Abstract
STUDY QUESTION
Are preconception phthalate and phthalate replacements associated with sperm differentially methylated regions (DMRs) among men undergoing IVF?
SUMMARY ANSWER
Ten phthalate metabolites were associated with 131 sperm DMRs that were enriched in genes related to growth and development, cell movement and cytoskeleton structure.
WHAT IS KNOWN ALREADY
Several phthalate compounds and their metabolites are known endocrine disrupting compounds and are pervasive environmental contaminants. Rodent studies report that prenatal phthalate exposures induce sperm DMRs, but the influence of preconception phthalate exposure on sperm DNA methylation in humans is unknown.
STUDY DESIGN, SIZE, DURATION
An exploratory cross-sectional study with 48 male participants from the Sperm Environmental Epigenetics and Development Study (SEEDS).
PARTICIPANTS/MATERIALS, SETTING, METHODS
The first 48 couples provided a spot urine sample on the same day as semen sample procurement. Sperm DNA methylation was assessed with the HumanMethylation 450 K array. Seventeen urinary phthalate and 1,2-Cyclohexane dicarboxylic acid diisononyl ester (DINCH) metabolite concentrations were measured from spot urine samples. The A-clust algorithm was employed to identify co-regulated regions. DMRs associated with urinary metabolite concentrations were identified via linear models, corrected for false discovery rate (FDR).
MAIN RESULTS AND ROLE OF CHANCE
Adjusting for age, BMI, and current smoking, 131 DMRs were associated with at least one urinary metabolite. Most sperm DMRs were associated with anti-androgenic metabolites, including mono(2-ethylhexyl) phthalate (MEHP, n = 83), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP, n = 16), mono-n-butyl phthalate (MBP, n = 22) and cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl (MCOCH, n = 7). The DMRs were enriched in lincRNAs as well as in regions near coding regions. Functional analyses of DMRs revealed enrichment of genes related to growth and development as well as cellular function and maintenance. Finally, 13% of sperm DMRs were inversely associated with high quality blastocyst-stage embryos after IVF.
LIMITATIONS, REASONS FOR CAUTION
Our modest sample size only included 48 males and additional larger studies are necessary to confirm our observed results. Non-differential misclassification of exposure is also a concern given the single spot urine collection.
WIDER IMPLICATIONS OF THE FINDINGS
To our knowledge, this is the first study to report that preconception urinary phthalate metabolite concentrations are associated with sperm DNA methylation in humans. These results suggest that paternal adult environmental conditions may influence epigenetic reprogramming during spermatogenesis, and in turn, influence early-life development.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by grant K22-ES023085 from the National Institute of Environmental Health Sciences. The authors declare no competing interests.
Keywords: phthalates, DNADNA methylation, sperm, epigenetics, embryo, endocrine disruptors, blastocyst, early life, preconception
Introduction
Human spermatogenesis, a 72 day process by which diploid spermatogonia progress to haploid spermatozoa, requires several epigenetic reprogramming events, which may provide a final opportunity for sperm to epigenetically respond to their current environment prior to fertilization (Wu et al., 2015a,b). Indeed, two intriguing rodent studies found that sperm DNA methylation can be influenced by adult exposures to pre-diabetic conditions (Wei et al., 2014) and fear conditioning (Dias and Ressler, 2014), which subsequently produced phenotypic changes in the offspring of affected fathers. In humans, adult sperm DNA methylation patterns were associated with body weight and was modifiable within individuals after gastric bypass surgery (Donkin et al., 2016). Similarly, sperm DNA methylation was responsive to high dose folic acid supplementation (5 mg/day) (Aarabi et al., 2015), but not to low dose supplementation (400 μg/day) or food fortification (Chan et al., 2017). With respect to environmental toxicants, adult exposures to particulate air pollution (Yauk et al., 2008), Chromium III chloride (Shiao et al., 2005) and methoxychlor (Stouder and Paoloni-Giacobino, 2011) altered sperm DNA methylation patterns in rodents. Together, these studies provide compelling data that the sperm epigenome can respond to environmental conditions experienced in adulthood.
Phthalates are a ubiquitous class of compounds found in many commercial products such as medical equipment, food packaging, and personal care products. Select phthalate metabolites have been associated with decreased sperm concentration and motility, increased sperm DNA damage, and increased sperm apoptosis (Duty et al., 2003a,b; Hauser et al., 2007; Pant et al., 2008; Wirth et al., 2008; Wang et al., 2015, 2016; You et al., 2015). In a cohort of the US general population, select male phthalate metabolites were associated with decreased fecundity as evidenced by a 20% increase in time to pregnancy (Buck Louis et al., 2014). Similarly, among couples undergoing fertility treatment, we have previously shown that male, and not female, phthalate metabolites were associated with diminished blastocyst quality (Wu et al., 2017), while others have shown decreased odds of implantation and live birth (Dodge et al., 2015). Such results suggest a sperm-derived effect because IVF protocols eliminate all other paternal inputs such as seminal plasma. However, the mechanism by which male preconception phthalates affect these early-life outcomes has not been fully resolved.
Several animal studies report that in utero phthalate exposure alters sperm DNA methylation. For example, maternal exposure to bis(2-ethylhexyl)phthalate (DEHP) during gestation altered sperm DNA methylation in subsequent generations of offspring (Manikkam et al., 2013; Iqbal et al., 2015; Prados et al., 2015), although it is unclear if the effects persist for more than one generation (Manikkam et al., 2013; Iqbal et al., 2015).
Given the compelling data from animal studies showing that the sperm epigenome is responsive to adult environmental conditions, we conducted a cross-sectional study to explore the relationship of preconception urinary phthalate and 1,2-cyclohexane dicarboxylic acid diisononyl ester (DINCH) metabolite concentrations with sperm DNA methylation in men undergoing fertility treatment.
Materials and Methods
Study population
Forty-eight couples were recruited from the Baystate Reproductive Medicine in Springfield, MA, as part of the Sperm Environmental Epigenetics and Development Study (SEEDS). Inclusion criteria were: male partners were 18–55 years old without vasectomy, female partners were ≤ 40 years old with expected delivery at Baystate Medical Center and fresh ejaculate sperm was used for IVF treatment. Relevant demographics (race, age, height, weight), lifestyle factors (current and past alcohol and cigarette use), medical history (diagnoses of infertility) data were collected by clinic personnel during the IVF cycle for both partners. Written consent from eligible males and females who were interested in participating was obtained by attending physicians. This study was approved by the institutional review boards at Baystate Medical Center and at the University of Massachusetts Amherst.
Urinary exposure biomarker measurements
A spot urine sample was collected from couples in a sterile polypropylene collection cup on the same day as semen sample procurement and oocyte retrieval. Urine samples were vortexed, divided into several aliquots, and stored at −80°C before being shipped overnight on dry ice to the National Center for Environmental Health of the Center for Disease Control (CDC), where the urinary biomarkers were quantified via published methods (Kato et al., 2005; Silva et al., 2013). Analytical standards, quality control (QC) materials (prepared from spiked pooled urine) and reagent blank samples were included in each batch along with study samples. The QC concentrations—averaged to obtain one measurement of high-concentration QC and one of low-concentration QC for each batch—were evaluated by using standard statistical probability rules (Caudill et al., 2008).
In total, seventeen urinary metabolites were quantified: mono(2-ethylhexyl) phthalate (MEHP); mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP); mono(2-ethyl-5-oxohexyl) phthalate (MEOHP); mono(2-ethyl-5-carboxypentyl) phthalate (MECPP); monocarboxy-isooctyl phthalate (MCOP); mono-isononyl phthalate (MNP); monobenzyl phthalate (MBzP); mono (3-carboxypropyl) phthalate (MCPP); monocarboxy-isononyl phthalate (MCNP); mono-n-butyl phthalate (MBP); mono-3-hydroxybutyl phthalate (MHBP); mono-isobutyl phthalate (MiBP); mono-hydroxyisobutyl phthalate (MHiBP); monoethyl phthalate (MEP); monomethyl phthalate (MMP); cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester (MCOCH); and cyclohexane-1,2-dicarboxylic acid-mono(hydroxy-isononyl) ester (MHINCH). The limits of detection (LODs) ranged from 0.2 to 0.6 ng/mL, depending on the metabolite. Specific gravity (SG) was measured at room temperature using a digital handheld refractometer (Atago Co., Ltd., Tokyo, Japan).
Sperm collection and DNA isolation
Semen samples were collected in a sterile plastic specimen cup after a recommended 2–3 day abstinence period, per standard IVF protocol. Motile sperm cells were isolated using a two-step gradient fractionation and DNA was isolated using our previously published protocol (Wu et al., 2015a,b).
450 K beadchip analysis
Genomic sperm DNA (400 ng) was bisfulfite converted and employed on the 450 K Infinium Methylation Beadchip Array (Illumina) at Wayne State University's genomic core. The 450 K array provides genome-wide coverage of 485 577 methylation sites. Samples were randomized within and across beadchip to minimize any potential batch effects.
450 K data analyses
The minfi package in R was used to correct for technical variation in background signals (Triche et al., 2013), to remove probes below the background fluorescence level, and to adjust for differences in Type I and Type II probes (Niu et al., 2016). The ComBat function in the sva package (Leek et al., 2012) was used to correct for batch effects. Cross-hybridizing probes and sex chromosome probes were also removed using the DMRcate package (Peters et al., 2015). The raw and processed data can be found on the GEO Accession Viewer (Accession Number GSE102970).
Statistical analyses
We used the A-clustering algorithm (Sofer et al., 2013) to identify co-regulated regions by generating clusters of ≥2 CpG sites ≤1000 base pairs apart. These CpG clusters formed the unit of our analyses. To balance both validity and interpretability, we conducted statistical analyses using both M-values and β-values. First, to identify differentially methylated regions (DMRs) associated with urinary exposure concentrations, we used M-values due to their better adherence to homoscedasticity in linear models (Du et al., 2010). Next, we used β-values, which are the ratio of the methylated probe intensity to the overall intensity (sum of methylated and unmethylated probe intensities), to generate CpG methylation values between 0% and 100% to facilitate the biological interpretation of our results.
General estimating equations (GEE) were used to identify DMRs from CpG clusters associated with paternal exposure concentrations. For each model, we specified Gaussian distribution and an exchangeable correlation structure. The 15 phthalate metabolites were fitted as continuous variables (after SG-correction and log-transformation), while the two DINCH metabolites were fitted as dichotomous variables (above or below the LOD) due to their low detection rates. To account for multiple comparisons, we used the Benjamini–Hochberg method, which corrects the P-values generated from linear GEE models using the total number of comparisons and the rank order of the P-values (Benjamini and Hochberg, 1995). Statistical significance was set at a false discovery rate (FDR) q-value < 0.05.
Inclusion of covariates in multivariable models was based on biological plausibility and statistical significance in bivariate models (P < 0.1). Covariates considered for inclusion were age, BMI, race (white versus non-white), alcohol use, cigarette smoking and season of biological collections. Bivariate analyses showed that age, BMI and current smoking status were associated with ≥1 metabolite and ≥1 DMR and were included in all models. In contrast, race and seasonality did not fulfill this criteria and were not included in the models. Alcohol was not included in the model as 92% of those who had available data reported current alcohol use (data not shown).
Analysis was performed with R (v3.3.0, R Foundation for Statistical Computing, Vienna, Austria) using ‘aclust’ package (v2.0.1) the ‘gee’ package (v4.13.19).
Bioinformatics analyses
Prior to gene set enrichment analysis (GSEA) and Ingenuity Pathway Analysis (IPA), each cluster was assigned the closest gene using GRCh37 assembly data from ENSEMBL via the ChIPpeakAnno R package (version 3.6.5). Similarly, we determined genic (exons, introns, promoters, enhancers, intergenic regions), CpG (island, shelves, shores), functional (protein coding, pseudogene, lincRNA) and protamine (protamine versus nucleosome) features of clusters via annotations from Ensembl. Binding site locations for transcription factors EZH2 and CTCF were retrieved from ENCODE. Fisher's exact test was used to test for significant enrichment or depletion of each feature in the DMRs versus the entire set of clusters.
We used IPA (www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis/) and GSEA (www.broadinstitute.org/gsea) (Subramanian et al., 2005) to determine whether the observed exposure-associated DMR was affiliated with sets of genes with similar biological function, chromosomal location or regulation. For IPA, we restricted results to include only networks with score ≥20 or functional groups with P < 0.05 and an available activation Z-score. For GSEA, we set the cut-off to be normalized enrichment score ≥1.8 or ≤−1.8 and q-value <0.20. IPA and GSEA analyses were restricted to clusters within 1500 bp of a gene.
Sequenom validation
To validate the results from the 450 K array, eight CpG sites from five clusters were assayed on the Sequenom MassARRAY system (van den Boom and Ehrich, 2009). The sites were randomly selected from all clusters.
Embryo quality assessment
A full description of embryo quality assessment methods can be found elsewhere (Wu et al., 2017). In brief, blastocysts were graded by trained embryologists at the IVF clinic 5 days post-fertilization based on standard morphological characteristics. For analysis, blastocysts were classified as high versus low quality embryos.
Results
Table I presents the demographic and semen parameter data. The majority of the men were non-Hispanic white, over the age of 30 years, and overweight. Four of the 48 participants were current smokers. Although all men were seeking fertility treatment with their female partners, only 12 (25%) were diagnosed by attending physicians with male factor infertility based on World Health Organization (WHO) semen reference levels (Cooper et al., 2010). Of the remaining 36 men, 20 sought fertility treatment due to female factor infertility and 16 couples were diagnosed with unexplained infertility. In our study, 42.2% and 18.2% of participants had values below the WHO semen reference levels for percent morphologically normal sperm and semen volume, respectively. In contrast, less than 10% of our population were below the WHO semen reference levels for percent motile sperm, total sperm count and sperm concentration.
Table I.
Demographics and semen parameters of 48 SEEDS participants.
| n | % | |
|---|---|---|
| Age (years) | ||
| <30 | 10 | 21% |
| 30–40 | 25 | 52% |
| 40+ | 13 | 27% |
| BMI (kg/m2) | ||
| <25 | 10 | 21% |
| 25–30 | 19 | 40% |
| 30+ | 18 | 38% |
| Missing | 1 | 2% |
| Current smoking | ||
| Yes | 4 | 8% |
| No | 44 | 92% |
| Diagnosed infertility | ||
| Male factor | 6 | 13% |
| Female factor | 20 | 42% |
| Both | 6 | 13% |
| Unexplained | 16 | 33% |
| Race | ||
| White (non-Hispanic) | 37 | 77% |
| All others | 4 | 8% |
| Missing | 7 | 15% |
| Median | % < WHO reference | |
| Normal morphology (%) | 5.5 | 42.2 |
| % Motile | 59.0 | 9.1 |
| Sperm count (106) | 90.8 | 4.6 |
| Semen volume (mL) | 3.1 | 18.2 |
| Sperm concentration (106/mL) | 47.8 | 9.1 |
SEEDS, Sperm Environmental Epigenetics and Development Study; WHO, World Health Organization.
The distributions of SG-corrected urinary phthalate and DINCH metabolite concentrations for male partners are summarized in Table II. Fourteen of the 15 measured urinary phthalate metabolite concentrations (MEHP, MEHHP, MEOHP, MECPP, MBP, MHBP, MBzP, MiBP, MHiBP, MEP, MCPP, MCNP, MCOP and MMP) had detection frequencies above 75%, while urinary MNP concentrations were detected in 67% of the samples. Concentrations of MHINCH and MCOCH, metabolites of the phthalate-alternative DINCH, were only detected in 50% and 17% of the samples, respectively.
Table II.
Distribution of specific gravity-adjusted urinary phthalate metabolite concentrations (ng/mL) among SEEDS participants (N = 48).
| Parent metabolite | Metabolite | Detection rate | Geometric mean | Percentiles | ||
|---|---|---|---|---|---|---|
| 25th | 50th | 75th | ||||
| HMW | ||||||
| DEHP | MEHP | 77% | 1.15 | 0.72 | 1.14 | 1.86 |
| MEOHP | 100% | 4.3 | 3.23 | 4.41 | 5.62 | |
| MEHHP | 100% | 6.53 | 4.52 | 6.84 | 9.27 | |
| MECPP | 100% | 9.51 | 7.12 | 9.87 | 13.83 | |
| DiNP | MCOP | 100% | 26.39 | 11.17 | 22.21 | 51.53 |
| MNP | 67% | 0.99 | 0.44 | 0.75 | 1.74 | |
| BBzP | MBzP | 100% | 4.06 | 2.15 | 3.94 | 8.69 |
| DOP | MCPP | 100% | 2.38 | 1.22 | 1.98 | 4.09 |
| DiDP | MCNP | 100% | 3.1 | 1.94 | 2.83 | 4.42 |
| LMW | ||||||
| DBP | MBP | 98% | 7.42 | 6.04 | 7.99 | 11.76 |
| MHBP | 77% | 0.65 | 0.46 | 0.64 | 1.00 | |
| DiBP | MHiBP | 98% | 2.09 | 1.58 | 2.01 | 2.79 |
| MiBP | 100% | 6.69 | 4.53 | 7.11 | 10.98 | |
| DEP | MEP | 100% | 26.72 | 9.84 | 19.28 | 54.46 |
| DMP | MMP | 96% | 2.28 | 1.43 | 2.07 | 3.80 |
| Phthalate alternatives | ||||||
| DINCH | MHiNCH | 50% | 0.48 | 0.30 | 0.39 | 0.65 |
| MCoCH | 17% | 0.61 | 0.34 | 0.53 | 0.89 | |
HMW, high molecular weight phthalates; LMW, low molecular weight phthalates Bis(2-ethylhexyl) phthalate (DEHP); diisononyl phthalate (DiNP); benzyl butyl phthalate (BBzP); dioctyl phthalate (DOP); diisodecyl phthalate (DiDP); dibutyl phthalate (DBP); diisobutyl phthalate (DiBP); diethyl phthalate (DEP); dimethyl phthalate (DMP); 1,2-cyclohexane dicarboxylic acid diisononyl ester (DINCH); mono(2-ethylhexyl) phthalate (MEHP); mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP); mono(2-ethyl-5-oxohexyl) phthalate (MEOHP); mono(2-ethyl-5-carboxypentyl) phthalate (MECPP); monocarboxy-isooctyl phthalate (MCOP); mono-isononyl phthalate (MNP); monobenzyl phthalate (MBzP); mono (3-carboxypropyl) phthalate (MCPP); monocarboxy-isononyl phthalate (MCNP); mono-n-butyl phthalate (MBP); mono-3-hydroxybutyl phthalate (MHBP); mono-isobutyl phthalate (MiBP); mono-hydroxyisobutyl phthalate (MHiBP); monoethyl phthalate (MEP); monomethyl phthalate (MMP); cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester (MCOCH); and cyclohexane-1,2-dicarboxylic acid-mono(hydroxy-isononyl) ester (MHINCH).
DMR identification
Of the original 485 577 interrogated CpGs, 74 193 were removed, leaving 411 384 available for analysis. The A-clust algorithm identified 6479 clusters spanning 22 420 CpG sites, with a range of 2–46 sites per cluster over a length of 3–4456 bp. Adjusted for age, BMI and current smoking status, we observed that urinary phthalate metabolite concentrations were associated with 138 sperm DMRs (q < 0.05), comprising 131 unique DMRs (Table III). Seven overlapping DMRs were identified between individual metabolites (Supplementary Table S1), all of which had identical direction of effects across metabolites.
Table III.
Number of DMRs associated with individual phthalate metabolites.
| Parent metabolite | Metabolite | DMRs (q < 0.05) | Shared DMRs |
| HMW |  |
||
| DEHP | MEHP | 83 | |
| MEOHP | 16 | ||
| MEHHP | 0 | ||
| MECPP | 1 | ||
| DiNP | MCOP | 0 | |
| MNP | 0 | ||
| BBzP | MBzP | 2 | |
| DOP | MCPP | 0 | |
| DiDP | MCNP | 2 | |
| LMW | |||
| DBP | MBP | 22 | |
| MHBP | 2 | ||
| DiBP | MHiBP | 2 | |
| MiBP | 0 | ||
| DEP | MEP | 0 | |
| DMP | MMP | 1 | |
| Phthalate alternatives | |||
| DINCH | MHiNCH | 0 | |
| MCoCH | 7 |
DMR, differentially methylated regions.
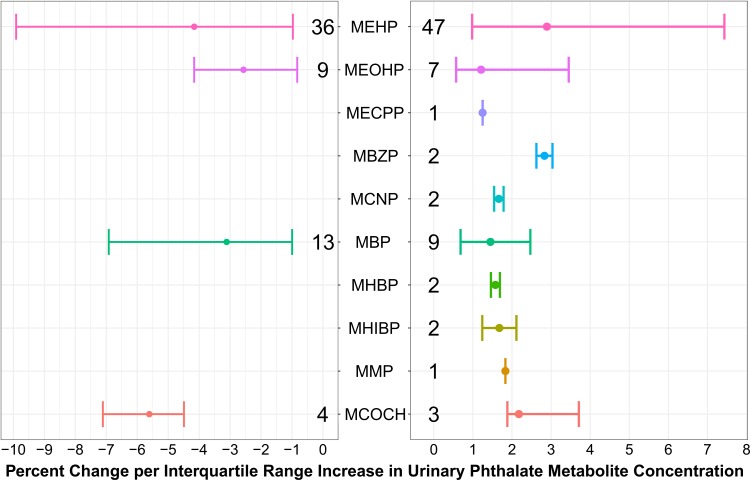
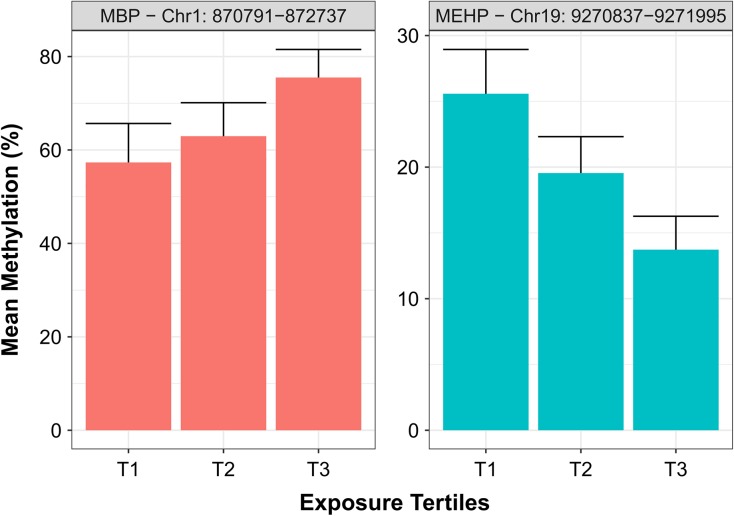
To aid in the interpretations of the magnitude of associations, we also report effect estimates based on models built from beta-values, which directly correspond to percent methylation of DMRs. Figure 1 shows the minimum, median, and maximum percent difference associated with one interquartile range (IQR) increase in urinary phthalate metabolite concentrations for all 131 DMRs. For example, for DMRs (q < 0.05) positively associated with MEHP concentrations, the median beta-value difference per IQR was 2.9% and ranged from 1% to 7.4%. Results of the GEE models for all DMRs, including annotations, are included in Supplementary Table S2. Furthermore, Fig. 2 provides a graphical representation of the mean methylation of two significant sperm DMRs by tertiles of urinary phthalate metabolite concentrations.
Figure 1.
Associations of urinary phthalate and phthalate-alternative metabolite concentrations with sperm differentially methylated regions. Sperm DNA methylation clusters (n = 6479) were generated by A-clust and modeled with phthalate metabolite concentrations as continuous variables using general estimation equation models adjusting for age, BMI, and current smoking and were corrected for false discovery rate (FDR). The dots represent the median percent difference in sperm DNA methylation associated with interquartile range (IQR) increase in phthalate metabolite concentrations, except for MCOCH, where the analysis was binary (those with detectable levels versus those below limits of detection). The error bars represent the minimum and maximum percent methylation change per IQR, and the numbers above/below metabolites represent the number of differentially methylated regions (DMRs) in each group. MEHP (mono(2-ethylhexyl) phthalate); MEOHP (mono(2-ethyl-5-oxohexyl) phthalate); MECPP (mono(2-ethyl-5-carboxypentyl) phthalate); MBzP (monobenzyl phthalate); MCNP (monocarboxy-isononyl phthalate); MBP (mono-n-butyl phthalate); MHBP (mono-3-hydroxybutyl phthalate); MHIBP (mono-hydroxyisobutyl phthalate); MMP (monomethyl phthalate); MCOCH (cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester).
Figure 2.
Mean percentage methylation of two DMRs by tertiles of urinary MBP and MEHP concentrations. Using GRCh37 annotations, the region Chr1:870791–872737 is located within the gene body of SAMD11 while the region Chr19:9270837–9271995 is located within the promoter region of ZNF317 gene. Error bars are SDs.
To validate our DMR findings from the 450k array, we chose eight CpGs from five randomly selected clusters. Seven of the eight CpGs showed a high concordance of methylation between the two platforms (Supplementary Figure S1, Supplementary Table S3). Moreover, GEE models using MassARRAY data provided similar estimated effects compared to those statistical models using 450 K data (data not shown).
Imprinted genes
To further validate our results, we examined the potential for somatic cell contamination by analyzing methylation levels (e.g. beta-values) of 203 probes across 18 imprinted loci identified previously (Nazor et al., 2012). Our analyses showed that all maternal and paternal imprinted regions were <10% or >85%, respectively, indicating negligible somatic cell contamination in the sperm samples.
Given that imprinted genes are known to escape the reprogramming event in pre-implantation embryos, we examined them independently from the main statistical analysis described above. Using the average M-values across the 18 imprinted loci, we found that MCNP was associated with increased methylation at eight (PLAGL1/HYMAI, KCNQ1/KCNQ1OT1, PEG3/ZIM2, MESTIT1/MEST, GNAS-AS1/GNAS, NAP1L5, PEG10/SGCE, L3MBTL) of the 14 maternally imprinted loci and with decreased methylation at one (H19) of the four paternally imprinted loci, adjusting for age, BMI, current smoking status and multiple comparisons (Supplementary Table S4). No statistically significant results were observed for any of the other urinary phthalate metabolites.
Enrichment analysis
To determine the functional significance of the significant DMRs, we conducted enrichment analyses (Table IV). To investigate if our sperm DMRs were enriched in DNA regions known to retain nucleosomes, we used previously published mnase-seq data (Donkin et al., 2016). Although there was a 7% increase in nucleosome retention in our significant DMRs, compared to all DNA methylation clusters, it was not statistically significant (P = 0.13). The proportion of lincRNA was twice as high in DMRs as compared to all clusters (8% versus 4%, P = 0.03) while no differences were observed for the proportion of pseudogenes (P = 0.60) and protein coding genes (P = 0.24). DMRs were enriched in CpG islands (P = 0.08), shelves (P = 0.01) and shores (P < 0.01) at the expense of open sea regions (P < 0.01). This was also reflected by the fact that DMRs were enriched in exons (P = 0.03) and introns (P = 0.02) whereas intergenic regions were depleted (P = 0.10). We observed no significant enrichment of DMRs for predicted binding sites for transcription factors CTCF (P = 0.74) and EZH2 (P = 0.25).
Table IV.
Enrichment analysis of DMRs compared to all clusters.
| DMRs (n = 131*) | All (n = 6479*) | ||||
|---|---|---|---|---|---|
| n | % | n | % | P-value** | |
| Chromatin features | |||||
| Nucleosome | 35 | 27% | 1356 | 21% | 0.1287 |
| Protamine | 96 | 73% | 5123 | 79% | |
| Gene features | |||||
| lincRNA | 11 | 8% | 277 | 4% | 0.0300 |
| Pseudogene | 5 | 4% | 196 | 3% | 0.6009 |
| Protein coding | 86 | 66% | 3904 | 60% | 0.2409 |
| CpG features | |||||
| Island | 39 | 30% | 1496 | 23% | 0.0760 |
| Shelves | 28 | 21% | 860 | 13% | 0.0131 |
| Shores | 75 | 57% | 2744 | 42% | 0.0009 |
| Open sea | 38 | 29% | 2901 | 45% | 0.0003 |
| Genic regions | |||||
| Exons | 49 | 37% | 1728 | 27% | 0.0408 |
| Introns | 47 | 36% | 1872 | 29% | 0.0218 |
| Promoters | 32 | 24% | 1404 | 22% | 0.4540 |
| Enhancers# | 6 | 5% | 192 | 3% | 0.2905 |
| Intergenic | 30 | 23% | 1933 | 30% | 0.1002 |
| Transcription factors | |||||
| CCCTC-binding factor (CTCF) | 29 | 22% | 1344 | 21% | 0.744 |
| Histone-lysine N-methyltransferase (EZH2) | 15 | 11% | 509 | 8% | 0.249 |
*Categories are not exclusive, the numbers may not all add to 100%.
**Calculated from Fisher's exact test.
#Taken from FANTOM5 project.
Pathway analysis
Network and functional analyses via IPA examined three sets of DMRs—(i) those associated with DEHP, (ii) DBP or (iii) all known or suspected anti-androgenic parent compounds (DEHP, BBzP, DiNP, DBP, DiBP and DINCH). For DEHP alone and anti-androgenic compounds, most of the DMRs were associated with one of three general pathway categories—‘cancer’, ‘cellular function and maintenance’, and ‘growth and development’ (Fig. 3, Supplementary Tables 5–8). For ‘cellular function and maintenance’ and ‘growth and development’, more pathways were associated with an increase in methylation compared to loss of methylation, as determined by the Activation Z-score (Fig. 3). In contrast, the number of DMRs within the ‘cancer’ pathway was more balanced with respect to gain or loss of methylation. Network analysis also revealed alterations in genes related to overall themes of ‘development’ and ‘cellular function’ (Supplemental Tables 5A and 5C). DBP-associated DMRs were not statistically related to specific diseases or functional groups, but were found to be related to genes involved in the cell cycle (Supplementary Table 9). Consistent with the results from IPA, GSEA showed that all four tested metabolites (MEHP, MEOHP, MBP, MCOCH) were associated with gene sets related to early development (Supplementary Table 10).
Figure 3.
Ingenuity pathway analysis of DMRs associated with urinary concentrations of DEHP metabolites and all anti-androgenic metabolites (MEHP, MEOHP, MBzP, MBP, MHBP, MHiBP, MCOCH). The general categories of disease and functional groups are shown by their calculated activation Z-score, which is an overall measure of loss or gain of methylation at genes associated with this functional category. The bars represent the mean activation Z-score in each category while the error bar represents the absolute maximum. The number in each bar represents the number of DMRs in each group. DEHP, bis(2-ethylhexyl)phthalate.
Blastocyst quality
We previously reported that paternal anti-androgenic phthalate metabolite concentrations (MEHP, MHBP, MBP, MMP and MCOCH) were associated with diminished blastocyst quality (Wu et al., 2017). Here, we find that three of these anti-androgenic metabolites (MEHP, MBP, MCOCH) are associated with the majority of sperm DMRs. Of the 57 DMRs inversely associated with urinary phthalate metabolite concentrations, two were positively associated with high blastocyst quality while one was negatively associated. Conversely, of the 74 DMRs positively associated with urinary phthalate metabolite concentrations, 16 were inversely associated with high blastocyst quality and none were positively associated (Supplementary Table 11). All GEE models were adjusted for age, BMI and current smoking. About 4 of these 19 DMRs associated with both urinary metabolite concentrations and embryo quality were located in nucleosomes and 17 were located on exonic, intronic or promoter regions.
Discussion
In our investigation of 48 males undergoing fertility treatment, 131 sperm DMRs were associated with at least one urinary phthalate and DINCH metabolite concentration. Functional analyses revealed that sperm DMRs were enriched in pathways related to development and general cell function and maintenance. In particular, urinary concentrations of MEHP, MEOHP, MBP and MCOCH were associated with the greatest number of sperm DMRs. Interestingly, the parent compounds of these metabolites, DEHP, DBP and DINCH all have known or suspected effects on androgens (Pan et al., 2006; Meeker et al., 2009; Boisvert et al., 2016). Our previous findings have shown that these same metabolites in the male, but not the female partner, were also associated with diminished blastocyst quality (Wu et al., 2017). These results suggest that phthalates may be associated with sperm DNA methylation in or near genes relevant to early embryogenesis, providing a pathway linking the observed inverse associations between anti-androgenic phthalates and blastocyst quality. More broadly, our results support the growing evidence that preconception paternal environmental health may contribute to both male reproductive potential and offspring development.
Functional enrichment analyses showed that many DMRs associated with both DEHP alone and all anti-androgenic compounds as a whole are within or near genes related to ‘growth and development’ and ‘cellular function and maintenance’. It is generally believed that proper sperm DNA methylation is important for embryogenesis and one recent study reported that DNA methylation patterns are predictive of embryo quality in an IVF setting (Aston et al., 2015). Alterations in methylation of genes associated with growth/development and cellular function/maintenance in sperm may have potential implications for embryo development. Aside from cell death and survival, most of the genes within the cellular function and maintenance group are related to cytoskeleton structure and cell migration. Cytoskeleton structure is known to play an important role during fertilization and pre-implantation embryo development (Schatten and Sun, 2014) while many genes related to cell migration are similarly important for early-life development. For example, DMRs associated with DEHP (and thus also anti-androgenic compounds as a whole) were found on or near genes C-C motif chemokine 11 (CCL11) (Chau et al., 2013) and Wnt Family Member 7b (Wnt7b) (Knofler and Pollheimer, 2013), known regulators of trophoblast development and migration. The potential for phthalate and DINCH metabolites associated DMRs to influence embryogenesis is further supported by the observation that lincRNAs were enriched in our DMRs compared to the background of all clusters. LincRNAs are a class of non-coding RNAs with diverse functions that include gene regulation (Ulitsky and Bartel, 2013) and roles in early development, including myogenesis, haematopoiesis, adipogenesis, and neurogenesis (Fatica and Bozzoni, 2014),
The observation that 19 of the 131 DMRs were associated with phthalates and DINCH as well as with poor blastocyst quality extend our previous findings that paternal MEHP, MHBP, MBP, MMP, and MCOCH were associated with diminished blastocyst quality (Wu et al., 2017) by suggesting that sperm DNA methylation is a pathway linking paternal exposures with embryo quality. These observed associations with blastocyst quality coincide with the timing of embryonic genome activation, which marks the earliest time in the developing embryo when the paternal genome and its methylation patterns may be relevant.
Despite the global epigenetic reprogramming that occurs shortly post-fertilization, imprinted sites and certain other regions may escape reprogramming (Monk, 2015). In our study, we observed that methylation at nine imprinted genes are associated with urinary concentrations of MCNP, implying that these imprinted DMRs may have the potential to escape the epigenetic reprogramming event and thus be inheritable. Furthermore, we observed that the 131 sperm DMRs associated with metabolites of phthalates and DINCH were enriched in exons and introns, regions previously reported to be preferentially maintained during the transient hypomethylation of pre-implantation embryos (Smith et al., 2014). It is unknown if our sperm DMRs at non-imprinted regions can escape, at least in part, reprogramming in the developing embryo. However, compelling animal data supports the notion that some sperm DMRs may be resistant to this reprogramming event (Dias and Ressler, 2014; Wei et al., 2014).
In regard to the interpretation of sperm DMRs, we need to consider that each sperm carries a haploid genome with a binary option (methylated or unmethylated) at each CpG site and that differences in percent methylation (beta-values) represent differences in the frequency of sperm with methylation at those specific CpG sites and/or regions. For example, a 5% increase in beta-values is interpreted as a 5% increase in the frequency of sperm containing methylation at that particular CpG site and/or region. Given that only a single sperm is needed for fertilization, even modest changes in frequency of methylation in motile sperm may be important for early-life development. Thus, while ≤10% methylation changes may not be biologically relevant in somatic cells such as leukocytes, such changes in frequency could be biologically significant in the ‘winner takes all’ scenario of fertilization.
We recognize that there are some limitations to our study. First, our modest sample size only included 48 males and additional larger studies are necessary to confirm our observed results. Second, as our population was recruited from the IVF clinic, our findings may not be generalizable to the broader population. Third, non-differential misclassification of exposure remains a concern given the single spot urine collection and the short half-life of phthalates. Though studies have reported temporal variations in urinary concentrations of phthalate metabolites (Hauser et al., 2004; Preau et al., 2010; Christensen et al., 2012; Frederiksen et al., 2013; Aylward et al., 2016), most have concluded that spot urine samples are comparable to the more intensive 24-h sample. Most importantly, a study using a population very similar to our study population showed that a single urine sample adequately represents exposure over 3 months, which spans over one spermatogenesis cycle (Hauser et al., 2004). The non-differential misclassification of exposure due to temporal variability likely biased our results toward the null and could have led to fewer DMRs being detected. Additionally, while we did collect information on lifestyle factors such as smoking and alcohol use, we do not have information on socioeconomic status and other related variables. Lastly, due to confidentiality considerations, we do not have information on refusal rates or characteristics of non-participants, and thus cannot rule out potential selection bias related to factors associated with both phthalates and sperm DNA methylation.
Despite these limitations, our study has notable strengths. First, our study utilized state-of-the-art exposure assessment methods for profiling urinary metabolite concentrations of phthalates and new emerging non-phthalate replacements. Second, we measured DNA methylation from the motile fraction of sperm, which represents those sperm with the highest fertilization potential and may better approximate relevant phthalate-related impacts on sperm DNA methylation in IVF and non-IVF populations. Third, the same cohort of sperm in our DNA methylation analyses was used for IVF; thus, our study design allowed us to connect sperm DMRs directly with embryo quality to facilitate our understanding of the influence of sperm DMRs on early-life development, which otherwise would not be possible among couples from the general population.
Conclusion
Our study is the first, to our knowledge, to examine the associations between preconception phthalates and sperm DNA methylation profiles in humans. Overall, we found that select preconception anti-androgenic phthalate metabolites are associated with 131 sperm DMRs, which are enriched in genes related to growth and development and basic cellular functions such as cell movement and cytoskeleton structure. This provides a critical step towards our understanding of the paternal preconception contributions to reproductive success and offspring development. Future studies are needed to replicate such findings and further clarify the role of phthalate-associated sperm DNA methylation on subsequent offspring health and development.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Acknowledgements
We gratefully acknowledge all the members of the SEEDS research team, specifically Suzanne Labrie and Ellen Tougias and the nurses and physicians in the Division of Reproductive Endocrinology and Infertility at Baystate Medical Center. We thank Xiaoyun Ye, Manori Silva, Ella Samandar, Jim Preau, Tao Jia and Antonia Calafat from the CDC for measuring urinary phthalate and phthalate replacement biomarkers. We would also like to thank all of the study participants for whom without this study would not be possible.
Authors’ roles
H.W. was responsible for the processing of the samples, analysis of the data, interpretation of the results, and the draft and revision of the manuscript. M.S.E., A.Su., and A.Sh. were responsible for analysis of the data. S.A.K. and B.W.W. advised on the analysis approach and the interpretation of the data, and revised the manuscript. H.D. was responsible for the recruitment of the participants, sample processing, and data collection. T.R. and C.K.S. advised on the design of the study and the interpretation of the data and revised the manuscript. J.R.P. was responsible for the design of the study, interpretation of the results, and the draft and revision of the manuscript.
Funding
This work was supported by grant K22-ES023085 from the National Institute of Environmental Health Sciences.
Conflict of interest
None declared.
References
- Aarabi M, San Gabriel MC, Chan D, Behan NA, Caron M, Pastinen T, Bourque G, MacFarlane AJ, Zini A, Trasler J. High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Hum Mol Genet 2015;24:6301–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston KI, Uren PJ, Jenkins TG, Horsager A, Cairns BR, Smith AD, Carrell DT. Aberrant sperm DNA methylation predicts male fertility status and embryo quality. Fertil Steril 2015;104:1388–1397 e1381-1385. [DOI] [PubMed] [Google Scholar]
- Aylward LL, Hays SM, Zidek A. Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: implications for exposure assessment and reverse dosimetry. J Expo Sci Environ Epidemiol 2016. http://www.nature.com/jes/journal/vaop/ncurrent/full/jes201654a.html?foxtrotcallback=true. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 1995;57:289–300. [Google Scholar]
- Boisvert A, Jones S, Issop L, Erythropel HC, Papadopoulos V, Culty M. In vitro functional screening as a means to identify new plasticizers devoid of reproductive toxicity. Environ Res 2016;150:496–512. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) study. Fertil Steril 2014;101:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med 2008;27:4094–4106. [DOI] [PubMed] [Google Scholar]
- Chan D, McGraw S, Klein K, Wallock LM, Konermann C, Plass C, Chan P, Robaire B, Jacob RA, Greenwood CM et al. Stability of the human sperm DNA methylome to folic acid fortification and short-term supplementation. Hum Reprod 2017;32:272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau SE, Murthi P, Wong MH, Whitley GS, Brennecke SP, Keogh RJ. Control of extravillous trophoblast function by the eotaxins CCL11, CCL24 and CCL26. Hum Reprod 2013;28:1497–1507. [DOI] [PubMed] [Google Scholar]
- Christensen KL, Lorber M, Koch HM, Kolossa-Gehring M, Morgan MK. Population variability of phthalate metabolites and bisphenol A concentrations in spot urine samples versus 24- or 48-h collections. J Expo Sci Environ Epidemiol 2012;22:632–640. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231–245. [DOI] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 2014;17:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge LE, Williams PL, Williams MA, Missmer SA, Souter I, Calafat AM, Hauser R, Team ES. Associations between paternal urinary phthalate metabolite concentrations and reproductive outcomes among couples seeking fertility treatment. Reprod Toxicol 2015;58:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkin I, Versteyhe S, Ingerslev LR, Qian K, Mechta M, Nordkap L, Mortensen B, Appel EV, Jorgensen N, Kristiansen VB et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab 2016;23:369–378. [DOI] [PubMed] [Google Scholar]
- Du P, Zhang X, Huang CC, Jafari N, Kibbe WA, Hou L, Lin SM. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics 2010;11:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani DC, Hauser R. Phthalate exposure and human semen parameters. Epidemiology 2003. a;14:269–277. [PubMed] [Google Scholar]
- Duty SM, Singh NP, Silva MJ, Barr DB, Brock JW, Ryan L, Herrick RF, Christiani DC, Hauser R. The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environ Health Perspect 2003. b;111:1164–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 2014;15:7–21. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Kranich SK, Jorgensen N, Taboureau O, Petersen JH, Andersson AM. Temporal variability in urinary phthalate metabolite excretion based on spot, morning, and 24-h urine samples: considerations for epidemiological studies. Environ Sci Technol 2013;47:958–967. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 2004;112:1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, Calafat AM. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod 2007;22:688–695. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Tran DA, Li AX, Warden C, Bai AY, Singh P, Wu X, Pfeifer GP, Szabo PE. Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming. Genome Biol 2015;16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of total phthalates in urine by isotope-dilution liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2005;814:355–360. [DOI] [PubMed] [Google Scholar]
- Knofler M, Pollheimer J. Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling. Front Genet 2013;4:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012;28:882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PloS one 2013;8:e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J Androl 2009;30:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk D. Germline-derived DNA methylation and early embryo epigenetic reprogramming: the selected survival of imprints. Int J Biochem Cell Biol 2015;67:128–138. [DOI] [PubMed] [Google Scholar]
- Nazor KL, Altun G, Lynch C, Tran H, Harness JV, Slavin I, Garitaonandia I, Muller FJ, Wang YC, Boscolo FS et al. Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell 2012;10:620–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L, Xu Z, Taylor JA. RCP: a novel probe design bias correction method for Illumina Methylation BeadChip. Bioinformatics 2016;32:2659–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect 2006;114:1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant N, Shukla M, Kumar Patel D, Shukla Y, Mathur N, Kumar Gupta Y, Saxena DK. Correlation of phthalate exposures with semen quality. Toxicol Appl Pharmacol 2008;231:112–116. [DOI] [PubMed] [Google Scholar]
- Peters TJ, Buckley MJ, Statham AL, Pidsley R, Samaras K, V Lord R, Clark SJ, Molloy PL. De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin 2015;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados J, Stenz L, Somm E, Stouder C, Dayer A, Paoloni-Giacobino A. Prenatal exposure to DEHP affects spermatogenesis and sperm DNA methylation in a strain-dependent manner. PLoS One 2015;10:e0132136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preau JL Jr, Wong LY, Silva MJ, Needham LL, Calafat AM. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ Health Perspect 2010;118:1748–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H, Sun QY. Posttranslationally modified tubulins and other cytoskeletal proteins: their role in gametogenesis, oocyte maturation, fertilization and Pre-implantation embryo development. Adv Exp Med Biol 2014;759:57–87. [DOI] [PubMed] [Google Scholar]
- Shiao YH, Crawford EB, Anderson LM, Patel P, Ko K. Allele-specific germ cell epimutation in the spacer promoter of the 45S ribosomal RNA gene after Cr(III) exposure. Toxicol Appl Pharmacol 2005;205:290–296. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Jia T, Samandar E, Preau JL Jr, Calafat AM. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000–2012). Environ Res 2013;126:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZD, Chan MM, Humm KC, Karnik R, Mekhoubad S, Regev A, Eggan K, Meissner A. DNA methylation dynamics of the human preimplantation embryo. Nature 2014;511:611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer T, Schifano ED, Hoppin JA, Hou L, Baccarelli AA. A-clustering: a novel method for the detection of co-regulated methylation regions, and regions associated with exposure. Bioinformatics 2013;29:2884–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouder C, Paoloni-Giacobino A. Specific transgenerational imprinting effects of the endocrine disruptor methoxychlor on male gametes. Reproduction 2011;141:207–216. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triche TJ Jr, Weisenberger DJ, Van Den Berg D, Laird PW, Siegmund KD. Low-level processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res 2013;41:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell 2013;154:26–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom D, Ehrich M. Mass spectrometric analysis of cytosine methylation by base-specific cleavage and primer extension methods. Methods Mol Biol 2009;507:207–227. [DOI] [PubMed] [Google Scholar]
- Wang YX, You L, Zeng Q, Sun Y, Huang YH, Wang C, Wang P, Cao WC, Yang P, Li YF et al. Phthalate exposure and human semen quality: results from an infertility clinic in China. Environ Res 2015;142:1–9. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zeng Q, Sun Y, You L, Wang P, Li M, Yang P, Li J, Huang Z, Wang C et al. Phthalate exposure in association with serum hormone levels, sperm DNA damage and spermatozoa apoptosis: a cross-sectional study in China. Environ Res 2016;150:557–565. [DOI] [PubMed] [Google Scholar]
- Wei Y, Yang CR, Wei YP, Zhao ZA, Hou Y, Schatten H, Sun QY. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc Natl Acad Sci U S A 2014;111:1873–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth JJ, Rossano MG, Potter R, Puscheck E, Daly DC, Paneth N, Krawetz SA, Protas BM, Diamond MP. A pilot study associating urinary concentrations of phthalate metabolites and semen quality. Syst Biol Reprod Med 2008;54:143–154. [DOI] [PubMed] [Google Scholar]
- Wu H, Ashcraft L, Whitcomb BW, Rahil T, Tougias E, Sites CK, Pilsner JR. Parental contributions to early embryo development: influences of urinary phthalate and phthalate alternatives among couples undergoing IVF treatment. Hum Reprod 2017;32:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, de Gannes MK, Luchetti G, Pilsner JR. Rapid method for the isolation of mammalian sperm DNA. BioTechniques 2015. a;58:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Hauser R, Krawetz SA, Pilsner JR. Environmental susceptibility of the sperm epigenome during windows of male germ cell development. Curr Environ Health Rep 2015. b;2:356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauk C, Polyzos A, Rowan-Carroll A, Somers CM, Godschalk RW, Van Schooten FJ, Berndt ML, Pogribny IP, Koturbash I, Williams A et al. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc Natl Acad Sci U S A 2008;105:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, Wang YX, Zeng Q, Li M, Huang YH, Hu Y, Cao WC, Liu AL, Lu WQ. Semen phthalate metabolites, spermatozoa apoptosis, and DNA damage: a cross-sectional study in China. Environ Sci Technol 2015;49:3805–3812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.