Abstract
Introduction:
A bacterial infection by Borrelia burgdorferi referred to as Lyme disease (LD) or borreliosis is transmitted mostly by a bite of the tick Ixodes scapularis in the USA and Ixodes ricinus in Europe. Various tests are used for the diagnosis of LD, but their results are often unreliable. We compiled a list of clinically visible and patient-reported symptoms that are associated with LD. Based on this list, we developed a novel scoring system.
Methodology:
Nutech functional Score (NFS), which is a 43 point positional (every symptom is subgraded and each alternative gets some points according to its position) and directional (moves in direction bad to good) scoring system that assesses the patient's condition.
Results:
The grades of the scoring system have been converted into numeric values for conducting probability based studies. Each symptom is graded from 1 to 5 that runs in direction BAD → GOOD.
Conclusion:
NFS is a unique tool that can be used universally to assess the condition of patients with LD.
Keywords: Borrelia burgdorferi-antibodies, human embryonic stem cells, Lyme disease, Nutech functional score
INTRODUCTION
“Lyme disease” (LD) or “borreliosis” is a bacterial infection caused by the spirochete Borrelia burgdorferi (Bb) and is transmitted to humans mostly by the bite of a tick (in Europe mostly by Ixodes ricinus). In Europe, approximately 5%–25% of the people are found to be positive for Bb-antibodies.[1,2] According to the Centers for Disease Control and Prevention (CDC) in the USA, the number of cases with LD has increased greatly, i.e., from 19,931 in 2006 to >300,000 in 2013.[3,4]
The course of LD mostly consists of three phases: early localized stage, early disseminated stage, and late disseminated stage.[5] Initially, after the tick bite or in the early localized stage (within the first 30 days), a person can develop an erythema migrans, a red and expanding rash, which is a typical and sure sign of the infection. Another early and sure but more rare sign of infection is a lymphocytoma, a bluish swelling of the earlobe, the nipple, or on the scrotum. Other general symptoms after an infection with Bb include marked fatigue, headache, fever, chills, swollen lymph nodes, and muscle and joint aches.[5] In the second stage, referred as early disseminated stage, the bacteria can affect the central and peripheral nervous system, and/or the heart and/or the musculoskeletal and/or the gastrointestinal and/or urogenital system.[5,6,7] The third or chronic stage of LD can last from months to years after the infection and shows equal manifestations in young and adults.[7]
An erythema migrans (the red rash on the skin) is the key indicator of borrelia infection, which is unfortunately only present in 50% of the cases. If left untreated, the infection leads to conditions such as peripheral neuropathy, encephalopathy with impaired cognitive abilities or to migrating mono-or polyarticular arthritis.[8] Many other signs may occur such as heart block, headache, myalgia, and facial paralysis or paralysis of extremities. Thus, several diagnostic tests are developed to confirm the infection with Bb, the reason for LD. Serological testing is done using enzyme-linked immunosorbent assay (ELISA) and Western blots. Tests to exclude other reasons than Bb for the observed central nervous impairments are the magnetic resonance imaging (MRI) and the single photon emission computed tomography (SPECT) scan, but the latter is rarely done.[9,10] Unfortunately, the results of these tests cannot be completely relied on to confirm the presence of LD. Therefore, the CDC recommends that the diagnosis of LD should be based on clinical signs and symptoms, the results of blood tests should only be used as supporting evidence as they may give false results.[11]
There are scoring systems for several other medical conditions such as spinal cord injury and cerebral palsy that assess the condition of patients based on the symptoms.[12,13] However, there is no such discrete system to assess the patients with LD. Nutech Mediworld, a center offering human embryonic stem cells for incurable and terminal conditions has developed a novel numeric approach, the Nutech functional score (NFS) to assess the condition of patients with LD based on clinical symptoms. NFS for LD is a 43 point positional (every symptom is subgraded and each alternative gets some points according to its position) and directional (moves in direction bad to good) scoring system that can be used to assess the diagnosis of LD and the effects of any given treatment.
METHODOLOGY
We have been treating patients with LD since the year 2000. The cases with LD admitted at our facility visited directly or were referred by other hospitals/institutions. They were either previously diagnosed with LD or underwent diagnosis at our facility. We evaluated each patient for their presented symptoms, common, or rare and recorded them in the diagnostic history.
Thus, over the years, a list of symptoms was prepared which included all the observed symptoms so far and was used thereafter to stage the patients with LD. This list of symptoms has been revised from time to time to maintain accuracy according to literature studies and our own experiences. Each symptom is evaluated on the basis of five ordinal grades running in BAD → GOOD direction. We used this scoring system to define patients with LD who were previously assessed with various other diagnostic tests including ELISA, Western blot, MRI, and SPECT scan.
RESULTS
We developed a 43-point scoring system that includes many possible presently known symptoms associated with LD. NFS grades for all the assessed symptoms are presented in Appendix 1 (9.2MB, tif) . NFS for LD has been organized into three groups depending on the kind of symptoms. Group 1 includes symptoms of the central nervous system with neurological and cognitive deficits; Group 2 includes symptoms associated with the muscular and skeletal system, and Group 3 includes all other symptoms such as those associated with the sensory system (vision, hearing), the cardiac and respiratory system, the urogenital and gastrointestinal system. Hormonal changes caused by LD have not been included as they are not clinically visible. The symptoms that are found not to be associated with the infection by Bb are scored as not afflicted in the ailment (NA). If a symptom is not present in the individual patient, then it is graded as not existing.
Nutech functional score for Lyme disease
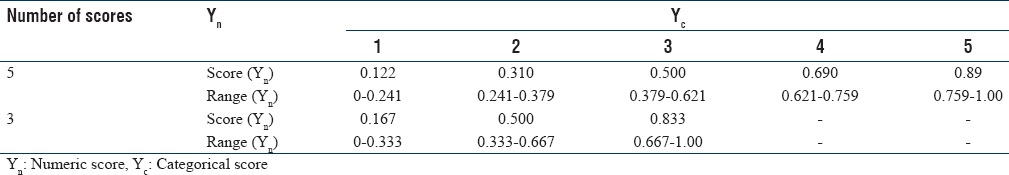
The five ordinal scores (1, 2, 3, 4, and 5) run in the direction of 1 → 5 i.e., BAD → GOOD. These five scores that lie in a range of (0.5, 5.5) are equidistant to each other and are continuous. The scores have been converted into numeric values to facilitate the conduct of probability based studies which require a range of (−1, 1) or (0, 1). This configuration can be used universally for one symptom. The polynomial smoothing and graphical methods have been used to derive an equation for converting categorical scores into numeric scores. The equation is as follow:
Yn = 0.096 × (Yc + 0.5)– 0.166
Where Yn = numeric score and Yc = categorical score.
Table 1 shows how five/three categorical scores (0.5–5.5) for symptoms that can be converted to five/three numeric scores in the range (0, 1).
Table 1.
Conversion table from categorical scores to numeric range for Nutech functional score
DISCUSSION
LD is reported to be a highly misdiagnosed condition. The first factor responsible for misdiagnosis is that the symptoms of LD are similar to a wide range of other medical conditions including chronic fatigue syndrome, fibromyalgia, Alzheimer's disease, Parkinson's disease, multiple sclerosis, and others. Co-infections caused by the tick transmitting Bb at the same time contribute to a misdiagnosis too. They make the clinical recognition of the underlying disease more difficult.[14] Interpretations of serology and other tests for the diagnosis of chronic LD do not give reliable validity. The modern tests include immune fluorescent staining for cell wall deficient forms of Bb, lymphocyte transformation tests and polymerase-chain reaction of different tissues and urine.[15,16] The CDC still recommends the use of a two-tier approach, i.e., ELISA and Western blot, even if it only has a sensitivity of 44% to 56%, if tested 4–6 weeks after infection.[17] At present, tests for LD in the USA are against only one strain of Bb, whereas there are more than 100 strains of Borrelia worldwide, of which 9 of them are known to be pathogenic to humans and cause LD-like illnesses. Normal laboratories are unable to isolate and identify these species in daily routine. Only specialized research laboratories can differentiate the different species. This could be one of the reasons why patients can get a false-negative laboratory result, even if they suffer from LD. The other crucial factor is that most of the diagnostic tests are indirect as they look for antibodies to Bb, but not for the organism itself. Because antibodies often persist even after the organism is no longer present, the tests will show positive results and lead to the therefore false diagnosis of a still active infection. On the other hand, sometimes no antibodies at all can be found, i.e., if antibiotics or immunosuppressant were given in the early course of the infection. Dark-field microscopy is another but direct method which is used in research and by some doctors treating LD to allow them a direct microscopic approach to the blood with the Bb spirochetes. However, until now, there is no 100% safe and valid method for confirming the presence of LD by tests. Therefore, the diagnosis of LD should predominantly be based on the clinical picture as it has been already suggested by the CDC in the USA. Physicians should try to get the history of the patient and his many and often fluctuating symptoms.[11]
The NFS scoring system developed by our facility seems to be a simple and appropriate method to confirm the diagnosis of patients with LD based solely on clinical symptoms. It has enlisted many of the possible clinical symptoms that are associated with LD. Although symptom-based questionnaires or checklists are available for LD, a numeric scoring system is not yet available. Renowned work in this field is done by Dr. Burrascano Jr., who has given a checklist of symptoms that help in identifying whether these symptoms are associated with LD or other co-infections caused by a tick bite. He has categorized the symptoms of LD as none, mild, moderate, and severe.[18] The Horowitz Lyme-MSIDS Questionnaire also enlists all those symptoms that we have included in the NFS; however, this questionnaire is not validated statistically.[19] Although the NFS system is inspired from their work; it is a numeric newly developed system that has been validated statistically.
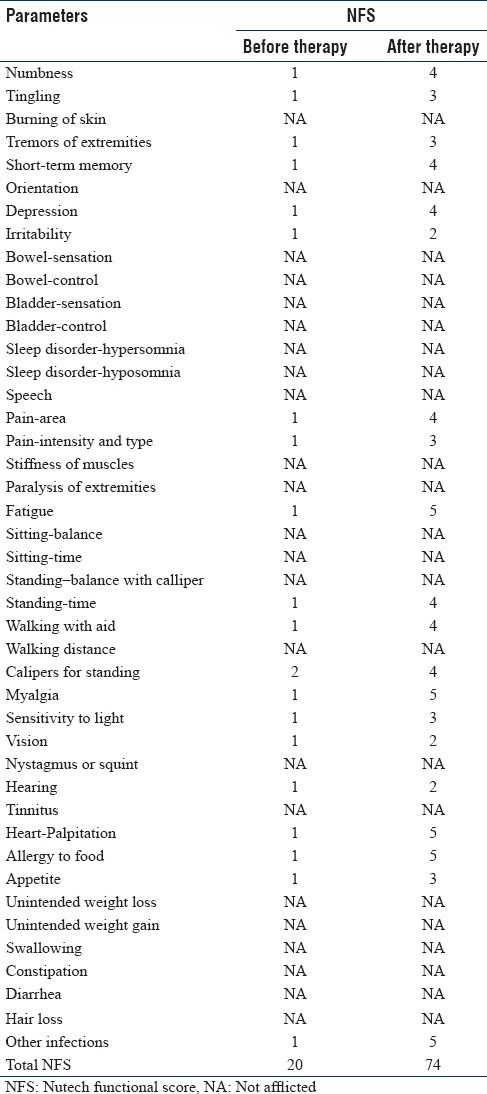
We illustrate an example on how the NFS-system is used to grade a patient with LD in a differentiated manner in Table 2. The total NFS score is calculated by counting the grades of all the symptoms. Suppose, this female patient scored 20 before treatment and the score increased to 74 after the therapy. This signifies a considerable improvement in her condition. As we can add or subtract grades in NFS, it can help in recognizing even the slightest improvements/deterioration in the condition of the patient. This number of grades can furthermore converted into numeric values to facilitate the conduct of probability based studies.
Table 2.
A hypothetical example showing Nutech functional score of a patient before and after therapy
CONCLUSION
There is a lack of a discrete and exhaustive clinical scoring system for patients with LD until now. The serological testing and other laboratory tests have low specificity and sensitivity. A numeric scoring system like NFS can be a novel tool that can help doctors worldwide to validate and confirm the diagnosis of LD for patients. This scoring system can also be used only in parts according to the clinical condition of the patient, i.e., only describing the changes of the individual symptoms that are present in the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge all the patients, doctors, and staff members of Nutech Mediworld. The authors also acknowledge Knowledge Isotopes Pvt. Ltd.
REFERENCES
- 1.Biesiada G, Czepiel J, Leśniak MR, Garlicki A, Mach T. Lyme disease: Review. Arch Med Sci. 2012;8:978–82. doi: 10.5114/aoms.2012.30948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derdáková M, Lencáková D. Association of genetic variability within the Borrelia burgdorferi sensu lato with the ecology, epidemiology of Lyme borreliosis in Europe. Ann Agric Environ Med. 2005;12:165–72. [PubMed] [Google Scholar]
- 3.Seibel MM, Smith DM, Levesque L, Borten M, Taymor ML. The temporal relationship between the luteinizing hormone surge and human oocyte maturation. Am J Obstet Gynecol. 1982;142:568–72. doi: 10.1016/0002-9378(82)90763-3. [DOI] [PubMed] [Google Scholar]
- 4.Stanke JJ, Fischer AJ. Embryonic retinal cells and support to mature retinal neurons. Invest Ophthalmol Vis Sci. 2010;51:2208–18. doi: 10.1167/iovs.09-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 6.Manzoor K, Aftab W, Choksi S, Khan IA. Lyme carditis: Sequential electrocardiographic changes in response to antibiotic therapy. Int J Cardiol. 2009;137:167–71. doi: 10.1016/j.ijcard.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Girschick HJ, Morbach H, Tappe D. Treatment of lyme borreliosis. Arthritis Res Ther. 2009;11:258. doi: 10.1186/ar2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacon RM, Kugeler KJ, Mead PS. Centers for Disease Control and Prevention (CDC). Surveillance for lyme disease – United States, 1992-2006. MMWR Surveill Summ. 2008;57:1–9. [PubMed] [Google Scholar]
- 9.Seltzer EG, Shapiro ED. Misdiagnosis of lyme disease: When not to order serologic tests. Pediatr Infect Dis J. 1996;15:762–3. doi: 10.1097/00006454-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 11.Spalton DJ. Posterior capsular opacification after cataract surgery. Eye. 1999;13:489–92. doi: 10.1038/eye.1999.127. [DOI] [PubMed] [Google Scholar]
- 12.Waring WP, 3rd, Biering-Sorensen F, Burns S, Donovan W, Graves D, Jha A, et al. _2009 review and revisions of the international standards for the neurological classification of spinal cord injury. J Spinal Cord Med. 2010;33:346–52. doi: 10.1080/10790268.2010.11689712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 14.Kozeis N. Brain visual impairment in childhood: Mini review. Hippokratia. 2010;14:249–51. [PMC free article] [PubMed] [Google Scholar]
- 15.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of lyme disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–1. [PubMed] [Google Scholar]
- 17.Stricker RB, Johnson L. Lyme disease: The next decade. Infect Drug Resist. 2011;4:1–9. doi: 10.2147/IDR.S15653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph J, Burrascano Advanced Topics in Lyme Disease. Diagnostic Hints and Treatments Guidelines for Lyme and Other Tick Borne Illnesses. 2008. [Last accessed on 2015 Aug 26]. Available from: http://www.lymenet.org/Burrguide200810.pdf .
- 19.Mohand-Said S, Hicks D, Simonutti M, Tran-Minh D, Deudon-Combe A, Dreyfus H, et al. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 1997;29:290–7. doi: 10.1159/000268027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nutech functional score for Lyme disease


