been set at >0.3 mm, with measurements >0.7 mm predicting a positive TAB result [11].
For the AX, a wall thickness (or intima–media complex; IMC) of >1.0 mm may indicate large vessel involvement, and is defined to be diagnostic of vasculitis if >1.5 mm [36].
A recent study evaluating the intima–media thickness in 40 GCA patients compared with controls confirmed that the halo size, besides the morphological parameters, can effectively distinguish normal from inflamed arteries with specific cut-offs according to the vessel assessed. The cut-off was set at 0.42 mm for the common superficial TA, 0.34 mm for the frontal branch, 0.29 mm for the parietal branch, 0.37 mm for the facial artery and 1.0 mm for AX [41].
Stenosis is demonstrated by finding a localized over 2-fold increase in flow velocity, while occlusion is the absence of flow [11, 12]. However, these findings do not add information in the diagnosis of GCA over the detection of halo.
Other abnormalities not exclusive to GCA, but important to be recorded are the presence of concomitant arteriosclerosis visualized as heterogeneous, hyperechoic, irregularly delineated wall alteration.
While a moderate level of tortuosity is physiological, significant tortuosity should be recorded because this can influence the reliability of the assessment. Supplementary Fig. S1, available at Rheumatology Online, shows a proforma US scan record for patients with suspected or confirmed GCA.
Specific findings in Takayasu arteritis
The role of CDS in assessing patients with Takayasu arteritis (TAK) has been investigated to a lesser extent compared with GCA [42]; nevertheless, some differences have emerged. In patients with TAK, the presence of inflammatory oedema appears as an increased, diffuse, circumferential IMC thickness, referred to in transverse sections as the Macaroni sign [43]. Compared with the halo in GCA and large-vessel GCA, the Macaroni sign is usually mid-echoic or hyperechoic (therefore brighter), probably reflecting less acute inflammation [44]. Occlusion, dilatation and dissection are other features of TAK [45, 46]. CDS of the carotid arteries has been shown to effectively detect a thickened IMC in 28 of 44 carotid arteries from patients with TAK, whereas conventional angiography detected stenosis in only 21 arteries [46]. The involvement of the proximal subclavian and the common carotid artery is more frequent in TAK compared with large-vessel GCA, usually characterized by involvement of AXs, proximal brachial and distal subclavian arteries [39]. CDS has been proposed to monitor patients with TAK; however, there are limitations in the accessibility of some arterial segments that might influence its usefulness. While the echogenecity of the thickened vessel wall is not helpful in distinguishing between acute or chronic involvement, it has been reported that the IMC is significantly thicker in active lesions [47]. A recent study by Germanò et al. [48] reported a good correlation of contrast-enhanced US findings with PET-CT evidence of carotid artery involvement in patients with LVV. Another study [49] reported five types of ultrasonographic changes in the carotid walls of TAK patients and a correlation between the morphological alterations and the activity of the disease; less thickened and less stenotic lesions were associated with younger age and inactive disease. During follow-up, the wall thickness and outer diameter of the carotid increased in patients who relapsed and decreased in patients who remained in remission.
Potential pitfalls
There are several common pitfalls that can decrease the accuracy of the CDS examination.
Applying the lowest possible pressure with the transducer to the examined areas is essential to avoid compression of the branches and a false elimination of the colour flow signal.
Significant tortuosity should be assessed critically, as vessel curves might be wrongly interpreted as halo signals if the transducer is not carefully swept side to side to visualize the vessel wall limits.
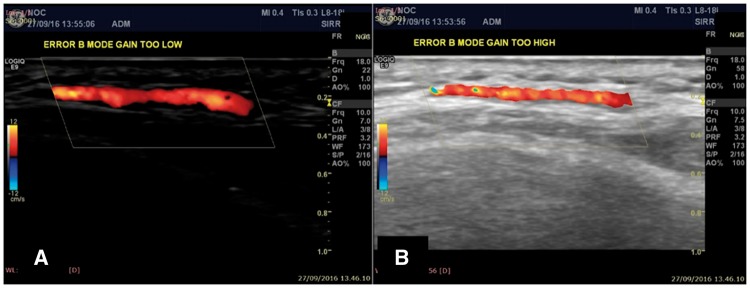
B-mode gain needs to be adjusted to correctly assess halo. A gain that is too low might enhance the false identification of halos in the presence of slightly thickened IMC as in the case of arteriosclerosis. On the other hand, an excessively high gain might underestimate the presence of hypoechoic halo (Fig. 2).
Fig. 2.
US image of potential pitfalls due to incorrect B mode gain adjustments
B mode gain adjustments errors that can affect the visualization of a proper halo sign: (A) B mode gain is too low (20 dB); (B) B mode gain is too high (63 dB).
Colour flow gain needs to be adjusted to just fill the vessel lumen, avoiding a blooming or bleeding effect caused by an excessively high gain with colour bleeding over the vessel wall limit (Fig. 3), or a potentially misleading false halo image if the gain is too low.
Fig. 3.
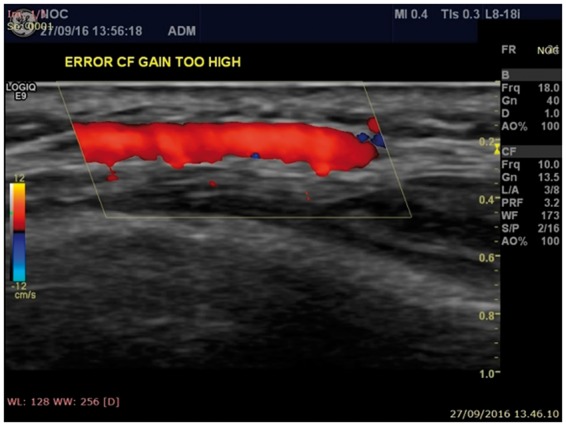
US image of potential pitfalls due to incorrect colour flow adjustments
Colour flow adjustment errors. In this case the colour gain is too high, causing blooming (also known as bleeding) outside the vessel walls limits.
Another common pitfall is the misinterpretation of a false halo due to low peripheral flow velocity leading to an image (particularly in the transverse views), known as pseudo-halo, with a valid flow visible only at the centre of the vessel lumen. In these cases the compression sign is particularly useful in identifying true pathological cases. Coded harmonics imaging may enhance the signal intensity of the vessel’s wall and potentially lead to a wrong assessment of the vessel in some equipment.
Conclusions
The role of CDS in the diagnosis and follow-up of GCA is supported by increasing robust evidence. The minimum CDS assessment of patients with suspected GCA should include the evaluation of the whole length of the TAs and the AXs. This reliably represents the presence of extra-cranial involvement in other vascular areas. The compression sign to confirm the presence of a halo of the TA has shown an excellent inter-observer agreement and should always be performed. The evaluation of stenosis or occlusion has been demonstrated to be neither specific nor sensitive for GCA. A detailed description of the technical parameters to optimize CDS and the most relevant pitfalls to be avoided are presented to support a reproducible and reliable use of CDS in the field of LVV.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: W.A.S. has received consultancies, research support and was a member of a speakers’ bureau for Roche, and has received consultancies and research support from GlaxoSmithKline (GSK). R.L. has received grants from Arthritis Research UK, GSK, Medical Research Council, University Challenge Seed Fund/Oxford Invention Fund, Canadian Institutes of Health Research, The Vasculitis Foundation, and has received consultancy fees from GSK, Medpace, MedImmune and Roche. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
Supplementary Material
References
- 1. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 2. Salvarani C, Pipitone N, Versari A. et al. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol 2012;8:509–21. [DOI] [PubMed] [Google Scholar]
- 3. Nesher G, Breuer GS.. Giant cell arteritis and polymyalgia rheumatica: 2016 update. Rambam Maimonides Med J 2016;7, doi: 10.5041/RMMJ.10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ponte C, Rodrigues AF, O’Neill L. et al. Giant cell arteritis: current treatment and management. World J Clin Cases 2015;3:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies CG, May DJ.. The role of temporal artery biopsies in giant cell arteritis. Ann R Coll Surg Engl 2011;93:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karassa FB, Matsagas MI, Schmidt WA. et al. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 2005;142:359–69. [DOI] [PubMed] [Google Scholar]
- 7. Khan A, Dasgupta B.. Imaging in giant cell arteritis. Curr Rheumatol Rep 2015;17:52. [DOI] [PubMed] [Google Scholar]
- 8. Bharadwaj A, Dasgupta B, Wolfe K. et al. Difficulties in the development of histological scoring of the inflamed temporal arteries in giant cell arteritis. Rheumatology 2005;44:1579–80. [DOI] [PubMed] [Google Scholar]
- 9. Buttgereit F, Dejaco C, Matteson EL. et al. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016;315:2442–58. [DOI] [PubMed] [Google Scholar]
- 10. Salvarani C, Cimino L, Macchioni P. et al. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Rheum 2005;53:293–7. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt WA, Kraft HE, Vorpahl K. et al. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997;337:1336–42. [DOI] [PubMed] [Google Scholar]
- 12. Arida A, Kyprianou M, Kanakis M. et al. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord 2010;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ball EL, Walsh SR, Tang TY. et al. Role of ultrasonography in the diagnosis of temporal arteritis. Br J Surg 2010;97:1765–71. [DOI] [PubMed] [Google Scholar]
- 14. Karahaliou M, Vaiopoulos G, Papaspyrou S. et al. Colour duplex sonography of temporal arteries before decision for biopsy: a prospective study in 55 patients with suspected giant cell arteritis. Arthritis Res Ther 2006;8:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chrysidis S, Duftner C, Dejaco C. et al. Ultrasound definitions for cranial and large vessel giant cell arteritis: Results of a reliability exercise on images and videos of the Omeract Ultrasound Large Vessel Vasculitis Task Force. Arthritis Rheumatol 2016;68 (Suppl 10):3197. [Google Scholar]
- 16. Aschwanden M, Daikeler T, Kesten F. et al. Temporal artery compression sign – a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med 2013;34:47–50. [DOI] [PubMed] [Google Scholar]
- 17. Aschwanden M, Imfeld S, Staub D. et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015;33:S-113–5. [PubMed] [Google Scholar]
- 18. Diamantopoulos AP, Haugeberg G, Lindland A. et al. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology 2016;55:66–70. [DOI] [PubMed] [Google Scholar]
- 19. Patil P, Williams M, Maw WW. et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol 2015;33:S-103–6. [PubMed] [Google Scholar]
- 20. Diamantopoulos AP, Haugeberg G, Hetland H. et al. Diagnostic value of color Doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res 2014;66:113–9. [DOI] [PubMed] [Google Scholar]
- 21. Linxweiler M, Hasenfus A, Wolf G. et al. Perivascular marginal zone lymphoma mimicking temporal arteritis. Otolaryngol Head Neck Surg 2015;152:187–8. [DOI] [PubMed] [Google Scholar]
- 22. Nesher G, Shemesh D, Mates M. et al. The predictive value of the halo sign in color Doppler ultrasonography of the temporal arteries for diagnosing giant cell arteritis. J Rheumatol 2002;29:1224–6. [PubMed] [Google Scholar]
- 23. Pfenninger L, Horst A, Stuckmann G. et al. Comparison of histopathological findings with duplex sonography of the temporal arteries in suspected giant cell arteritis. Klin Monatsblätter Für Augenheilkd 2012;229:369–73. [DOI] [PubMed] [Google Scholar]
- 24. Salvarani C, Silingardi M, Ghirarduzzi A. et al. Is duplex ultrasonography useful for the diagnosis of giant-cell arteritis? Ann Intern Med 2002;137:232–8. [DOI] [PubMed] [Google Scholar]
- 25. Germanò G, Muratore F, Cimino L. et al. Is colour duplex sonography-guided temporal artery biopsy useful in the diagnosis of giant cell arteritis? A randomized study. Rheumatology 2015;54:400–4. [DOI] [PubMed] [Google Scholar]
- 26. Muratore F, Boiardi L, Restuccia G. et al. Comparison between colour duplex sonography findings and different histological patterns of temporal artery. Rheumatology 2013;52:2268–74. [DOI] [PubMed] [Google Scholar]
- 27. Hauenstein C, Reinhard M, Geiger J. et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology 2012;51:1999–2003. [DOI] [PubMed] [Google Scholar]
- 28. De Miguel E, Roxo A, Castillo C. et al. The utility and sensitivity of colour Doppler ultrasound in monitoring changes in giant cell arteritis. Clin Exp Rheumatol 2012;30:S34–8. [PubMed] [Google Scholar]
- 29. Serafim AS, Singh S, Piper J. et al. Early halo sign features on ultrasound examination of treated patients with giant cell arteritis. Rheumatology 2015;54 (Suppl 1):i32. [Google Scholar]
- 30. Luqmani R, Lee E, Singh S. et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess 2016;20:1–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmidt WA, Moll A, Seifert A. et al. Prognosis of large-vessel giant cell arteritis. Rheumatology 2008;47:1406–8. [DOI] [PubMed] [Google Scholar]
- 32. Czihal M, Piller A, Schroettle A. et al. Impact of cranial and axillary/subclavian artery involvement by color duplex sonography on response to treatment in giant cell arteritis. J Vasc Surg 2015;61:1285–91. [DOI] [PubMed] [Google Scholar]
- 33. Agard C, Barrier J-H, Dupas B. et al. Aortic involvement in recent-onset giant cell (temporal) arteritis: a case-control prospective study using helical aortic computed tomodensitometric scan. Arthritis Rheum 2008;59:670–6. [DOI] [PubMed] [Google Scholar]
- 34. Blockmans D, de Ceuninck L, Vanderschueren S. et al. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum 2006;55:131–7. [DOI] [PubMed] [Google Scholar]
- 35. Czihal M, Zanker S, Rademacher A. et al. Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol 2012;41:231–6. [DOI] [PubMed] [Google Scholar]
- 36. Schmidt WA, Seifert A, Gromnica-Ihle E. et al. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology 2008;47:96–101. [DOI] [PubMed] [Google Scholar]
- 37. Czihal M, Brendel T, Seibold C. et al. Listen to the axillary artery: diagnosis of occult giant cell arteritis. J Clin Rheumatol 2011;17:214–5. [DOI] [PubMed] [Google Scholar]
- 38. Aschwanden M, Kesten F, Stern M. et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2x11 arterial regions. Ann Rheum Dis 2010;69:1356–9. [DOI] [PubMed] [Google Scholar]
- 39. Schmidt WA, Natusch A, Möller DE. et al. Involvement of peripheral arteries in giant cell arteritis: a color Doppler sonography study. Clin Exp Rheumatol 2002;20:309–18. [PubMed] [Google Scholar]
- 40. Iagnocco AM, Hachulla E, Bijlsma H (eds). Eular Textbook on Musculoskeletal Ultrasound in Rheumatology. London: BMJ Publishing Group, 2016, 1–27. [Google Scholar]
- 41. Schäfer VS, Juche A, Ramiro S, Krause A, Schmidt WA.. Ultrasound cut-off values for intima-media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatology 2017;56:1479–83. [DOI] [PubMed] [Google Scholar]
- 42. Pipitone N, Versari A, Hunder GG. et al. Role of imaging in the diagnosis of large and medium-sized vessel vasculitis. Rheum Dis Clin North Am 2013;39:593–608. [DOI] [PubMed] [Google Scholar]
- 43. Nicoletti G, Mannarella C, Nigro A. et al. The "Macaroni Sign" of Takayasu’s arteritis. J Rheumatol 2009;36:2042–3. [DOI] [PubMed] [Google Scholar]
- 44. Pipitone N, Versari A, Salvarani C.. Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an update. Rheumatology 2008;47:403–8. [DOI] [PubMed] [Google Scholar]
- 45. Wang J, Lee YZ, Cheng Y. et al. Sonographic characterization of arterial dissections in takayasu arteritis. J Ultrasound Med 2016;35:1177–91. [DOI] [PubMed] [Google Scholar]
- 46. Taniguchi N, Itoh K, Honda M. et al. Comparative ultrasonographic and angiographic study of carotid arterial lesions in Takayasu’s arteritis. Angiology 1997;48:9–20. [DOI] [PubMed] [Google Scholar]
- 47. Park SH, Chung JW, Lee JW. et al. Carotid artery involvement in Takayasu’s arteritis: evaluation of the activity by ultrasonography. J Ultrasound Med 2001;20:371–8. [DOI] [PubMed] [Google Scholar]
- 48. Germanò G, Macchioni P, Possemato N. et al. Contrast-enhanced ultrasound of the carotid artery in patients with large vessel vasculitis: Correlation with positron emission tomography findings. Arthritis Care Res 2017;69:143–9. [DOI] [PubMed] [Google Scholar]
- 49. Fan W, Zhu J, Li J. et al. Ultrasound morphological changes in the carotid wall of Takayasu’s arteritis: monitor of disease progression. Int Angiol 2016;35:586–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.