Abstract
Phenotypic plasticity involves adaptive responses to predictable environmental fluctuations and may promote evolutionary change. We studied the regulation of phenotypic plasticity in an important agricultural pollinator, the solitary alfalfa leafcutting bee (Megachile rotundata F.). Specifically, we investigated how larval nutrition affects M. rotundata diapause plasticity and how diapause plasticity affects adult female reproductive behavior. Field surveys and laboratory manipulations of aspects of larval diet demonstrated nutritional regulation of M. rotundata diapause plasticity. Manipulation of larval diet quality through the addition of royal jelly, the caste-determining substance of the honey bee Apis mellifera L., increased the probability of diapause in M. rotundata. We also found that larval nutrition and diapause status affected M. rotundata adult female reproductive behavior. Nutritional effects on larval diapause that also impact adult fitness have intriguing implications for the evolution of developmental plasticity in bees. In particular, as the solitary lifestyle of M. rotundata is considered to be the ancestral condition in bees, nutritionally regulated plasticity may have been an ancestral condition in all bees that facilitated the evolution of other forms of phenotypic plasticity, such as the castes of social bees.
Keywords: developmental plasticity, diapause, nutrition
Phenotypic plasticity can be an adaptive response to environmental variation (Simpson et al. 2011, Kivelä et al. 2013) and an important facilitator of evolution (West-Eberhard 1989, 2005; Moczek 2005; Moczek et al. 2011). Plasticity allows selection to act independently on alternative phenotypes, potentially allowing for more rapid evolution and greater divergence (West-Eberhard 1989, Tomkins and Moczek 2009, Snell-Rood et al. 2010). Plastic phenotypes present in ancestral populations may affect the evolutionary trajectory of novel traits by influencing which phenotypes are exposed to selection and constraining the mechanisms through which evolutionary change can occur (Shaw et al. 2007, Ledon-Rettig et al. 2008, Moczek et al. 2011, Rajakumar et al. 2012). Identifying the mechanisms of plasticity is important for understanding how species adapt to environmental change and how plasticity may influence evolutionary dynamics.
We studied plasticity in larval diapause strategy in the alfalfa leafcutting bee, Megachile rotundata F. (Hymenoptera: Megachilidae). Megachile rotundata is a gregarious, cavity-nesting, bee that is commercially managed for the alfalfa (Medicago sativa L., Fabaceae) seed industry in the northwestern United States and central Canada (Pitts-Singer and Cane 2011). Megachile rotundata is the most important pollinator of alfalfa, accounting for ∼57 million pounds of seed production in 2012 in the United States (National Agricultural Statistics Service [NASS] 2014).
Megachile rotundata is mass-reared by the tens of thousands under controlled conditions, so adult emergence can be synchronized to alfalfa bloom. Adults readily nest in large aggregations in artificial nesting domiciles placed in commercial alfalfa fields. Females nest in preexisting cavities and build brood cells in a linear series. They forage for alfalfa leaves, which they cut and carry to their nest to construct a cell cup; they then forage for pollen and nectar to form a provision mass inside the cup. A single egg is laid on the provision, and the cell is sealed with leaf discs before construction begins on the next cell. The provision mass is the only source of nutrition provided in the cell, and a larva almost always consumes the entire provision (Trostle and Torchio 1994). After feeding is complete, the larva constructs a cocoon and is referred to as a prepupa. The prepupa then pupates and ecloses into an adult inside the cocoon. The eclosed adult remains inside the cocoon for a few days before chewing a hole through the cocoon and leaf cell to emerge from the nest. For scientific or commercial purposes, nests containing prepupae are harvested at the end of the season, and progeny can be stored over the winter and managed for pollination services the following season.
Megachile rotundata exhibits phenotypic plasticity via a partially bivoltine life cycle. The majority of prepupae diapause over the winter, and adults emerge to reproduce the following year (“diapausers”), while some prepupae undergo direct development to adulthood without diapause and reproduce in the year of their birth (“nondiapausers”).
Nondiapausers present a problem for the U.S. M. rotundata commercial industry. Nondiapausing females emerge and begin to nest when late summer floral resources are sparse (Pitts-Singer 2013), and these females probably suffer low reproductive success or disperse from the alfalfa fields. Also, in order to exit the nest, nondiapausing individuals sometimes must chew through and kill diapausing nestmates closer to the nest entrance (Tepedino and Frohlich 1984, Pitts-Singer and Cane 2011). Furthermore, this chewing behavior may increase the spread of the fungus Ascosphaera aggregata Skou (Ascosphaeraceae), which causes chalkbrood disease and may contribute up to ∼20% of progeny mortality in U.S. populations (Stephen 1981, Pitts-Singer and Cane 2011). The presence of nondiapausers adds to the poor reproduction of M. rotundata pollinator populations in much of the U.S. alfalfa seed-growing regions (James and Pitts-Singer 2013), and many U.S. alfalfa seed growers must purchase bees annually from Canada.
Diapause in M. rotundata is likely regulated by multiple factors mediated through maternal and larval responses to seasonal cues such as temperature, humidity, and photoperiod, as well as genotypic effects (Pitts-Singer and Cane 2011). In addition, correlational evidence suggests that larval nutrition plays a role in diapause regulation. Larval provision size is correlated with adult weight (Klostermeyer et al. 1973), and upon adult emergence, individuals that underwent diapause weigh more than those that did not (Tepedino and Parker 1988, Pitts-Singer and Bosch 2010). However, a causal relationship between larval nutrition and diapause has not yet been shown for M. rotundata, or any other bee species.
Nutritional regulation of diapause has been found in other insect species (Munyiri et al. 2004, Hahn and Denlinger 2011), as entering diapause is typically more energetically costly than direct development to adulthood without diapause (Hahn and Denlinger 2007). Additionally, larval nutrition is known to regulate many other forms of plasticity in insects (Koyama et al. 2013), including caste determination in many social hymenopterans (Wilson 1971), horn expression in dung beetles (Emlen 1997, Snell-Rood and Moczek 2012), reproductive performance in ladybeetles (Xie et al. 2015), and wing formation in some aphid species (Müller et al. 2001). In some cases, the effects of preadult nutrition carry over life stages to influence adult fitness, as has been observed in invertebrates (Hahn and Denlinger 2007, Barrett et al. 2009, Tigreros 2013, May et al. 2015) and vertebrates (Lindström 1999, Blount et al. 2003, Aihie Sayer and Cooper 2007). Due to the significant and long-lasting effects of early nutrition in many organisms, we were interested in investigating how larval nutrition might affect diapause plasticity and adult fitness in M. rotundata.
We used surveys of larval provisions in natural nests and manipulations of larval nutrition in the laboratory to investigate how aspects of larval nutrition affect M. rotundata body weight, diapause probability, and adult female reproductive behavior. In addition, because the lifestyle exhibited by M. rotundata is considered to be the ancestral condition in bees, the group from which eusociality has evolved more frequently than any other group (Wilson 1971, Schwarz et al. 2007), we speculate on the possible implications of our results for understanding the evolution of nutritionally regulated caste determination in eusocial bees.
Materials and Methods
Effects of Larval Nutrition on Diapause
Relationship Between Larval Provision Size, Adult Body Weight, and Diapause Probability in Field-Collected Nests
Using field surveys of natural nests, we asked whether larvae provisioned with larger masses of food were more likely to enter diapause. Nests were collected from commercial nesting shelters with thousands of actively nesting females in alfalfa seed production monocultures in the summers of 2010 (Year 1) and 2011 (Year 2). Alfalfa seed growers established these commercial nesting shelters on their farms according to standard practices in the summers of Year 1 and Year 2 (Richards 1984, Pitts-Singer 2008). In Year 1, the alfalfa field was located in North Logan, UT, and in Year 2, the field was in Tremonton, UT. In these shelters, females are provided with artificial nesting cavities in polystyrene “bee boards.” At the USDA ARS Pollinating Insects Research Unit (PIRU), commercial bee boards were cut into smaller “collection boards” (Beaver Plastics, Ltd., Alberta, Canada; collection board size: 30–40 cm long; 11–15 cm high; 9 cm deep), and nesting cavities were lined with custom-made paper straws so that nests could be extracted intact by removing the straw lining. Females made nests in the small boards placed in active bee-nesting shelters in the alfalfa fields. Nest-filled boards were removed and replaced with empty boards 1–2 times weekly. Filled boards were taken to the laboratory where straws containing newly made nests were removed and labeled for monitoring of individual cells.
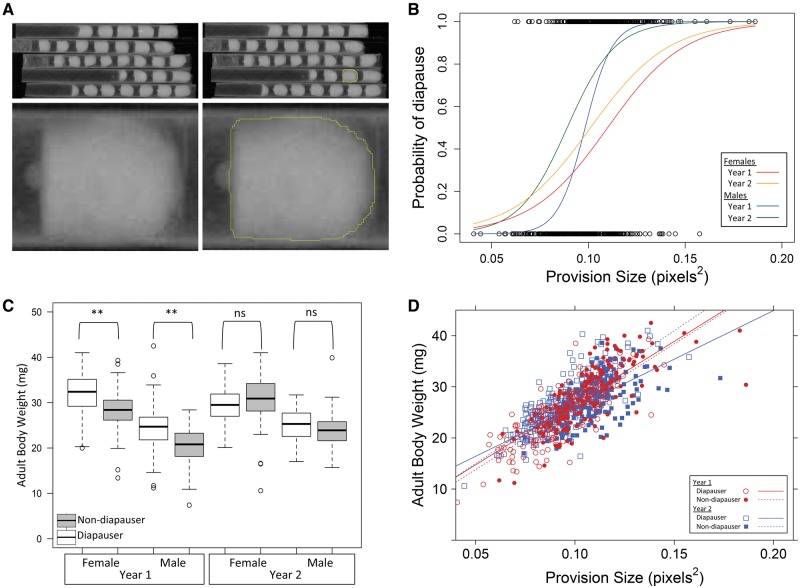
X-radiographs (X-rays) of collected nests were taken to allow for nondisruptive digital measurement of provisions inside nest cells, as seen in Fig. 1A. In Year 1, each X-ray was taken with a Faxitron 43855A (product no longer sold), printed on film and then scanned to create a digital file of the image. In Year 2, each X-ray was taken with a Faxitron MX-20 with a computed radiography high resolution system (Faxitron X-Ray LLC, Linconshire, IL) that directly creates a digital image file. The freehand selection tool in the imaging processing software ImageJ (Rasband 1997-2016) was used to manually trace the outline of the provision (Fig. 1A), and the area (in square pixels) enclosed by the outline was calculated using the “Measure” function. Each provision was traced three times, and the mean of these measurements was used as the estimate of provision size for each cell.
Fig. 1.
Megachile rotundata provision size and adult body weight of diapausers and nondiapausers from field-collected nests. (A) Example of an x-radiograph of collected nests. Individual provisions were traced, and the area enclosed in the outline was calculated. (B) The relationship between provision size and diapause probability. Each line represents the logistic regression for one year and sex combination. (C) Adult body weight of male and female diapausers and nondiapausers from nests collected in Year 1 and Year 2. Boxes extend to upper and lower quartiles, line within box represents median, whiskers extend to 1.5 × interquartile distance (white boxes: Diapausers, gray boxes: Nondiapausers, **: Tukey HSD P < 0.001, ns: not significant). (D) The relationship between provision size and adult body weight. Each line represents the linear regression for one year and diapause status combination.
Nests were maintained at ambient outdoor conditions at PIRU to allow for natural pupation and adult emergence of nondiapausing bees. According to standard practices (Richards, 1984), individuals that had not pupated by the end of September were presumed to be diapausers, stored for the winter at 4 °C starting in early October, and then incubated at 29 °C for pupation and adult emergence the following summer. Both diapausing and nondiapausing adults were weighed within 8 h of emergence.
Provision size, adult body weight, and diapause status were measured for 754 bees from field-collected nests (Year 1: female diapausers = 83, female nondiapausers = 83, male diapausers = 76, male nondiapausers = 79; Year 2: female diapausers = 113, female nondiapausers = 71, male diapausers = 87, male nondiapausers = 162). The effects of year, sex, provision size, and their interactions on diapause probability were evaluated using a logistic regression model (glm in R). The effects of year, sex, diapause status (diapauser or nondiapauser), and their interactions on adult body weight were evaluated using ANOVA (aov in R). Diapause status was considered an independent variable affecting adult weight because, compared to nondiapausers, diapausers have a prolonged prepupal period during which metabolic energy must be reserved, presumably as stored fat (Pitts-Singer and James 2009). Post hoc, pairwise comparisons were performed with Tukey’s HSD test. The correlation between provision size and adult body weight was evaluated using the Pearson correlation coefficient (cor.test in R), and ANCOVA (in R) was used to compare the correlation among sex, year, and diapause status.
Effect of Larval Diet Manipulations on Diapause Probability
To isolate the effects of larval nutrition on diapause regulation, in Year 2 we directly manipulated two aspects of larval diet in a controlled laboratory setting and monitored development to adulthood (Fig. 2A). We predicted that larvae reared on diets with higher quantity or quality of nutrition would be more likely to enter diapause. Using six treatments (see details below), we manipulated 1) the nutritional quantity of diets by changing the amount of food (by weight) provided to developing larvae and 2) the nutritional quality of the larval diet by including honey bee royal jelly (RJ) in some diets. Royal jelly is a nutrient-rich substance found only in honey bees that regulates caste determination in multiple ways, including through active compounds that cause epigenetic reprogramming (Wilson 1971, Michener 1974, Spannhoff et al. 2011). It also causes increased weight in the fruit fly Drosophila melanogaster Meigen (Diptera: Drosophilidae) (Kamakura 2011, Shorter et al. 2015), which led us to speculate that although royal jelly is a novel substance never naturally encountered by M. rotundata, it may also influence M. rotundata diapause plasticity.
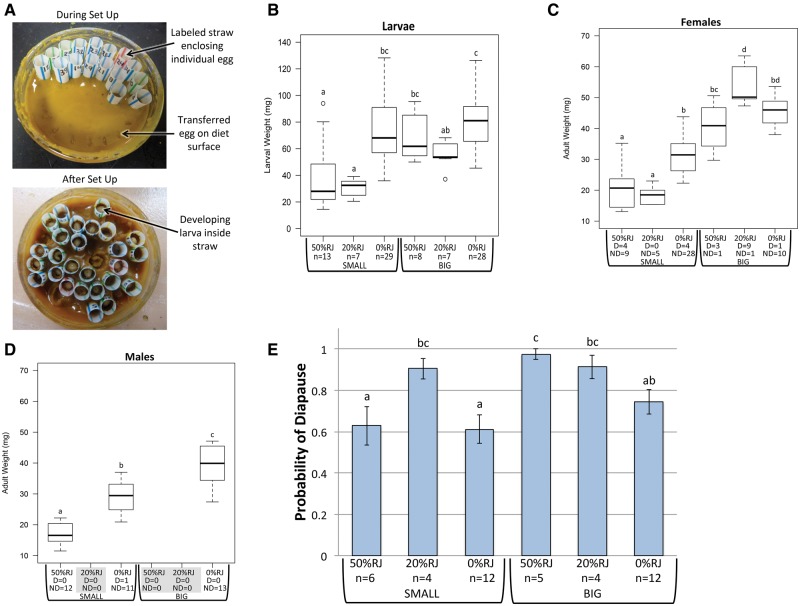
Fig. 2.
Effects of M. rotundata larval diet treatments on larval weight, adult weight, and diapause incidence. (A) Example of dish set up used for rearing of larvae on manipulated diets. (B) Weight of fifth-instar larvae reared on different diets. n: Number of larvae. (C, D) Weights of adult females (C) and adult males (D) reared on different diets. D/ND: Number of diapausers/nondiapausers weighed. Boxes extend to upper and lower quartiles, line within box represents median, whiskers extend to 1.5 × interquartile distance. (E) Probability of diapause of larvae reared on different diets. Bars represent mean ± standard error of mean. Groups with different letters have significantly different means (Tukey HSD P < 0.05). n: Sample size (number of rearing dishes).
Because M. rotundata larvae have been shown previously to have poor survival on artificial diets (Nelson et al. 1972, Goettel et al. 1993, personal observation), natural provisions were collected from nests in the field and used to rear groups of larvae in the laboratory. Nests were collected in boards as described before from commercial nesting shelters in alfalfa monocultures from July-August in Year 2. Thousands of cells were dissected out of nests, the egg was removed, and the provision was squeezed out of the cell into a collection dish. There were three provision collection periods throughout the summer, and all provisions from each collection period were mixed before use, resulting in three separate batches of provisions used over the course of the experiment. Because pathogens, especially the chalkbrood-causing fungus A. aggregata are common in M. rotundata, provisions were sterilized before use by γ-irradiation at a dose of 28 kGy for 12 h (after Xu and James 2009; courtesy of G. Hallman, USDA-ARS Subtropical Agricultural Research Center, Weslaco, TX). Provisions were aliquoted into round, flat-bottom “rearing dishes” (plastic dishes 2.54 cm high and 3.81 cm in diameter with screw-on lids; Widget Supply, Albany, OR, XSX4-8744SBB). After adding provisions to the dish, we pipetted 0.5 ml of molecular biology grade water onto the surface of the provision mass. This prevented desiccation of the diet and simulated the shallow nectar layer often found on the provision surface in natural nests (Trostle and Torchio 1994). Unhatched eggs were obtained by dissecting nest cells, and eggs were transferred from the cell to the provision surface in the rearing dish using a honey bee larval “grafting” tool (Fig. 2A). To increase the proportion of females used in the experiment, eggs were only taken from the back three cells of the nest, which typically contain female brood (Pitts-Singer and Cane 2011, Yocum et al. 2014). To avoid genotypic bias, only one egg was taken from each nest. Eggs were collected in daily batches grouped by the day the nests were harvested from the field and randomly assigned to a diet treatment. Each rearing dish held 28–33 eggs. Within the rearing dish, each egg was enclosed by an individually labeled, short segment of plastic drinking straw (∼2 cm tall by 0.6 cm diameter) pushed to the bottom of the dish to encapsulate each egg with a known and uniform amount of provision (Fig. 2A).
Rearing dishes were covered, and individuals were kept in a dark incubator at 26 °C for the duration of larval development. Larvae were monitored daily through observation under a microscope to record the date of larval hatching, maturation to the final instar as indicated by larval defecation, any incidence of mortality (collapsed or unhatched egg, or dead larvae) and any evidence of chalkbrood mortality due to fungal infection (based on gray, chalky appearance). Molecular biology grade water was pipetted into straws as needed to prevent desiccation of provisions and larvae.
A small subset of larvae (7–29 individuals per diet) from each of the six diet treatments were removed from their cells and weighed during the early fifth instar before cocoon spinning had begun. Once a larva completed spinning its cocoon, the straw segment containing the now prepupa was transferred to a No. 0 gelatin capsule (Eli Lilly and Co., Indianapolis, IN). The capsules were X-rayed every other day to determine the date of pupation for nondiapausers. Cells were monitored daily for adult emergence of nondiapausers through the end of September. Remaining nonemerged prepupae were presumed to be diapausers, and were cooled to 4 °C for winter storage as per industry management standards (Richards 1984). Due to logistical circumstances, cells with unemerged diapausing individuals were maintained in winter storage for an extended time period and were incubated at 29 °C to induce adult eclosion in February 2013. Adults were weighed within 24 h of emergence from the cell.
The six larval diet treatments were administered to different sets of rearing dishes (Table 1) in a 2 × 3 factorial design to investigate effects of diet quantity (by weight) and diet quality (by % RJ) on diapause probability. There were two quantities: “Big” and “Small.” Big diet dishes contained 11.5 g of food with ∼0.13 g of food in each straw, and Small diet dishes contained 3.5 g of food with ∼0.03 g of food in each straw. Diet quantities were chosen so that the weight of food contained inside a straw segment in a Big (0.13 g) and Small (0.03 g) rearing dish was equivalent to upper and lower provision weights measured from cells collected from natural nests (data not shown). There were three quality categories: “0% RJ,” “20% RJ,” and “50% RJ.” In these diets, 0%, 20%, or 50% of the total diet weight was honey bee RJ, and the rest consisted of natural provisions. Fresh, frozen RJ obtained from a commercial supplier (GloryBee Foods, Eugene, OR) was thawed just before use and thoroughly mixed with the natural provisions directly in the rearing dish.
Table 1.
Description of M. rotundata larval diet treatments for evaluating the effects of larval nutrition on probability of diapause
| Diet treatment | Natural provisions weight (g) | Royal jelly weight (g) | Total diet weight (g) | Total dishes | Total adults (total eggs transferred) |
|---|---|---|---|---|---|
| 50% RJ Small | 1.75 | 1.75 | 3.50 | 6 | 63 (141) |
| 20% RJ Small | 2.80 | 0.70 | 3.50 | 4 | 51 (90) |
| 0% RJ Small | 3.50 | 0.00 | 3.50 | 12 | 117 (299) |
| 50% RJ Big | 5.75 | 5.75 | 11.50 | 5 | 40 (129) |
| 20% RJ Big | 9.20 | 2.30 | 11.50 | 4 | 37 (107) |
| 0% RJ Big | 11.50 | 0.00 | 11.50 | 12 | 111 (306) |
Total adults refers to the total number of individuals that survived to adulthood, out of the total number of eggs transferred from nests to rearing dishes (in parentheses), many of which did not thrive. RJ: royal jelly.
A generalized linear mixed model was used to evaluate the effects of diet quantity, diet quality, and the interaction between diet quantity and quality on diapause incidence, overall mortality and mortality due to chalkbrood. Rearing dish was included as a random effect, and a binomial distribution with a logit link function was assigned for the response variables (PROC GLIMMIX in SAS). ANOVA (in R) was used to evaluate the effects of diet quantity, diet quality, the interaction between diet quantity and quality, sex (for adult weight only), and diapause status (for adult weight only) on larval and adult weight. Post hoc, pairwise comparisons were performed with Tukey’s HSD test.
Effects of Larval Nutrition on Adult Reproductive Behavior
We used weight upon adult emergence from the cell as a measure of the quantity and quality of larval nutrition (Klostermeyer et al. 1973, Bosch and Vicens 2002) and compared nesting success of females across a range of body weights in both competitive and noncompetitive nesting conditions. We predicted that heavier M. rotundata females would have greater reproductive success. While controlling for weight differences, we also compared nesting success of M. rotundata diapausers and nondiapausers to determine if they represent discrete adult variants with different reproductive strategies and capabilities.
We developed competitive and noncompetitive nesting assays to evaluate multiple measurements of nesting success, using diapausers and nondiapausers with synchronized eclosion. Diapausing prepupae in their natural cells were obtained from commercial bee suppliers (JWM Leafcutters, Nampa, ID) in the winter and maintained in diapause at 4 °C. Nondiapausing cells were obtained by collecting nests from the aforementioned commercial nesting shelters and maintaining nests at ambient outdoor conditions at the PIRU. These nests were periodically X-rayed to identify cells with pupae, indicating that these individuals were destined to emerge as nondiapausers. These cells were dissected out of the nests, put into individual No. 0 gelatin capsules (Eli Lilly and Co., Indianapolis, IN) and monitored for adult emergence. To coordinate adult emergence of diapausers and nondiapausers, we slightly delayed diapauser adult emergence by keeping cells containing diapausing prepupae in winter storage until early July (instead of early June). Starting 3 wk before expected nondiapauser emergence (in early August), batches of diapausing cells were placed in an incubator every few days to assure that diapausers would emerge at the same time as nondiapausers. Incubation at 29 °C breaks diapause and initiates the ∼3-wk period needed for development to the adult stage. This delay allowed us to synchronize diapauser with nondiapauser emergence within 24 h of each other.
On the first day of an assay, an equal number of synchronized female diapausers and nondiapausers were weighed, chilled on ice, and uniquely paint-marked. Females were released together with ∼10 males in a screened enclosure (length by width by height = 6 by 6 by 2 m) over a plot of blooming alfalfa in North Logan, UT. Several enclosures were used for different cohorts of age-matched bees, and each enclosure was equipped with a portion of commercial polystyrene nesting board housed in a small wooden domicile suspended ∼1 m above the ground on a metal post. Nesting holes were lined with removable paper straws to facilitate observations and later remove nests. Nesting activity was monitored for 8 d to determine the day of nest initiation, daily nest ownership, and daily nest progression. To monitor nest progression, the paper straw was removed so that the location of the end of the most recently created cell could be marked and dated, and the nest was replaced. All nesting assays ran for 8 d, at which time nests were collected and x-rayed to show the number of cells built.
In the noncompetitive assay, there were at least two nesting holes provided for each female released in the enclosure. This assay was run as six replicates by releasing one cohort of 20–25 age-matched females into separate field enclosures on three successive days in August in both Year 1 and Year 2. In the competitive assay, there was one nesting hole per two released females. The competitive assay was run as four replicates by releasing two cohorts of 18–24 age-matched bees into separate field enclosures on two successive days in August of Year 2.
For the noncompetitive assays, human observers, blind to the identity of the bees, sat in the field cage next to the nesting domicile and recorded nesting activity to determine nest ownership. A female was considered to own a nest if she was seen carrying leaf material and pollen into a nesting hole at least three times during the observation period. Megachile rotundata have high nest fidelity, and in the field enclosures it is very rare that a female deposits nesting material into a nest that is not her own (personal observation). Observations continued until nest ownership was determined for each active nest each day.
For the competitive assays, human observers could not be used because females without nests would actively investigate any novel object brought into the enclosure as a potential nesting site, including any exposed human orifice or clothing items with the appearance of a hole (e.g., grommet in belt or hat). Instead, video recordings were used. Tripods were set up in the enclosures and covered in black felt before bees were released and left in the cage for the duration of the experiment so the bees would acclimate to their presence. Each day a video camera was brought into the cage and installed on the tripod, the camera was covered with black felt and left to record nesting activity for 1 h. Nest ownership was assigned if a female was seen bringing leaf material or pollen into a nesting hole at least once during the observation period (though most bees were seen many more times) and no other bee was observed bringing nesting material into that hole.
A generalized linear mixed model was used to evaluate the effects of diapause status (diapauser or nondiapauser) and weight on the response variables. Replicate was included as a random effect, and we specified a Poisson distribution with a log link function for the response variables. For the noncompetitive assay, the response variables were total cells built during the experiment and the number of days until a bee initiated nesting. To investigate the relationship between weight and total cells built in Year 2, a pseudo-R2 was calculated using a general linear model with a Poisson distribution, ignoring the random effect of replicate. For the competitive assay, the response variable was the number of days a female controlled a nesting hole. In order to pool the data from the four replicates in the competitive assays, weights were standardized using z-scores to account for variation in the distributions of weights among replicates.
Results
Effects of Larval Nutrition on Diapause
Effects of Provision Size on Diapause Probability
In field-collected nests, the probability of entering diapause was significantly affected by provision size (z = 3.69, P < 0.001), sex (z = −3.86, P < 0.001), and their interaction (z = 4.02, P < 0.001). Within each sex, relatively larger provisions increased diapause probability, but females required larger provision sizes than males to achieve the same diapause probability (Fig. 1B).
Adult body weight was significantly affected by sex (F1,746 = 460, P < 0.001), diapause status (F1,746 = 28.5, P < 0.001), and their interactions with year (Year × Sex: F1,746 = 12.0, P < 0.001; Year × Diapause Status: F1,746 = 34.3, P < 0.001). Pairwise comparisons revealed that diapausers weighed significantly more than nondiapausers in Year 1, but not Year 2 (Fig. 1C, Tukey HSD P < 0.001). Provision size was highly correlated with adult body weight (R2 = 0.56, Fig. 1D). There was a significant effect of the interaction between diapause status and year on the relationship between provision size and adult body weight (F3,749=3.53, P < 0.05). This effect was driven by diapausers in Year 2, which did not gain as much weight per unit provision as other groups. Perhaps differences in environmental factors (such as fall temperature and the duration under those fall conditions before storage at constant wintering temperature) between Years 1 and 2 caused more prewinter and winter weight loss in diapausers in Year 2 than in Year 1 (Pitts-Singer and James 2009); however, no environmental data were recorded for these bees in the fall. The relationship between provision size and body weight in Year 2 diapausers had a higher intercept (95% confidence interval: Year 2 diapausers = 0.0485–0.0682; Others = 0.0260–0.0350) and a lower slope (95% confidence interval: Year 2 diapausers = 0.00149–0.00219; Others = 0.00236–0.00269) than all other groups. Provision size and body weight were also less correlated in Year 2 diapausers compared to other groups (R2: Year 2 diapausers = 0.35; Others = 0.62).
Effects of Larval Diet Manipulations on Larval and Adult Weight
Larval weight was significantly affected by diet quantity (F1,87 = 14.02, P < 0.01) and quality (F2,87 = 20.09, P < 0.01; Fig. 2B). Pairwise comparisons revealed that larvae reared on Small diets containing 20% or 50% RJ weighed significantly less (Tukey HSD P < 0.01) than larvae reared on the Small diet without RJ (0% RJ) and larvae reared on Big diets (Tukey HSD P < 0.05; Fig. 2B). The effect of RJ within the Big diets was only seen in larvae reared on the 20% RJ diet, which weighed significantly less (Tukey HSD P < 0.05) than larvae reared on the 0% RJ Big diet (Fig. 2B). The reason why the effects of RJ on larval weight appear stronger in the Small than Big diets may be that at the time larvae were weighed, those on the Small diet had consumed all the food provided to them, while those given the Big diet were still eating.
Adult weight also was significantly affected by diet quantity (F1,95 = 292, P < 0.01), diet quality (F2,95 = 27.60, P < 0.01), sex (F1,95 = 18.06, P < 0.01), and diapause status (F1,95 = 7.61, P < 0.01). Pairwise comparisons of adult body weight among individuals reared on different diets were conducted within each sex using Tukey’s HSD test. Due to low sample sizes in some groups (Fig. 2B–D), all relevant pairwise comparisons within sex and diapause status among diets could not be analyzed. However, the general pattern observed in comparisons that could be analyzed show that individuals reared on Small diets weighed significantly less as adults than individuals reared on Big diets, and individuals reared on diets containing royal jelly weighed less as adults than individuals reared on diets without royal jelly or with lower % RJ (Fig. 2C and D). The weights of diapausers and nondiapausers within each sex and diet were combined for further pairwise comparisons because sample size was low for most diapauser groups. These comparisons revealed that within each sex and diet, the weight of diapausers and nondiapausers did not differ (Tukey HSD P > 0.01).
Mortality during development to adulthood was 60% (1 s.d. = 8%) on average among the diets. This is comparable to observed mortality (40–60%) in natural nests in the United States (Huntzinger et al. 2008, Pitts-Singer and James 2008, Pitts-Singer and Cane 2011, James and Pitts-Singer 2013). The natural level of mortality has been used to determine tolerated mortality level in experiments involving artificial rearing of Apis mellifera L. (Hymenoptera: Apidae) larvae (Crailsheim et al. 2012). On average, 29% (1 s.d. = 4%) of total mortality occurred prior to egg hatching and was likely due to egg damage during transfer from cell to rearing dish (Whitfield et al. 1987). The remaining posthatch mortality was likely due to multiple factors including desiccation, mold, and chalkbrood, which are also main causes of mortality in the field (Pitts-Singer and James 2008, James and Pitts-Singer 2013).
Diet quantity had a significant effect on mortality (F1,37 =7.15, P < 0.05), with Small diets having a lower mortality rate (54%) than Big diets (67%). The 20% RJ Small diet had a significantly lower mortality rate (43%) than all other diets except the 50% RJ Small diet (Tukey HSD P < 0.05). The average incidence of chalkbrood was 22% and did not significantly differ among diets (Tukey HSD P > 0.1).
Effects of Larval Diet Manipulations on Diapause Probability
There were significant effects of diet quantity (F1,37 = 6.38, P < 0.02) and quality (F2,37 = 6.15, P < 0.01) on diapause probability, and the interaction between diet quantity and quality was marginally significant (F2,37 = 2.51, P = 0.095). In general, individuals reared on Big diets had a higher likelihood of entering diapause than those reared on Small diets. In addition, individuals reared on diets containing RJ had a higher likelihood of entering diapause than those reared on diets that did not contain RJ (Fig. 2E). This result was striking because the above results indicated that RJ decreased the nutritional quality of the diet for M. rotundata.
Effects of Larval Nutrition on Adult Reproductive Behavior
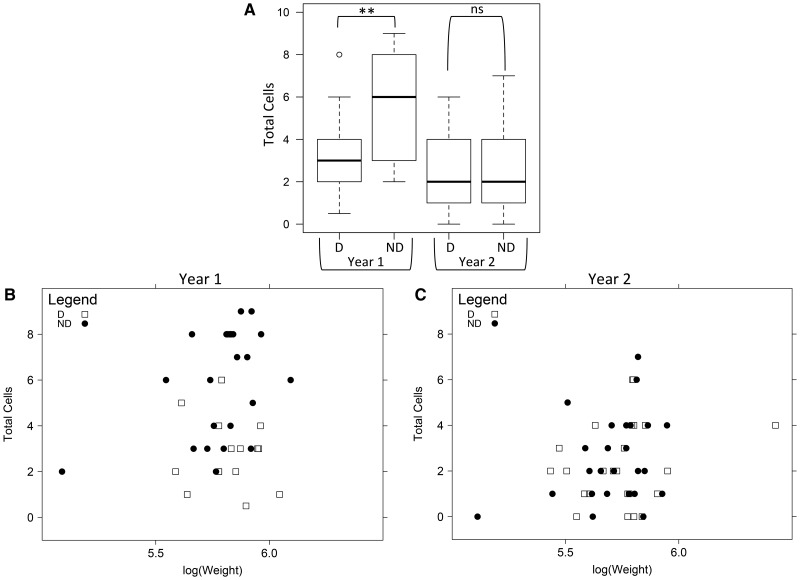
In the noncompetitive nesting assay, larval nutrition affected female nesting success, but results differed across the two years. In Year 1, nondiapausers built significantly more cells than diapausers (P < 0.001, N = 17 diapausers and 21 nondiapausers; Fig. 3A). There was no effect of female weight on total cells built (P > 0.05, Fig. 3B). In Year 2, diapause status did not affect total cells built (N = 24 diapausers and 25 nondiapausers), but there was a significant effect of weight (P < 0.05), though it explained very little of the variation in total cells built (Fig. 3C; pseudo-R2 for the relationship between weight and total cells built was 0.064 in Year 2). There was no effect of diapause status or weight on the latency to begin nesting in either year (P > 0.05). Total cells built per female reflected those documented in previous field cage studies in the same alfalfa field (Pitts-Singer and Bosch 2010, Pitts-Singer and Barbour 2016), where the number of cells made by diapausers ranged from 2–8 cells per females; however, in one year the mean was 5–8 cells per female, and in the next year was 2–4 cells per female (Pitts-Singer and Bosch 2010). Just as in the other studies, differences in weather conditions and the condition of the alfalfa bloom between years may explain why nesting was generally reduced in Year 2 relative to Year 1, with bees taking longer to initiate nests (5.0 vs. 2.7 d) and building fewer total cells per bee (2.4 vs. 4.6 cells) on average in Year 2 relative to Year 1. These results suggest that nondiapausers had a nesting advantage in the environmental conditions of Year 1, but lost that advantage due to the conditions of Year 2.
Fig. 3.
Total cells built by M. rotundata females in noncompetitive nesting assays. In both Year 1 and Year 2, three cohorts of 20–25 age-matched females were released into separate field enclosures containing at least two nesting holes for each female. (A) Total cells built by diapausers [D] and nondiapausers [ND] in Year 1 and Year 2. Diapause status had a significant effect on total cells built in Year 1, but not in Year 2 (**: P < 0.001, ns: not significant). (B) The relationship between female adult weight and total cells built by diapausers [D] and nondiapausers [ND] in Year 1. Weight did not significantly affect total cells built (P > 0.05). (C) The relationship between female weight and total cells built by diapausers [D] and nondiapausers [ND] in Year 2. There was a statistically significant effect of weight on total cells built (P < 0.05); however, the pseudo-R2 for the correlation was 0.064, indicating that weight explains very little of the variation in total cells built.
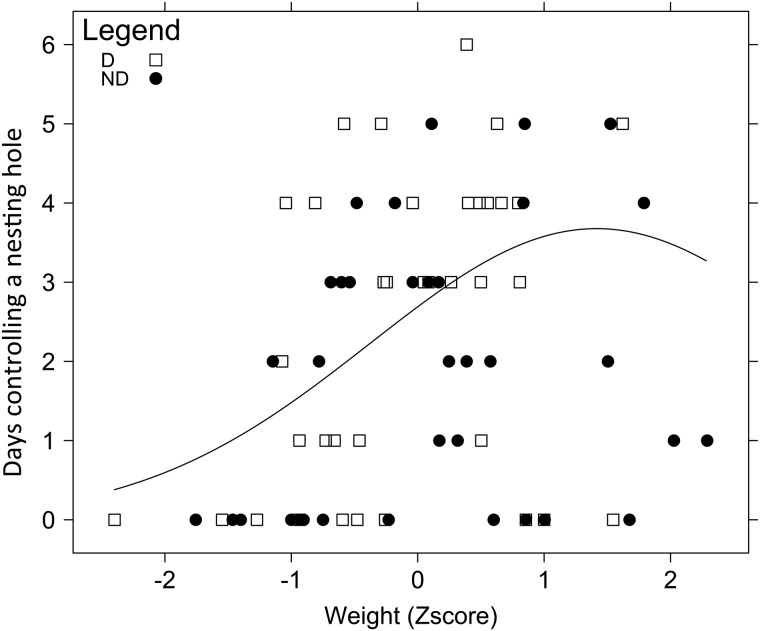
In the competitive nesting assay, there was a high turnover in nest ownership, and females had to vigilantly defend their nests and expel intruders in order to maintain nest ownership throughout the experiment. Weight significantly affected the number of days a female controlled a nest (P < 0.001, N = 36 diapausers and 35 nondiapausers; Fig. 4). Because the relationship between weight and the number of days a female controlled a nest was nonlinear, we added a quadratic effect of weight to the model. The quadratic effect was significant (P < 0.001, Fig. 4), and a model with a quadratic effect was more supported than a model with a linear effect (ΔAIC = 14.38 for the linear model). In general, heavier bees tended to outcompete lighter bees in controlling nests, but the very heaviest bees did not compete as well as bees of intermediate weight.
Fig. 4.
The relationship between the number of days an M. rotundata female controlled a nesting hole and her weight in competitive nesting assays. In Year 2, four cohorts of 18–24 age-matched females were released into separate field enclosures containing one nesting hole per two females. Data were pooled from the four replicates, and weights were standardized using z-scores to account for variation in the distributions of weights among replicates. There was a quadratic effect of weight on the number of days a female controlled a nesting hole (P < 0.001). D: diapausers, ND: nondiapausers.
Discussion
Our results show that larval nutrition influences diapause and adult reproductive success in M. rotundata. There are likely multiple factors involved in regulating M. rotundata diapause plasticity (Hobbs and Richards 1976, Rank and Rank 1989, Pitts-Singer and Cane 2011), and our findings now indicate that larval nutrition is one of them. Our results support previous findings that preadult nutrition affects the manifestation of phenotypic plasticity and adult fitness and provide an example of how these effects can be mediated through maternal manipulation (e.g., Strohm and Linsenmair 2000, Kim and Thorp 2001, Bosch 2008).
The results of the royal jelly diet manipulations on M. rotundata diapause plasticity were more complex. It appears that this substance may be able to act directly on molecular mechanisms that regulate diapause, rather than simply altering the nutritional state of an individual. Royal jelly increased the likelihood of diapause despite the fact that it generally resulted in a decrease in larval and adult weights, suggestive of decreases in food quality. We speculate that in M. rotundata, royal jelly was able to override input from natural cues of nutritional status and induce diapause in larvae with lower than normal energy reserves perhaps by acting on pathways involved in developmental plasticity shared between M. rotundata and A. mellifera. Royal jelly causes complex, dose-dependent effects on various physiological and anatomical traits in the even more distantly related D. melanogaster (Shorter et al. 2015). Future detailed analysis is needed to understand how royal jelly is able to cause diverse effects in species that never encounter this substance naturally.
Larval nutrition also influences adult female reproduction, especially under conditions when individuals need to compete for nesting sites. Female body size is often positively associated with fecundity in insects (Honěk 1993). However, in solitary bees, a positive correlation between body size and nesting success has been found in some studies (Sugiura and Maeta 1989, Kim 1997, Rehan and Richards 2010), but not others (Tepedino and Torchio 1982, Johnson 1990, Alcock et al. 2006, Bosch and Vicens 2006). We showed that M. rotundata female body size, which is highly correlated to larval nutrition, can affect nesting success. However, the effect is dependent on environmental conditions and may also be mediated by other intrinsic differences in addition to body size.
In facultatively bivoltine species like M. rotundata, diapausers and nondiapausers nest at different points in the season (although the generations often partially overlap). Selection may favor different suites of adaptations in each group to optimize fitness under the environmental conditions and internal states in which each group emerges (Friberg and Wiklund, 2007, Taborsky et al. 2008). A study of another leafcutting bee, Megachile apicalis, found that diapausers and nondiapausers differ in their maternal investment strategies; diapausers provided offspring with smaller provisions than nondiapausers (Kim and Thorp 2001). Our results are consistent with these findings and suggest that M. rotundata diapausers and nondiapausers represent discrete adult variants with different reproductive strategies and capabilities.
The expression of alternative reproductive behaviors under some environmental conditions suggests that the potential exists for selection to act independently on diapauser and nondiapauser phenotypes. This finding supports the possibility that diapause plasticity could facilitate evolutionary change in bees. A developmental mechanism that translates differences in larval nutrition into alternative adult phenotypes with different consequences for reproduction is a plausible model for the general mechanism of caste evolution in many eusocial insects (Smith et al. 2008, Moczek 2010).
It is unknown whether the solitary lineages from which groups of social bees arose exhibited diapause; however, the capacity for diapause is extremely widespread in insects (Meuti and Denlinger 2013). Although all species within Megachilidae are solitary, this family is the sister clade to Apidae, a bee family that contains many eusocial species, including the well-studied honey bee, A. mellifera (Danforth et al. 2006, Schwarz et al. 2007). Our evidence that, like caste determination, diapause is also regulated by larval nutrition in M. rotundata, provides suggestive support for an evolutionary link between these two forms of plasticity in bees.
This is of particular relevance because of the “diapause groundplan hypothesis” (Hunt and Amdam 2005), which proposes that mechanisms involved in regulating facultative diapause in solitary species were coopted during eusocial evolution to regulate caste determination. According to this hypothesis, the typically well-nourished, reproductive queen caste arose from an ancestral developmental pathway that induces diapause, while the typically poorly nourished, sterile worker caste arose from a pathway that averts diapause (Hunt and Amdam 2005; Hunt et al. 2007, 2010). This hypothesis was developed for wasps, but in the tropics where most social bees evolved (Roubik 1989), facultative diapause in response to wet/dry seasonal changes is well documented for many insects, including bees and other hymenopterans (Nishida 1955, Claret and Carton 1980, Denlinger 1986). It is unknown whether diapause plasticity and caste determination share common regulatory pathways, but our findings evoke a fruitful line of study.
It is important to extend analyses of diapause to other solitary bee species that are closely related to social lineages, such as those within the tribes Allodapini and Halictini, which contain relatively recent transitions from solitary to eusocial living (Schwarz et al. 2007) or those within the tribes Centridini and Euglossini, which contain the most closely related solitary species to highly eusocial corbiculate bees (Cardinal et al. 2010). In addition, the genomes of several solitary bee species recently have been published, which should facilitate some aspects of such comparative analyses (Kocher et al. 2013, Kapheim et al. 2015).
A practical implication of our findings is that nutritional enhancement may reduce the frequency of nondiapausers, which would be a benefit for the alfalfa pollination industry. Understanding under what circumstances females alter the size of their provision mass will also help to devise methods to reduce the number of nondiapausing brood. Comparative analyses of phenotypic plasticity across bee species may help better manage M. rotundata for pollination purposes and shed new light on the evolution of eusociality in this group.
Acknowledgments
We thank Ellen Klomps, Ellen Klinger, Craig Huntzinger, Amy Spielmaker, Martin Welker, Tabitha Pitts, Adam Kriska, and Jacob Herman for help with field and lab work. Thanks to Bradley J. Cosentino for statistical consulting. We also appreciate the helpful comments given by members of the Robinson Lab for improving the manuscript and the supportive and directive critiques from two anonymous reviewers. This research was supported by the National Science Foundation (NSF) Grant DEB07-43154 and an NIH Pioneer Award (to G.E.R), and the University of Illinois Program in Ecology, Evolution, and Conservation Biology and an NSF Predoctoral Fellowship (to B.J.F).
References
- Aihie Sayer A., Cooper C. 2007. Early diet and growth: impact on ageing. Proc. Nutr. Soc. 61: 79–85. [DOI] [PubMed] [Google Scholar]
- Alcock J., Simmons L. W., Beveridge M. 2006. Does variation in female body size affect nesting success in Dawson’s burrowing bee, Amegilla dawsoni (Apidae: Anthophorini)? Ecol. Entomol. 31: 352–357. [Google Scholar]
- Barrett E.L.B., Hunt J., Moore A. J., Moore P. J. 2009. Separate and combined effects of nutrition during juvenile and sexual development on female life-history trajectories: The thrifty phenotype in a cockroach. Proc. Biol. Sci. 276: 3257–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount J. D., Metcalfe N. B., Arnold K. E., Surai P. F., Devevey G. L., Monaghan P. 2003. Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proc. Biol. Sci. 270: 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J. 2008. Production of undersized offspring in a solitary bee. Anim. Behav. 75: 809–816. [Google Scholar]
- Bosch J., Vicens N. 2002. Body size as an estimator of production costs in a solitary bee. Ecol. Entomol. 27: 129–137. [Google Scholar]
- Bosch J., Vicens N. 2006. Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behav. Ecol. Sociobiol. 60: 26–33. [Google Scholar]
- Cardinal S., Straka J., Danforth B. N. 2010. Comprehensive phylogeny of apid bees reveals the evolutionary origins and antiquity of cleptoparasitism. Proc. Natl. Acad. Sci. USA. 107: 16207–16211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret J., Carton Y. 1980. Diapause in a tropical species, Cothonaspis boulardi (Parasitic Hymenoptera). Oecologia 45: 32–34. [DOI] [PubMed] [Google Scholar]
- Crailsheim K., Brodschneider R., Aupinel P., Behrens D., Genersch E., Vollmann J., Riessberger-Gallé U. 2012. Standard methods for artificial rearing of Apis mellifera larvae In Dietemann V., Ellis J. D., Neumann P. (ed.), The COLOSS BEEBOOK, Volume I: standard methods for Apis mellifera research. J. Apic. Res. 52: http://dx.doi.org/10.3896/IBRA.1.52.1.05. [Google Scholar]
- Danforth B. N., Sipes S., Fang J., Brady S. G. 2006. The history of early bee diversification based on five genes plus morphology. Proc. Natl. Acad. Sci. USA. 103: 15118–15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger D. L. 1986. Dormancy in tropical insects. Annu. Rev. Entomol. 31: 239–264. [DOI] [PubMed] [Google Scholar]
- Emlen D. J. 1997. Diet alters male horn allometry in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Proc. Biol. Sci. 264: 567–574. [Google Scholar]
- Friberg M., Wiklund C. 2007. Generation-dependent female choice: behavioral polyphenism in a bivoltine butterfly. Behav. Ecol. 18: 758–763. [Google Scholar]
- Goettel M. S., Vandenberg J. D., Duke G. M., Schaalje G. B. 1993. Susceptibility to chalkbrood of alfalfa leafcutter bees, Megachile rotundata, reared on natural and artificial provisions. J. Invertebr. Pathol. 61: 58–61. [Google Scholar]
- Hahn D. A., Denlinger D. L. 2007. Meeting the energetic demands of insect diapause: nutrient storage and utilization. J. Insect Physiol. 53: 760–773. [DOI] [PubMed] [Google Scholar]
- Hahn D. A., Denlinger D. L. 2011. Energetics of insect diapause. Annu. Rev. Entomol. 56: 103–121. [DOI] [PubMed] [Google Scholar]
- Hobbs G. A., Richards K. W. 1976. Selection for a univoltine strain of Megachile (Euthricharea) pacifica (Hymenoptera: Megachilidae). Can. Entomol. 108: 165–167. [Google Scholar]
- Honěk A. 1993. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 66: 483–492. [Google Scholar]
- Hunt J. H., Amdam G. V. 2005. Bivoltinism as an antecedent to eusociality in the paper wasp genus Polistes. Science 308: 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. H., Kensinger B. J., Kossuth J. A., Henshaw M. T., Norberg K., Wolschin F., Amdam G. V. 2007. A diapause pathway underlies the gyne phenotype in Polistes wasps, revealing an evolutionary route to caste-containing insect societies. Proc. Natl. Acad. Sci. USA. 104: 14020–14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. H., Wolschin F., Henshaw M. T., Newman T. C., Toth A. L., Amdam G. V. 2010. Differential gene expression and protein abundance evince ontogenetic bias toward castes in a primitively eusocial wasp. PLoS ONE 5: e10674.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntzinger C., James R. R., Bosch J., Kemp W. P. 2008. Laboratory bioassays to evaluate fungicides for chalkbrood control in larvae of the alfalfa leafcutting bee, Megachile rotundata (Hymenoptera: Megachilidae). J. Econ. Entomol. 101: 660–667. [DOI] [PubMed] [Google Scholar]
- James R. R., Pitts-Singer T. L. 2013. Health status of alfalfa leafcutting bee larvae (Hymenoptera: Megachilidae) in United States alfalfa seed fields. Environ. Entomol. 42: 1166–1173. [DOI] [PubMed] [Google Scholar]
- Johnson M. D. 1990. Female size and fecundity in the small carpenter bee, Ceratina calcarata (Robertson) (Hymenoptera: Anthophoridae). J. Kans. Entomol. Soc. 63: 414–419. [Google Scholar]
- Kapheim K. M., Pan H., Li C., Salzberg S. L., Puiu D., Magoc T., Robertson H. M., Hudson M. E., Venkat A., et al. 2015. Genomic signatures of evolutionary transitions from solitary to group living. Science 348: 1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher S. D., Li C., Yang W., Tan H., Yi S. V., Yang X., Hoekstra H. E., Zhang G., Pierce N. E., Yu D. W. 2013. The draft genome of a socially polymorphic halictid bee, Lasioglossum albipes. Genome Biol. 14: R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura M. 2011. Royalactin induces queen differentiation in honeybees. Nature 473: 478–483. [DOI] [PubMed] [Google Scholar]
- Kim J.-Y. 1997. Female size and fitness in the leaf-cutter bee Megachile apicalis. Ecol. Entomol. 22: 275–282. [Google Scholar]
- Kim J.-Y., Thorp R. W. 2001. Maternal investment and size-number trade-off in a bee, Megachile apicalis, in seasonal environments. Oecologia 126: 451–456. [DOI] [PubMed] [Google Scholar]
- Kivelä S. M., Välimäki P., Gotthard K. 2013. Seasonality maintains alternative life-history phenotypes. Evolution 67: 3145–3160. [DOI] [PubMed] [Google Scholar]
- Klostermeyer E. C., Mech S. J. Jr, Rasmussen W. B. 1973. Sex and weight of Megachile rotundata (Hymenoptera: Megachilidae) progeny associated with provision weights. J. Kans. Entomol. Soc. 46: 536–548. [Google Scholar]
- Koyama T., Mendes C. C., Mirth C. K. 2013. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front. Physiol. 4: 263.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledon-Rettig C. C., Pfennig D. W., Nascone-Yoder N. 2008. Ancestral variation and the potential for genetic accommodation in larval amphibians: implications for the evolution of novel feeding strategies. Evol. Dev. 10: 316–325. [DOI] [PubMed] [Google Scholar]
- Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14: 343–348. [DOI] [PubMed] [Google Scholar]
- May C. M., Doroszuk A., Zwaan B. J. 2015. The effect of developmental nutrition on life span and fecundity depends on the adult reproductive environment in Drosophila melanogaster. Ecol. Evol. 5: 1156–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuti M. E., Denlinger D. L. 2013. Evolutionary links between circadian clocks and photoperiodic diapause in insects. Integr. Comp. Biol. 53: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener C. D. 1974. The social behavior of bees. Belknap Press, Cambridge, MA. [Google Scholar]
- Moczek A. P. 2005. The evolution and development of novel traits, or how beetles got their horns. BioScience 55: 937–951. [Google Scholar]
- Moczek A. P. 2010. Phenotypic plasticity and diversity in insects. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 365: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczek A. P., Sultan S., Foster S., Ledón-Rettig C., Dworkin I., Nijhout H. F., Abouheif E., Pfennig D. W. 2011. The role of developmental plasticity in evolutionary innovation. Proc. Biol. Sci. 278: 2705–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C. B., Williams I. S., Hardie J. 2001. The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol. Entomol. 26: 330–340. [Google Scholar]
- Munyiri F., Shintani Y., Ishikawa Y. 2004. Evidence for the presence of a threshold weight for entering diapause in the yellow-spotted longicorn beetle, Psacothea hilaris. J. Insect. Physiol. 50: 295–301. [DOI] [PubMed] [Google Scholar]
- (NASS) National Agricultural Statistics Service 2014. Census of agriculture summary and state data 2012. United States Department of Agriculture. U.S. Gov. Print. Office, Washington, DC. [Google Scholar]
- Nelson E. V., Roberts R. B., Stephen W. P. 1972. Rearing larvae of the leaf-cutter bee Megachile rotundata on artificial diets. J. Apic. Res. 11: 153–156. [Google Scholar]
- Nishida T. 1955. The phenomenon of arrested insect development in the Hawaiian Islands. Proc. Hawai. Entomol. Soc. 15: 575–582. [Google Scholar]
- Pitts-Singer T. L. 2008. Past and present management of alfalfa bees, pp 105–123. InPitts-Singer T. L., James R. R. (eds.), Bee pollination in agricultural ecosystems. Oxford University Press, New York, NY. [Google Scholar]
- Pitts-Singer T. L. 2013. Intended release and actual retention of alfalfa leafcutting bees (Hymenoptera: Megachilidae) for pollination in commercial alfalfa seed fields. J. Econ. Entomol. 106: 576–586. [DOI] [PubMed] [Google Scholar]
- Pitts-Singer T. L., Bosch J. 2010. Nest establishment, pollination efficiency, and reproductive success of Megachile rotundata (Hymenoptera: Megachilidae) in relation to resource availability in field enclosures. Environ. Entomol. 39: 149–158. [DOI] [PubMed] [Google Scholar]
- Pitts-Singer T. L., Barbour J. D. 2016. Effects of residual novaluron on reproduction in alfalfa leafcutting bees, Megachile rotundata. Pest Manag. Sci. 73: 153–159. [DOI] [PubMed] [Google Scholar]
- Pitts-Singer T. L., Cane J. H. 2011. The alfalfa leafcutting bee, Megachile rotundata: The world’s most intensively managed solitary bee. Ann. Rev. Entomol. 56: 221–237. [DOI] [PubMed] [Google Scholar]
- Pitts-Singer T. L., James R. R. 2008. Do weather conditions correlate with findings in failed, provision-filled nest cells of Megachile rotundata (Hymenoptera: Megachilidae) in western North America? J. Econ. Entomol. 101: 674–685. [DOI] [PubMed] [Google Scholar]
- Pitts-Singer T. L., James R. R. 2009. Prewinter management affects Megachile rotundata (Hymenoptera: Megachilidae) prepupal physiology and adult emergence and survival. J. Econ. Entomol. 102: 1407–1416. [DOI] [PubMed] [Google Scholar]
- Rajakumar R., San Mauro D., Dijkstra M. B., Huang M. H., Wheeler D. E., Hiou-Tim F., Khila A., Cournoyea M., Abouheif E. 2012. Ancestral developmental potential facilitates parallel evolution in ants. Science 335: 79–82. [DOI] [PubMed] [Google Scholar]
- Rank G. H., Rank F. P. 1989. Diapause intensity in a French univoltine and a Saskatchewan commercial strain of Megachile rotundata (Fab.). Can. Entomol. 121: 141–148. [Google Scholar]
- Rasband W. S. 1997-2016. ImageJ, U.S. National Institutes of Health, Bethesda, MD, (http://imagej.nih.gov/ij/)
- Rehan S. M., Richards M. H. 2010. The influence of maternal quality on brood sex allocation in the small carpenter bee, Ceratina calcarata. Ethology 116: 876–887. [Google Scholar]
- Richards K. W. 1984. Alfalfa leafcutter bee management in Western Canada. Agriculture Canada Publication 1495/E. Agriculture Canada, Ottawa. [Google Scholar]
- Roubik D. W. 1989. Ecology and natural history of tropical bees. Cambridge University Press, Cambridge, United Kingdom. [DOI] [PubMed] [Google Scholar]
- Schwarz M. P., Richards M., Danforth B. N. 2007. Changing paradigms in insect social evolution: Insights from halictine and allodapine bees. Annu. Rev. Entomol. 52: 127–150. [DOI] [PubMed] [Google Scholar]
- Shaw K., Scotti M., Foster S. A. 2007. Ancestral plasticity and the evolutionary diversification. Anim. Behav. 73: 415–422. [Google Scholar]
- Shorter J. R., Geisz M., Özsoy E., Magwire M. M., Carbone M. A., et al. 2015. The effects of royal jelly on fitness traits and gene expression in Drosophila melanogaster. PLoS ONE 10: e0134612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. J., Sword G. A., Lo N. 2011. Polyphenism in insects. Curr. Biol. 21R738–21R749. [DOI] [PubMed] [Google Scholar]
- Smith C. R., Toth A. L., Suarez A. V., Robinson G. E. 2008. Genetic and genomic analyses of the division of labour in insect societies. Nat. Rev. Genet. 9: 735–748. [DOI] [PubMed] [Google Scholar]
- Snell-Rood E. C., Moczek A. P. 2012. Insulin signaling as a mechanism underlying developmental plasticity: The role of FOXO in a nutritional polyphenism. PLoS ONE 7: e34857.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell-Rood E. C., Van Dyken J. D., Cruickshank T., Wade M. J., Moczek A. P. 2010. Toward a population genetic framework of developmental evolution: the costs, limits, and consequences of phenotypic plasticity. BioEssays 32: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spannhoff A., Kim Y. K., Raynal N. J.-M., Gharibyan V., Su M.-B., Zhou Y.-Y., Li J., Castellano S., Sbardella G., Issa J.-P. J., et al. 2011. Histone deacetylase inhibitor activity in royal jelly might facilitate caste switching in bees. EMBO Rep. 12: 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen W. P. 1981. The design and function of field domiciles and incubators for leafcutting bee management (Megachile rotundata (F.)). Oregon State Coll. Agric. Exp. Stn. Bull. 654: 1–13. [Google Scholar]
- Strohm E., Linsenmair K. E. 2000. Allocation of parental investment among individual offspring in the European beewolf Philantus triangulum F. (Hymenoptera: Sphecidae). Biol. J. Linnean. Soc. 69: 173–192. [Google Scholar]
- Sugiura N., Maeta Y. 1989. Parental investment and offspring sex ratio in a solitary mason bee, Osmia cornifrons (Radoszkowski) (Hymenoptera, Megachilidae). Jpn, J. Entomol. 57: 861–875. [Google Scholar]
- Taborsky M., Oliveira R. F., Brockmann H. J. 2008. The evolution of alternative reproductive tactics: concepts and questions, pp. 1–21. InOliveira R. F., Taborsky M., Brockmann H. J. (eds.), Alternative reproductive tactics: An integrative approach. Cambridge University Press, Cambridge, MA. [Google Scholar]
- Tepedino V. J., Frohlich D. R. 1984. Fratricide in a parsivoltine bee (Osmia texana). Anim. Behav. 32: 1265–1266. [Google Scholar]
- Tepedino V. J., Parker F. 1988. Alternation of sex-ratio in a partially bivoltine bee, Megachile rotundata (Hymenoptera, Megachilidae). Ann. Entomol. Soc. Am. 81: 467–476. [Google Scholar]
- Tepedino V. J., Torchio P. F. 1982. Phenotypic variability in nesting success among Osmia lignaria propinqua females in a glasshouse environment: (Hymenoptera: Megachilidae). Ecol. Entomol. 7: 453–462. [Google Scholar]
- Tigreros N. 2013. Linking nutrition and sexual selection across life stages in a model butterfly system. Funct. Ecol. 27: 145–154. [Google Scholar]
- Tomkins J. L., Moczek A. P. 2009. Patterns of threshold evolution in polyphenic insects under different developmental models. Evolution 63: 459–468. [DOI] [PubMed] [Google Scholar]
- Trostle G., Torchio P. F. 1994. Comparative nesting behavior and immature development of Megachile rotundata (Fabricius) and Megachile apicalis Spinola (Hymenoptera: Megachilidae). J. Kans. Entomol. Soc. 67: 53–72. [Google Scholar]
- West-Eberhard M. J. 1989. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20: 249–278. [Google Scholar]
- West-Eberhard M. J. 2005. Developmental plasticity and the origin of species differences. Proc. Natl. Acad. Sci. USA. 102: 6543–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield G. H., Richards K. W., Kveder T. M. 1987. Number of instars of larvae of the alfalfa leafcutter bee, Megachile rotundata (F.) (Hymenoptera: Megachildae). Can. Entomol. 119: 859–865. [Google Scholar]
- Wilson E. O. 1971. The insect societies. Belknap Press, Cambridge, MA. [Google Scholar]
- Xie J., De Clercq P., Pan C., Li H., Zhang H. Y., Pang H. 2015. Larval nutrition-induced plasticity affects reproduction and gene expression of the ladybeetle, Cryptolaemus montrouzieri. BMC Evol. Biol. 15: 276.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., James R. R. 2009. Genes related to immunity, as expressed in the alfalfa leafcutting bee, Megachile rotundata, during pathogen challenge. Inst. Mol. Biol. 18: 785–794. [DOI] [PubMed] [Google Scholar]
- Yocum G. D., Rinehart J. P., Kemp W. P. 2014. Cell position during larval development affects postdiapause development in Megachile rotundata (Hymenoptera: Megachilidae). Environ. Entomol. 43: 1045–1052. [DOI] [PubMed] [Google Scholar]